CHAPTER 46 Tumors of the Esophagus
Esophageal tumors can be divided into benign and malignant tumors based on their biological behavior and into epithelial and nonepithelial tumors based on histopathology (Table 46-1).
Table 46-1 Classification of Esophageal Tumors
| EPITHELIAL TUMORS | NONEPITHELIAL TUMORS |
|---|---|
| Malignant
Benign |
Malignant
Benign |
GIST, gastrointestinal stromal cell tumor.
CARCINOMA
EPIDEMIOLOGY
A vast majority of all esophageal cancers are malignant epithelial tumors (carcinomas). The two most common types of esophageal carcinomas are squamous cell cancer and adenocarcinoma (Figs. 46-1 and 46-2). Malignant tumors of the esophagus are one of the commonest types of cancer. More than half a million patients were newly diagnosed with esophageal cancer worldwide in 2007 alone. Globally the incidence of esophageal cancer is sixth and ninth among cancers in men and women, respectively, and is the fifth and ninth leading causes of cancer deaths.1 The American Cancer Society estimated that approximately 16,470 new esophageal cancer cases would be diagnosed in the United States in 2008 and that 14,280 patients would die from esophageal cancer.2 In the United States from 2001 to 2005, the median age at diagnosis for cancer of the esophagus was 69 years. Approximately 3% of all patients with esophageal cancer were diagnosed in individuals younger than age 45; 36% between 45 and 64; 29% between 65 and 74; approximately 30% older than age 75. In 2005 the age-adjusted incidence rate of esophageal cancer in the United States for all races and both sexes was 4.3 per 100,000 and varied from 2.9 in Hispanics to 4.9 in blacks. The age-adjusted incidence rates were higher in men than women for all races (overall, 7.5 versus 1.8 per 100,000 in men and women, respectively). The highest incidence rate in the United States was in black men. Based on data from 2003 to 2005, it is estimated that 0.5% of all men and women born today in the United States will be diagnosed with cancer of the esophagus at some time during their lifetime (lifetime risk, 1 in 198). The overall five-year relative survival rates of patients with esophageal cancer by race and sex, reported for the period from 1996 to 2004 from 17 geographic areas were 16.5% for white men, 17.6% for white women, 9.2% for black men, and 12.9% for black women.3
Squamous cell cancer is the commonest type of esophageal carcinoma worldwide. There are marked geographic variations in the incidence of different types of squamous cell esophageal cancer, with more than 80% of these cancers occurring in developing countries. The highest incidence of esophageal cancers, with incidence rates greater than 100 per 100,000, is in the “Asian esophageal cancer belt,” extending from northern Iran through the central Asian republics to north-central China. The intermediate risk regions, with incidence rates from 20 to 50 cases per 100,000, are in parts of east and southeast Africa (e.g., eastern Kenya, Zimbabwe, and the Transkei region of South Africa), in southeastern South America (southern Brazil, Uruguay, Paraguay, northern Argentina), and in certain parts of western Europe such as northern France and Switzerland. The rest of the world including the United States is considered a low-incidence area, with rates below 10 per 100,000 of the population.1 In high-incidence areas, there are unexplained shifts in incidence rates within countries and regions. For example, within the esophageal cancer belt, the Chinese counties with the highest cancer rates are located in the north-central provinces of Shanxi and Henan, whereas in central Asia the high-risk areas are in parts of Turkmenistan and Kazakhstan. In northern Iran there is quite a dramatic difference in the relatively small area of the Caspian littoral; the relatively dry regions east of the Caspian Sea have a high incidence with a much lower incidence in the more humid western parts.4
In Western countries, squamous cell cancers are more common in blacks. In low-incidence areas such as the United States, there is a male preponderance in the incidence of squamous cell cancers. However, such gender specificity is lost in areas with a high incidence of squamous cell cancers, such as China, where women are nearly equally affected. Persons with low socioeconomic status as well as unskilled manual workers are at almost two-fold higher risk of developing squamous cell cancers. Living without a life-partner increases the risk of squamous cell cancers, even after adjustment of confounding variables.5
Although the incidence of squamous cell esophageal cancer has decreased over the past two decades in most Western countries and in parts of Asia, including certain high-risk areas of China, there has been a disturbing upward trend in the incidence of esophageal and gastroesophageal junctional adenocarcinoma in the United States and in northern Europe including Denmark, Finland, Norway, Sweden, Scotland, and Switzerland.6–8 In the 1960s squamous cell esophageal cancers comprised approximately 90% of all esophageal cancers. However, because of an alarming rise in the incidence of esophageal adenocarcinoma, esophageal adenocarcinoma is now the predominant type of esophageal carcinoma in the United States. This reversal pattern has also been recently noted in some European countries such as Denmark and Scotland. In a longitudinal study of annual incidence of adenocarcinoma of the esophagus reporting from 43 tumor registries in North America, Europe, and Australia since 1960, the average increase in annual incidence ranged from 15% to 42% in European countries, 23.5% in Australia, and 20.6% in the United States.9
ETIOLOGY AND RISK FACTORS (SQUAMOUS CELL CANCER)
Dietary and Nutritional Factors
An association with dietary intake of N-nitroso compounds has been implicated in the pathogenesis of squamous cell cancer, particularly in high-incidence areas. N-nitroso compounds are derived from reduced dietary nitrates, often by ubiquitous fungal toxins, and certain food material are particularly susceptible to be contaminated by N-nitroso compounds, such as smoked pickles and a bread-like food called qocho or kocho in Ethiopia.10 It has been hypothesized that in black South Africans, the rising incidence of squamous cell cancer could be partly related to a recent dietary shift to maize from sorghum.11 Fusarium fungi, which grow sparsely on sorghum, grow freely on maize, producing fumonisins, which reduce nitrates to nitrites and synthesize cancer-producing nitrosamines. N-nitroso compounds are mutagenic in animal models and act by inducing alkyl adducts in deoxyribonucleic acid (DNA).12 Chewing betel-quid with areca nut and use of gutka (pan masala, a dry powdered mixture of areca nut, catechu, lime, unspecified spices, and flavoring agents), which is very common in Southeast Asia, has been proposed to have a synergistic carcinogenic effect in association with alcohol and tobacco.13 In areas of the world endemic for squamous cell cancers, drinking very hot beverages has been shown to have carcinogenic attributes, probably by causing chronic esophageal damage related to repeated thermal injury.
Certain dietary factors such as selenium have been shown to be protective against squamous cell cancers. Low levels of selenium have been associated with squamous cell cancers in high-risk areas of China, and selenium supplementation has been shown to be protective in controlled trials from China.14,15 Similarly, zinc deficiency, which potentiates the carcinogenic effects of nitrosamines as well as causes overexpression of the cyclo-oxygenase pathway in esophageal carcinogenesis, has been recognized as an independent risk factor of squamous cell cancers.16
Evidence suggests that low dietary folate intake and impaired folate metabolism due to functional polymorphisms in folate-metabolizing pathways, particularly polymorphisms in the 5,10-methylenetetrahydrofolate reductase (MTHFR) gene, play an important role in the causation of many gastrointestinal cancers, including esophageal cancer. In a recent meta-analysis of seven case-control studies, relative cancer risks for individuals in the highest compared with the lowest category of dietary folate intake were 0.66 for esophageal squamous cell carcinoma and 0.50 for esophageal adenocarcinoma.17 Also other investigators have shown that the MTHFR 677TT gene variant, which is associated with reduced enzyme activity, was associated with an increased risk of esophageal squamous cell carcinoma.18
Coffee drinking and increased intake of fruit, fish, and white meat has a protective effect on squamous cell cancers. In contrast, red meat, salted meat, and meat boiled at high temperature may increase the risk of squamous cell cancers.19,20
Alcohol and Tobacco
Alcohol and tobacco use, particularly in the form of pipe, cigar, or cigarette smoking, is a dominant risk factor of squamous cell cancers in low-incidence areas, such as the United States. On the other hand, in high-incidence areas, tobacco or alcohol does not seem to play a dominant causative role, although in Asian countries, where smoking is becoming increasingly popular, particularly among men, its relative importance as a risk factor is increasing. Epidemiologic studies suggest that the amount of alcohol consumed is more important than type of alcohol consumed, and the most prevalent alcoholic beverage consumed in a particular region tends to be the one with the highest risk of squamous cell esophageal cancers in that population.21 Multiple epidemiological studies carried out in regions with different incidences of squamous cell cancers suggest that specific polymorphisms in genes encoding for alcohol metabolizing enzymes may determine individual susceptibility to the carcinogenic role of alcohol.22,23
Preexisting Diseases of the Esophagus
Several underlying esophageal diseases are known to increase risk of subsequent development of squamous cell cancers of the esophagus. There is a fairly well-established association of esophageal cancer with achalasia. In a recent epidemiologic study from Sweden involving 2896 patients with achalasia, excess risks for squamous cell carcinoma and adenocarcinoma of the esophagus were observed, predominantly in men. There was no association with esophagomyotomy.24 Esophageal strictures caused by ingestion of lye, a caustic corrosive agent, are associated with a very high risk of squamous cell cancer, which develops three to five decades after the initial event. In achalasia and lye strictures, relative stasis, stagnation of food, and chronic inflammation have been implicated, but no definite carcinogenic mechanisms have been established. Tylosis, particularly the inherited form, which manifests in an autosomal dominant fashion with hyperkeratosis of palms and soles, has been associated with squamous cell cancer. For an affected family member, the estimated lifetime risk of esophageal cancer varied from 40% to 92% by the age of 70 (see Chapter 22). Although rare, the carcinogenetic events in tylosis has received recent attention in that a tylosis esophageal cancer (TOC) gene locus has been mapped to chromosome 17q25 by linkage analyses. Interestingly, loss of heterozygosity at this same locus, which contains the promoter sequence of the cytoglobin gene, has been detected in a significant number of patients with sporadic squamous cell cancers.25 The association of chronic mucocutaneous candidiasis with squamous cell carcinoma of the oral cavity and esophagus has been described in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED).26
A rare syndrome of iron deficiency anemia, dysphagia, and postcricoid esophageal web known as Plummer-Vinson syndrome in the United States and Patterson-Kelly syndrome in the United Kingdom has been reported to have an association with squamous cell cancers (see Chapter 41). The anecdotally reported association of squamous cell cancers with partial gastrectomy has not been substantiated.
Patients with history of squamous cell cancer of the upper aero-digestive tract have an increased risk of synchronous or metachronous squamous cell carcinoma of the esophagus, most likely due to shared risk factors. In prospective controlled studies, 3% to 14% of patients with squamous cell cancers of head and neck area developed synchronous or metachronous squamous cell cancers of the esophagus.27,28 Radiation therapy following mastectomy moderately increases the risk of squamous cell esophageal cancer in the upper and middle thirds of the esophagus, starting approximately 5 years after exposure, with the risk persisting after 10 years; no similar increase in the risk of esophageal adenocarcinoma has been reported. This finding appears to be a function of the portals used for postmastectomy radiation therapy, which typically do not expose the lowest third of the esophagus, where adenocarcinoma commonly arises.29
Given that human papillomavirus (HPV) has been implicated in the etiopathogenesis of squamous cell cancers of the oropharynx, its association with squamous cell cancers of the esophagus has been studied. Patients with squamous cell cancers of the esophagus from the United States, Europe, and Japan are infrequently HPV positive. On the other hand, in certain high-incidence areas, such as China and South Africa, HPV DNA can be demonstrated in a significant proportion of patients with squamous cell cancer of the esophagus. The interactive effect of genetic, environmental, and dietary risk factors in determining geographic susceptibility to the oncogenetic potential of chronic HPV infection needs further study.30 There is no clear association of esophageal cancer with chronic infection with other viruses such as herpes simplex virus or Epstein-Barr virus.
ETIOLOGY AND RISK FACTORS (ADENOCARCINOMA)
Dietary and Nutritional Factors
Increased intake of cereal fiber has been shown to have a dose-dependent inverse association with gastric adenocarcinoma and, to a lesser extent, with distal esophageal adenocarcinoma, but not with squamous cell cancer of the esophagus. Diets high in fiber, beta-carotene, folate, and vitamins C, E, and B6 may be protective, whereas diets high in cholesterol, animal protein, and vitamin B12 may be associated an with increased risk of esophageal adenocarcinoma.31 Antioxidant intake appears to be protective against esophageal adenocarcinoma but not gastric adenocarcinoma.32 Carbonated soft drinks may have an inverse relationship, but drinking tea and coffee is not associated with esophageal adenocarcinoma.33
Alcohol and Tobacco
Numerous epidemiologic studies, including prospective studies, suggest that association between alcohol and tobacco use is less consistent with esophageal adenocarcinoma than with squamous cell cancers. In general, smoking is considered a moderate risk factor for esophageal adenocarcinoma, whereas alcohol use has no association with esophageal adenocarcinoma. Relative to persons who had never smoked, current cigarette smoking was found in a prospective study to be associated with increased risk of squamous cell cancers (hazard ratio [HR], 9.27), esophageal adenocarcinoma (HR, 3.70), adenocarcinoma of the gastric cardia (HR, 2.86), and noncardia gastric adenocarcinoma (HR, 2.04).34
Obesity
Pooled results from observational studies support a positive association between an increased body mass index (BMI) greater than 25 kg/m2 and the risk for esophageal adenocarcinoma and possibly for adenocarcinoma of the cardia. In one study, increasing abdominal waist size was associated with an increased risk of esophageal adenocarcinoma, independent of BMI.35 Most recent published information support the hypothesis that obesity, in particular, abdominal obesity, contributes to GERD, which may in turn increase the risk of Barrett’s esophagus (see Chapters 43 and 44).36–38 In contrast to adenocarcinoma, in a large population-based prospective study of Chinese men, a low BMI was associated with an increased risk of squamous cell carcinoma of the esophagus.39
Gastroesophageal Reflux Disease
Barrett’s esophagus, which is a complication of gastroesophageal reflux disease (GERD), is one of the strongest risk factors for esophageal adenocarcinoma (see Chapter 44). However, there is controversy whether GERD without Barrett’s esophagus is by itself a risk factor for esophageal adenocarcinoma. Many patients with esophageal adenocarcinoma do not report preexisting GERD symptoms and only a minority of patients with GERD develop Barrett’s esophagus. However, it is also thought that chronic mucosal inflammation in patients with recurrent GERD may predispose to esophageal adenocarcinoma. A large population-based Swedish study showed that in persons with recurrent symptoms of reflux, as compared with people without such symptoms, the odds ratio for esophageal adenocarcinoma was 7.7 and was 2 for adenocarcinoma of the cardia. The more frequent, more severe, and longer-lasting the symptoms of reflux, the greater the risk.40 A recent Australian study suggested that obesity and GERD may play a synergistic role in the pathogenesis of esophageal adenocarcinoma.41 Overall, epidemiologic evidence suggests that chronic, severe GERD may predispose susceptible individuals to esophageal adenocarcinoma.
Barrett’s Esophagus (see also Chapter 44)
The reported prevalence of Barrett’s esophagus in the general population varies widely from 0.9% to 4.5%.42,43 Such fivefold variations in prevalence are mostly related to the type of population studied and also the definition of Barrett’s esophagus that is used. For example, experts disagree whether histopathologic demonstration of intestinal metaplasia is a requirement for diagnosing Barrett’s esophagus.44,45 Recent studies have suggested an increasing incidence of Barrett’s esophagus.46,47
The reported annual risk esophageal adenocarcinoma in patients with Barrett’s esophagus varies from 0.2% to 2% (see Chapter 44). In more recent studies with long-term follow-up of patients with histologically confirmed long-segment Barrett’s esophagus, the annual risk of developing high-grade dysplasia (HGD) or adenocarcinoma is approximately 1%.48,49 Although the annual incidence of cancer in those with low-grade dysplasia (LGD) was 0.6% to 1.3%, the incidence rate in patients with HGD is almost 10 times higher.50,51 In a meta-analysis of 236 patients with Barrett’s esophagus and HGD, esophageal adenocarcinoma was reported in 69 patients over 1241 patient-years of follow-up, with a weighted incidence rate of 6.58 per 100 patient-years.52 In another recently published meta-analysis, the overall pooled risk for developing adenocarcinoma in all patients with Barrett’s esophagus was 6.1 per 1000 person-years and was just 4.1 per 1000 person-years when early incident cancers and HGD at baseline were excluded.53 The risk of esophageal adenocarcinoma in patients with short segment Barrett’s esophagus or with specialized intestinal metaplasia of the esophagogastric junction is not known, but may be less compared with long-segment Barrett’s esophagus. However, it is important to note that these two entities are several-fold more common than long-segment Barrett’s esophagus and could account for a large number of patients with gastroesophageal junctional adenocarcinoma.
In addition to segment length and dysplasia, hiatal hernia, central obesity, and possibly smoking may be other factors favoring progression from nondysplastic Barrett’s esophagus to adenocarcinoma.54,55
Helicobacter pylori Infection
Epidemiologic studies show that as the prevalence of Helicobacter pylori infection has decreased in Western societies, the prevalence of GERD, Barrett’s esophagus, and distal esophageal and gastroesophageal junctional adenocarcinoma has rapidly increased. Although there are no data to suggest that H. pylori plays any role in esophageal mucosal resistance to acid-induced injury, in esophageal acid clearance, or in competence of the lower esophageal sphincter, it has been suggested that eradication of H. pylori in patients with corpus-predominant gastritis may exacerbate acid reflux. It has been postulated that the alkaline ammonia produced by H. pylori colonizing the cardiac mucosa could protect the neighboring distal esophageal squamous mucosa from damage by acidic reflux. There appears to be a negative association between H. pylori infection and GERD symptoms, although an association between successful H. pylori therapy and development of new or recurrent reflux symptoms has not been established.56,57 In a recent meta-analysis, patients with Barrett’s esophagus and adenocarcinoma, but not those with squamous cell esophageal cancer, were less likely to be infected with H. pylori, particularly cagA-positive strains.58 The clinical implication of such inverse correlations is unclear and will require careful evaluation, given that H. pylori infection is considered an important etiologic factor for gastric cancer.
Diverse Preexisting Conditions
Patients with acid hypersecretory states or conditions associated with severe GERD (e.g., scleroderma) may be at increased risk for esophageal adenocarcinoma. In a population-based study from Sweden, cholecystectomy was associated with a moderately increased risk of subsequent esophageal adenocarcinoma, but not squamous cell cancer. Increased duodeno-gastroesophageal reflux and a toxic effect on bile acids and bile salts on the esophageal mucosa was thought to be the mechanism. A case control study failed to identify an association between diabetes and esophageal adenocarcinoma.59 GERD is common in premature infants and in infants who are small for gestational age, and epidemiologic data suggest that preterm birth and low birth weight may be risk factors for development of esophageal adenocarcinoma. Down syndrome is not associated with esophageal cancer.60
Drugs
There is conflicting evidence regarding whether long-term use of drugs that relax the lower esophageal sphincter, such as anticholinergics, β-adrenergic agonists, theophylline or aminophylline, and benzodiazepines increase risk of esophageal adenocarcinoma.61 A few epidemiologic studies and a recent meta-analysis reported a protective effect of aspirin use (and to a lesser extent nonsteroidal anti-inflammatory drug [NSAID] use) with both types of esophageal cancer, an effect that appears to be dose dependent.62
FAMILY HISTORY AND GENETIC FACTORS
In areas with a high incidence of squamous cell esophageal cancer, family history is a strong risk factor for this disease. Gene expression profiling studies in these families have found consistent ribonucleic acid (RNA) expression patterns.63 Furthermore, the possibility of an esophageal squamous cell cancer (ESCC) susceptibility gene has been considered based on frequent allelic loss on chromosome 13 in these patients.64
Familial aggregation of Barrett’s esophagus, and related esophageal and junctional adenocarcinoma, has been described and a study reported that in up to 7% patients with Barrett’s esophagus, a familial aggregation can be confirmed (see Chapter 44). It has been suggested that familial Barrett’s esophagus is likely a complex genetic disorder consistent with a major mendelian autosomal dominant gene with relatively high penetrance.65,66
Individual variations in risk of developing esophageal cancer of any type may be partly explained by the presence of specific variant alleles (polymorphisms) of different genes that are present in a significant proportion of the population and likely play important roles in the multistep process of carcinogenesis (see Chapter 3). Such genetic polymorphisms that may increase susceptibility to esophageal cancer have been described in genes involved in alcohol metabolism, folate metabolism, carcinogen metabolism, DNA repair, cell cycle control, and oncogenes.67
MOLECULAR BIOLOGY
Similar to other cancers, esophageal cancers of both types develop through a multistep progressive process, which at the cellular level is reflected by disorders of the control of cell proliferation, differentiation, and controlled cell death (apoptosis) (see Chapter 3). There has been increasing interest in understanding the molecular mechanisms of these carcinogenetic events, not only for understanding etiologic factors but also for potential therapeutic targeting and determining prognosis.
Autonomous Growth (Growth Factors)
Increased expression of epidermal growth factor (EGF) and transforming growth factor-α (TGF-α) has been demonstrated in esophageal adenocarcinoma and has been associated with invasive disease and possibly poorer outcome.68,69 Furthermore, increased concentrations of TGF-β in azygos vein blood and increased expression of connective tissue growth factor (CTGF) and endoglin, a member of the TGF-β receptor family, have been correlated with angiogenesis, angiolymphatic invasion, and metastasis in patients with esophageal cancer.70 On the other hand, increased expression of SMAD has been associated with better survival in patients with squamous cell esophageal cancer.71 C-erbB2, a proto-oncogene coded by the HER2/neu gene and sharing a significant homology with EGF receptor, is amplified in patients with Barrett’s esophagus and adenocarcinoma, is clinically associated with a poorer outcome, and may play an early role in disease invasiveness.72,73 HER2/neu gene overexpression may promote carcinogenesis through multiple pathways, such as inhibition of apoptosis and enhanced cell proliferation, increased mitogen activated protein kinase (MAPK) activity and increased matrix metalloproteinase (MMP) activity, and by potent induction of vascular endothelial growth factor (VEGF).
The restriction point (R-point) is a critical gatekeeping checkpoint that controls G1- to S-phase transformation in the cell cycle (see Chapter 3). Cyclin D and E are proteins that are important regulators of the R-point and serve as the final step in many proliferation cascades. CCND1, the gene that encodes cyclin D1, is associated with an increased risk for esophageal adenocarcinoma.74 Also cyclin B has been shown to be overexpressed in esophageal squamous cell cancer, with possibly with a negative prognosis.75
Attenuation of Antiproliferative Pathways
Attenuation of antiproliferative cellular signals is a hallmark of cancer cells. Most cellular antiproliferative pathways converge on the retinoblastoma protein (Rb) pathway, which plays an important antiproliferative role by having a regulatory role on transition of cells from G0 to S phase (see Chapter 3). Loss of heterozygosity (LOH) of Rb protein and its functional repression by hypermethylation of p16, a tumor suppressor gene, has been described in patients with esophageal adenocarcinoma.76,77 Expression of p21, another cell cycle regulator that is activated after DNA damage by ionizing radiation, has been correlated with responsiveness of esophageal cancers to chemoradiotherapy.78 Although mutations of the APC gene is uncommon in patients with esophageal cancer, allelic deletion of 5q where APC resides, as well as functional repression of APC by hypermethylation of the promoter region of this gene, is quite common in patients with esophageal adenocarcinoma.79 In fact, plasma levels of hypermethylated APC DNA may be a biomarker of esophageal cancer.80
Disordered Apoptosis
Programmed cell death, or apoptosis, is an important defense mechanism against cancer and is regulated by TP53 and bcl-2 family of genes and their proteins (see Chapter 3). Alterations in TP53 in patients with esophageal cancer are seen in up to 50% to 99% of patients and are usually associated with a more aggressive form of tumor.81 Similarly, the bcl-2 family of proteins (Bcl-2, Bcl-xl, Bax) maintains an intricate balance of pro- and antiapoptotic factors in the cellular milieu and may play an important yet unclear role in the progression of non-dysplastic Barrett’s esophagus to high-grade dysplasia and adenocarcinoma.82 Nuclear factor kappa B (NF-κB), an antiapopotic factor, is expressed in 60% of patients with esophageal adenocarcinoma and has prognostic significance.83 The protein kinase Akt (also known as protein kinase B), a recently described serine-threonine kinase, is an important mediator of growth and antiapoptotic signals in esophageal adenocarcinoma. Increased Akt activation in the basal epithelium has been associated with metaplastic progression from squamous epithelium to nondysplastic Barrett’s esophagus, and eventual progression to high-grade dysplasia and adenocarcinoma.84 The hormone leptin and short-term acid exposure activate Akt in esophageal adenocarcinoma cells in vitro.85
Unlimited Replication
Telomeres are composed of several thousand repeats of short six-base-pair sequence elements and are located at the ends of chromosomes. At each cell replication the telomeres are shortened so that telomere length serves as counters of cycles of replication. After a finite number of cell replications, the telomeres are short enough to trigger the cell to exit from G1 to G0 phase, thus arresting further replication. Cancer cells, which are characterized by ability for limitless replication, achieve this ability partly by increased expression of telomerase, a ribonucleoprotein reverse transcriptase that counteracts shortening of telomeres. Increased expression of telomerase has been associated with progression of metaplastic epithelium to dysplasia to adenocarcinoma in patients with Barrett’s esophagus.86
Factors Promoting Neoangiogenesis, Invasion, and Metastasis
The ability of cancer cells to invade and disseminate is determined by their ability to disrupt intercellular adhesion as well as by altering the delicate balance between extra cellular matrix degrading proteases, such as urokinase-type plasminogen activator (uPA) of the serine protease system, MMP, and antiproteinases, such as tissue inhibitory metalloproteinase (TIMP) (see Chapter 3). Not unexpectedly, esophageal cancer, one of the most aggressive cancers with respect to invasiveness and propensity to metastasis, is associated with molecular abnormalities related to cell-cell adhesion molecules (CAMs), such as E-cadherins, integrins, and CD44 transmembrane glycoproteins, and overexpression or altered localization of uPA, the cysteine protease, cathepsin B (CTSB) protein, MMP and TIMP. Evidence suggests that many of these may be useful prognostic markers.87,88
Alteration of the Cyclooxygenase Pathway
Cyclooxygenases (COX) are key enzymes in the prostaglandin metabolism. COX-2, an inducible enzyme, has been shown to be an important mediator of tumorigenesis and angiogenesis. Selective inhibition with COX-2 inhibitors induces apoptosis and reduces angiogenesis. Activation of the COX-2 pathway can inhibit apoptosis by lowering the intracellular level of arachidonic acid, a fatty acid precursor of prostaglandins, which is known to promote apoptosis. A second mechanism that can be targeted by COX-2 inhibitors is prostaglandin-E2 (PGE2) dependent, and appears to be mediated by increase in intracellular Bcl-2 as well as intracellular cyclic adenosine monophosphate (cAMP), both of which suppress apoptosis. The angiogenetic potential of the COX-2 pathway occurs through increased expression of VEGF, increased endothelial survival by bcl-2 and Akt signaling, induction of MMP, EGF receptor–mediated angiogenesis, and suppression of interleukin 12 (IL-12) expression. Studies have shown that patients with esophageal cancer who express a high level of COX-2 in their tumors are more likely to have metastatic disease at presentation, to have more local tumor recurrences after definitive treatment, and to have a worse survival than patients not overexpressing COX-2.89,90
Microsatellite Instability
Several investigators have reported microsatellite instability (MSI) in less than 20% of esophageal and gastroesophageal junctional adenocarcinomas. Early unconfirmed evidence, however, suggests that MSI may be an early event in the progression of intestinal metaplasia to esophageal adenocarcinoma.91
Chromosomal Abnormalities
Defects in mitotic checkpoint genes lead to aneuploidy, or an abnormal number of chromosomes. Although a vast majority of esophageal cancers have aneuploidy, the exact gene defects are not characterized. DNA aneuploidy detected by flow cytometry has been considered to be a marker of progression of intestinal metaplasia to esophageal adenocarcinoma.92 A host of specific chromosomal abnormalities have been described in patients with esophageal cancer, and recent studies based on a comparative genomic hybridization technique using different types of esophageal cancer and gastric cardiac adenocarcinoma may lead to identification of genes important for disease-specific pathogenesis.93
Molecular Events in Neoplastic Progression of Barrett’s Esophagus
Research has focused on the complex molecular events that lead to development of esophageal adenocarcinoma in the setting of Barrett’s esophagus.94 It is postulated that the initial turn-on event, which is related to chronic exposure to noxious stimuli, such as acid reflux and bile salt exposure, is the activation of the inflammatory cascade mediated by cytokines such as tumor necrosis factor-α (TNF-α) and IL-1b. This cytokine activation eventually leads to ectopic expression of CDX1 and CDX2 homeobox proteins that play a major role in the development of intestinal epithelium in the embryonic stage and in this setting cause intestinal metaplasia to develop.95–97 With the development of metaplastic intestinal epithelium, multiple poorly characterized genetic (e.g, aneuploidy, loss of heterozygosity, mutation) and epigenetic events (e.g., hypermethylation) set off the metaplasia to dysplasia to adenocarcinoma sequence, which eventually leads to genetic instability with clonal expansion of cells with genetic errors. The exact sequence of this multistep process is not known, but several early events in this transition sequence have been identified.98 Abnormalities of p16 and possibly overexpression of cyclin D1 are relatively early events that lead to loss of control of cell cycle regulatory genes. Telomerase may also play in early role. Prevalence of TP53 damage such as loss of heterozygosity and mutation increases with advancing grade of dysplasia. Similarly, progression of dysplasia is characterized by decreased expression of E-cadherin and membranous β-catenin expression. Growth signals such TNF-α and COX-2 expression also increase with progression of grades of dysplasia and warrant further evaluation as potential biomarkers.
PATHOLOGY
Squamous Cell Cancer
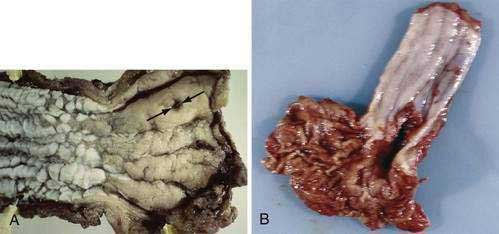
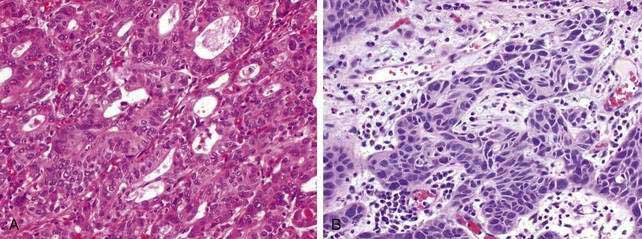
Esophageal squamous cell cancer typically develops by progression from premalignant (dysplastic) precursor lesions (Fig. 46-2). Many pathologists describe dysplasia using a two-tiered system: low-grade dysplasia, which includes mild and moderate dysplasia; and high-grade dysplasia, which includes severe dysplasia and cancer in situ.99 In the World Health Organization (WHO) classification the term “intraepithelial neoplasia” is preferred over “dysplasia,” although dysplasia is by far more popular.100 Dysplasia includes both cytologic and architectural abnormalities of varying severity and extent. Low-grade dysplasia often involves the basal part of the epithelium and high-grade dysplasia usually involves the entire epithelial layer and, in a small proportion of patients, dysplasia extends into the ducts of the esophageal glands, simulating stromal invasion or spread in a horizontal pagetoid pattern.101 Dysplasia is often multifocal, supporting a field defect hypothesis in the pathogenesis of esophageal squamous cancer. Cytologic changes are typically seen as coarse chromatin, increased nuclear to cytoplasmic ratio, nuclear hyperchromasia, nuclear pleomorphism, and mitotic figures. Architectural changes of dysplasia include disorganization, loss of polarity, overlapping nuclei, and lack of surface maturation. The presence of these features is often used to distinguish dysplasia from non-neoplastic (reactive) processes typically seen with esophageal mucosal inflammation.
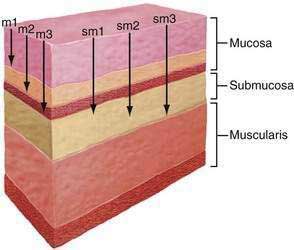
Squamous cell cancers are aggressive and local lymph nodal metastasis occurs early, partly related to the presence of lymphatic channels in the esophageal lamina propria. Because of the prognostic importance of lymph nodal metastasis and its effect in deciding management options, early squamous cell cancer has been categorized into six subcategories based on depth of tumor infiltration. Intramucosal carcinomas are divided into three groups (m1, m2, and m3), and carcinomas invading the submucosa also are divided into three groups (sm1, sm2, and sm3) (Fig. 46-3). Data from Japan suggest that although m1 and m2 early squamous cell cancers do not usually have nodal metastasis, m3 disease has up to an 8% incidence of nodal metastasis, and this incidence progressively increases as the tumor infiltrates the submucosa, with sm1, sm2, and sm3 tumors having incidences of nodal involvement of 17%, 28%, and 49%, respectively. Similarly the incidence of vascular invasion progressively increases, with m1 tumors having none, whereas almost 90% of sm3 tumors show vascular involvement.102
Invasion of local structures, such as mediastinal pleura, the trachea, the bronchi, and the aorta as well as distant metastases to liver, lung, bone, and other sites may be present in more than a third of these patients at presentation and signifies a poor prognosis. Relatively rapid invasion of the tumor into neighboring structures in the mediastinum has been attributed to the absence of a true serosal layer in the esophageal wall.103
Adenocarcinoma
Compared with squamous cell esophageal cancer, a larger body of information exists related to the pathobiology of dysplasia in Barrett’s esophagus and its progression to esophageal adenocarcinoma. Grossly, esophageal dysplasia in Barrett’s esophagus is usually flat and inconspicuous. Dysplasia may be described as low grade or high grade and generally follows the same patterns of cytological or architectural changes described earlier with squamous cell dysplasia. There is considerable interobserver variation in the interpretation and grading of dysplasia and adenocarcinoma among pathologists.104 Furthermore, progression of dysplasia from low grade to high grade is a morphologic continuum and thus may not follow identifiable features of orderly progression for definitive categorization.105 “Adenoma-like” dysplastic changes show minimal crypt distortion and mild cytological abnormalities. This is in contrast to the much less common “nonadenoma-like” dysplasia that typically shows crowded glands containing cuboidal cells with a high nuclear to cytoplasmic ratio, round nuclei with irregular contours, vesicular chromatin, and prominent nucleoli.106 Goblet cells are usually depleted in dysplastic epithelium, and even the surrounding nondysplastic mucosa may show a decrease in goblet cells.107
Many pathologists often use the term “indefinite for dysplasia” when true dysplasia cannot be accurately differentiated from reactive changes in the presence of esophageal inflammation. Although lack of surface maturation is normally considered a cardinal feature of dysplasia, this has been recently debated.108 Adjunct immunohistochemical markers (e.g., proliferating cell nuclear antigen (PCNA) and Ki67, cyclin D1, and TP53) have been used to improve the accuracy of diagnosis of dysplasia in this setting.109,110 Recent studies have reported the utility of immunostaining for alpha-methylacyl-CoA racemase (AMACR) for differentiating non-dysplastic epithelium from low-grade and high-grade dysplasia and also from adenocarcinoma.111,112 In one study, AMACR staining was negative in all cases of Barrett’s esophagus considered negative for dysplasia, whereas 38% of cases of low-grade dysplasia, 81% of cases of high-grade dysplasia, and 72% of cases of adenocarcinoma were positive.111 Another recent study also reported a high negative predictive value for AMACR immunostaining.112
Almost all esophageal adenocarcinomas arise in the setting of Barrett’s esophagus and typically occur in the distal third of the esophagus, including the esophagogastric junction (see Chapter 44). Adenocarcinomas unrelated to Barrett’s esophagus are extremely rare and usually arise from foci of gastric heterotopia in the cervical esophagus (inlet patch; see Chapter 41). Esophageal adenocarcinoma may have a flat, inconspicuous appearance or can be polypoid, ulcerated, or infiltrative. The majority are well or moderately differentiated, usually comprising cystic or tubular glands in solid nests and irregular clusters and often in a cribriform pattern with considerable stratification. In poorly differentiated carcinomas, the tumor cells infiltrate the esophageal wall with sheets of poorly formed glands with a prominent desmoplastic stroma. Signet ring cells and bizarre pleomorphic tumor cells may be present. One of the common problems encountered by pathologists is distinguishing a tumor of the gastric cardiac from an esophageal adenocarcinoma. In these instances, correlation of the biopsy site with endoscopic anatomic landmarks is crucial.
The incidence of lymph node metastasis in esophageal adenocarcinoma is related to depth of tumor infiltration and appears to be equal or less than in squamous cell esophageal cancer. Whereas nodal disease is rare with adenocarcinomas limited to the esophageal mucosa, the rate of nodal metastases for tumors invading into the submucosa is reported to be 27% to 41% for all patients, and 67% to 78% for those with tumors infiltrating the deep submucosa.113–115 Involvement of celiac and perihepatic lymph nodes is more common with esophageal adenocarcinoma than squamous cell carcinoma because of the more common occurrence of these former tumors at or near the gastroesophageal junction.
CLINICAL FEATURES
Patients with squamous cell cancer and adenocarcinoma of the esophagus present with similar symptoms. The most common is progressive dysphagia, often accompanied by disproportionate weight loss. The esophagus can distend up to a diameter of 40 mm in normal adults; solid food dysphagia can occur at luminal diameter of 25 mm, but at or below a diameter of 13 mm dysphagia is always present. Dysphagia initially can be subtle and reported by patients as a sensation of transient sticking of food. Odynophagia usually coincides with presence of an ulcerated tumor. Many patients with early esophageal cancer are asymptomatic. Some may present with iron deficiency anemia. Chest pain or pain radiating to the back is an ominous symptom, often indicating invasion into periesophageal structures. People with esophageal adenocarcinoma are almost eight times as likely to report at least weekly symptoms of reflux or regurgitation as control subjects; no association of GERD symptoms and squamous cell cancer was reported.40 Regurgitation of undigested food material proximal to the area of obstruction and symptoms related to aspiration pneumonia are often present at advanced stages of the disease. Hoarseness can result from recurrent laryngeal nerve involvement by the tumor per se or metastatic lymph nodes. Esophagorespiratory fistula develops in approximately 5% to 15% of all patients with advanced esophageal cancer and is associated with a particularly poor prognosis. Fistula usually manifests with intractable cough and recurrent pneumonia. Uncommon sites of fistulae from esophageal carcinoma include extension to the aorta, pleura, pericardium, and mediastinum. Hematemesis can be due to local hemorrhage from an ulcerated tumor; rarely, exsanguination can occur from development of an aortoesophageal fistula. Many patients have symptoms related to metastases in the lung, liver, bone, or brain, either at presentation or during the course of the disease.
DIAGNOSIS
Imaging Tests
Contrast Esophagography
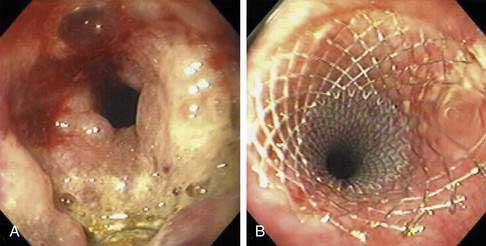
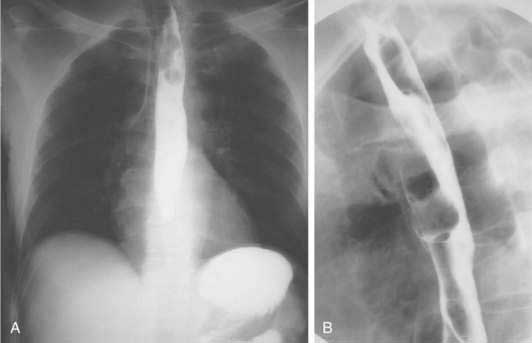
The role of contrast esophagography in diagnosis of esophageal cancer has diminished over the years with the increasing use of endoscopy for primary diagnosis. Esophagography should be performed only when this test is likely to affect decision making. Double-contrast barium radiographs often show early cancers as small polypoid lesions, plaque-like lesions, or focal irregularity of the wall. Advanced cancers commonly appear as areas of irregular luminal narrowing, ulceration, and stricture, with abrupt shoulders. Esophagogastric junctional cancers typically extend into the gastric cardia, and for this reason the fundus and cardia of the stomach should always be included in an optimal contrast radiographic study of the esophagus. To evaluate a suspected esophagorespiratory fistula, contrast radiographs are very useful for delineation of the anatomy prior to endoscopic stenting. Such contrast radiographic studies should be performed using barium instead of a hyperosmolar contrast agent such as meglumine ditrizoate because of the risk of pulmonary edema with the hyperosmolar contrast agent (Fig. 46-4).
Computed Tomography
Three-dimensional multidetector CT imaging and CT esophagography appear to be promising techniques in early reports, reproducing a contrast radiograph–like image with fairly accurate depiction of length of the tumor and its location.116
Endoscopy and Biopsy
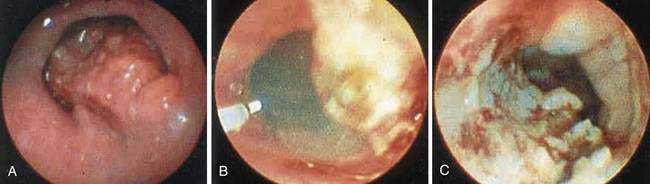
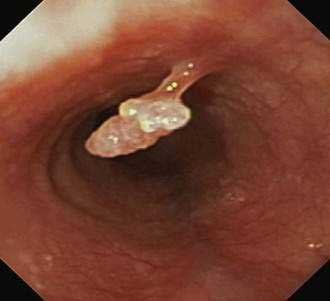
During endoscopy, the location of the esophageal tumor, its relation to anatomic landmarks such as the upper esophageal sphincter and the esophagogastric junction, and the degree of luminal obstruction are typically assessed. Also endoscopic visualization combined with endoscopic biopsy provides an accurate assessment of malignant (and premalignant) lesions of the esophagus. A Paris classification has been proposed for endoscopic assessment of superficial neoplasia of the gastrointestinal tract, including the esophagus, but its clinical acceptance has been limited particularly in North America.117 Advanced esophageal cancers appear endoscopically as fungating, friable, often ulcerated mass lesions occupying some or all of the luminal circumference, usually with indistinct margins (Fig. 46-5). Less frequently, both types of cancers (squamous and adenocarcinoma) can present with a submucosal infiltrative pattern and may not have a prominent luminal component. If the esophagogastric junction is involved by such a tumor, the clinical presentation is often suggestive of peseudoachalasia (see Chapter 42). Typically a higher number of biopsies translates to a higher accuracy rate. Tumors with a submucosal infiltrative pattern may require an aggressive biopsy technique to obtain deeper tissue, using a bite-on-bite technique or endoscopic ultrasonography (EUS)-guided fine-needle aspiration of submucosal tissue.
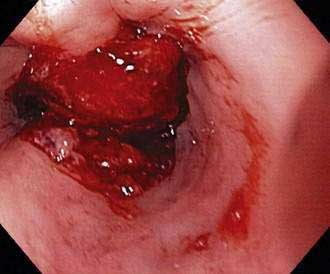
Figure 46-5. Advanced squamous cell carcinoma of the esophagus that is nearly completely occluding the lumen.
Brush cytology is a useful and quick technique to increase the yield in establishing a diagnosis of esophageal cancer, particularly with the availability of advanced cytologic techniques such as fluorescence in situ hybridization (FISH).118
Endoscopic Detection of Dysplasia and Early Cancer
Conventional Chromoendoscopy
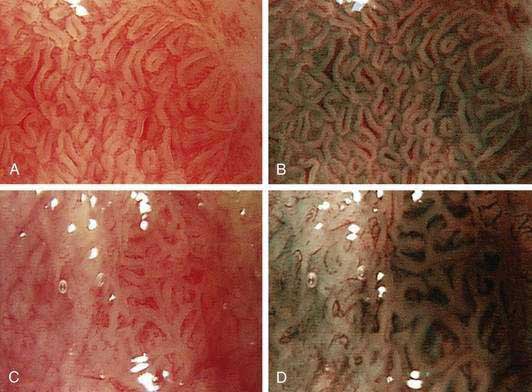
Chromoendoscopy with topical application of stains or pigments has been used for improved visualization of subtle mucosal pathology. Lugol’s iodine, when sprayed on the esophageal mucosa during endoscopy, stains the glycogen-rich squamous epithelium black, dark brown, or green-brown, whereas abnormal (neoplastic or inflamed) glycogen-depleted squamous epithelium does not pick up the stain, providing good visual contrast. Lugol’s iodine increases detection of dysplasia and early squamous cell cancers compared with endoscopic visualization alone, and this method may be particularly useful in screening patients at high risk for squamous cell cancer.119 Lugol’s iodine may also be useful in identifying residual islands of Barrett’s epithelium after endoscopic mucosal ablative therapy and development of neosquamous epithelium. For endoscopic detection of high-grade dysplasia and early adenocarcinoma in the setting of Barrett’s esophagus, a number of chromoendoscopic techniques, based on application of vital staining with dyes such as methylene blue, toluidine blue, and cresyl violet and contrast stains such indigo carmine, have been used. The largest experience is with methylene blue–based chromoendoscopy, although the reported results of chromoendoscopy in this setting are quite variable, leading to controversy on the utility of this technique in clinical practice.120 A meta-analysis concluded that targeted biopsies after methylene blue–based chromoendoscopy is not superior to random biopsy in detecting specialized intestinal metaplasia and dysplasia of any grade in patients with Barrett’s esophagus who are undergoing endoscopic surveillance and thus cannot be recommended for routine clinical practice.121 Chromoendoscopy using acetic acid, which dissolves the superficial mucus layer by breaking the glycoproteins’ disulfide bonds and reversibly acetylates cellular proteins, has been used to highlight the mucosal vasculature pattern in patients with Barrett’s esophagus.122
Electronic Chromoendoscopy
Electronic chromoendoscopy by spectral manipulation of white light, such as narrow band imaging using narrow bandwidth filters or the use of postprocessing spectral estimation technique, has been used during esophageal endoscopy to highlight the surface texture such as pit pattern, as well as the mucosal capillary vascular pattern (Fig. 46-6).123,124 Although initial reports of higher accuracy for early detection of dysplasia and early esophageal cancer were encouraging,125–128 a later report questioning the effect of electronic chromoendoscopy in this setting has dampened the initial enthusiasm.129
High-Resolution Endoscopic Imaging
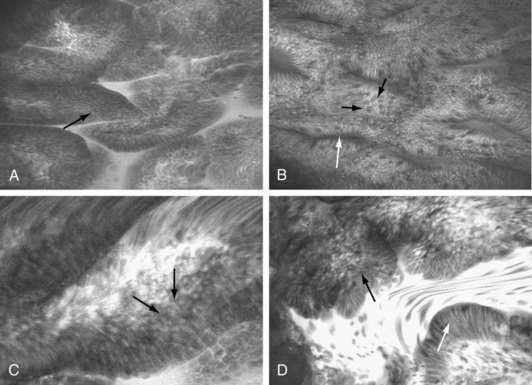
High-resolution endoscopes with pixel densities of approximately 850,000 (compared with approximately 100,000 to 300,000 in conventional endoscopes) and magnification endoscopes, which can optically zoom the image from 1.5 to 150 times, have been used in prospective studies to increase the yield of detection of early esophageal cancers and foci of high-grade dysplasia, often performed in conjunction with chromoendoscopy.130 Fluorescent-aided confocal endomicroscopy, which uses principles of confocal imaging, has been made commercially available, as an endoscope and as an endoscopic probe. Initial experience in early esophageal neoplasia has been encouraging, particularly for subsurface imaging (Fig. 46-7).131 One of the limitations of confocal endomicroscopy is the requirement of a fluorescent dye administered either topically or parenterally for fluorescent contrast. Endocytoscopy is based on the technology that incorporates optical contact microscopy on a probe-based platform for real-time microscopic examination of the esophageal epithelial cells at a magnification ranging from 450 to 1100 times.132,133
EUS and optical coherence tomography (OCT) are endoluminal cross-sectional imaging techniques. The former uses acoustic waves and the latter coherent light waves to quantitatively measure the degree of back-scattering from the tissue to image tissue. High-resolution EUS using catheter miniprobes has limited accuracy for detecting invasive adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia or intramucosal carcinoma.134,135 The utility of EUS seems mostly to be for staging esophageal cancer rather than early detection. Several investigators have reported the utility of OCT in detecting areas of high-grade dysplasia and early esophageal cancer; further technical developments, such as availability of ultrahigh-resolution and Doppler-enabled OCT, as well as optical coherence microscopy, which marries features of OCT and confocal microscopy for 3-D imaging, may further enhance the usefulness of endoscopic OCT.136–138
Spectroscopic Imaging
Tissue fluorescence is characterized by absorption of incident light by tissue fluorophores and re-emission of absorbed light at a different wavelength. Autofluorescence of neoplastic tissue is substantially different from normal tissue, a principle that has been used for imaging early esophageal cancers and areas of high grade dysplasia, with limited success. Spectroscopic assessment, including light-scattering spectroscopy and fluorescence spectroscopy, have been evaluated. A recent study showed that trimodal spectroscopy, which combines fluorescence (providing information of tissue chemistry and reflectance) and light-scattering spectroscopy (providing morphologic information such as nuclear size and density), were complementary and provided better results in detecting high-grade dysplasia than either technique alone.139 Raman spectroscopy, which assesses energy shifts due to interaction with different molecules, provides a fingerprint of molecular composition of neoplastic tissue and has been used to study the molecular progression of Barrett’s epithelium to carcinoma, with acceptable accuracy.140
SCREENING AND SURVEILLANCE
Most of the published information on screening for Barrett’s esophagus suggests that reflux symptoms are an insensitive marker of Barrett’s esophagus. Also, only a small proportion of patients with Barrett’s esophagus eventually develops esophageal adenocarcinoma (see Chapter 44). Likewise, only a minority of patients diagnosed with adenocarcinoma were known to have Barrett’s esophagus.141,142 Moreover, a diagnosis of Barrett’s esophagus, made by screening, may have long-term emotional and financial implications.143,144 On the other hand, studies using decision analysis models suggest that endoscopic screening for esophageal adenocarcinoma in high-risk groups, such as white men with GERD symptoms of long duration may be cost-effective.145–147 Published professional guidelines, such as the American College of Gastroenterology guidelines, do not recommend screening for Barrett’s esophagus in the general population and recommend that the use of screening in selective populations at higher risk should be individualized.
Screening techniques for esophageal cancer are nonendoscopic and endoscopic. Nonendoscopic techniques include cytology and molecular screenings. Cytologic techniques use a modification of a balloon-based technique or a sponge-based technique, using polyurethane mesh compressed inside dissolvable gelatin capsule and is based on the principle of scraping the esophageal mucosa to obtain exfoliative cytology samples. In areas of China and elsewhere with a very high incidence of squamous cell cancer, long-term follow-up studies have demonstrated that cytologic screening techniques may be a viable option for low cost nonendoscopic screening.148 Experience with the use of balloon-based exfoliative cytologic techniques in the area of Barrett’s esophagus is very limited. There is no reliable molecular screening test for esophageal cancer, although a number of molecular markers are being studied for potential application as a screening test in these patients. There has been interest in detecting the plasma level of p16 and hypermethylated APC and also the level of Mcm5 in gastric aspirates, each of which are markers of early epithelial carcinogenesis; small studies have reported a positive predictive value for esophageal cancer.80,149
Endoscopy is by far the most common technique used for screening for esophageal cancer. Endoscopy is performed in conjunction with endoscopic biopsies, often targeted by conventional or electronic chromoendoscopy, or other emerging techniques as described in the preceding section. Studies have shown unsedated, ultrathin esophagoscopy to be tolerable, safe, and accurate in screening in patients for Barrett’s esophagus.150 Esophageal capsule endoscopy and its modifications such as string capsule endoscopy or tethered capsule endoscopy also has been used for screening for Barrett’s esophagus.151,152
Surveillance refers to testing persons who are known to have cancer precursor lesions, such as metaplasia or dysplasia. Although the theoretical argument for surveillance to detect advanced dysplasia and early cancers to allow an appropriate timely intervention to improve outcomes is attractive, the evidence supporting this approach is limited and often contradictory.153,154 Randomized controlled trials have not been performed and are probably impossible to conduct. Some recent retrospective and case-control studies suggest that surveillance endoscopy can detect early-stage cancers and improve survival. In contrast, other studies suggest that the majority of patients with Barrett’s esophagus do not benefit from endoscopic surveillance.155–157 Available studies have been limited by lack of current knowledge on the natural history of dysplasia, as well as lack of agreement among pathologists in terms of diagnosis of dysplasia.104 Economic analyses, with their inherent limitation of uncertainty and assumptions, suggest that although surveillance of patients with nondysplastic Barrett’s esophagus may not be cost-effective, the periodic surveillance of patients with dysplastic Barrett’s esophagus is cost-effective.145,147
STAGING
Staging System
Staging of esophageal cancer is not only important for planning appropriate stage-specific therapy but also provides crucial information regarding prognosis. For example, Surveillance, Epidemiology, and End Results (SEER) data from 2005 showed that one-year survival in patients with local, regional, and distant (metastatic) disease were 68%, 54%, and 28%, respectively. The five-year survival for esophageal cancers confined to the mucosa (T1m, N0, M0) may approach 90%.158
The TNM staging system for esophageal cancer is outlined in Table 46-2. The prefixes “c,” and “p” can be used to designate whether the information for a given TNM stage grouping is based solely on clinical information or includes surgical pathology findings, respectively. Some investigators also use a “u” prefix to specify the use of EUS in determining the cancer stage.
Table 46-2 American Joint Committee on Cancer Staging System for Cancers of the Esophagus
| Tumor Node Metastasis Definitions |
| Primary Tumor (T) |
* For tumors of the midthoracic esophagus, use only M1b because tumors with metastasis in nonregional lymph nodes have an equally poor prognosis as those with metastasis in other distant sites.
Modified from American Joint Committee on Cancer (AJCC) Cancer Staging Manual. 6th ed. New York: Springer; 2002. p 91.
Endoscopic Staging
Standard endoscopic evaluation of esophageal cancer can provide very useful information on the extent of tumor. For example, the ability to lift a nodular lesion with submucosal injection of saline suggests early mucosal disease. Endoscopic mucosal resection of early esophageal cancer and pathologic examination of the resected specimen provide excellent information on the depth of tumor invasion and has been used for accurate pathologic staging of early esophageal squamous cell cancer as well as early adenocarcinoma complicating high-grade dysplasia in Barrett’s esophagus.159 At the other extreme, esophageal cancers that are 5 cm or greater in length, or are sufficiently stenotic to prevent passage of a customary endoscope, are likely to be T3 or higher-stage lesions.160
Computed Tomography Staging
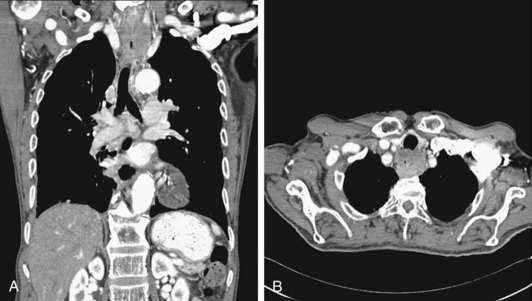
CT is typically the initial staging modality once a diagnosis has been established by endoscopy. CT is valuable in detecting metastatic disease in the liver, lungs, and periaortic lymph nodes. CT has a reasonable accuracy in detecting invasion of mediastinal structures in locally advanced tumors and has accuracy rates of up to 90% in detecting aortic, tracheobronchial, and pericardial invasion (Fig. 46-8). Despite improvements in CT technology, the constituent layers of the esophageal wall cannot be easily differentiated from each other, which explains the poor accuracy (50% to 60%) of CT in assessing tumor stage. CT has a sensitivity of 50% to 79% in detecting nodal disease with reported specificities of 25% to 67%. Accuracy of CT in assessment of periesophageal abdominal lymph nodes is superior to detection of thoracic adenopathy. CT (even helical CT) is insensitive for detection of involvement of celiac lymph nodes in esophageal cancer. In a prospective trial, positive and negative predictive values for helical CT assessing celiac lymph nodes were only 67% and 77%, respectively, using the gold standard of EUS with fine-needle aspiration (FNA).161 Although magnetic resonance imaging (MRI) can easily delineate the margins of the air-filled esophagus from the surrounding mediastinal fat, MRI does not offer any significant advantage over CT, even with the use of experimental endoscopic MRI techniques.162
Positron Emission Tomography Staging
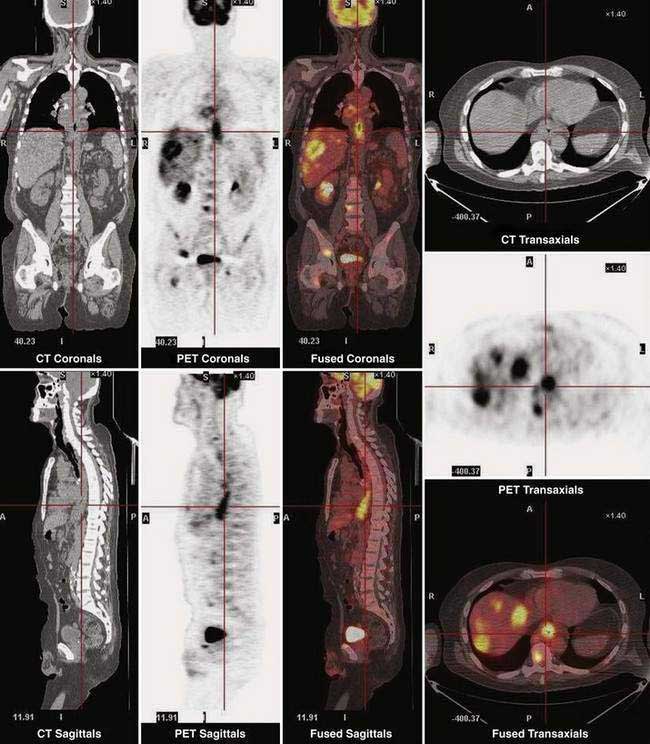
Fluorine 18 fluoro-deoxyglucose (FDG) positron emission tomography (PET) is being increasingly used for staging esophageal cancer. PET has the ability to image the entire body and thus provide information on both locoregional and metastatic disease (Fig. 46-9). A recent review reported that FDG PET is more sensitive and specific than CT for detecting distant metastases in patients with esophageal carcinoma. However, the sensitivity of FDG PET for detecting locoregional disease is low and inferior to EUS due to a number of factors, including (1) limitation of spatial resolution with currently available PET scanners; (2) masking of adjacent involved lymph nodes due to FDG accumulation in the primary tumor; and (3) low FGD uptake by poorly differentiated esophageal and EG junctional adenocarcinomas.163
Endoscopic Ultrasonography Staging
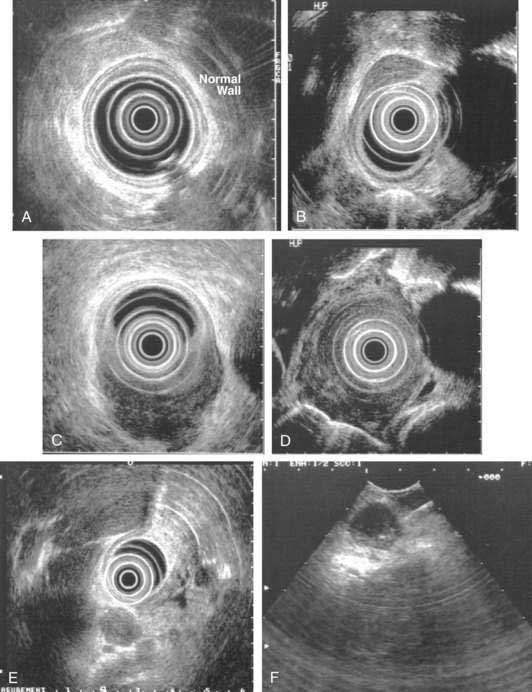
EUS is the cornerstone in the pretreatment staging evaluation of esophageal cancer. EUS images of the normal esophageal wall at typical scanning frequencies (7.5 to 12, or even 20 MHz) show characteristic delineation of the different echo layers of the esophageal wall. EUS is the best imaging modality for tumor staging, with an overall accuracy for tumor staging of up to 85% to 90% in the hands of experienced endosonographers (Fig. 46-10).164 Accuracy seems to be the lowest (≈ 80%) for T2 cancers. In addition to imaging the esophageal wall itself, EUS also obtains high-resolution views of the periesophageal tissue, including the celiac trunk, the left lobe of the liver, the left adrenal gland, the thoracic and proximal abdominal aorta, the azygos vein, the right and left pleura, the left atrium, and the subcarinal region. The overall accuracy of EUS for defining the presence of nodal metastasess ranges between 65% and 86%. Lymph nodes that are hypoechoic, round, and 1 cm or larger, with clearly demarcated borders, are more likely to be malignant than nodes that are elongated and hyperechoic with poorly demarcated borders, which are most often reactive nodes. The higher the number of criteria a lymph node acquires, the more likely it is to be malignant, although all four of these criteria are found only in a minority of malignant nodes. The addition of EUS FNA to EUS imaging increases the accuracy of nodal assessment, and more liberal use of EUS-guided FNA has been advocated.165 Adjunctive analytic techniques, such as digital image analysis and elastography may enhance the ability of EUS in differentiating benign from malignant periesophageal lymph nodes.
There is no difference in staging accuracy between curved linear array and radial scanning echoendoscopes. High-frequency catheter-based ultrasound probes that provide higher resolution images but with limited depth of imaging, may be particularly useful to assess the feasibility of endoscopic mucosal resection by differentiating T1m cancers from T1sm cancers (see Table 46-2). However, the reported accuracy of these probes in demarcating mucosa from the submucosa has been variable.
Effect of Staging Approach on Outcome
There have been few analyses of the effect of EUS-based staging on outcomes in patients with esophageal cancer. In a prospective study of 204 patients pretreatment, EUS predicted survival in esophageal cancer based on initial T-category and N-category; celiac nodes and lymphadenopathy at EUS were shown to be important predictors of survival.166 Another study assessed the clinical impact of EUS on esophageal cancer in 107 patients with esophageal cancer and reported improved survival in patients who were evaluated with EUS for staging.167 In an analysis of the SEER-Medicare–linked database, receipt of EUS was independently associated with improved survival, possibly because of improved stage-appropriate management in a cohort of 2830 patients with esophageal cancer.168 Studies have also evaluated the effect on patient outcome of FDG PET–based staging, with divergent results.169,170
There are other possible explanations for the increased survival associated with receipt of EUS and perhaps PET. The concept of stage migration, also known as the Will Rogers phenomenon, is well known in oncology and occurs with more accurate staging of cancer.171 If a population of patients with cancer is staged more accurately by any newer staging modality, then some patients with subtle advanced disease will be upstaged; this upstaging will appear to improve survival in patients with early and advanced cancers.
A decision analysis study favored a strategy of initial staging of esophageal cancer by PET and EUS FNA over a strategy based on CT and EUS FNA in terms of higher effectiveness, although the PET-based strategy was more expensive.172
Restaging
There has been resurgence of interest in restaging of esophageal cancer after preoperative chemoradiotherapy (neoadjuvant therapy). Currently, there are no reliable biomarkers or histopathologic or morphologic predictors of response to preoperative chemoradiotherapy. In clinical practice, in order to decide whether to attempt curative surgical resection, response to preoperative chemoradiotherapy is assessed by different imaging modalities. Although commonly performed for restaging, CT has a poor ability to discriminate responders from nonresponders; in a meta-analysis, the overall sensitivity and specificity of CT in predicting pathological response varied from 33% to 55% and 50% to 70%, respectively.173
EUS is one of the common modalities for restaging esophageal cancer after chemoradiotherapy. Similar to other morphologic imaging modalities, EUS suffers from its inability to discriminate viable tumor from post-chemoradiotherapy fibrosis and scarring. Primarily for this reason, studies of EUS in restaging of esophageal cancer have consistently yielded poor accuracy for T category and investigators often relied more on surrogate markers of tumor volume, such as maximal cross-sectional areas or tumor thickness during assessment of response to neoadjuvant therapy.174 Patients with recalcitrant nodal disease after chemoradiotherapy are particularly likely to have a poor long-term outcome. EUS, particularly with its ability to sample suspected lymph nodes by FNA, is considered a useful modality in this regard, with an overall sensitivity and specificity of approximately 50% to 100% and 36% to 100%, respectively, with the lower estimates derived from studies in which EUS FNA was not used.
FDG PET is increasingly being used for restaging of esophageal cancer. This imaging modality has two basic advantages over EUS: the ability to image the entire body in one examination for evaluation of metastatic disease, and, when combined with CT, the ability to provide structural and metabolic information with high reproducibility and internal validity. Serial quantitative measurement of tumor FDG uptake (by standardized uptake value, or SUV) provides an objective means to monitor response to chemoradiotherapy and has been correlated with survival in these patients. Given that almost half of patients with esophageal cancer have a minimal or suboptimal response to chemoradiotherapy, there has been considerable interest in predicting response early in the course of chemoradiotherapy in order to prevent continued toxic, futile, and costly treatment in nonresponders. In a prospective study it was shown that performing FDG PET two weeks after initiation of systemic chemotherapy, a metabolic response in the tumor can be objectively identified by a reduction of SUV compared to baseline, and this response predicted an eventual clinical response to completed chemoradiotherapy with a high degree of accuracy.175
There have been only a few studies that have directly compared EUS and FDG PET head to head in patients undergoing chemoradiotherapy. In a prospective study of 41 consecutive patients, it was shown that PET CT (using a hybrid PET CT scanner) was superior to EUS and CT scan in evaluating nodal status after chemoradiotherapy. PET CT was also the more accurate modality for identifying nodal status.176 In a systematic review of published information, the overall joint sensitivity-specificity (Q-point) values for FDG PET was reported to be 85% which was no different than EUS at 86% and significantly better than CT at 54%.173 Thus, subject to local availability of resources and expertise, it appears that for restaging of esophageal cancer patients after chemoradiotherapy, conventional CT, particularly the single detector variety, has no role and FDG PET and EUS FNA are likely to provide complementary information. For example, if hybrid PET CT shows suspected nodal disease or residual primary tumor, this finding should be confirmed by EUS FNA. On the other hand, it is not known whether, in a patient with a completely negative PET CT, additional evaluation with EUS FNA will improve accuracy of assessment of restaging evaluation.
PREVENTION
Based on data from epidemiologic studies and supported by insight in the molecular pathobiology of esophageal cancers, selenium supplementation and use of COX inhibitors have received attention as strategies for chemoprevention of esophageal cancer. Unfortunately, two randomized trials have reported rather disappointing results. In one trial of 100 patients with Barrett’s dysplasia, administration of 200 mg of celecoxib twice daily for 48 weeks did not prevent progression of the dysplasia to adenocarcinoma.177 In a second randomized placebo-controlled trial involving 238 Chinese patients with mild or moderate esophageal squamous dysplasia, selenomethionine (200 µg/day) and/or celecoxib 200 mg twice daily did not prevent esophageal squamous carcinogenesis in high-risk subjects. Selenomethionine did result in a nonsignificant trend toward dysplasia regression (43% vs. 32%) and less dysplasia progression (14% vs. 19%) compared with no selenomethionine.178
TREATMENT
Early Esophageal Cancers
Early esophageal cancers include carcinoma in situ (Tis) or cancers that invades the mucosa (T1a or T1m) or submucosa (T1b or T1sm) but without nodal disease (i.e., T1, N0, M0; see Table 46-2).
Endoscopic Therapy
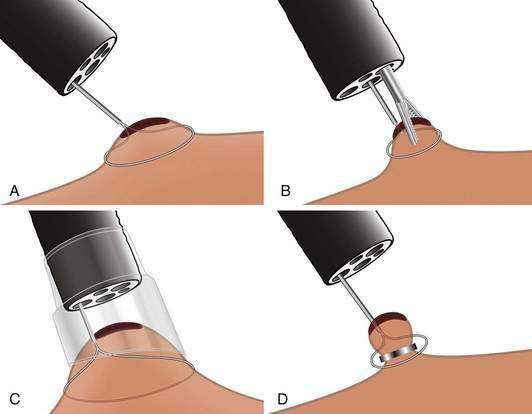
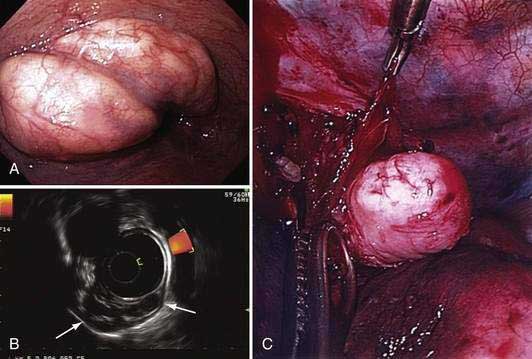
Esophageal resection has been the standard of care for early esophageal cancer. However, evidence has accumulated that early esophageal cancers with m1 or m2 depth of invasion have little or no risk of nodal disease. In such patients, endoscopic resection may be an attractive alternative to surgery. One endoscopic resection technique is similar to saline-assisted polypectomy using a simple polypectomy snare. A second technique (lift-and-cut) uses a double-channel endoscope and a biopsy forceps to lift up the lesion prior to polypectomy. A third and common technique is the cap-assisted technique, which uses a transparent cap attached to the endoscope to suction the target lesion (typically after submucosal injection of saline below the lesion to form a pseudopolyp), which is then resected using a pre-looped snare. A fourth ligate-and-cut (band-and-cut) technique uses a banding device to suction the target lesion, followed by application of a rubber band ligation prior to polypectomy. This particular technique has become popular with the introduction of a commercially available endoscopic mucosal resection (EMR) kit, which is a modified variceal band ligation device that allows multiple resections with a single endoscopic intubation of the esophagus (Figs. 46-11 and 46-12). The latter two techniques have been compared in a controlled trial and were similar in terms of size of the resected tissue specimens and complications.179
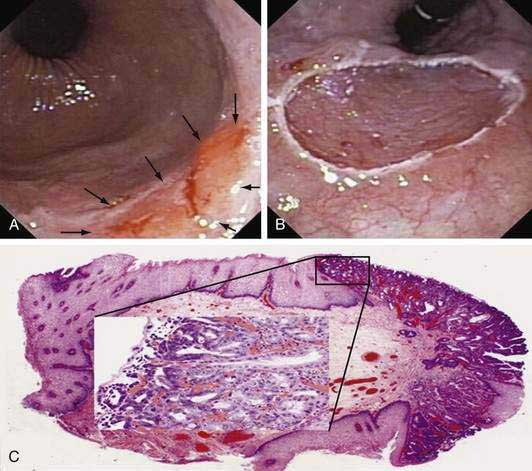
Figure 46-12. A, Endoscopic image of a nodular superficially ulcerated lesion at the gastroesophageal junction, which, on biopsy, was an adenocarcinoma in the setting of Barrett’s esophagus. B, An endoscopic mucosal resection (EMR) was performed using the ligate-and-cut technique (see Fig. 46-11D). C, Photomicrograph of the EMR specimen showed adenocarcinoma (Hematoxylin and eosin, ×2). The inset is a magnified view (Hematoxylin and eosin, ×40) of the area of the mucosa indicated by the square in which an intramucosal adenocarcinoma (m1; see Fig. 46-3) could be identified by the presence of fused glands with severe cytological atypia.
(Courtesy Giovanni De Petris, MD, Scottsdale, Ariz.)
To date, the largest series using EMR in the setting of Barrett’s adenocarcinoma reported 100 consecutive patients with early esophageal adenocarcinoma. These patients underwent 144 resections (maximum, 3 resections) using the ligate-and-cut technique; a complete local remission was achieved within 1.9 months (range, 1 to 18 months) in 99 of the 100 patients. During a mean follow-up period of 36.7 months, recurrent or metachronous carcinomas were detected in 11% of the patients, but successful repeat treatment with endoscopic resection was possible in each case. The calculated five-year survival rate was 98%. Minor bleeding, which was easily controlled with endoscopic therapy, occurred in 11% of patients.158
Other investigators have reported similar success with endoscopic resection of early esophageal cancer, including in patients with squamous cell cancers.180,181 The ideal lesion for EMR is a solitary, nodular lesion, less than 20 mm in diameter, limited to the mucosa and with a suitable macroscopic appearance (macroscopic types I, IIa, IIb, and IIc by the Paris classification). En-bloc resection is always preferred to piecemeal resection, the latter of which increases the risk of local recurrence in addition to making a histopathologic interpretation of complete resection more difficult.
Endoscopic submucosal dissection (ESD) is becoming popular in Japan and involves a deeper and larger resection of the esophageal wall by dissecting the submucosal connective tissue just beneath the target lesion from the underlying muscle layer using the hook knife or other electrocautery devices. ESD is usually reserved for larger lesions with evidence of some degree of submucosal invasion. ESD also requires high level of expertise on the part of the endoscopist.181 In a comparative trial, ESD using the hook knife had a higher en-bloc and curative resection rate than EMR using a transparent cap, but with a similar complication rate.182
Endoscopic resection is often combined with other forms of endoscopic ablative therapy such as photodynamic therapy (PDT), laser ablation, thermal ablation with argon plasma coagulation (APC), or radiofrequency ablation. Combination therapy is particularly applicable for larger lesions, multifocal lesions, or for treatment of flat high-grade dysplasia in patients with Barrett’s esophagus. Experience with EMR combined with other endoscopic ablative therapy is limited, but may be a viable alternative to surgery.183 PDT has been studied as single-modality therapy. In a recent report, a complete response could be achieved in all 31 patients with early adenocarcinoma and Barrett’s esophagus treated with 5-aminolevulinic acid–based PDT, but local recurrence occurred in almost one third of patients.184
Surgical Therapy
Although recent studies have reported excellent outcomes with endoscopic therapy for early esophageal cancers, there is a relative paucity of long-term follow-up data. Thus, the role of endoscopic therapy for early esophageal cancer is still a matter of controversy, particularly in the United States. Also, excellent outcomes after endoscopic therapy of early esophageal cancer are typically seen in specialized centers treating a small number of patients; it is unclear whether such excellent outcomes can be achieved in community practice.185 In a recent study of early esophageal cancer that analyzed data from a large population-based cancer registry, cancer-free survival was similar in patients undergoing endoscopic therapy or surgical resection.186 Obviously, management decisions for treatment of early esophageal cancer should be individualized. Although younger, otherwise healthy patients with larger mutifocal lesions with submucosal invasion may benefit from surgery, selected older patients with limited disease and multiple comorbidities may be more appropriate candidates for endoscopic therapy. When surgical resection is considered for treatment of early esophageal cancer without submucosal involvement, less morbid surgical techniques such as vagal-sparing esophagectomy, which has fewer postoperative complications, may be considered.187
Radiation Therapy and Chemotherapy
The role of radiotherapy, with or without chemotherapy, as definitive treatment of early esophageal cancer has been evaluated. In a series of 141 Japanese patients with superficial esophageal cancer who were treated with external beam radiotherapy, the three-year survival rates were 90% and 70% for tumors limited to the mucosa and submucosa, respectively, and were comparable with reported surgical outcomes.188 It is likely that concurrent chemotherapy may be effective in decreasing local recurrence, particularly in tumors with submucosal invasion. Addition of high-dose brachytherapy may decrease the rate of local recurrence, but at a higher risk of radiation toxicity.189 Some investigators have reported excellent results using a combination of endoscopic resection followed by chemoradiotherapy or even using chemoradiotherapy after EMR for management of nodal recurrence.190,191 Until controlled studies are conducted, radiotherapy (with or without concurrent chemotherapy) should be reserved for those patients with early esophageal cancer who cannot undergo endoscopic resection due to contraindications to this procedure (e.g., esophageal varices or severe cervical spinal disease) in whom endoscopic procedures may be more risky.
Locally Advanced Cancer
Surgical Therapy
Different surgical techniques have been used for primary management of esophageal cancer, the choice of which depend on factors such as tumor location, stage, extent of lymphadenectomy, replacement conduit planned, use of neoadjuvant therapy, and the preference of the surgeon. Overall, 30% to 59% of patients with esophageal cancer are candidates for potentially curative resection, with five-year survival rates ranging from 15% to 24%. However, the operative mortality and perioperative morbidity rates (e.g., cardiopulmonary events, infection, anastomotic leaks or strictures) vary from 4% to 10% and 26% to 41%, respectively.192
Transhiatal esophagectomy and Ivor-Lewis transthoracic esophagectomy are the most common techniques used in the United States. Modifications of transthoracic esophagectomy, such as transthoracic total esophagectomy with node dissection and cervical esophagogastric anastomosis (the three-field technique) and left thoracoabdominal incision with gastric pull-up into the left chest, are also used frequently. These techniques have their own advantages and disadvantages. For example, a complete mediastinal exploration and lymphadenectomy is not possible with the transhiatal approach. With the transthoracic technique, there is greater likelihood of a troublesome intrathoracic anastomotic leakage and a higher incidence of postoperative bile reflux. In a prospective randomized trial, transhiatal esophagectomy was associated with lower morbidity than transthoracic esophagectomy with extended en-bloc lymphadenectomy.193 However, a recent update of this cohort showed a 14% five-year survival advantage in patients with distal esophageal adenocarcinomas if they had been randomized to the transthoracic approach.194 Despite this finding, an earlier meta-analysis of published trials, and a more recent population-based outcomes study found that transthoracic and transhiatal resections yielded similar long-term survival rates.195,196 Published data also suggest that patients undergoing esophagectomy in high-volume hospitals may have less perioperative morbidity, lower mortality, and improved early clinical outcomes, although there are conflicting data on the effect of hospital volume on long-term survival.197,198
Radiotherapy
Radiation therapy has been a cornerstone of treatment of esophageal cancer. Modern radiotherapy techniques such as three-dimensional conformal radiotherapy [3D-CRT] and intensity modulated radiotherapy [IMRT] have reduced toxicity from radiotherapy. Radiotherapy alone can result in long-term survival in patients with esophageal cancer, and reports of five-year survival of 21% to 59% have been published in selected patients with early disease. In a recent Chinese trial, 269 patients with resectable squamous esophageal cancer type were randomized to surgery versus late-course accelerated hyperfractionated conformal radiotherapy alone; the five-year survival rates were similar: 36.9% versus 34.7%, respectively.199
Combined Chemoradiotherapy
In a Cochrane review of randomized trials, concomitant (but not sequential) chemoradiotherapy provided a significant reduction in mortality at one and two years. Combined chemoradiotherapy provided an absolute risk reduction for mortality by 9% and 4% after one year and two years, respectively, with a 12% reduction in local recurrence rate.200 However, there was significant toxicity related to such treatment. Thus, it appears that combined concurrent chemoradiotherapy, particularly using cisplatinum-based chemotherapy, is superior to radiotherapy alone in the setting of definitive therapy for locoregional disease (without surgical intervention), but better outcomes come with a cost of higher toxicity. Also, use of higher dosage of radiotherapy did not increase survival, but added to toxicity.201
Given the poor long-term results with surgical resection alone, investigators have studied the effect of adding preoperative chemoradiotherapy on long-term survival. This neoadjuvant approach carries the theoretical promise of improved local tumor control and eradication of micrometastasis. Several randomized controlled trials have been published comparing neoadjuvant chemoradiotherapy with surgery alone in patients with localized cancer of the thoracic esophagus or esophagogastric junction. Although it appears that neoadjuvant chemoradiotherapy, particularly administered concurrently, may impart a modest survival advantage, the results from the various trials are not uniform. Two meta-analyses have addressed this issue. The first showed that compared with surgery alone, neoadjuvant chemoradiation followed by surgery improved three-year survival and reduced locoregional cancer recurrence.202 The overall rate of resection attempt was reduced, but when resection was attempted, a higher rate of complete (R0) resection was achieved. There was also a trend toward increased treatment complications with chemoradiotherapy, and benefits were restricted to those undergoing concurrent chemoradiotherapy administration. In a second, more recent meta-analysis, pooled results from 10 randomized trials comparing neoadjuvant chemoradiotherapy with surgery alone in more than 1000 patients showed that the relative risk for all-cause mortality with neoadjuvant chemoradiotherapy versus surgery alone was 0.81 (95% CI 0.70 to 0.93), resulting in a 13% survival difference at two years. Results were similar in patients with squamous cell or adenocarcinoma of the esophagus.203 In order to consolidate the modest gains achieved by neoadjuvant therapy in this situation, several investigators have attempted intensification of the preoperative regimen by including cycles of induction chemotherapy prior to concurrently administered chemoradiotherapy. Also, other chemotherapeutic drugs with better radiosensitizing potential (such as paclitaxel, carboplatin, and oxaliplatin) have been studied, with some initial encouraging results. However, the strategy of intensification of the preoperative regimen has not been proven in randomized controlled trials. The concern remains whether the increased complications seen with such aggressive regimens will be justified by a corresponding increase in long-term survival.
Few trials have compared neoadjuvant chemotherapy (without radiation) with surgery alone. In the same meta-analysis referred to earlier, neoadjuvant chemotherapy was favored over surgery alone, albeit with a lower reduction in mortality compared with neoadjuvant chemoradiotherapy.203
Several issues remain unanswered in the management of locally advanced esophageal cancer and esophagogastric junctional cancers. It is not known if there is any difference in outcome in patients treated with primary chemoradiotherapy or with neoadjuvant chemoradiotherapy followed by surgery. Experience from few controlled studies suggests that patients treated with neoadjuvant chemoradiotherapy followed by surgery had better locoregional control and needed fewer palliative procedures, but had similar overall survival and quality of life compared with those treated with primary (definitive) therapy.204,205 It is important to note that these conclusions are based on studies in which most patients had squamous cell cancer; in patients with adenocarcinoma, the case for the necessity of surgery after chemoradiotherapy is stronger. Salvage esophagectomy in patients with recurrence after primary (definitive) chemoradiotherapy may be a feasible option in a minority of patients, but such a strategy is plagued by two problems: first, difficulty in identifying recurrent disease in these patients and second, the increased risk of perioperative complications in patients undergoing salvage esophagectomy compared with primary esophagectomy or a planned esophagectomy after combined modality treatment.
Locally Advanced Unresectable Cancer
Metastatic Cancer Chemotherapy
Similar to other advanced gastrointestinal cancers, systemic chemotherapy has a role in palliative treatment in patients with advanced, unresectable esophageal cancer, particularly those with distant metastatic disease. Although a large volume of published information exists in this area evaluating a large number of chemotherapy regimens, there is no convincing evidence that one particular regimen is superior to others. Based on randomized trials, it appears that a combination of cisplatinum, 5-fluorouracil (5-FU), and either epirubicin (an anthracyclic antineoplastic agent) or docitaxel is appropriate for first-line therapy. Capecitabine, the orally administered fluoropyrimidine, has attracted attention as a replacement agent for 5-FU infusion, because it eliminates the need for central venous access and an ambulatory infusion pump. Recent evidence suggests that capecitabine and oxaliplatin are as effective as 5-FU and cisplatin.206 Irinotecan-containing regimens have also been studied recently in advanced esophageal and esophagogastric junctional cancers.
Biological agents such as cetuximab (a monoclonal antibody against the EGF receptor), erlotinib (an orally active tyrosine kinase inhibitor), and bevacizumab (which targets VEGF) have been studied in a preliminary fashion as single agents or parts of combination chemotherapeutic regimens. In the recent Southwest Oncology Group (SWOG) trial, patients with unresectable or metastatic esophagogastric junctional adenocarcinoma had a reasonable response to erlotinib, with a very attractive toxicity profile.207 Experience with second-line chemotherapy in patients who fail initial chemotherapy regimens has been generally disappointing.
Endoscopic Palliative Treatment
Laser Therapy
Laser photoablation using neodymium:yttrium-aluminum-garnet (Nd:YAG), potassium titanyl phosphate (KTP), and argon lasers has been used extensively in the palliation of malignant dysphagia associated with esophageal cancers (Fig. 46-13). The bulk of the experience has been attained with the Nd:YAG laser in the noncontact mode. The endoscopist usually passes the laser fiber through the accessory channel of the endoscope and with the tip of the fiber placed about 10 mm from the tumor tissue, a combination of tissue vaporization and coagulation necrosis is achieved with a typical Nd:YAG laser setting of 40 to 100 W, used either in a pulsed or continuous mode. The ablation is typically started at the distal margin of the tumor, moving proximally in a circumferential manner. However, if there is complete luminal obstruction, the laser ablation is started at the proximal extent of the tumor in order to try to carefully bore through the obstructive tumor tissue. In the majority of patients, more than one session is required to establish reasonable luminal patency, and the procedure is typically repeated at 48- to 72-hour intervals. Chest pain, odynophagia, low-grade fever, and leukocytosis may occur after the procedure. Follow-up endoscopy with a “touch-up” session in three to four weeks is often required, depending on the response to laser ablation. Reasonable improvement in dysphagia can be achieved in up to 70% of patients, which typically lasts three to six weeks and rarely beyond 12 months in absence of retreatment. Complication rates are approximately 4% in experienced hands, with a 1% procedural mortality rate. Polypoid, fleshy, noncircumferential lesions in the body of the esophagus are easier to treat with laser ablation. Anastomotic recurrences and tumor overgrowth in a previously placed endoprosthesis can also be treated by laser photoablation.
Photodynamic Therapy
PDT has a role in palliation of dysphagia in patients with advanced esophageal cancer (Fig. 46-14). Comparative trials suggest that PDT may be better than endoscopic laser ablation in terms of fewer perforations and better tumor response, particularly in proximal tumors and long tumors. Photosensitivity is a significant problem in up to 20% of patients. PDT has also been used for ablation of tumor in-growth after stent placement.
Esophageal Stent Placement
Use of esophageal stents for palliation of malignant dysphagia has increased rapidly and compares favorably with other endoscopic palliative techniques. Self-expanding covered metallic stents are the most effective palliative therapy for tracheoesophageal fistula. However, palliative stent placement in patients with esophageal cancer is not always followed by improvement of nutritional parameters.208
The rigid plastic esophageal stents have been replaced by self-expanding metal stents (SEMS) because of their familiarity, ease of placement, improved palliation of dysphagia, and better safety profile. The covered SEMS are the only effective practical treatment available for management of esophagorespiratory fistula (Fig. 46-15). SEMS are typically placed as an outpatient procedure under conscious sedation. The patient is placed in left lateral or supine position and an initial endoscopic examination is carefully performed to estimate the lesion’s location, length, configuration, luminal diameter, and relationship to the upper esophageal sphincter and esophagogastric junction. A recent contrast esophagogram is often helpful to plan the placement of the SEMS. Esophageal dilation is often performed prior to stent placement, but may not be always recommended or required. Most endoscopists use fluoroscopic guidance for placement, although one study questioned the utility of such a practice.209 Accurate marking of the tumor margins in relation to the distal and proximal end of the SEMS, and consideration of the stent foreshortening are critical to optimal placement. A post-placement barium contrast study should be performed routinely in patients with esophagorespiratory fistula to document effective sealing of the fistula. Most patients can be started on oral intake immediately after the endoscopic procedure. Patients should have individualized dietary instruction to prevent stent occlusion by food impaction. Antiemetic and antitussive drugs may be needed if the patient experiences significant postprocedure retching, coughing, or hiccups, both to relieve those symptoms and prevent stent dislodgement.
The Z-stent (Cook Medical, Inc., Winston-Salem, N.C.) is made of stainless steel wires shaped in a Z configuration and releases without shortening to a diameter of 18 mm. The version preferred by many endoscopists, because of a lower risk of stent migration, has uncovered segments in the proximal and distal sections. A model designed with a latex “wind-sock” extending from the distal opening of the stent (Dua stent) and intended to prevent gastroesophageal reflux has been marketed for esophagogastric junction and distal esophageal cancers, tumors in which the stent must bridge the esophagogastric junction. Another commercially available antireflux stent (FerX-Ella; Dr Karel Volenec, Ella CS, Hradec Králové, Czech Republic) has been used to minimize reflux of gastric contents. Clinical experience with these antireflux stents is limited, and randomized studies with small numbers of patients failed to show a difference in reflux symptoms or global quality of life.210,211 Another introduced version of the Z-stent (Evolution Controlled-Release Stent, Cook Medical, Inc., Winston-Salem, N.C.) allows more controlled stepwise release and better ability to recapture and reposition the stent.
A covered hybrid esophageal metallic stent has been marketed (Allimax-E, Alveolus, Charlotte, N.C.). Some experts believe that covered metal stents are more likely to migrate compared with the bare metal uncovered stent. A double-layered stent, the Niti-S stent (Taewong Medical, Seoul, South Korea), with an inner polyurethane layer (to prevent tumor in-growth) and an outer uncovered nitinol wire tube (to allow the mesh of the stent to embed itself in the esophageal wall to minimize stent migration) has been marketed; initial experience with this stent has been promising.212
The Polyflex (Boston Scientific, Inc., Natick, Mass) is a completely coated self-expanding nonmetallic (plastic) stent introduced for palliation of malignant and benign strictures. Advantages are its lower cost and potential of removability. Published series have shown the Polyflex stent to be safe and effective in relieving malignant dysphagia. In a randomized prospective trial of 101 patients with unresectable esophageal cancer, there was no difference between the Polyflex and Ultraflex stents in palliating dysphagia. However, Polyflex stents had a higher rate of complications such as tumor overgrowth, hyperplastic granulomatous reaction, food bolus impaction, and stent migration and may be more technically difficult to place.213
There is little comparative information on the utility of different types of SEMS. A randomized trial comparing Flamingo, Ultraflex, and Z-stents failed to show superiority of one stent over another.214 In another randomized study comparing Ultraflex, Niti-S, and Polyflex stents, all three stents adequately palliated malignant dysphagia, although Polyflex stents had a higher rate of migration.215 The decision to place a particular type of stent should be individualized and is typically determined by assessment factors such as tumor length, location, and configuration; the patient’s performance status and overall prognosis; plans for further palliative therapy, such as chemoradiotherapy; and local expertise.
Available information reports good results with SEMS in palliating dysphagia and patients with esophagorespiratory fistula, with 70% to 100% reported success rates in sealing the fistula.216 Complications occur in 30% to 40% of patients with SEMS, although most are minor. Common complications include tumor in-growth or tumor overgrowth (5% to 20%), stent migration (10%), chest pain, procedure-related perforation, food bolus impaction, bleeding, foreign body sensation, and reflux esophagitis. Also, aspiration of gastric contents is commonly seen in patients with a stent placed across the esophagogastric junction.
Brachytherapy
Brachytherapy administered intraluminally by a nasoesophageal applicator has gained attention for palliation of malignant dysphagia. In a randomized trial, single-dose brachytherapy was more effective than metal stent placement for palliation of dysphagia in patients with unresectable esophageal cancer, although dysphagia improved more rapidly in patients who were treated with the Ultraflex SEMS. The brachytherapy group also had fewer complications than the stent group, and costs were similar.217 A prognostic score based on age, gender, WHO performance score, tumor length, and presence of metastasis has been suggested to identify patients with a poor prognosis in whom stent placement is at least equivalent to brachytherapy.218 In a Markov model analysis using current levels of reimbursements, brachytherapy (and not SEMS) was shown to be the most cost-effective intervention for palliation of malignant dysphagia.219 In a randomized trial of 53 patients with advanced esophageal cancer from China, a SEMS loaded with radioactive iodine (125I) seeds yielded better palliation of malignant dysphagia and better survival than a conventional SEMS.220
Nutritional Therapy
Most patients with esophageal cancer are nutritionally compromised and need specific nutritional management for improvement of functional status before and after surgery, during chemoradiotherapy, and as an adjunct to other palliative measures. Success of endoscopic therapy in palliation of dysphagia does not necessarily translate into improved nutritional status, and many of these patients need establishment of enteral access by endoscopic, surgical, or radiologic procedures for maximizing nutritional intake. A surgical feeding jejunostomy should be performed in all patients undergoing esophagectomy; percutaneous endoscopic gastrostomy (PEG) is contraindicated in those patients who may be candidates for gastric pull-up surgery. In many patients with esophagectomy, or those with a SEMS placed across the esophagogastric junction, feeding the patient through a PEG tube may lead to recurrent aspiration, and in these patients a PEG with a jejunal feeding tube extension or even direct percutaneous endoscopic jejunostomy may be preferred. Some patients with markedly compromised nutritional status may benefit for short-term parenteral nutrition, especially during the perioperative period (see Chapters 4 and 5).
Multidisciplinary Care
It is clear that appropriate management of patients with esophageal cancer needs input from many providers from different specialties. A multidisciplinary team approach with a tailored treatment plan for each patient with esophageal cancer has been shown to result in improved staging, lower operative mortality, and improved five-year survival when compared with a group of patients managed by surgeons who were working independently.221
OTHER MALIGNANT EPITHELIAL TUMORS (see Table 46-1)
MALIGNANT NONEPITHELIAL TUMORS (see Table 46-1)
SARCOMA
Malignant mesenchymal esophageal tumors are uncommon and represent about 5% of all gastrointestinal sarcomas. Leiomyosarcomas are the most common and can be difficult to distinguish from leiomyomas. Others include malignant gastrointestinal stromal tumors (see following and Chapter 30), rhabdomyosarcoma, fibrosarcoma, liposarcoma, fibrous histiocytoma, and choriocarcinoma. Tumor characteristics include spindle-shaped smooth muscle cells, high mitotic rates, local invasion, and, infrequently, distant metastasis. Most patients exhibit dysphagia. Superficial mucosal biopsy specimens are often nondiagnostic, and EUS-guided FNA is usually required for a definitive diagnosis. Cytologic needle aspiration specimens, however, cannot reliably exclude malignancy. Treatment is based on surgical resection, often followed by radiotherapy. Kaposi’s sarcoma (KS) has been reported in the esophagus usually concomitant with oral and skin lesions in patients with AIDS. When symptomatic, KS can present with dysphagia, odynophagia, and, rarely, gastrointestinal hemorrhage. In symptomatic patients, endoscopic laser ablation or injection sclerotherapy may be attempted for hemorrhage or obstruction.
BENIGN NONEPITHELIAL TUMORS
GASTROINTESTINAL STROMAL TUMOR (see also Chapter 30)
Gastrointestinal stromal tumors (GISTs) are rare benign tumors of the esophagus and account for approximately 1% of all GISTs (Fig. 46-17). Most esophageal GISTs arise from the distal third of the esophagus and are solitary. Most GISTs express CD117 antigen in contrast to leiomyomas or other spindle-cell tumors of the gastrointestinal tract, which are typically CD117-negative. Histopathologic evaluation reveals that these tumors are firm, round, gray or yellow, unencapsulated, and composed of spindle-shaped cells with cigar-shaped elongated nuclei. The risk of malignant transformation of GISTs is variable and depends on the size of the GIST and the mitotic rate. Lesions less than 2 cm in size with a mitotic rate of less than five per 50 high-power fields are considered to have a very low risk of malignancy.
Management of an esophageal GIST should take into consideration the presence or absence of symptoms and the risk of eventual malignant transformation. Most small, incidentally detected GISTs can be followed conservatively. Surgery should be considered in symptomatic patients, in those in whom the diagnosis is uncertain (particularly if there is suspicion of malignant transformation), and in patients in whom the lesion shows interval growth during surveillance endoscopy. Surgical excision, particularly using a minimally invasive approach, has been reported. Endoscopic resection may be feasible in a minority of patients with small GISTs arising from the muscularis mucosae. Administration of orally active tyrosine kinase inhibitors such as imatinib mesylate (Gleevec) and sunitinib (Sutent) has become an option in patients with resected GISTs as an adjuvant therapy and in patients with unrespectable GIST as a cytoreductive therapy (see Chapter 30).222
FIBROVASCULAR POLYP
Large, benign fibrovascular polyps occur most commonly on the upper third of the esophagus, near the cricopharyngeus muscle (Fig. 46-18). They may contain a mixture of fibrovascular tissue, adipose cells, and stroma, but are uniformly covered by squamous epithelium. Although most are asymptomatic, bizarre symptoms of polyp regurgitation and asphyxiation have been reported. Barium esophagography and endoscopy are usually sufficient for diagnosis, but MRI can help determine the origin of these polyps and plan for surgery. The latter is recommended for large symptomatic polyps. EUS-assisted endoscopic snare resection of fibrovascular polyps may be feasible for smaller lesions.
Atherfold PA, Jankowski JA. Molecular biology of Barrett’s cancer. Best Pract Res Clin Gastroenterol. 2006;20:813-27. (Ref 98.)
Coorley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: A systematic review and meta-analysis. Gastroenterology. 2003;124:47-56. (Ref 62.)
Das A, Singh V, Fleischer DE, Sharma VK. A comparison of endoscopic treatment and surgery in early esophageal cancer: An analysis of surveillance epidemiology and end results data. Am J Gastroenterol. 2008;103:1340-5. (Ref 186.)
Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: A meta-analysis. Lancet Oncol. 2007;8:226-34. (Ref 203.)
Guo JH, Teng GJ, Zhu GY, et al. Self-expandable esophageal stent loaded with 125I seeds: Initial experience in patients with advanced esophageal cancer. Radiology. 2008;247:574-81. (Ref 220.)
Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;18(340):825-31. (Ref 40.)
Larsson SC, Giovannucci E, Wolk A. Folate intake, MTHFR polymorphisms, and risk of esophageal, gastric, and pancreatic cancer: A meta-analysis. Gastroenterology. 2006;131:1271-83. (Ref 17.)
Ngamruengphong S, Sharma VK, Das A. Diagnostic yield of methylene blue chromoendoscopy for detecting specialized intestinal metaplasia and dysplasia in Barrett’s esophagus: A meta-analysis. Gastrointest Endosc. 2009;69:1021-8. (Ref 121.)
Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: A meta-analysis. Gastrointest Endosc. 2008;67:394-8. (Ref 52.)
Stein HJ, Feith M, Bruecher BL, et al. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg. 2005;242:566-73. (Ref 115.)
Steyerberg EW, Homs MY, Stokvis A, et al. Stent placement or brachytherapy for palliation of dysphagia from esophageal cancer: A prognostic model to guide treatment selection. Gastrointest Endosc. 2005;62:333-40. (Ref 218.)
Takubo K, Aida J, Sawabe M. Early squamous cell carcinoma of the oesophagus: The Japanese viewpoint. Histopathology. 2007;51:733-42. (Ref 102.)
Wang KK, Sampliner RE, Parameters Practice. Committee of the American College of Gastroenterology. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788-97. (Ref 44.)
Westerterp M, van Westreenen HL, Reitsma JB, et al. Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy—Systematic review. Radiology. 2005;236:841-51. (Ref 173.)
Wolfsen HC, Crook JE, Krishna M, et al. Prospective, controlled tandem endoscopy study of narrow band imaging for dysplasia detection in Barrett’s esophagus. Gastroenterology. 2008;135:24-31. (Ref 128.)
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108.
2. Garcia M, Jemal A, Ward EM, et al. Global cancer facts & figures 2007. Atlanta: American Cancer Society; 2007.
3. Ries LAG, Melbert D, Krapcho M, et al, editors. SEER Cancer statistics review, 1975-2005. 2008. National Cancer Institute, Bethesda, MD. http://seer.cancer.gov/csr/1975_2005/ based on November 2007 SEER data submission, posted to the SEER website
4. Saidi F, Sepehr A, Fahimi S, et al. Oesophageal cancer among the Turkomans of northeast Iran. Br J Cancer. 2000;83:1249-54.
5. Jansson C. Socioeconomic factors and risk of esophageal adenocarcinoma: A nationwide Swedish case-control study. Cancer Epidemiol Biomarkers Prev. 2005;14:1754-61.
6. Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184-7.
7. Pera M, Manterola C, Vidal O, Grande L. Epidemiology of esophageal adenocarcinoma. J Surg Oncol. 2005;92:151-9.
8. Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin North Am. 2002;11:235-56.
9. Bollschweiler E, Wolfgarten E, Gutschow C, Hölscher AH. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer. 2001;92:549-55.
10. Mengesha B, Ergete W. Staple Ethiopian diet and cancer of the oesophagus. East Afr Med J. 2005;82:353-6.
11. Isaacson C. The change of the staple diet of black South Africans from sorghum to maize (corn) is the cause of the epidemic of squamous carcinoma of the oesophagus. Med Hypotheses. 2005;64:658-60.
12. de Boer JG, Yang H, Holcroft J, Skov K. Chemoprotection against N-nitrosomethylbenzylamine-induced mutation in the rat esophagus. Nutr Cancer. 2004;50:168-73.
13. Wu IC, Lu CY, Kuo FC, et al. Interaction between cigarette, alcohol and betel nut use on esophageal cancer risk in Taiwan. Eur J Clin Invest. 2006;36:236-41.
14. Mark SD, Qiao YL, Dawsey SM, et al. Prospective study of serum selenium levels and incident esophageal and gastric cancers. J Natl Cancer Inst. 2000;92:1753-63.
15. Blot WJ, Li JY, Taylor PR, et al. Nutrition intervention trials in Linxian, China: Supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85:1483-92.
16. Abnet CC, Lai B, Qiao YL, et al. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst. 2005;97:301-6.
17. Larsson SC, Giovannucci E, Wolk A. Folate intake, MTHFR polymorphisms, and risk of esophageal, gastric, and pancreatic cancer: a meta-analysis. Gastroenterology. 2006;131:1271-83.
18. Li D, Diao Y, Li H, Fang X, Li H. Association of the polymorphisms of MTHFR C677T, VDR C352T, and MPO G463A with risk for esophageal squamous cell dysplasia and carcinoma. Arch Med Res. 2008;39:594-600.
19. Tavani A, Bertuzzi M, Talamini R, et al. Coffee and tea intake and risk of oral, pharyngeal and esophageal cancer. Oral Oncol. 2003;39:695-700.
20. De Stefani E, Deneo-Pellegrini H, Ronco AL, et al. Food groups and risk of squamous cell carcinoma of the oesophagus: A case-control study in Uruguay. Br J Cancer. 2003;89:1209-14.
21. Altieri A, Bosetti C, Gallus S, et al. Wine, beer and spirits and risk of oral and pharyngeal cancer: A case-control study from Italy and Switzerland. Oral Oncol. 2004;40:904-9.
22. Guo YM, Wang Q, Liu YZ, et al. Genetic polymorphisms in cytochrome P4502E1, alcohol and aldehyde dehydrogenases and the risk of esophageal squamous cell carcinoma in Gansu Chinese males. World J Gastroenterol. 2008;14:1444-9.
23. Yokoyama A, Kato H, Yokoyama T, et al. Genetic polymorphisms of alcohol and aldehyde dehydrogenases and glutathione S-transferase M1 and drinking, smoking, and diet in Japanese men with esophageal squamous cell carcinoma. Carcinogenesis. 2002;23:1851-9.
24. Zendehdel K, Nyrén O, Edberg A, Ye W. Risk of esophageal adenocarcinoma in achalasia patients, a retrospective cohort study in Sweden. Am J Gastroenterol. 2007;102:1-5.
25. VonBrevern M, Hollstein MC, Risk JM, et al. Loss of heterozygosity in sporadic oesophageal tumours in the tylosis oesophageal cancer (TOC) gene region of chromosome 17q. Oncogene. 1998;17:2101-5.
26. Rosa DD, Pasqualotto AC, Denning DW. Chronic mucocutaneous candidiasis and oesophageal cancer. Med Mycol. 2008;46:85-91.
27. Erkal HS, Mendenhall WM, Amdur RJ, et al. Synchronous and metachronous squamous cell carcinomas of the head and neck mucosal sites. J Clin Oncol. 2001;19:1358-62.
28. Petit T, Georges C, Jung GM, et al. Systematic esophageal endoscopy screening in patients previously treated for head and neck squamous-cell carcinoma. Ann Oncol. 2001;12:643-6.
29. Zablotska LB, Chak A, Das A, Neugut AI. Increased risk of squamous cell esophageal cancer after adjuvant radiation therapy for primary breast cancer. Am J Epidemiol. 2005;161:330-7.
30. Syrjänen KJ. HPV infections and oesophageal cancer. J Clin Pathol. 2002;55:721-8.
31. Kubo A, Levin TR, Block G, et al. Dietary antioxidants, fruits, and vegetables and the risk of Barrett’s esophagus. Am J Gastroenterol. 2008 May 20. [Epub ahead of print]
32. Kubo A, Corley DA. Meta-analysis of antioxidant intake and the risk of esophageal and gastric cardia adenocarcinoma. Am J Gastroenterol. 2007;102:2323-30.
33. Ibiebele TI, Hughes MC, O’Rourke P, et al. Cancers of the esophagus and carbonated beverage consumption: A population-based case-control study. Cancer Causes Control. 2008;19:577-84.
34. Freedman ND, Abnet CC, Leitzmann MF, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol. 2007;165:1424-33.
35. Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev. 2008;17:352-8.
36. Corley DA, Kubo A, Levin TR, et al. Abdominal obesity and body mass index as risk factors for Barrett’s esophagus. Gastroenterology. 2007;133:34-41.
37. Edelstein ZR, Farrow DC, Bronner MP, et al. Central adiposity and risk of Barrett’s esophagus. Gastroenterology. 2007;133:403-11.
38. Cook MB, Greenwood DC, Hardie LJ, et al. A systematic review and meta-analysis of the risk of increasing adiposity on Barrett’s esophagus. Am J Gastroenterol. 2008;103:292-300.
39. Smith M, Zhou M, Whitlock G, et al. Esophageal cancer and body mass index: Results from a prospective study of 220,000 men in China and a meta-analysis of published studies. Int J Cancer. 2008;122:1604-10.
40. Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;18(340):825-31.
41. Whiteman DC, Sadeghi S, Pandeya N, et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57:173-80.
42. Hirota WK, Loughney TM, Lazas DJ, et al. Specialized intestinal metaplasia, dysplasia, and cancer of the esophagus and esophagogastric junction: prevalence and clinical data. Gastroenterology. 1999;116:277-85.
43. Zagari RM, Fuccio L, Wallander MA, et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett’s oesophagus in the general population: Loiano-Monghidoro study. Gut. 2008 Apr 18. [Epub ahead of print]
44. Wang KK, Sampliner RE, Practice Parameters Committee of the American College of Gastroenterology. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788-97.
45. Playford RJ. New British Society of Gastroenterology (BSG) guidelines for the diagnosis and management of Barrett’s oesophagus. Gut. 2006;55:442.
46. van Soest EM, Dieleman JP, Siersema PD, et al. Increasing incidence of Barrett’s oesophagus in the general population. Gut. 2005;54:1062-6.
47. Post PN, Siersema PD, Van Dekken H. Rising incidence of clinically evident Barrett’s oesophagus in the Netherlands: A nation-wide registry of pathology reports. Scand J Gastroenterol. 2007;42:17-22.
48. Hage M, Siersema PD, van Dekken H, et al. Oesophageal cancer incidence and mortality in patients with long-segment Barrett’s oesophagus after a mean follow-up of 12.7 years. Scand J Gastroenterol. 2004;39:1175-9.
49. Sharma P, Falk GW, Weston AP, et al. Dysplasia and cancer in a large multicenter cohort of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2006;4:566-72.
50. Dulai GS, Shekelle PG, Jensen DM, et al. Dysplasia and risk of further neoplastic progression in a regional Veterans Administration Barrett’s cohort. Am J Gastroenterol. 2005;100:775-83.
51. Wani S, Choi W, Sharma P. Low-grade dysplasia in Barrett’s esophagus—An innocent bystander? Pro. Endoscopy. 2007;39:643-6.
52. Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: A meta-analysis. Gastrointest Endosc. 2008;67:394-8.
53. Yousef F, Cardwell C, Cantwell MM, et al. The incidence of esophageal cancer and high-grade dysplasia in Barrett’s esophagus: A systematic review and meta-analysis. Am J Epidemiol. 2008;168:237-49.
54. Anandasabapathy S, Jhamb J, Davila M, et al. Clinical and endoscopic factors predict higher pathologic grades of Barrett dysplasia. Cancer. 2007;109:668-74.
55. Smith KJ, O’Brien SM, Smithers BM, et al. Interactions among smoking, obesity, and symptoms of acid reflux in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2005;14:2481-6.
56. Raghunath A, Hungin AP, Wooff D, Childs S. Prevalence of Helicobacter pylori in patients with gastro-oesophageal reflux disease: Systematic review. BMJ. 2003;326:737.
57. Raghunath AS, Hungin AP, Wooff D, Childs S. Systematic review: The effect of Helicobacter pylori and its eradication on gastro-oesophageal reflux disease in patients with duodenal ulcers or reflux oesophagitis. Aliment Pharmacol Ther. 2004;20:733-44.
58. Rokkas T, Pistiolas D, Sechopoulos P, et al. Relationship between Helicobacter pylori infection and esophageal neoplasia: A meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1413-17.
59. Rubenstein JH, Davis J, Marrero JA, Inadomi JM. Relationship between diabetes mellitus and adenocarcinoma of the oesophagus and gastric cardia. Aliment Pharmacol Ther. 2005;22:267-71.
60. Moreels TG, van Vliet EP, et al. Down syndrome and esophageal cancer. Dis Esophagus. 2007;20:183-6.
61. Fortuny J, Johnson CC, Bohlke K, et al. Use of anti-inflammatory drugs and lower esophageal sphincter-relaxing drugs and risk of esophageal and gastric cancers. Clin Gastroenterol Hepatol. 2007;5:1154-1159.e3.
62. Coorley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: A systematic review and meta-analysis. Gastroenterology. 2003;124:47-56.
63. Su H, Hu N, Shih J, et al. Gene expression analysis of esophageal squamous cell carcinoma reveals consistent molecular profiles related to a family history of upper gastrointestinal cancer. Cancer Res. 2003;63:3872-6.
64. Hu N, Su H, WJ Li, et al. Allelotyping of esophageal squamous-cell carcinoma on chromosome 13 defines deletions related to family history. Genes Chromosomes Cancer. 2005;44:271-8.
65. Chak A, Faulx A, Kinnard M, et al. Identification of Barrett’s esophagus in relatives by endoscopic screening. Am J Gastroenterol. 2004;99:2107-14.
66. Chak A, Ochs-Balcom H, Falk G, et al. Familiality in Barrett’s esophagus, adenocarcinoma of the esophagus, and adenocarcinoma of the gastroesophageal junction. Cancer Epidemiol Biomarkers Prev. 2006;15:1668-73.
67. Hiyama T, Yoshihara M, Tanaka S, Chayama K. Genetic polymorphisms and esophageal cancer risk. Int J Cancer. 2007;121:1643-58.
68. von Rahden BH, Stein HJ, Feith M, et al. Overexpression of TGF-beta1 in esophageal (Barrett’s) adenocarcinoma is associated with advanced stage of disease and poor prognosis. Mol Carcin. 2006;45:786-94.
69. Gibson MK, Abraham SC, Wu TT, et al. Epidermal growth factor receptor, p53 mutation, and pathological response predict survival in patients with locally advanced esophageal cancer treated with preoperative chemoradiotherapy. Clin Cancer Res. 2003;9:6461-8.
70. Fukuchi M, Miyazaki T, Fukai Y, et al. Plasma level of transforming growth factor beta1 measured from the azygos vein predicts prognosis in patients with esophageal cancer. Clin Cancer Res. 2004;10:2738-41.
71. Natsugoe S, Xiangming C, Matsumoto M, et al. Smad4 and transforming growth factor beta1 expression in patients with squamous cell carcinoma of the esophagus. Clin Cancer Res. 2002;8:1838-42.
72. Brien TP, Odze RD, Sheehan CE, et al. HER-2/neu gene amplification by FISH predicts poor survival in Barrett’s esophagus-associated adenocarcinoma. Hum Pathol. 2000;31:35-9.
73. Wang KL, Wu TT, Choi IS, et al. Expression of epidermal growth factor receptor in esophageal and esophagogastric junction adenocarcinomas: Association with poor outcome. Cancer. 2007;109:658-67.
74. Casson AG, Zheng Z, Evans SC, et al. Cyclin D1 polymorphism (G870A) and risk for esophageal adenocarcinoma. Cancer. 2005;104:730-9.
75. Song Y, Zhao C, Dong L, et al. Overexpression of cyclin B1 in human esophageal squamous cell carcinoma cells induces tumor cell invasive growth and metastasis. Carcinogenesis. 2008;29:307-15.
76. Vieth M, Schneider-Stock R, Röhrich K, et al. INK4a-ARF alterations in Barrett’s epithelium, intraepithelial neoplasia and Barrett’s adenocarcinoma. Virchows Arch. 2004;445:135-41.
77. Bian YS, Osterheld MC, Fontolliet C, et al. p16 Inactivation by methylation of the CDKN2A promoter occurs early during neoplastic progression in Barrett’s esophagus. Gastroenterology. 2002;122:1113-21.
78. Nakamura T, Hayashi K, Ota M, et al. Expression of p21(Waf1/Cip1) predicts response and survival of esophageal cancer patients treated by chemoradiotherapy. Dis Esophagus. 2004;17:315-21.
79. Kawakami K, Brabender J, Lord RV, et al. Hypermethylated APC DNA in plasma and prognosis of patients with esophageal adenocarcinoma. J Natl Cancer Inst. 2000;92:1805-11.
80. Clément G, Braunschweig R, Pasquier N, et al. Methylation of APC, TIMP3, and TERT: A new predictive marker to distinguish Barrett’s oesophagus patients at risk for malignant transformation. J Pathol. 2006;208:100-7.
81. Schneider PM, Stoeltzing O, Roth JA, et al. P53 Mutational status improves estimation of prognosis in patients with curatively resected adenocarcinoma in Barrett’s esophagus. Clin Cancer Res. 2000;6:3153-8.
82. Iravani S, Zhang HQ, Yuan ZQ, et al. Modification of insulinlike growth factor 1 receptor, c-Src, and Bcl-XL protein expression during the progression of Barrett’s neoplasia. Hum Pathol. 2003;34:975-82.
83. Abdel-Latif MM, O_Riordan J, Windle HJ, et al. NF-kappa B activation in esophageal adenocarcinoma: Relationship to Barrett’s metaplasia, survival, and response to neoadjuvant chemoradiotherapy. Ann Surg. 2004;239:491-500.
84. Sagatys E, Garrett CR, Boulware D, et al. Activation of the serine/threonine protein kinase Akt during the progression of Barrett neoplasia. Hum Pathol. 2007;38:1526-31.
85. Beales IL, Ogunwobi O, Cameron E, et al. Activation of Akt is increased in the dysplasia-carcinoma sequence in Barrett’s oesophagus and contributes to increased proliferation and inhibition of apoptosis: A histopathological and functional study. BMC Cancer. 2007 Jun 8;7:97.
86. Lord RV, Salonga D, Danenberg KD, et al. Telomerase reverse transcriptase expression is increased early in the Barrett’s metaplasia, dysplasia, adenocarcinoma sequence. J Gastrointest Surg. 2000;4:135-42.
87. Nair KS, Naidoo R, Chetty R. Expression of cell adhesion molecules in oesophageal carcinoma and its prognostic value. J Clin Pathol. 2005;58:343-51.
88. Tanioka Y, Yoshida T, Yagawa T, et al. Matrix metalloproteinase-7 and matrix metalloproteinase-9 are associated with unfavourable prognosis in superficial oesophageal cancer. Br J Cancer. 2003;89:2116-21.
89. Buskens CJ, van Rees BP, Sivula A. Prognostic significance of elevated cyclooxygenase 2 expression in patients with adenocarcinoma of the esophagus. Gastroenterology. 2002;122:1800-7.
90. Buskens CJ, Sivula A, van Rees BP, et al. Comparison of cyclooxygenase 2 expression in adenocarcinomas of the gastric cardia and distal oesophagus. Gut. 2003;52:1678-83.
91. Evans SC, Gillis A, Geldenhuys L, et al. Microsatellite instability in esophageal adenocarcinoma. Cancer Lett. 2004;212:241-51.
92. Fang M, Lew E, Klein M, et al. DNA abnormalities as marker of risk for progression of Barrett’s esophagus to adenocarcinoma: Image cytometric DNA analysis in formalin-fixed tissues. Am J Gastroenterol. 2004;99:1887-94.
93. Su M, Chin SF, Li XY, et al. Comparative genomic hybridization of esophageal adenocarcinoma and squamous cell carcinoma cell lines. Dis Esophagus. 2006;19:10-14.
94. Zagorowicz E, Jankowski J. Molecular changes in the progression of Barrett’s oesophagus. Postgrad Med J. 2007;83:529-35.
95. Wong NA, Wilding J, Bartlett S, et al. CDX1 is an important molecular mediator of Barrett’s metaplasia. Proc Natl Acad Sci U S A. 2005;102:7565-70.
96. Kazumori H, Ishihara S, Rumi MA, et al. Bile acids directly augment caudal related homeobox gene Cdx2 expression in oesophageal keratinocytes in Barrett’s epithelium. Gut. 2006;55:16-25.
97. Eda A, Osawa H, Yanaka I, et al. Expression of homeobox gene CDX2 precedes that of CDX1 during the progression of intestinal metaplasia. J Gastroenterol Hepatol. 2002;37:94-100.
98. Atherfold PA, Jankowski JA. Molecular biology of Barrett’s cancer. Best Pract Res Clin Gastroenterol. 2006;20:813-27.
99. Schlemper RJ, Dawsey SM, Itabashi M, et al. Differences in diagnostic criteria for esophageal squamous cell carcinoma between Japanese and Western pathologists. Cancer. 2000;88:996-1006.
100. Gabbert HE, Shimoda T, Hainaut P, et al. Squamous cell carcinoma of the oesophagus. In: Hamilton SR, Aaltonen LA, editors. World Health Organization of Tumours. Pathology and genetics. Tumours of the digestive system. Lyon (France): IARC Press; 2000:9-30.
101. Tajima Y, Nakanishi Y, Tachimori Y, et al. Significance of involvement by squamous cell carcinoma of the ducts of esophageal submucosal glands: Analysis of 201 surgically resected superficial squamous cell carcinoma. Cancer. 2000;89:248-54.
102. Takubo K, Aida J, Sawabe M. Early squamous cell carcinoma of the oesophagus: The Japanese viewpoint. Histopathology. 2007;51:733-42.
103. Ludeman L, Shepherd NA. Serosal involvement in gastrointestinal cancer: Its assessment and significance. Histopathology. 2005;47:123-31.
104. Downs-Kelly E, Mendelin JE, Bennett AE, et al. Poor interobserver agreement in the distention of high-grade dysplasia and adenocarcinoma in pretreatment Barrett’s esophagus biopsies. Am J Gastroenterol. 2008;103:2333-40.
105. Hornick JL, Odze RD. Neoplastic precursor lesions in Barrett’s esophagus. Gastroenterol Clin North Am. 2007;36:775-96.
106. Odze RD. Diagnosis and grading of dysplasia in Barrett’s oesophagus. J Clin Pathol. 2006;59:1029-38.
107. Srivastava A, Hornick JL, Li X, et al. Loss of goblet cell differentiation occurs with the progression of dysplasia in Barrett’s esophagus [abstract]. Gastroenterology. 2006;130:A-264.
108. Lomo LC, Blount PL, Sanchez CA, et al. Crypt dysplasia with surface maturation: a clinical, pathologic, and molecular study of a Barrett’s esophagus cohort. Am J Surg Pathol. 2006;30:423-35.
109. Lörinc E, Jakobsson B, Landberg G, Veress B. Ki67 and p53 immunohistochemistry reduces interobserver variation in assessment of Barrett’s oesophagus. Histopathology. 2005;46:642-8.
110. Skacel M, Petras RE, Rybicki LA, et al. p53 Expression in low grade dysplasia in Barrett’s esophagus: Correlation with interobserver agreement and disease progression. Am J Gastroenterol. 2002;97:2508-13.
111. Dorer R, Odze RD. AMACR immunostaining is useful in detecting dysplastic epithelium in Barrett’s esophagus, ulcerative colitis, and Crohn’s disease. Am J Surg Pathol. 2006;30:871-7.
112. Lisovsky M, Falkowski O, Bhuiya T. Expression of alpha-methylacyl-coenzyme A racemase in dysplastic Barrett’s epithelium. Hum Pathol. 2006;37:1601-6.
113. Westerterp M, Koppert L, Buskens CJ, et al. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch. 2005;446:497-504.
114. Bollschweiler E, Baldus SE, Schröder W, et al. High rate of lymph-node metastasis in submucosal esophageal squamous-cell carcinomas and adenocarcinomas. Endoscopy. 2006;38:149-56.
115. Stein HJ, Feith M, Bruecher BL, et al. Early esophageal cancer: Pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg. 2005;242:566-73.
116. Kim SH, Lee JM, Han JK, et al. Three-dimensional MDCT imaging and CT esophagography for evaluation of esophageal tumors: preliminary study. Eur Radiol. 2006;16:2418-26.
117. The Paris endoscopic classification of superficial neoplastic lesions: Esophagus, stomach, and colon. Gastrointest Endosc. 2003;58:S3-43.
118. Fritcher EG, Brankley SM, Kipp BR, et al. A comparison of conventional cytology, DNA ploidy analysis, and fluorescence in situ hybridization for the detection of dysplasia and adenocarcinoma in patients with Barrett’s esophagus. Hum Pathol. 2008;39:1128-35.
119. Dubuc J, Legoux JL, Winnock M, et al. Endoscopic screening for esophageal squamous-cell carcinoma in high-risk patients: A prospective study conducted in 62 French endoscopy centers. Endoscopy. 2006;38:690-5.
120. Canto MI, Kalloo A. Chromoendoscopy for Barrett’s esophagus in the twenty-first century: to stain or not to stain? Gastrointest Endosc. 2006;64:200-5.
121. Ngamruengphong S, Sharma VK, Das A. Diagnostic yield of methylene blue chromoendoscopy for detecting specialized intestinal metaplasia and dysplasia in Barrett’s esophagus: A meta-analysis. Gastrointest Endosc. 2009;69:1021-8.
122. Hoffman A, Kiesslich R, Bender A, et al. Acetic acid-guided biopsies after magnifying endoscopy compared with random biopsies in the detection of Barrett’s esophagus: A prospective randomized trial with crossover design. Gastrointest Endosc. 2006;64:1-8.
123. Hamamoto Y, Endo T, Nosho K, et al. Usefulness of narrow-band imaging endoscopy for diagnosis of Barrett’s esophagus. J Gastroenterol. 2004;39:14-20.
124. Pohl J, May A, Rabenstein T, et al. Comparison of computed virtual chromoendoscopy and conventional chromoendoscopy with acetic acid for detection of neoplasia in Barrett’s esophagus. Endoscopy. 2007;39:594-8.
125. Kara MA, Peters FP, Rosmolen WD, et al. High-resolution endoscopy plus chromoendoscopy or narrow-band imaging in Barrett’s esophagus: A prospective randomized crossover study. Endoscopy. 2005;37:929-36.
126. Sharma P, Bansal A, Mathur S, et al. The utility of a novel narrow band imaging endoscopy system in patients with Barrett’s esophagus. Gastrointest Endosc. 2006;64:167-75.
127. Goda K, Tajiri H, Ikegami M, et al. Usefulness of magnifying endoscopy with narrow band imaging for the detection of specialized intestinal metaplasia in columnar-lined esophagus and Barrett’s adenocarcinoma. Gastrointest Endosc. 2007;65:36-46.
128. Wolfsen HC, Crook JE, Krishna M, et al. Prospective, controlled tandem endoscopy study of narrow band imaging for dysplasia detection in Barrett’s esophagus. Gastroenterology. 2008;135:24-31.
129. Curvers W, Baak L, Kiesslich R, et al. Chromoendoscopy and narrow-band imaging compared with high-resolution magnification endoscopy in Barrett’s esophagus. Gastroenterology. 2008;134:670-9.
130. Guelrud M, Ehrlich EE. Enhanced magnification endoscopy in the upper gastrointestinal tract. Gastrointest Endosc Clin North Am. 2004;14:461-73.
131. Kiesslich R, Gossner L, Goetz M, et al. In vivo histology of Barrett’s esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2006;4:979-87.
132. Pohl H, Koch M, Khalifa A, et al. Evaluation of endocytoscopy in the surveillance of patients with Barrett’s esophagus. Endoscopy. 2007;39:492-6.
133. Fujishiro M, Takubo K, Sato Y, et al. Potential and present limitation of endocytoscopy in the diagnosis of esophageal squamous-cell carcinoma: a multicenter ex vivo pilot study. Gastrointest Endosc. 2007;66:551-5.
134. Chemaly M, Scalone O, Durivage G, et al. Miniprobe EUS in the pretherapeutic assessment of early esophageal neoplasia. Endoscopy. 2008;40:2-6.
135. May A, Günter E, Roth F, et al. Accuracy of staging in early oesophageal cancer using high resolution endoscopy and high resolution endosonography: A comparative, prospective, and blinded trial. Gut. 2004;53:634-40.
136. Evans JA, Poneros JM, Bouma BE, et al. Optical coherence tomography to identify intramucosal carcinoma and high-grade dysplasia in Barrett’s esophagus. Clin Gastroenterol Hepatol. 2006;4:38-43.
137. Isenberg GA, Sivak MVJr, Chak A, et al. Accuracy of endoscopic optical coherence tomography in the detection of dysplasia in Barrett’s esophagus: A prospective, double-blinded study. Gastrointest Endosc. 2005;62:825-31.
138. Vakoc BJ, Shishko M, Yun SH, et al. Comprehensive esophageal microscopy by using optical frequency-domain imaging. Gastrointest Endosc. 2007;65:898-905.
139. Georgakoudi I, Jacobson BC, Van Dam J, et al. Fluorescence, reflectance, and light-scattering spectroscopy for evaluating dysplasia in patients with Barrett’s esophagus. Gastroenterology. 2001;120:1620-9.
140. Shetty G, Kendall C, Shepherd N, et al. Raman spectroscopy: elucidation of biochemical changes in carcinogenesis of oesophagus. Br J Cancer. 2006;94:1460-4.
141. Shaheen NJ, Provenzale D, Sandler RS. Upper endoscopy as a screening and surveillance tool in esophageal adenocarcinoma: A review of the evidence. Am J Gastroenterol. 2002;97:1319-27.
142. Dulai GS, Guha S, Kahn KL, et al. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: A systematic review. Gastroenterology. 2002;122:26-33.
143. Gerson LB, Ullah N, Hastie T, Goldstein MK. Does cancer risk affect health-related quality of life in patients with Barrett’s esophagus? Gastrointest Endosc. 2007;65:16-25.
144. Shaheen NJ, Dulai GS, Ascher B, et al. Effect of a new diagnosis of Barrett’s esophagus on insurance status. Am J Gastroenterol. 2005;100:577-80.
145. Inadomi JM, Sampliner R, Lagergren J, et al. Screening and surveillance for Barrett esophagus in high-risk groups: A cost-utility analysis. Ann Intern Med. 2003;138:176-86.
146. Soni A, Sampliner RE, Sonnenberg A. Screening for high-grade dysplasia in gastroesophageal reflux disease: Is it cost effective? Am J Gastroenterol. 2000;95:2086-93.
147. Gerson LB, Groeneveld PW, Triadafilopoulos G. Cost-effectiveness model of endoscopic screening and surveillance in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2004;2:868-79.
148. Wang LD, Yang HH, Fan ZM, et al. Cytological screening and 15 years’ follow-up (1986-2001) for early esophageal squamous cell carcinoma and precancerous lesions in a high-risk population in Anyang County, Henan Province, Northern China. Cancer Detect Prev. 2005;29:317-22.
149. Williams GH, Swinn R, Prevost AT, et al. Diagnosis of oesophageal cancer by detection of minichromosome maintenance 5 protein in gastric aspirates. Br J Cancer. 2004;91:714-19.
150. Atkinson M, Das A, Faulx A, et al. Ultrathin esophagoscopy in screening for Barrett’s esophagus at a Veterans Administration hospital: Easy access does not lead to referrals. Am J Gastroenterol. 2008;103:92-7.
151. Galmiche JP, Sacher-Huvelin S, Coron E, et al. Screening for esophagitis and Barrett’s esophagus with wireless esophageal capsule endoscopy: A multicenter prospective trial in patients with reflux symptoms. Am J Gastroenterol. 2008;103:538-45.
152. Ramirez FC, Shaukat MS, Young MA, et al. Feasibility and safety of string, wireless capsule endoscopy in the diagnosis of Barrett’s esophagus. Gastrointest Endosc. 2005;61:741-6.
153. Sharma P, Sidorenko EI. Are screening and surveillance for Barrett’s oesophagus really worthwhile? Gut. 2005;54:i27-32.
154. Garside R, Pitt M, Somerville M, et al. Surveillance of Barrett’s oesophagus: Exploring the uncertainty through systematic review, expert workshop and economic modeling. Health Technol Assess. 2006;10:1-142.
155. Corley DA, Levin TR, Habel LA, et al. Surveillance and survival in Barrett’s adenocarcinoma: A population-based study. Gastroenterology. 2002;122:633-40.
156. Cooper GS. Endoscopic screening and surveillance for Barrett’s esophagus: Can claims data determine its effectiveness? Gastrointest Endosc. 2003;57:914-15.
157. Macdonald CE, Wicks AC, Playford RJ, et al. Final results from 10 year cohort of patients undergoing surveillance for Barrett’s oesophagus: Observational study. BMJ. 2000;321:1252-5.
158. Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer). Gastrointest Endosc. 2007;65:3-10.
159. Larghi A, Lightdale CJ, Memeo L, et al. EUS followed by EMR for staging of high-grade dysplasia and early cancer in Barrett’s esophagus. Gastrointest Endosc. 2005;62:16-23.
160. Bhutani MS, Barde CJ, Markert RJ, Gopalswamy N. Length of esophageal cancer and degree of luminal stenosis during upper endoscopy predict T stage by endoscopic ultrasound. Endoscopy. 2002;34:461-3.
161. Romagnuolo J, Scott J, Hawes RH, et al. Helical CT versus EUS with fine needle aspiration for celiac nodal assessment in patients with esophageal cancer. Gastrointest Endosc. 2002;55:648-54.
162. Dave UR, Williams AD, Wilson JA, et al. Esophageal cancer staging with endoscopic MR imaging: pilot study. Radiology. 2004;230:281-6.
163. van Vliet EP, Heijenbrok-Kal MH, Hunink MG, et al. Staging investigations for oesophageal cancer: A meta-analysis. Br J Cancer. 2008;98:547-57.
164. Das A, Chak A. Role of endoscopic ultrasonography in the staging of esophageal cancer: A review. Curr Opin Gastroenterol. 2003;19:474-6.
165. Eloubeidi MA. Routine EUS-guided FNA for preoperative nodal staging in patients with esophageal carcinoma: Is the juice worth the squeeze? Gastrointest Endosc. 2006;63:212-14.
166. Pfau PR, Ginsberg GG, Lew RJ, et al. Endoscopic ultrasound predictors of long term survival in esophageal carcinoma. Gastrointest Endosc. 2001;53:463-9.
167. Harewood GC, Kumar KS. Assessment of clinical impact of endoscopic ultrasound on esophageal cancer. Gastroenterol Hepatol. 2004;19:433-9.
168. Das A, Chak A, Sivak MVJr, et al. Endoscopic ultrasonography and prognosis of esophageal cancer. Clin Gastroenterol Hepatol. 2006;4:695-700.
169. Duong CP, Hicks RJ, Weih L, et al. FDG-PET status following chemoradiotherapy provides high management impact and powerful prognostic stratification in oesophageal cancer. Eur J Nucl Med Mol Imaging. 2006;33:770-8.
170. McDonough PB, Jones DR, Shen KR, et al. Does FDG-PET add information to EUS and CT in the initial management of esophageal cancer? A prospective single center study. Am J Gastroenterol. 2008;103:570-4.
171. Christensen D. The Will Rogers phenomenon roping the effects of a new cancer staging system. J Natl Cancer Inst. 2003;95:1105-6.
172. Wallace MB, Nietert PJ, Earle C, et al. An analysis of multiple staging management strategies for carcinoma of the esophagus: Computed tomography, endoscopic ultrasound, positron emission tomography, and thoracoscopy/laparoscopy. Ann Thorac Surg. 2002;74:1026-32.
173. Westerterp M, van Westreenen HL, Reitsma JB, et al. Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy—Systematic review. Radiology. 2005;236:841-51.
174. Das A, Chak A. Reassessment of patients with esophageal cancer after neoadjuvant therapy. Endoscopy. 2006;38:S13-7.
175. Weber WA, Ott K, Becker K, et al. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol. 2001;19:3058-65.
176. Cerfolio RJ, Bryant AS, Ohja B, et al. The accuracy of endoscopic ultrasonography with fine-needle aspiration, integrated positron emission tomography with computed tomography, and computed tomography in restaging patients with esophageal cancer after neoadjuvant chemoradiotherapy. J Thorac Cardiovasc Surg. 2005;29:1232-41.
177. Heath EI, Canto MI, Piantadosi S, et al. Secondary chemoprevention of Barrett’s esophagus with celecoxib: Results of a randomized trial. J Natl Cancer Inst. 2007;99:545-57.
178. Limburg PJ, Wei W, Ahnen DJ, et al. Randomized, placebo-controlled, esophageal squamous cell cancer chemoprevention trial of selenomethionine and celecoxib. Gastroenterology. 2005;129:863-73.
179. May A, Gossner L, Behrens A, et al. A prospective randomized trial of two different endoscopic resection techniques for early stage cancer of the esophagus. Gastrointest Endosc. 2003;58:167-75.
180. Ciocirlan M, Lapalus MG, Hervieu V, et al. Endoscopic mucosal resection for squamous premalignant and early malignant lesions of the esophagus. Endoscopy. 2007 Jan;39:24-9.
181. ASGE Technology committeeKantsevoy SV, Adler DG, et al. Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:11-18.
182. Ishihara R, Iishi H, Uedo N, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc. 2008 Jul 10. [Epub ahead of print]
183. Pacifico RJ, Wang KK, Wongkeesong LM, et al. Combined endoscopic mucosal resection and photodynamic therapy versus esophagectomy for management of early adenocarcinoma in Barrett’s esophagus. Clin Gastroenterol Hepatol. 2003;1:252-7.
184. Pech O, Gossner L, May A, et al. Long-term results of photodynamic therapy with 5-aminolevulinic acid for superficial Barrett’s cancer and high-grade intraepithelial neoplasia. Gastrointest Endosc. 2005;62:24-30.
185. DeMeester SR. EMR for intramucosal adenocarcinoma of the esophagus: Does one size fit all? Gastrointest Endosc. 2007;65:14-15.
186. Das A, Singh V, Fleischer DE, Sharma VK. A comparison of endoscopic treatment and surgery in early esophageal cancer: An analysis of surveillance epidemiology and end results data. Am J Gastroenterol. 2008;103:1340-5.
187. Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: Outcomes in 222 patients. Ann Surg. 2003;238:486-94.
188. Nemoto K, Yamada S, Nishio M, et al. Results of radiation therapy for superficial esophageal cancer using the standard radiotherapy method recommended by the Japanese Society of Therapeutic Radiology and Oncology (JASTRO) Study Group. Anticancer Res. 2006;26:1507-12.
189. Pasquier D, Mirabel X, Adenis A, et al. External beam radiation therapy followed by high-dose-rate brachytherapy for inoperable superficial esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2006;65:1456-61.
190. Shimizu Y, Kato M, Yamamoto J, et al. EMR combined with chemoradiotherapy: A novel treatment for superficial esophageal squamous-cell carcinoma. Gastrointest Endosc. 2004;59:199-204.
191. Nemoto K, Takai K, Ogawa Y, et al. Salvage radiation therapy for residual superficial esophageal cancer after endoscopic mucosal resection. Int J Radiat Oncol Biol Phys. 2005;63:1290-4.
192. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-52.
193. Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662-9.
194. Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: Five-year survival of a randomized clinical trial. Ann Surg. 2007;246:992-1000.
195. Hulscher JB, Tijssen JG, Obertop H, van Lanschot JJ. Transthoracic versus transhiatal resection for carcinoma of the esophagus: A meta-analysis. Ann Thorac Surg. 2001;72:306-13.
196. Chang AC, Ji H, Birkmeyer NJ, et al. Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg. 2008;85:424-9.
197. Chang AC, Birkmeyer JD. The volume-performance relationship in esophagectomy. Thorac Surg Clin. 2006;16:87-94.
198. Santin B, Kulwicki A, Price P. Mortality rate associated with 56 consecutive esophagectomies performed at a “low-volume” hospital: Is procedure volume as important as we are trying to make it? J Gastrointest Surg. 2008;12:1346-50.
199. Sun XD, Yu JM, Fan XL, et al. Randomized clinical study of surgery versus radiotherapy alone in the treatment of resectable esophageal cancer in the chest. Zhonghua Zhong Liu Za Zhi. 2006;28:784-7.
200. Wong R, Malthaner R. Combined chemotherapy and radiotherapy (without surgery) compared with radiotherapy alone in localized carcinoma of the esophagus. Cochrane Database Syst Rev 2006; CD002092.
201. Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. Clin Oncol. 2002;20:1167-74.
202. Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003;185:538-43.
203. Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: A meta-analysis. Lancet Oncol. 2007;8:226-34.
204. Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310-17.
205. Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160-8.
206. Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36-46.
207. Dragovich T, McCoy S, Fenoglio-Preiser CM, et al. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol. 2006;24:4922-7.
208. Lecleire S, Di Fiore F, Antonietti M, et al. Undernutrition is predictive of early mortality after palliative self-expanding metal stent insertion in patients with inoperable or recurrent esophageal cancer. Gastrointest Endosc. 2006;64:479-84.
209. Barrichello SA, De Moura EG, Couto DS, Sakai P. Comparison of the safety and efficacy of two methods of stent placement in esophageal malignant strictures: With fluoroscopy and without fluoroscopy taken in consideration [Abstract]. Gastrointest Endosc. 2008;67:A283-4.
210. Wenger U, Johnsson E, Arnelo U, et al. An antireflux stent versus conventional stents for palliation of distal esophageal or cardia cancer: a randomized clinical study. Surg Endosc. 2006;20:1675-80.
211. Sabharwal T, Gulati MS, Fotiadis N, et al. Randomised comparison of the FerX Ella antireflux stent and the ultraflex stent: Proton pump inhibitor combination for prevention of post-stent reflux in patients with esophageal carcinoma involving the esophago-gastric junction. J Gastroenterol Hepatol. 2008;23:723-8.
212. Verschuur EM, Homs MY, Steyerberg EW, et al. A new esophageal stent design (Niti-S stent) for the prevention of migration: A prospective study in 42 patients. Gastrointest Endosc. 2006;63:134-40.
213. Conio M, Repici A, Battaglia G, et al. A randomized prospective comparison of self-expandable plastic stents and partially covered self-expandable metal stents in the palliation of malignant esophageal dysphagia. Am J Gastroenterol. 2007;102:2667-77.
214. Siersma P, Hop W, van Blankenstein M, et al. A comparison of 3 types of covered metal stents for the palliation of patients with dysphagia caused by esophagogastric carcinoma: A prospective, randomized study. Gastrointest Endosc. 2001;54:145-53.
215. Verschuur EM, Repici A, Kuipers EJ, et al. New design esophageal stents for the palliation of dysphagia from esophageal or gastric cardia cancer: A randomized trial. Am J Gastroenterol. 2008;103:304-12.
216. Balazs A, Kupcsulik PK, Galambos Z. Esophagorespiratory fistulas of tumorous origin: Non-operative management of 264 cases in a 20-year period. Eur J Cardiothorac Surg. 2008 Aug 2. [Epub ahead of print]
217. Homs MY, Steyerberg EW, Eijkenboom WM, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: Multicentre randomised trial. Lancet. 2004;364:1497-504.
218. Steyerberg EW, Homs MY, Stokvis A, et al. Stent placement or brachytherapy for palliation of dysphagia from esophageal cancer: A prognostic model to guide treatment selection. Gastrointest Endosc. 2005;62:333-40.
219. da Silveira EB, Artifon EL. Cost-effectiveness of palliation of unresectable esophageal cancer. Dig Dis Sci. 2008 Jun 4. [Epub ahead of print]
220. Guo JH, Teng GJ, Zhu GY, et al. Self-expandable esophageal stent loaded with 125I seeds: Initial experience in patients with advanced esophageal cancer. Radiology. 2008;247:574-81.
221. Jobe BA, Enestvedt CK, Thomas CRJr. Disease-specific multidisciplinary care: A natural progression in the management of esophageal cancer. Dis Esophagus. 2006;19:417-18.
222. D’Amato G, Steinert DM, McAuliffe JC, Trent JC. Update on the biology and therapy of gastrointestinal stromal tumors. Cancer Control. 2005;12:44-56.