32 Tumors of the Cervical Spine
KEY POINTS
Intramedullary Spinal Tumors
General Information, Clinical Presentation, and Imaging
Ependymomas
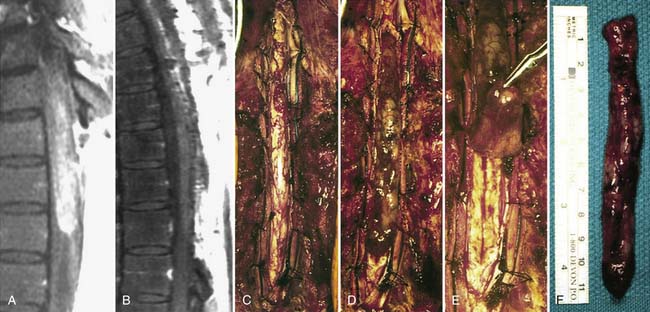
Intramedullary ependymomas most commonly occur in the cervical and cervicothoracic regions of the spinal cord. The mean age at presentation is 42 years, and there is a slight female predominance. The most common presenting symptom is neck pain localized to the region of the spine, but patients may also present with dysesthetic pain or numbness and, with larger tumors, with symptoms from neural compression. Given the slow growth and well-circumscribed quality of these tumors, symptoms generally progress slowly, and patients often have a long history prior to diagnosis.
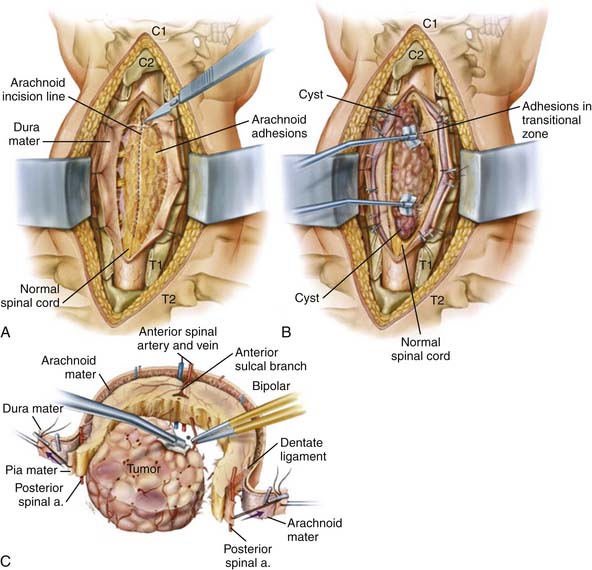
Operative Techniques (See Figures 32-1 and 32-2)
Intramedullary Tumors
After tumor resection, we achieve hemostasis, avoiding the temptation to coagulate any of the surface vessels. The tacked sutures on the pia and dura are removed. The dura is closed primarily, in a watertight fashion. The subarachnoid space is irrigated to remove any blood prior to final closure, and a Valsalva maneuver confirms lack of CSF egress. Fibrin glue is placed over the dural closure. The wound is closed in the standard fashion and we leave a subfascial drain until there is limited output. We allow the patient to ambulate and sit up immediately after surgery.
Intradural-Extramedullary Spinal Cord Tumors
General Information, Clinical Presentation, and Imaging
Meningiomas
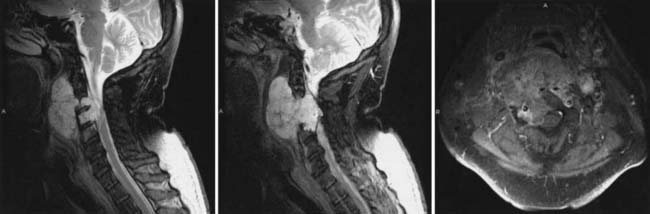
MRI is the best imaging technique for diagnosing spinal meningiomas. It clearly delineates the level of the tumor and its relation to the cord, which is useful in planning surgery. Typically, spinal meningiomas are isointense to the normal spinal cord parenchyma on T1- and T2-weighted images, and they display intense enhancement after gadolinium injection. The characteristic sign of a meningioma is a “dural tail” of enhancement.
Operative Techniques
Extradural Spinal Cord Tumors
General Information, Clinical Presentation, and Imaging
Primary tumors of the vertebral column are relatively infrequent, with the vast majority of extradural tumors being spinal column metastases. Metastatic tumors are the most common neoplastic lesions of the spine, and the vertebral column is the most common site of bone metastasis, but metastasis to the cervical spine (10%) occurs less often than to the thoracic (70%) and lumbar regions (20%). Nearly 5% to 10% of patients with systemic cancer suffer spinal metastases, and approximately 30% to 70% of patients with solid tumors have spinal metastatic disease on autopsy. Breast, lung, prostate, and renal cell carcinomas; lymphoma; and sarcoma account for 70% of all sources of spinal metastasis. The metastases occur in the vertebral body (60%), posterior elements (30%), or both (10%). The most common symptom is neck pain (90%); the pain is usually local with tenderness on palpation, but there can also be a radicular component. More than 50% of patients who present with symptomatic epidural spinal cord compression may be nonambulatory, may have bowel or bladder dysfunction, and may present with severe deficits, including acute weakness that may progress to quadriplegia. It is important to assess for mechanical pain secondary to instability, as this is common in the cervical spine, specifically with tumors with significant bone destruction associated with pathological fractures and deformity.
Operative and Postoperative Management
Spinal Metastatic Tumors
As most metastatic lesions originate in the vertebral body, an anterior cervical corpectomy offers the most direct approach for tumor excision, neurological decompression, and effective reconstruction of the weight-bearing vertebral column. This approach is especially appropriate in patients with significant vertebral body destruction resulting in neck pain or symptomatic spinal cord compression. When choosing spinal reconstructive materials and techniques, multiple biomechanical factors must be considered to achieve anatomical restoration of sagittal and coronal plane deformity and physiological load bearing. Stabilization and reconstruction of the cervical vertebral body defect after corpectomy can be performed with bone allograft, cement, pins or Silastic tubes, or titanium or PEEK interbody spacers and cages. Stabilization is then achieved with anterior instrumentation with cervical plate fixation, to prevent distraction failure and to provide increased rigidity. Additionally, posterior instrumentation with or without bone grafting may be necessary to supplement the anterior construct. As mentioned above, some tumors can also be addressed with anterior followed by posterior decompression or posterior decompression alone with laminectomy or more extensive bony decompression, with stabilization with lateral mass or pedicle screws.
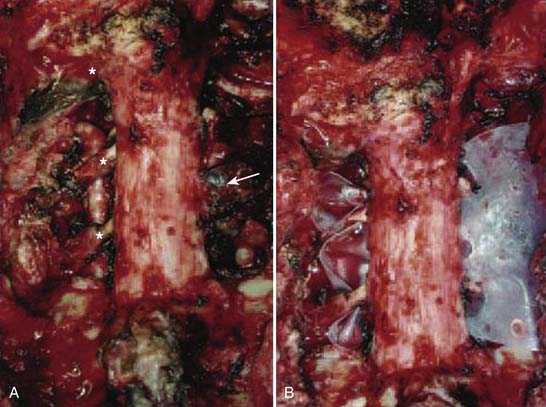
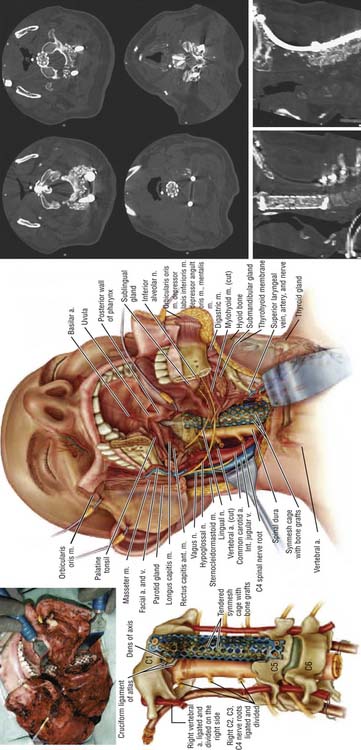
En Bloc Resection and Treatment of Cervical Chordoma (See Figures 32-3 through 32-5)
The operation was performed in two stages. The first stage, in the prone position, involved the resection of the posterior elements of the involved vertebrae, freeing neural elements that could be spared, completing the dissection around the VAs and the posterior margins of the tumor, and stabilizing the spine. After a tracheostomy was placed, the patient was placed in the prone position with head fixation, and lateral films confirmed the neutral position of the head and neck. Bilateral laminectomies and facetectomies of C2-4 (and a partial C1 right laminectomy) were performed to allow wide exposure of the exiting nerve roots. The right C2-4 roots were ligated and transected as they entered the tumor mass. Next, the right VA was exposed from C2-4 with drilling both rostral and caudal to the tumor. An initial plane of dissection was created on the right lateral side of the tumor, and a Silastic sheet was placed between the tumor and the ventral thecal sac to protect the neural structures during the subsequent anterior procedure. Occipito-cervicothoracic fixation was performed. At this point, prior to the second stage, MRA was performed to evaluate patency of the left VA to ensure safe sacrifice of the right VA for tumor resection.
On the left, the longus colli insertion on the transverse process of C2-4 was released to allow the transverse process to be drilled away, completing the circumferential exposure of the left VA from C2-5. The surgical margin on the left was thus freed from all structures. On the right, the longus colli muscles were mobilized above and below the tumor and the VA was dissected above C2 and below C4 so as not to violate the tumor. Additional dissection was performed around the lateral aspect of the tumor, medial to the carotid sheath, until this met with the dissection plane from the posterior approach. Temporary aneurysm clips were placed on the right VA and SSEPs were noted to remain stable for 30 minutes. The vessel was ligated and transected at both ends beyond the tumor, freeing the specimen along the right lateral aspect. Finally, a high-speed drill was used to cut across the base of the dens, and rongeurs were used to resect the ligamentous complex behind the dens. This established a superior margin for the resection. The entire tumor mass, including the C2-4 vertebral bodies, the right VA segment, and the right C2-4 nerve roots were removed en bloc, but the resection was marginal at the dura.
1. Cohen Z., Fourney D., Marco R., Rhines L., Gokaslan Z. Total cervical spondylectomy for primary osteogenic sarcoma. J. Neurosurg. Spine. 2002;97:386-392.
2. Gottfried O., Gluf W. Quinones-Hinojosa, Kan P, Schmidt M: Spinal meningiomas: surgical management and outcome. Neurosurg. Focus. 2003;14:1-7.
3. Gottfried O., Binning M., Schmidt M. Surgical approaches to spinal schwannomas. Contemp. Neurosurg.. 2005;27:1-8.
4. Hanbali F., Fourney D., Marmor E., Suki D., Rhines L., Weinberg J., McCutcheon I., Suk I., Gokaslan Z. Spinal cord ependymoma: radical surgical resection and outcome. Neurosurgery. 2002;51:1162-1174.
5. McGirt M., Goldstein I., Chaichana K., Tobias M., Kothbauer K., Jallo G. Extent of surgical resection of malignant astrocytomas of the spinal cord: outcome analysis of 35 patients. Neurosurgery. 2008;63:55-60.
6. Patchell R., Tibbs P., Regine W., Payne R., Saris S., Kryscio R., Mohiuddin M., Young B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomized trial. Lancet. 2005;366:643-648.
7. Rhines L., Fourney D., Siadati A., Suk I., Gokaslan Z. En bloc resection of multilevel cervical chordoma with C-2 involvement. J. Neurosurg. Spine. 2005;2:199-205.
8. Sciubba D., Chi J., Rhines L., Gokaslan Z. Chordoma of the spinal column. Neurosurg. Clin. N. Am.. 2008;19:5-15.
9. Vincent F., Fehlings M. Spinal column tumors. In Bernstein M., Berger M., editors: Neuro-oncology: the essentials, ed 2, New York: Thieme Medical Publishers, 2008.