Chapter 207 Tuberculosis (Mycobacterium tuberculosis)
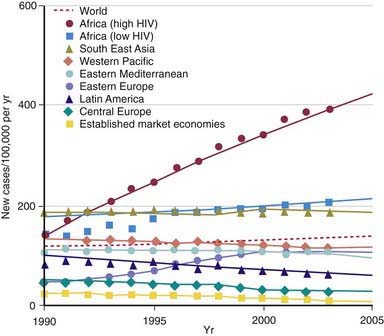
During the last decade of the 20th century the number of new cases of tuberculosis increased worldwide. Currently, 95% of tuberculosis cases occur in developing countries where HIV/AIDS epidemics have had the greatest impact and where resources are often unavailable for proper identification and treatment of these diseases (Figs. 207-1 and 207-2). In many industrialized countries, most cases of tuberculosis occur in foreign-born populations (Figs. 207-3 and 207-4). The World Health Organization (WHO) estimates that >8 million new cases of tuberculosis occur and that approximately 2 million people die of tuberculosis worldwide each year. Almost 1.3 million cases and 450,000 deaths occur in children each year. More than 30% of the world’s population is infected with Mycobacterium tuberculosis. If present trends continue, 10 million new cases are expected to occur annually by 2010, with Africa having more cases than any other region of the world (see Fig. 207-1). In the USA, after a resurgence in the late 1980s, the total number of cases of tuberculosis began to decrease in 1992, but tuberculosis continues to be a public health concern (see Fig. 207-3).
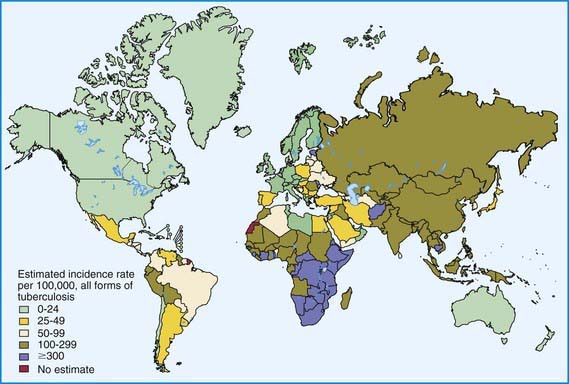
Figure 207-2 Distribution of tuberculosis in the world in 2003.
(From Dye C: Global epidemiology of tuberculosis, Lancet 367:938–940, 2006.)
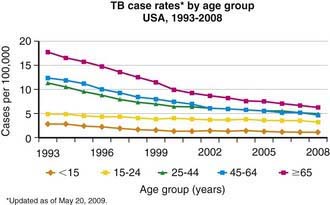
Figure 207-3 Case rates of tuberculosis (TB), by age—USA, 1993-2008.
(From the Centers for Disease Control and Prevention: Reported Tuberculosis in the United States, 2008. Atlanta, U.S. Department of Health and Human Services, September 2009.)
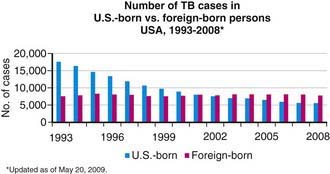
Figure 207-4 Number of tuberculosis (TB) cases in U.S.-born and foreign-born persons—USA, 1993-2008.
(From the Centers for Disease Control and Prevention: Reported Tuberculosis in the United States, 2008. Atlanta, U.S. Department of Health and Human Services, September 2009.)
Epidemiology
The World Health Organization estimates that 30% of the world’s population (2 billion people) are infected with M. tuberculosis. Infection rates are highest in Africa, Asia, and Latin America (see Fig. 207-2). The global burden of tuberculosis continues to grow owing to several factors, including the impact of HIV epidemics, population migration patterns, increasing poverty, social upheaval and crowded living conditions in developing countries and in inner city populations in developed countries, inadequate health coverage and poor access to health services, and inefficient tuberculosis control programs.
Tuberculosis case rates decreased steadily in the USA during the 1st half of the 20th century, long before the advent of antituberculosis drugs, as a result of improved living conditions and, likely, genetic selection favoring persons resistant to developing disease. A resurgence of tuberculosis in the late 1980s was associated primarily with the HIV epidemic and transmission of the organism in congregate settings, adding to increased immigration and poor tuberculosis control (see Fig. 207-3). Since 1992, the number of reported cases of tuberculosis has decreased each year, reaching a record low of 12,904 cases (rate of 4.2/100,000 population) in the year 2008. Of these, 786 (6.1%) cases occurred in children <15 yr of age (rate 1.3/100,000 population). The decline in overall incidence was mostly due to a substantial decrease in cases in persons born in the USA. About 59% of all cases were among foreign-born persons. The total number of cases among foreign-born persons increased 5% between 1992 and 2005 (see Fig. 207-4). In all age groups, the proportion of reported cases was strikingly higher in foreign-born and nonwhite persons, even though the number of cases among foreign-born children <15 yr of age has declined. In white populations in the USA, tuberculosis rates are highest among the elderly who acquired the infection decades ago. In contrast, among nonwhite populations, tuberculosis is most common in young adults and children <5 yr of age. The age range of 5-14 yr is often called the “favored age,” because in all human populations this group has the lowest rate of tuberculosis disease. Among adults two thirds of cases occur in men, but in children there is no significant difference in sex distribution.
In the USA, most children are infected with M. tuberculosis in their home by someone close to them, but outbreaks of childhood tuberculosis also occur in elementary and high schools, nursery schools, daycare centers and homes, churches, school buses, and sports teams. HIV-infected adults with tuberculosis can transmit M. tuberculosis to children, and children with HIV infection are at increased risk for developing tuberculosis after infection. Specific groups are at high risk for acquiring tuberculosis infection and progressing from LTBI to tuberculosis (Table 207-1).
Table 207-1 GROUPS AT HIGH RISK FOR ACQUIRING TUBERCULOSIS INFECTION AND DEVELOPING DISEASE IN COUNTRIES WITH LOW INCIDENCE
RISK FACTORS FOR TUBERCULOSIS INFECTION
RISK FACTORS FOR PROGRESSION OF LATENT TUBERCULOSIS INFECTION TO TUBERCULOSIS DISEASE
RISK FACTORS FOR DRUG-RESISTANT TUBERCULOSIS
Pathogenesis
The primary complex of tuberculosis includes local infection at the portal of entry and the regional lymph nodes that drain the area (Fig. 207-5). The lung is the portal of entry in >98% of cases. The tubercle bacilli multiply initially within alveoli and alveolar ducts. Most of the bacilli are killed, but some survive within nonactivated macrophages, which carry them through lymphatic vessels to the regional lymph nodes. When the primary infection is in the lung, the hilar lymph nodes usually are involved, although an upper lobe focus can drain into paratracheal nodes. The tissue reaction in the lung parenchyma and lymph nodes intensifies over the next 2-12 wk as the organisms grow in number and tissue hypersensitivity develops. The parenchymal portion of the primary complex often heals completely by fibrosis or calcification after undergoing caseous necrosis and encapsulation (Fig. 207-6). Occasionally, this portion continues to enlarge, resulting in focal pneumonitis and pleuritis. If caseation is intense, the center of the lesion liquefies and empties into the associated bronchus, leaving a residual cavity.
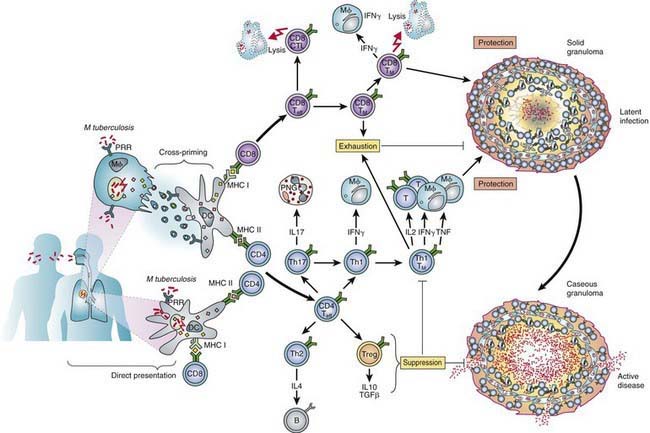
(From Kaufman SHE, Hussey G, Lambert PH: New vaccines for tuberculosis. Lancet 375:2110–2118, 2010.)
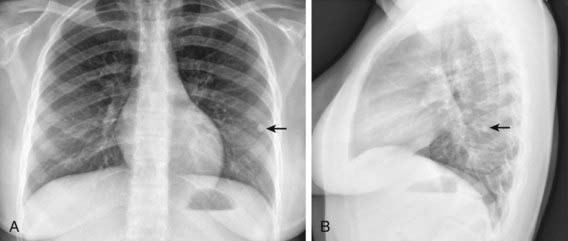
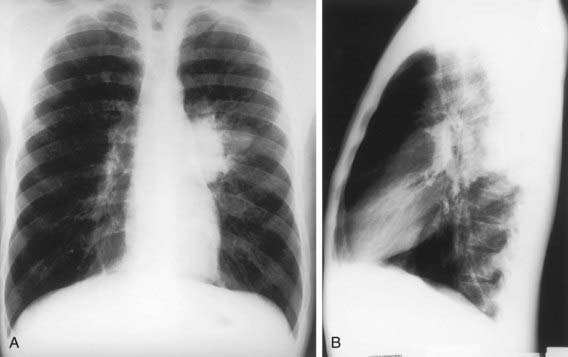
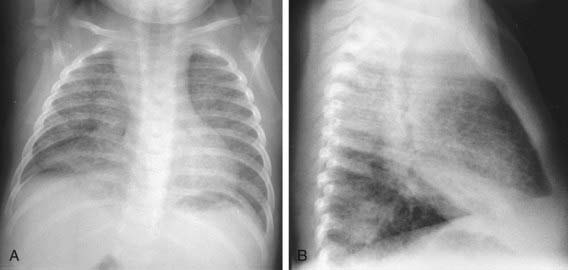
The foci of infection in the regional lymph nodes develop some fibrosis and encapsulation, but healing is usually less complete than in the parenchymal lesion. Viable M. tuberculosis can persist for decades within these foci. In most cases of initial tuberculosis infection, the lymph nodes remain normal in size. However, hilar and paratracheal lymph nodes that enlarge significantly as part of the host inflammatory reaction can encroach on a regional bronchus (Figs. 207-7 and 207-8). Partial obstruction of the bronchus caused by external compression can cause hyperinflation in the distal lung segment. Complete obstruction results in atelectasis. Inflamed caseous nodes can attach to the bronchial wall and erode through it, causing endobronchial tuberculosis or a fistula tract. The caseum causes complete obstruction of the bronchus. The resulting lesion is a combination of pneumonitis and atelectasis and has been called a collapse-consolidation or segmental lesion (Fig. 207-9).
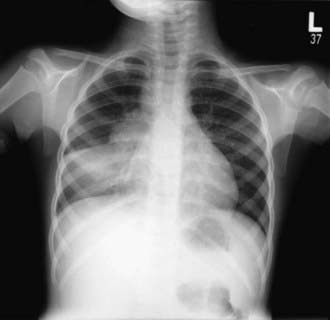
Figure 207-9 Right-sided hilar lymphadenopathy and collapse-consolidation lesions of primary tuberculosis in a 4 yr old child.
Immunity
Conditions that adversely affect cell-mediated immunity predispose to progression from tuberculosis infection to disease. Rare specific genetic defects associated with deficient cell-mediated immunity in response to mycobacteria include interleukin (IL)-12 receptor B1 deficiency and complete and partial interferon-γ (IFN-γ) receptor 1 chain deficiencies. Tuberculosis infection is associated with a humoral antibody response, which appears to play little role in host defense. Shortly after infection, tubercle bacilli replicate in both free alveolar spaces and within inactivated alveolar macrophages. Sulfatides in the mycobacterial cell wall inhibit fusion of the macrophage phagosome and lysosomes, allowing the organisms to escape destruction by intracellular enzymes. Cell-mediated immunity develops 2-12 wk after infection, along with tissue hypersensitivity (see Fig. 207-6). After bacilli enter macrophages, lymphocytes that recognize mycobacterial antigens proliferate and secrete lymphokines and other mediators that attract other lymphocytes and macrophages to the area. Certain lymphokines activate macrophages, causing them to develop high concentrations of lytic enzymes that enhance their mycobactericidal capacity. A discrete subset of regulator helper and suppressor lymphocytes modulates the immune response. Development of specific cellular immunity prevents progression of the initial infection in most persons.
Tuberculin Skin Testing
The appropriate size of induration indicating a positive Mantoux TST result varies with related epidemiologic and risk factors. In children with no risk factors for tuberculosis, skin test reactions are usually false-positive results. The American Academy of Pediatrics (AAP) and Centers for Disease Control and Prevention (CDC) discourage routine testing of children and recommend targeted tuberculin testing of children at risk identified through periodic screening surveys conducted by the primary care provider (Table 207-2). Possible exposure to an adult with or at high risk for infectious pulmonary tuberculosis is the most crucial risk factor for children. Reaction size limits for determining a positive tuberculin test result vary with the person’s risk for infection (Table 207-3). For adults and children at the highest risk for having infection progress to disease (those with recent contact with infectious persons, clinical illnesses consistent with tuberculosis, or HIV infection or other immunosuppression), a reactive area of ≥5 mm is classified as a positive result, indicating infection with M. tuberculosis. For other high-risk groups, a reactive area of ≥10 mm is considered positive. For low-risk persons, especially those residing in communities where the prevalence of tuberculosis is low, the cutoff point for a positive reaction is ≥15 mm. An increase of induration of ≥10 mm within a 2-yr period is considered a TST conversion at any age.
Table 207-2 TUBERCULIN SKIN TEST (TST) OR INTERFERON-γ RELEASE ASSAY (IGRA) RECOMMENDATIONS FOR INFANTS, CHILDREN, AND ADOLESCENTS*
Children for whom immediate TST or IGRA is indicated†:
Children who should have annual TST or IGRA:
CHILDREN AT INCREASED RISK FOR PROGRESSION OF LTBI TO TUBERCULOSIS DISEASE
Children with other medical conditions, including diabetes mellitus, chronic renal failure, malnutrition, and congenital or acquired immunodeficiencies deserve special consideration. Without recent exposure, these children are not at increased risk of acquiring tuberculosis infection. Underlying immunodeficiencies associated with these conditions theoretically would enhance the possibility for progression to severe disease. Initial histories of potential exposure to tuberculosis should be included for all of these patients. If these histories or local epidemiologic factors suggest a possibility of exposure, immediate and periodic TST should be considered. An initial TST or IGRA should be performed before initiation of immunosuppressive therapy, including prolonged steroid administration, use of tumor necrosis factor-alpha antagonists, or immunosuppressive therapy in any child requiring these treatments.
LTBI, latent tuberculosis infection.
* Bacille Calmette-Guérin immunization is not a contraindication to a TST.
† Beginning as early as 3 mo of age.
‡ If the child is well, the TST should be delayed for up to 10 wk after return.
From American Academy of Pediatrics: Red book: 2009 report of the Committee on Infectious Diseases, ed 28, Elk Grove Village, IL, 2009, American Academy of Pediatrics, p 684.
Table 207-3 DEFINITIONS OF POSITIVE TUBERCULIN SKIN TEST (TST) RESULTS IN INFANTS, CHILDREN, AND ADOLESCENTS*
INDURATION ≥5 mm
Children in close contact with known or suspected contagious people with tuberculosis disease
Children suspected to have tuberculosis disease:
INDURATION ≥10 mm
Children at increased risk of disseminated tuberculosis disease:
Children with increased exposure to tuberculosis disease:
INDURATION ≥15 mm
Children ≥4 yr of age without any risk factors
* These definitions apply regardless of previous bacille Calmette-Guérin (BCG) immunization; erythema at TST site does not indicate a positive test result. Tests should be read at 48 to 72 hr after placement.
† Evidence by physical examination or laboratory assessment that would include tuberculosis in the working differential diagnosis (e.g., meningitis).
‡ Including immunosuppressive doses of corticosteroids.
From American Academy of Pediatrics: Red book: 2009 report of the Committee on Infectious Diseases, ed 28, Elk Grove Village, IL, 2009, American Academy of Pediatrics, p 681.
Clinical Manifestations and Diagnosis
Primary Pulmonary Disease
The primary complex includes the parenchymal pulmonary focus and the regional lymph nodes. About 70% of lung foci are subpleural, and localized pleurisy is common. The initial parenchymal inflammation usually is not visible on chest radiograph, but a localized, nonspecific infiltrate may be seen before the development of tissue hypersensitivity. All lobar segments of the lung are at equal risk for initial infection. Two or more primary foci are present in 25% of cases. The hallmark of primary tuberculosis in the lung is the relatively large size of the regional lymphadenitis compared with the relatively small size of the initial lung focus (see Figs. 207-6 to 207-9). As DTH develops, the hilar lymph nodes continue to enlarge in some children, especially infants, compressing the regional bronchus and causing obstruction. The usual sequence is hilar lymphadenopathy, focal hyperinflation, and then atelectasis. The resulting radiographic shadows have been called collapse-consolidation or segmental tuberculosis (see Fig. 207-9). Rarely, inflamed caseous nodes attach to the endobronchial wall and erode through it, causing endobronchial tuberculosis or a fistula tract. The caseum causes complete obstruction of the bronchus, resulting in extensive infiltrate and collapse. Enlargement of the subcarinal lymph nodes can cause compression of the esophagus and, rarely, a bronchoesophageal fistula.
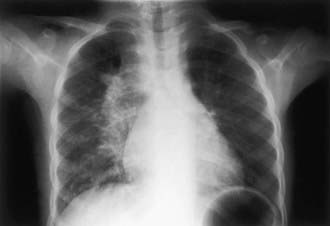
Children can have lobar pneumonia without impressive hilar lymphadenopathy. If the primary infection is progressively destructive, liquefaction of the lung parenchyma can lead to formation of a thin-walled primary tuberculosis cavity. Rarely, bullous tuberculous lesions occur in the lungs and lead to pneumothorax if they rupture. Erosion of a parenchymal focus of tuberculosis into a blood or lymphatic vessel can result in dissemination of the bacilli and a miliary pattern, with small nodules evenly distributed on the chest radiograph (Fig. 207-10).
Pleural Effusion
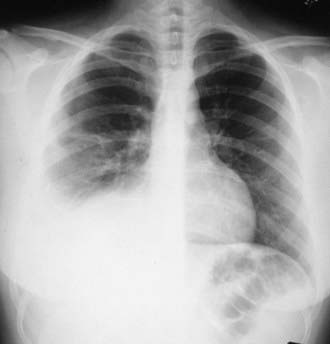
Tuberculous pleural effusions, which can be local or general, originate in the discharge of bacilli into the pleural space from a subpleural pulmonary focus or caseated lymph node. Asymptomatic local pleural effusion is so common in primary tuberculosis that it is basically a component of the primary complex. Larger and clinically significant effusions occur months to years after the primary infection. Tuberculous pleural effusion is uncommon in children <6 yr of age and rare in children <2 yr of age. Effusions are usually unilateral but can be bilateral. They are rarely associated with a segmental pulmonary lesion and are uncommon in disseminated tuberculosis. Often the radiographic abnormality is more extensive than would be suggested by physical findings or symptoms (Fig. 207-11).
Lymphohematogenous (Disseminated) Disease
The onset of miliary tuberculosis is sometimes explosive, and the patient can become gravely ill in several days. More often, the onset is insidious, with early systemic signs, including anorexia, weight loss, and low-grade fever. At this time, abnormal physical signs are usually absent. Generalized lymphadenopathy and hepatosplenomegaly develop within several weeks in about 50% of cases. The fever can then become higher and more sustained, although the chest radiograph usually is normal and respiratory symptoms are minor or absent. Within several more weeks, the lungs can become filled with tubercles, and dyspnea, cough, rales, or wheezing occur. The lesions of miliary tuberculosis are usually smaller than 2-3 mm in diameter when first visible on chest radiograph (see Fig. 207-10). The smaller lesions coalesce to form larger lesions and sometimes extensive infiltrates. As the pulmonary disease progresses, an alveolar-air block syndrome can result in frank respiratory distress, hypoxia, and pneumothorax, or pneumomediastinum. Signs or symptoms of meningitis or peritonitis are found in 20-40% of patients with advanced disease. Chronic or recurrent headache in a patient with miliary tuberculosis usually indicates the presence of meningitis, whereas the onset of abdominal pain or tenderness is a sign of tuberculous peritonitis. Cutaneous lesions include papulonecrotic tuberculids, nodules, or purpura. Choroid tubercles occur in 13-87% of patients and are highly specific for the diagnosis of miliary tuberculosis. Unfortunately, the TST is nonreactive in up to 40% of patients with disseminated tuberculosis.
Lymph Node Disease
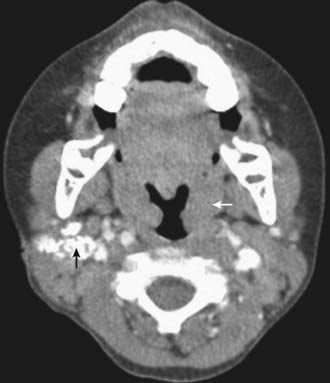
Tuberculosis of the superficial lymph nodes, often referred to as scrofula, is the most common form of extrapulmonary tuberculosis in children (Fig. 207-12). Historically, scrofula was usually caused by drinking unpasteurized cow’s milk laden with M. bovis. Most current cases occur within 6-9 mo of initial infection by M. tuberculosis, although some cases appear years later. The tonsillar, anterior cervical, submandibular, and supraclavicular nodes become involved secondary to extension of a primary lesion of the upper lung fields or abdomen. Infected nodes in the inguinal, epitrochlear, or axillary regions result from regional lymphadenitis associated with tuberculosis of the skin or skeletal system. The nodes usually enlarge gradually in the early stages of lymph node disease. They are discrete, nontender, and firm but not hard. The nodes often feel fixed to underlying or overlying tissue. Disease is most often unilateral, but bilateral involvement can occur because of the crossover drainage patterns of lymphatic vessels in the chest and lower neck. As infection progresses, multiple nodes are infected, resulting in a mass of matted nodes. Systemic signs and symptoms other than a low-grade fever are usually absent. The TST is usually reactive, but the chest radiograph is normal in 70% of cases. The onset of illness is occasionally more acute, with rapid enlargement, tenderness, and fluctuance of lymph nodes and with high fever. The initial presentation is rarely a fluctuant mass with overlying cellulitis or skin discoloration.
Cutaneous Disease
Cutaneous tuberculosis is rare in the USA but occurs worldwide and accounts for 1-2% of tuberculosis (Chapter 657).
Bone and Joint Disease
Bone and joint infection complicating tuberculosis is most likely to involve the vertebrae. The classic manifestation of tuberculous spondylitis is progression to Pott disease, in which destruction of the vertebral bodies leads to gibbus deformity and kyphosis (Chapter 671.4). Skeletal tuberculosis is a late complication of tuberculosis and has become a rare entity since the availability of antituberculosis therapy but is more likely to occur in children than in adults. Tuberculous bone lesions can resemble pyogenic and fungal infections or bone tumors. Multifocal bone involvement can occur. A bone biopsy is essential to confirm the diagnosis.
Treatment
The basic principles of management of tuberculosis disease in children and adolescents are the same as those in adults. Several drugs are used to effect a relatively rapid cure and prevent the emergence of secondary drug resistance during therapy (Tables 207-4 and 207-5). The choice of regimen depends on the extent of tuberculosis disease, the host, and the likelihood of drug resistance (Chapter 206 and Table 206-1). The standard therapy of intrathoracic tuberculosis (pulmonary disease and/or hilar lymphadenopathy) in children recommended by the CDC and AAP is a 6 mo regimen of isoniazid and rifampin supplemented in the 1st 2 mo of treatment by pyrazinamide and ethambutol. Several clinical trials have shown that this regimen yields a success rate approaching 100%, with an incidence of clinically significant adverse reactions of <2%. Nine month regimens of only isoniazid and rifampin are also highly effective for drug-susceptible tuberculosis, but the necessary length of treatment, the need for good adherence by the patient, and the relative lack of protection against possible initial drug resistance have led to the use of shorter regimens with additional medications. Most experts recommend that all drug administration be directly observed, meaning that a health care worker is physically present when the medications are administered to the patients. When directly observed therapy is used, intermittent (twice weekly) administration of drugs after an initial period as short as 2 wk of daily therapy is as effective in children as daily therapy for the entire course.
Table 207-4 COMMONLY USED DRUGS FOR THE TREATMENT OF TUBERCULOSIS IN INFANTS, CHILDREN, AND ADOLESCENTS
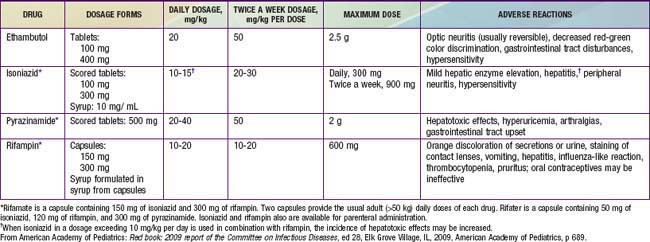
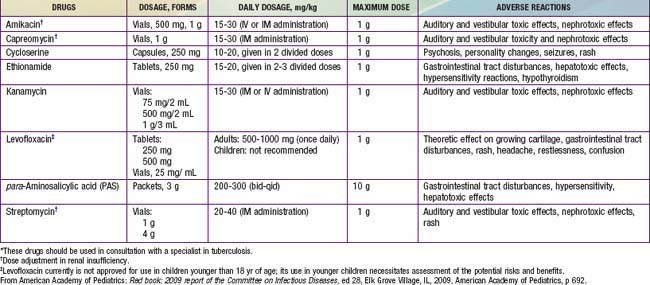
Table 207-5 LESS COMMONLY USED DRUGS FOR TREATING DRUG-RESISTANT TUBERCULOSIS IN INFANTS, CHILDREN, AND ADOLESCENTS*
Aziz MA, Wright A, Laszlo A, et al. Epidemiology of antituberculosis drug resistance (the Global Project on Anti-tuberculosis Drug Resistance Surveillance): an updated analysis. Lancet. 2006;368:2142-2154.
Bakir M, Dosanjh DPS, Deeks JJ, et al. Use of T-cell diagnosis of tuberculosis infection to optimize interpretation of tuberculin skin testing for child tuberculosis contacts. CID. 2009;48:302-312.
Battista MG. XDR tuberculosis in South Africa: old questions, new answers. Lancet. 2010;375:1760-1761.
Bertholet S, Ireton GC, Ordway DJ, et al. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci Trans Med. 2010;2(53):1-10.
Blomberg B, Langeland N. Tuberculous meningitis and resistance to isomiazid. BMJ. 2010;341:565-566.
Boehme CC, Nabeta P, Hillermann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363(11):1005-1015.
Borgdorff MW, Small PM. Scratching the surface of ignorance on MDR tuberculosis. Lancet. 2009;373:1822-1824.
Cain KP, Benoit SR, Winston CA, MacKenzie WR. Tuberculosis among foreign-born persons in the United States. JAMA. 2008;300:405-412.
Carcalho ACC, Codecasa L, Pinsi G, et al. Differential diagnosis of cervical mycobacterial lymphadenitis in children. Pediatr Infect Dis J. 2010;29(7):629-632.
Centers for Disease Control and Prevention. Trends in tuberculosis. MMWR. 2011;60(11):333-337.
Centers for Disease Control and Prevention. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR. 2010;59:1-25.
Centers for Disease Control and Prevention. Controlling tuberculosis in the United States—recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54:1-81.
Centers for Disease Control and Prevention: Reported Tuberculosis in the United States, 2008. Atlanta, GA, U.S. Department of Health and Human Services, CDC, September 2009.
Cox H, McDermid C. XDR tuberculosis can be cured with aggressive treatment. Lancet. 2008;372:1363-1364.
Cruz AT, Ong LT, Starke JR. Childhood pleural tuberculosis. Pediatr Infect Dis J. 2009;28:981-984.
Diel R, Loddenkemper R, Nienhaus AL. Evidence-based comparison of commercial interferon-γ release assays for detecting active TB: a meta-analysis. Chest. 2010;137:952-968.
Dorman SE, Johnson JL, Goldberg S, et al. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. Am J Respir Crit Care Med. 2009:273-280.
Dye C. Global epidemiology of tuberculosis. Lancet. 2006;367:938-940.
Gardy JL, Johnston JC, Ho Sui SJ, et al. Whole-genome sequencing and social-network analysis of a tuberculosis outbreak. N Engl J Med. 2011;364(8):730-738.
Ghebreyesus TA. Tuberculosis and HIV: time for an intensified response. Lancet. 2010;375:1757-1758.
Hesseling AC, Cotton MF, Jennings T, et al. High incidence of tuberculosis among HIV-infected infants: evidence from a South African population-based study highlights the need for improved tuberculosis control strategies. CID. 2009;48:108-114.
Hesseling AC, Cotton MF, von Reyn F, et al. Consensus statement on the revised World Health Organization recommendations for BCG vaccination in HIV-infected infants. Int J Tuberc Lung Dis. 2008;12:1376-1379.
Jafari C, Thijsen S, Sotgiu G, et al. Bronchoalveloar lavage enzyme-linked immunospot for a rapid diagnosis of tuberculosis. Am J Respir Crit Care Med. 2009;180:666-673.
Kaufman SHE, Hussey G, Lambert PH. New vaccines for tuberculosis. Lancet. 2010;375:2110-2118.
Lancet. Crunch time for tuberculosis control. Lancet. 2009;373:1145.
Laraque F, Griggs A, Stopen M, Munsiff SS. Performance of nucleic acid amplification tests for diagnosis of tuberculosis in a large urban setting. Clin Infect Dis. 2009;49:46-54.
Lewinsohn DA, Lobato MN, Jereb JA. Interferon-gamma release assays: new diagnostic tests for Mycobacterium tuberculosis infection and their use in children. Curr Opin Pediatr. 2010;22:71-76.
Lighter J, Rigaud M. Diagnosing childhood tuberculosis: traditional and innovative modalities, Curr Prob Pediatr Adolesc. Health Care. 2009;39:55-88.
Lighter J, Rigaud M, Eduardo R, et al. Latent tuberculosis diagnosis in children by using the quantiFERON-TB gold in-tube test. Pediatrics. 2009;123:30-37.
Liu Y, Weinberg MS, Ortega LS, et al. Overseas screening for tuberculosis in U.S. bound immigrants and refugees. N Engl J Med. 2009;360:2406-2414.
Lobato MN, Sun SJ, Moonan PK, et al. Underuse of effective measures to prevent and manage pediatric tuberculosis in the United States. Arch Pediatr Adolesc Med. 2008;162:426-431.
Magdor FK, Detjen AK. Proposed management of childhood tuberculosis in low-incidence countries. Eur J Pediatr. 2008;167:927-938.
Mandalakas AM, Kirchner L, Iverson S, et al. Predictors of Mycobacterium tuberculosis infection in international adoptees. Pediatrics. 2007;120:e610-e616.
Marais BJ, Gie RP, Hesseling AC, et al. Radiographic signs and symptoms in children treated for tuberculosis. Pediatr Infect Dis J. 2006;25:237-240.
Marais BJ, Gie RP, Schaaf HS, et al. Childhood pulmonary tuberculosis: old wisdom and new challenges. Am J Respir Crit Care Med. 2006;173:1078-1090.
Marais BJ, Gupta A, Starke JR, El Sony A. Tuberculosis in women and children. Lancet. 2010;375:2057-2059.
Nathanson E, Numm P, Uplekar M, et al. MDR tuberculosis—critical steps for prevention and control. N Engl J Med. 2010;363(11):1050-1058.
Newton SM, Brent AJ, Anderson S, et al. Paediatric tuberculosis. Lancet Infect Dis. 2008;8:498-510.
Raval A, Akhavan-Toyserkani G, Brinker A, Avigan M. Brief communication: characteristics of spontaneous cases of tuberculosis associated with infliximab. Ann Intern Med. 2007;147:699-702.
Sellar RS, Corbett EL, D’Sa S, et al. Treatment for lymph node tuberculosis. BMJ. 2010;340:c63.
Shaw JET, Pasipanodya JG, Gumbo T. Meningeal tuberculosis—high long-term mortality despite standard therapy. Medicine. 2010;89:189-195.
Spyridis NP, Spryidis PG, Gelesme A, et al. The effectiveness of a 9-month regimen of isoniazid alone versus 3- and 4-month regimens of isoniazid plus rifampin for treatment of latent tuberculosis infection in children: results of an 11-year randomized study. CID. 2007;45:715-722.
Starke JR. New concepts in childhood tuberculosis. Curr Opin Pediatr. 2007;19:306-313.
Trunz BB, Fine PEM, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173-1180.
World Health Organization. Guidelines for intensified tubersulosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings (PDF). http://whqlibdoc.who.int/publications/2011/9789241500708_eng.pdf. Accessed April 6, 2011
Zar HJ, Cotton MF, Strauss S, et al. Effect of isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: randomized controlled trial. BMJ. 2007;334:136-139.