181 Tuberculosis
• Despite advances in diagnosis and therapy, tuberculosis (TB) remains a leading cause of death worldwide.
• TB begins as a primary infection (usually in the lung, but other organ systems may be involved) that enters a latent period.
• Immunocompromised patients are at increased risk of reactivation of the disease and the development of active TB.
• The presentation of TB can be very broad and should remain in the differential diagnosis in all patients who present with systemic signs of infection.
• Therapy for TB should include multiple drugs to which the mycobacterium is susceptible and should continue for at least 6 to 12 months.
• Because of the risk of multidrug-resistant TB, an initial four-drug regimen with isoniazid, rifampin, ethambutol, and pyrazinamide is recommended.
Pathophysiology
Mycobacterium tuberculosis is a small, slow-growing bacterium that is transmitted by inhalation of droplet nuclei. As infected persons talk, cough, or sneeze, numerous nuclei are expelled into the surrounding air. Only a few inhaled droplets are needed to infect, so an increased length of exposure to the pathogen and the number of bacilli present correlate with infectivity.1
A delayed hypersensitivity response uses cytotoxic killer CD8 suppressor T cells to kill the other nonactivated macrophages with bacilli. This process creates local tissue destruction and the formation of a caseating granuloma. In immunocompromised patients, this caseous center may expand and then calcify to form a Ghon complex in the lung. If the infection is uncontrolled, primary tuberculous pneumonia may ensue, or infection may spread through blood and lymph, with resulting disseminated TB.2
Classic Presentation
The symptom constellation of cough, night sweats, and hemoptysis, commonly associated with TB, is of little benefit in the early identification of disease because TB often has no early symptoms. Late symptoms are wide ranging and do not always involve the respiratory tract. Extrapulmonary manifestations of TB are seen in 20% of patients when the host is immunocompetent and are even more common when the host is immunocompromised.3 In patients with one or more risk factors for TB (Table 181.1 and Box 181.1),4 a detailed history helps to refine the pretest probability for the diagnosis of TB.
Table 181.1 Tuberculosis Rates (per 100,000 Population) Among Five Racial and Ethnic Populations: United States, 2003
| RACE OR ETHNICITY | RATE* |
|---|---|
| White, non-Hispanic | 1.4 |
| American Indian/Alaska Native | 8.0 (5.7) |
| Hispanic | 10.5 (7.5) |
| Black, non-Hispanic | 11.5 (8.2) |
| Asian/Pacific Islander | 29.4 (21.0) |
* Numbers in parentheses represent risk for tuberculosis compared with white non-Hispanics.
From American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep 2003:52:1-77 [erratum appears in MMWR Recomm Rep 2005;53:1203; dosage error in text].
Box 181.1
Populations at Risk for Contracting Tuberculosis
Persons with human immunodeficiency infection
Persons with exposure to a known case
Persons of Asian, African, or Latin American descent
Adapted from American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society of America. Controlling tuberculosis in the United States. Am J Respir Crit Care Med 2005;172:1169-1227.
Early stages of infection are most often asymptomatic in the normal host. Unless cell-mediated immunity is impaired, symptoms may manifest only after reactivation of the infection. Patients may present with a combination of systemic symptoms including cough, fatigue, fever, anorexia, malaise, and weight loss. An indolent cough that has progressed over more than 2 weeks should raise the clinical suspicion of pulmonary TB.5 Hemoptysis signifies erosion into the bronchial tree and is unlikely to be an early symptom of pulmonary TB. When the clinical suspicion is raised, the physical findings in pulmonary TB may include fever, pleural effusion, focal pneumonic ausculatory findings, and adenopathy.
Variations
• Diffuse adenopathy of the neck (scrofula)
• Recurrent urinary tract infections, scrotal mass, prostatitis, epididymitis, or orchitis (genitourinary TB)
• Headache, fever, meningeal signs, or altered mental status (TB meningitis)
• Focal neurologic deficits, cranial nerve abnormalities, cerebellar dysfunction, or seizure (central nervous system tuberculomas)
• Joint or spinal pain (Pott disease)
Differential Diagnosis
Depending on the clinical symptoms at presentation, TB may mimic numerous systemic diseases. Radiographic findings, sputum smears, and skin tests help in the diagnosis of TB. The “gold standard” diagnostic test is culture, and results may not be available for several weeks (Table 181.2).
| Pulmonary Tuberculosis |
HIV, Human immunodeficiency virus.
![]() Red Flags
Red Flags
• Failure to consider extrapulmonary tuberculosis in the differential diagnosis
• Tuberculosis in immunocompromised patients or those at the extremes of age
• Tuberculosis in patients with risk factors for multidrug-resistant tuberculosis
• Patients with social concerns who may not be able to fulfill directly observed therapy
Diagnostic Testing
Latent TB in asymptomatic patients is clinically difficult to identify. Targeted population screening has been advocated as a means to identify early disease while it is still in the more easily treated latent period. Current recommendations are to perform a PPD skin test on patients who would benefit from therapy for latent TB and who are at high risk of developing active TB (Box 181.2).6 Currently, the foundation of screening is skin testing with PPD in patients at risk for TB. Patients who harbor latent TB and who have intact cellular immunity demonstrate a hypersensitivity reaction at the site of PPD placement that can be graded and evaluated with a well-established scale reflecting positivity or negativity (Table 181.3).6 This type of screening is not particularly sensitive in immunocompromised patients, older patients, newly infected patients, or very young children. Additionally, the PPD test lacks the specificity to distinguish among other Mycobacterium infections.7 Newer whole-blood antigen-stimulated interferon-γ release assays are available that have high sensitivity and specificity, are not affected by prior bacille Calmette-Guérin vaccination, and do not have a waning response over time.4,7 As genomic testing becomes more widely available, the ability to predict disease progression based on the genetic expression of an individual patient may play an important role in diagnosis.8
Box 181.2
Indications for a Purified Protein Derivative Skin Test
From Myers JP. New recommendations for the treatment of tuberculosis. Curr Opin Infect Dis 2005;18:133-40.
Table 181.3 Evaluation of Purified Protein Derivative Skin Testing Results
| INDURATION SIZE | CONSIDERED POSITIVE IN |
|---|---|
| ≥5 mm |
Recent immigrants from countries with a high prevalence of TB
Homeless and low-income populations
Residents or employees of nursing homes, hospitals, homeless shelters, correctional facilities
Risk factors for reactivation TB (diabetes, renal failure, malignant disease)
HIV, Human immunodeficiency virus; TB, tuberculosis.
From Myers JP. New recommendations for the treatment of tuberculosis. Curr Opin Infect Dis 2005;18:133-40.
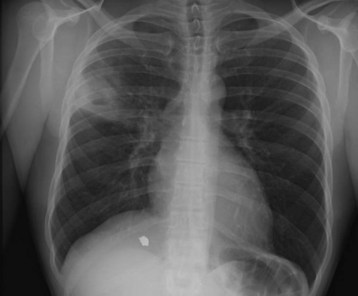
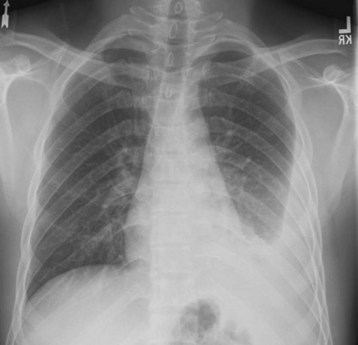
The advent of auscultation and radiography greatly advanced the diagnosis of TB in the eighteenth and nineteenth centuries.9 Despite technical advances in diagnostic tests, history, physical examination, and conventional radiography remain cornerstones in the diagnosis of TB in the emergency department (ED). Frequently, patients who present to the ED with symptoms of pulmonary or extrapulmonary TB have limited prior access to care, and heightened suspicion is required. Once a history is obtained that suggests active pulmonary disease, patients should be isolated (in a negative-pressure room, if possible), and a chest radiograph should be obtained. The identification of an upper lobe infiltrate suggests the presence of active disease (Figs. 181.1 and 181.2). Chest radiography is not highly sensitive, particularly in the immunocompromised patient, in whom reticulonodular and miliary patterns of disease may be present.10 The clinical syndrome of normal chest radiographs with sputum culture-positive TB is well described in patients with human immunodeficiency virus (HIV) infection and low CD4 counts.11 In these patient populations, a high clinical suspicion for disease mandates that sputum studies be ordered.
Unfortunately, sputum Gram stain with carbolfuchsin and phenol or auramine O staining for the identification of acid-fast bacilli (AFB) is unreliable in the ED evaluation of patients suspected to have TB. In addition to the variability in performance inherently linked to the skill of the laboratory technical staff, and the availability of rapid test results at all times of day, the sensitivity of a single AFB smear remains low (<50%), far too low for the safe discharge of the high-risk patient.12 This situation necessitates further management by the inpatient team or close follow-up with the local health department for further sputum smears in those patients who are deemed safe for discharge. The additive value of three morning sputum smears is helpful for excluding the diagnosis of active pulmonary TB. When AFB smears are identified, distinguishing among Mycobacterium species is not possible, and the definitive diagnosis should be confirmed with sputum culture.
Sputum culture is not part of the ED evaluation of a patient with suspected TB. Cultures traditionally take 4 to 8 weeks to grow. Cultures are sensitive in more than 80% of cases of infiltrative pulmonary TB and in more than 90% of cases of cavitary TB.13 Newer methods of radiometric culture systems with radiolabeled carbon-14 yield faster results (1 to 3 weeks).
The advent of nucleic acid amplification tests and of polymerase chain reaction identification greatly increased the sensitivity (97% to 99%) for the diagnosis of TB from a single sample, and accurate results can be obtained in hours. Additional strain typing for M. tuberculosis with restriction fragment length polymorphism is available to identify the organism’s DNA.14 Even for large centers, these tests are time and resource consuming to perform on an as-needed basis, and they are impractical for use in the ED work-up of patients with suspected TB. The utility of these newer tests in smear-negative individuals has been called into question with reports of lower sensitivities (70%) in this population.15
Extrapulmonary TB is often confirmed by biopsy of affected tissues and lymph nodes. Blood culture, urine culture, and culture of other body fluids are used to aid in the diagnosis of extrapulmonary TB, with varying sensitivities, depending on the host and the site of culture. The diagnosis of pleural TB is facilitated with pleural biopsy and culture; yields are 86% in immunocompetent patients and 47% in immunocompromised patients.16
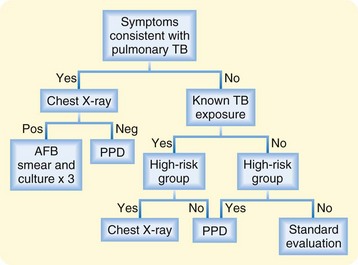
Ultimately, the diagnosis of TB remains firmly grounded in the clinician’s analysis. A high suspicion of disease, coupled with appropriate testing for the given presentation, will translate into clinical success (Fig. 181.3).17
Procedures
Historically, bleeding, leeches, cupping, application of animal excrement, “touching” by kings, pleurocentesis, and the isolation of patients in sanatoriums were used to treat TB. More modern medical times have seen the use of surgical pneumonectomy, thoracoplasty, pneumothorax, and phrenic nerve interruption as potential therapeutic procedures.9 The only procedure infrequently used today is surgical resection.
Treatment and Disposition
Pulmonary Tuberculosis
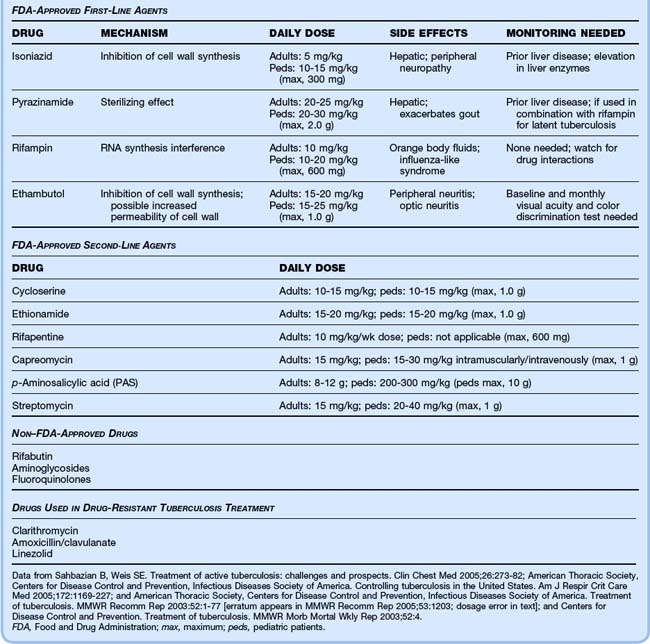
Treatment consists of two phases: the initiation (bactericidal) phase, lasting 2 months; and the continuation (sterilizing) phase, usually lasting 4 to 7 months. Because of the risk of multidrug-resistant organisms, at least two drugs should be used. The American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America recommend an initial four-drug cocktail of isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), and ethambutol (ETH) for 2 months (Table 181.4).4 Directly observed therapy increases completion rates for pulmonary TB and prevents the spread of disease.18–20
Immunocompetent patients become noninfectious 2 to 4 weeks after initiating therapy if the organism is susceptible. If the initial treatment regimen fails, then at least two additional drugs should be added.20 Usually, PZA or another first-line agent is coupled with a second-line agent such as ethionamide or a fluoroquinolone.5
Special Populations
Patients Infected With Human Immunodeficiency Virus
Because of specific drug interactions and resistance, HIV-infected patients present a challenge to standard treatment protocols. Many of the anti-TB drugs have overlapping toxicities with the antiretroviral agents, and some anti-TB drugs may decrease the concentration of certain antiretroviral agents as a result of cytochrome system interactions.21 HIV-infected patients should not receive once-weekly INH-RIF combinations because of high relapse rates. Patients with CD4 counts lower than 100 cells/mL should receive the anti-TB regimen at least three times a week because of the potential for RIF resistance. If sputum cultures are still positive at 2 months, the continuation phase of treatment may need to be extended to 7 months.20 Coordination with the infectious disease specialist during treatment of these patients is important.
Multidrug-Resistant Organisms
By definition, multidrug-resistant TB is disease that is resistant to two or more first-line agents, usually INH and RIF. Multidrug-resistant TB results from improper treatment regimens, noncompliance with therapy, and spontaneous mutations of the mycobacterium (Box 181.3).4 These patients should be treated with alternative drug regimens that include at least three drugs to which the organism is susceptible and that have not been previously administered. Ideally, one of these drugs should be injectable.4
Box 181.3
Risk Factors for Multidrug-Resistant Tuberculosis
Human immunodeficiency virus infection
Recent immigration from a country with a prevalence of multidrug-resistant TB
From American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society of America. Controlling tuberculosis in the United States. Am J Respir Crit Care Med 2005;172:1169–227.
Pregnancy
Pregnant patients may be treated with INH, RIF, and ETH. These drugs do cross the placenta, but they are not known to have any teratogenic effects.4 Adjuvant pyridoxine should be given to decrease the potential for the development of peripheral neuropathy.4
Children
![]() Priority Actions
Priority Actions
Isolate patients who have risk factors for pulmonary tuberculosis.
Recognize that immunocompromise may distort the classic presentation.
Maintain a high level of suspicion for tuberculosis across multiple organ systems.
Ensure that patients with suspected tuberculosis are managed in conjunction with your local health department.
Extrapulmonary Tuberculosis
Most patients with extrapulmonary TB infections may be treated with the same drug regimen as patients with pulmonary TB. In patients with TB pericarditis or meningitis, corticosteroids are added to prevent pericardial constriction and neurologic sequelae, respectively.12 A regimen of 6 to 9 months of treatment is adequate for most infections, but patients with meningeal infection require 9 to 12 months of therapy.4
Latent Tuberculosis
Patients with latent TB who are at high risk of developing active TB need empiric therapy. Before INH is initiated, patients require a chest radiograph and clinical evaluation to exclude active TB. The preferred treatment regimen for latent TB is daily INH for 9 months.4
Mycobacterium Bovis Bacille Calmette-Guérin Vaccine
Bacille Calmette-Guérin is an attenuated strain of Mycobacterium bovis that is the only current vaccine for TB. It was developed by Albert Calmette and Camille Guérin in the early twentieth century and remains the most commonly used vaccine in the world. Unfortunately, its efficacy may be waning as a result of mutations and deletions in the bacterium. Interest in creating alternative improved vaccines has been renewed.22,23
American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society of America. Controlling tuberculosis in the United States. Am J Respir Crit Care Med. 2005;172:1169–1227.
Blumberg HM, Leonard MK, Jr., Jasmer RM. Update on the treatment of tuberculosis and latent tuberculosis infection. JAMA. 2005;293:2776–2784. [erratum appears in JAMA 2005;294:182; dosage error in text]
National Tuberculosis Controllers Association, Centers for Disease Control and Prevention. Guidelines for the investigation of contacts of persons with infectious tuberculosis: recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Morb Mortal Recomm Rep. 2005;54:1–37.
1 Dye C, Watt CJ, Bleed DM, et al. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. JAMA. 2005;293:2767–2775.
2 Dannenberg AM, Jr. Delayed-type hypersensitivity and cell-mediated immunity in the pathogenesis of tuberculosis. Immunol Today. 1991;12:228–233.
3 Frieden TR, Munsiff SS. The DOTS strategy for controlling the global tuberculosis epidemic. Clin Chest Med. 2005;26:197–205.
4 American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society of America. Controlling tuberculosis in the United States. Am J Respir Crit Care Med. 2005;172:1169–1227.
5 Onyebujoh P, Zumla A, Ribeiro I, et al. Treatment of tuberculosis: present status and future prospects. Bull World Health Organ. 2005;83:857–865.
6 Myers JP. New recommendations for the treatment of tuberculosis. Curr Opin Infect Dis. 2005;18:133–140.
7 Whalen CC. Diagnosis of latent tuberculosis infection: measure for measure. JAMA. 2005;293:2785–2787.
8 Maertzdorf J, Repsilber D, Parida SK, et al. Human gene expression profiles of susceptibility and resistance in tuberculosis. Genes Immun. 2011;12:15–22.
9 Rubin SA. Tuberculosis: captain of all these men of death. Radiol Clin North Am. 1995;33:619–639.
10 Perlman DC, el-Sadr WM, Nelson ET, et al. Variation of chest radiographic patterns in pulmonary tuberculosis by degree of human immunodeficiency virus-related immunosuppression: the Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA). The AIDS Clinical Trials Group (ACTG). Clin Infect Dis. 1997;25:242–246.
11 Smith RL, Yew K, Berkowitz KA, Aranda CP. Factors affecting the yield of acid-fast sputum smears in patients with HIV and tuberculosis. Chest. 1994;106:684–686.
12 Woods GL, Petony E, Boxley MJ, Gatson AM. Concentration of sputum by cytocentrifugation for preparation of smears for detection of acid fast bacilli does not increase sensitivity of the fluorochrome stain. J Clin Microbiol. 1995;33:1915–1916.
13 Van den Brande P. Revised guidelines for the diagnosis and control of tuberculosis: impact on management in the elderly. Drugs Aging. 2005;22:663–686.
14 Drobniewski FA, Caws M, Gibson A, Young D. Modern laboratory diagnosis of tuberculosis. Lancet Infect Dis. 2003;3:141–147.
15 Lim TK, Zhu D, Gough A, et al. What is the optimal approach for using a direct amplification test in the routine diagnosis of pulmonary tuberculosis? A preliminary assessment. Respirology. 2002;7:351–357.
16 Relkin F, Aranda CP, Garay SM, et al. Pleural tuberculosis and HIV infection. Chest. 1994;105:1338–1341.
17 National Tuberculosis Controllers Association, Centers for Disease Control and Prevention. Guidelines for the investigation of contacts of persons with infectious tuberculosis: recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Morb Mortal Recomm Rep. 2005;54:1–37.
18 Sahbazian B, Weis SE. Treatment of active tuberculosis: challenges and prospects. Clin Chest Med. 2005;26:273–282.
19 Chaulk CP, Kazandjian VA. Directly observed therapy for treatment completion of pulmonary tuberculosis: consensus statement of the Public Health Tuberculosis Guidelines Panel. JAMA. 1998;279:943–948. [erratum appears in JAMA 1998;280:134]
20 Blumberg HM, Leonard MK, Jr., Jasmer RM. Update on the treatment of tuberculosis and latent tuberculosis infection. JAMA. 2005;293:2776–2784. [erratum appears in JAMA 2005;294:182; dosage error in text]
21 Burman WJ, Gallicano K, Peloquin C. Therapeutic implications of drug interactions in the treatment of human immunodeficiency virus–related tuberculosis. Clin Infect Dis. 1999;28:419–429.
22 Nor NM, Musa M. Approaches towards the development of a vaccine against tuberculosis: recombinant BCG and DNA vaccine. Tuberculosis. 2004;84:102–109.
23 Lahey T, Arbeit RD, Bakari M, et al. Immunogenicity of a protective whole cell mycobacterial vaccine in HIV-infected adults: a phase III study in Tanzania. Vaccine. 2010;28:7652–7658.