Chapter 47 Tubal Disease
ETIOLOGY AND CLASSIFICATION
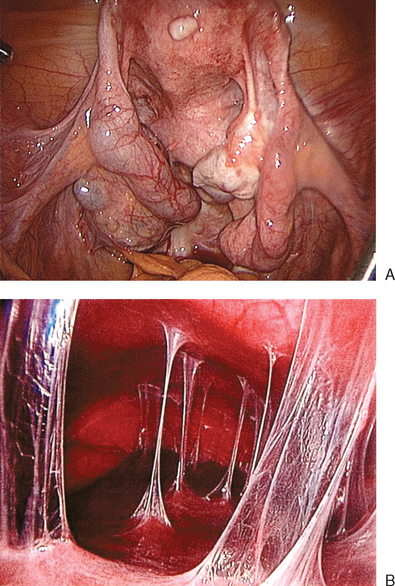
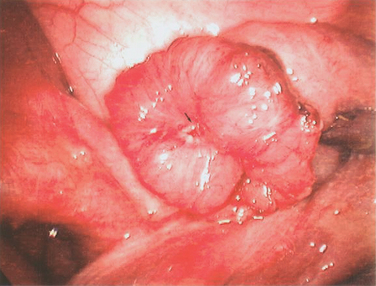
Tubal disease accounts for 25% to 35% of female infertility. The most common cause of tubal damage is pelvic inflammatory disease (PID) (Fig. 47-1A; Table 47-1). Furthermore, the incidence of subsequent tubal occlusion is proportional to the number of PID episodes.1 Other causes of tubal damage are ectopic pregnancy and iatrogenic tubal sterilization. A detailed discussion of the infectious causes of infertility is given in Chapter 33. In a smaller scale, in utero exposure to diethylstilbestrol (DES) has been implicated to cause fallopian tube abnormalities, such as foreshortened, sacculated, or convoluted tube.2
| Causes | Frequency |
|---|---|
| Pelvic inflammatory disease | 50% |
| Previous pelvic surgery | 27% |
| Iatrogenic posttubal sterilization | 1–3% |
| Endometriosis | 7–14% |
| Congenital anomalies | Rare |
EVALUATION OF TUBAL DISEASE
Hysterosalpingography
HSG is a contrast study of the fallopian tubes and the uterine cavity. It is widely used because it is simple, cost-effective, and associated with minimal side effects. Distal tubal blockage is usually associated with a hydrosalpinx. A potential limitation of HSG is tubal spasm, which can give a false impression of proximal or cornual occlusion. The incidence of false-positive results is estimated to be 50%.3 Hurd and colleagues reported that an apparent unilateral obstruction might be resolved in more than 50% of the patients by rotating the patient in a direction that places the obstructed tube in more inferior position.4
Pregnancy can occur after HSG. Perhaps, this is due to dislodging of a mucus plug or necrotic debris from the tubal lumen. In a randomized study, the pregnancy rate in women with patent tubes was 64% after HSG using oil-soluble contrast medium and 56% after the use of water-soluble contrast medium.5
Sonohysterosalpingography
Sonohysterosalpingography, also referred to as hystero-contrast-sonography or HyCoSy, is an alternative to HSG. The technique involves introduction of fluid into the uterine cavity. This is an outpatient procedure that usually lasts about 20 minutes. Saline solution is an echo-free contrast medium for assessment of the uterine cavity. The fallopian tubes can be evaluated by another contrast medium, such as air, albumin with micro-air bubbles, or galactose with micro-air bubbles. This contrast medium outlines the fallopian tube, giving a hyperechoic appearance.6
In a meta-analysis that included more than 1000 women, a similar rate of detecting tubal pathology was reported between sonohysterosalpingography and HSG or laparoscopy.7 Sonohysterosalpingography showed a false tubal occlusion in 10% of cases when compared to laparoscopy and chromopertubation. When sonohysterosalpingography was compared to HSG, a false-positive rate of 13% was found.
Laparoscopic Procedures
Laparoscopic Chromopertubation
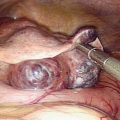
This has been the gold standard for investigating tubal patency. Furthermore, laparoscopy permits direct visualization of the whole peritoneal cavity, including other pathology such as perihepatic adhesions (see Fig. 47-1B). Details of this procedure are reviewed in Chapter 44.
Transhydrolaparoscopy
This technique involves the use of a culdoscopic approach and the use of a small endoscope to explore the pelvic cavity. It is performed with the patient in the dorsal lithotomy position. Using saline solution as a distension medium, the posterior aspect of the uterus and tubo-ovarian structures are inspected. The saline instillation continues throughout the procedure to float the tubes and the ovaries. The advantage of this procedure is that it can potentially be used in an office setting. The disadvantage of this procedure is that it does not allow evaluation of the whole abdominal or pelvic cavity. The main concern of transhydrolaparoscopy is bowel injury (about 0.65%).8
Salpingoscopy
This is an endoscopic approach to visualize the intraluminal part of the fallopian tube. The procedure is usually done with a rigid salpingoscope.9 The salpingoscope is introduced into the abdominal cavity through the operating channel of the laparoscope. An atraumatic forceps in the secondary port is used to grasp and align the ampullary segment of the tube. The salpingoscope is then slowly advanced into the tube from the fimbrial end. The inner portion of the tube is visualized until the tubal ostium. Saline solution is used as the distension medium.
Salpingoscopic findings can be classified into five grades. Grade I refers to normal mucosal folds; in grade II, major folds are separated and flattened; grade III refers to the presence of a focal lesion (adhesions, polyps, or strictures); in grade IV, there are extensive lesions with either preservation or loss of the mucosal folds; and grade V is complete loss of the mucosal folds. The cumulative pregnancy rate is inversely related to the degree of tubal damage.10,11 Salpingoscopy can also be performed through transvaginal hydrolaparoscopy.12
Falloposcopy
This transvaginal endoscopic technique13 involves the use of an extremely narrow microendoscope (0.45 to 0.5 mm in diameter) for transcervical tubal cannulation using local anesthesia. The entire fallopian tube can be visualized. There are two techniques. The first (coaxial technique) is a hysteroscopic approach where a flexible guidewire enclosed in a soft catheter is introduced into the uterotubal ostium. The catheter and the guidewire are slowly advanced for a distance of approximately 12 cm or until resistance is encountered. The guidewire is removed and the falloposcope is introduced through the catheter. In a multicenter trial, it was found that 40% of normal tubes diagnosed on HSG had abnormalities observed on falloposcopy.14 This coaxial approach lacks a uniform scoring system and the image quality is poor. The second technique involves the use of a linear everting catheter by a transvaginal approach that does not require hysteroscopy.15 This procedure is also limited by image quality. Neither procedure is commonly used.
MANAGEMENT OF TUBAL INFERTILITY
Basic Principles of Tubal Surgery
At operative laparoscopy, dissection is better performed using sharp dissection with minimal use of electrical or laser energy. Thermal damage is associated with higher risk of adhesion formation. The use of sutures should be limited as well. However, if needed the suture material should be fine and nonreactive (e.g., 6-0 polyglactin or polyglycolic acid). The sutures should be placed without strangulating the tissue. Hemostasis is achieved using microbipolar forceps.
Proximal Tubal Occlusion
Proximal tubal occlusion is found in 10% to 25% of women with tubal disease. It is mainly due to salpingitis isthmica nodosa. Other causes of failed filling of the tubes are chronic pelvic infection, congenital malformation, and tubal spasm. Tuberculosis may cause varying degrees of tubal damage from minimal to extensive proximal tubal block.16 The thick muscular wall of the proximal tube, with its physiologic sphincter and its narrow lumen, makes it susceptible for “obstruction” by mucous debris.17
Selective Salpingography and Tubal Catheterization under Fluoroscopy
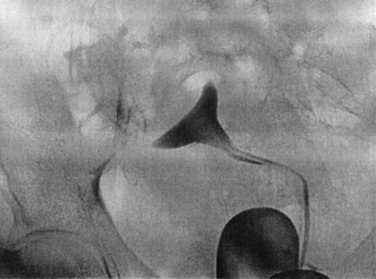
This is an established procedure for diagnosis and treatment of proximal tubal occlusion (Fig. 47-2). The procedure is performed by passing a catheter through the cervix into the proximal tubes. Radiocontrast medium is then injected under fluoroscopic guidance. Similar to HSG, the pressure induced by the injection may help in overcoming the obstruction. Otherwise, a guidewire is introduced into the fallopian tube to overcome the obstruction.
The procedure is usually performed under paracervical block with or without intravenous sedation. The first catheter is introduced through the cervix into the uterine cavity. A balloon is inflated to occlude the cervix. The second (ostial) catheter is then passed through the central lumen of the cervical catheter and advanced to the uterine cornua. Radiopaque contrast medium is injected into the tube directly (Fig. 47-3). Repeated radiographs are done to demonstrate the filling of the tube proximally and distally and whether peritoneal spillage occurs.
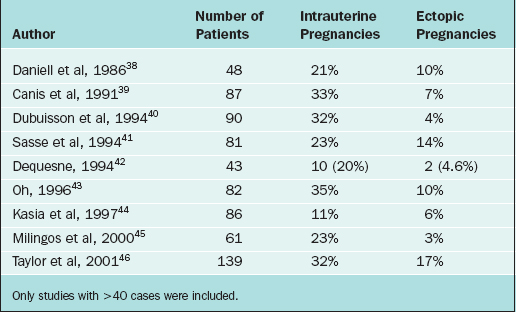
If selective salpingography is not successful, the third (uterine cornual) catheter with a guidewire is used. Threading of an atraumatic guidewire through the catheter into the ostium and the isthmus (tubal cannulation) unblocks the obstruction. In most cases (85%), the tubal occlusion can be overcome. Yet, reocclusion rate is high (30%). Tubal perforation can occur in 3% to 11% of cases; however, it heals spontaneously without any further treatment. The pregnancy rate after selective salpingography and tubal catheterization is about 50% (Table 47-2). The procedure can be repeated if the tubes reocclude. If the procedure fails, patients can be offered IVF or reconstructive tubal surgery.
Table 47-2 Pregnancy Rates after Selective Tubal Catheterization
| Technique | Pregnancy Rate (%) | Ectopic Rate (%) |
|---|---|---|
| Fluoroscopic technique | 50% | 2–9% |
| Hysteroscopic technique | 50% | 5% |
Hysteroscopic Transcervical Tubal Cannulation
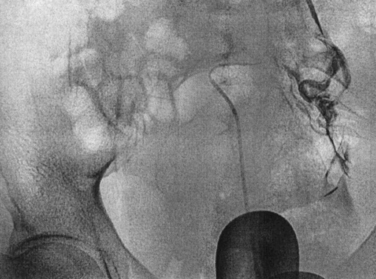
This hysteroscopic procedure is similar to selective salpingography and tubal catheterization. This procedure typically uses a Novy cornual cannulation set (Cook Ob/Gyn, Spencer, Ind.). A catheter is introduced into the working channel of an operative hysteroscope. The catheter is then advanced toward the tubal orifice and wedged against the ostium (Fig. 47-4). Dilute indigo carmine is then injected while observing laparoscopically for spillage from the fimbriated end of the tube. If there is no spill an inner catheter with a guidewire is then introduced. The guidewire is introduced through the uterotubal junction and then into the tube. Only then is the inner catheter introduced. No resistance should be met. The guidewire is then removed and dilute indigo carmine is injected.
Microsurgical Resection and Anastomosis
The edges of the transected tube are examined under the microscope or close-contact laparoscope. Depending on the laparoscope and the distance between the end of the scope and the tissue, magnification up to 10-fold can be obtained. The two cut ends of the tube are approximated with several interrupted sutures of 6-0 polyglactin in the mesosalpinx. Three to four interrupted 7-0 sutures are then placed in the mucosa and muscularis layers circumferentially. The pregnancy rate following cornual anastomosis is approximately 50% (Table 47-3). A meta-analysis of different techniques found that the pregnancy rate after microsurgical repair of cornual obstruction is 58.9%, with an ectopic pregnancy rate of 7.4 %.25 Most patients choose IVF after failed cannulation techniques rather than a surgical approach.
Midtubal Occlusion
Tubal sterilization is the most common permanent method of contraception in females. However, more than 1% of sterilized women subsequently seek restoration of their fertility. Data from 2253 women who had tubal sterilization showed a strong correlation between regret and age or change in marital status.26
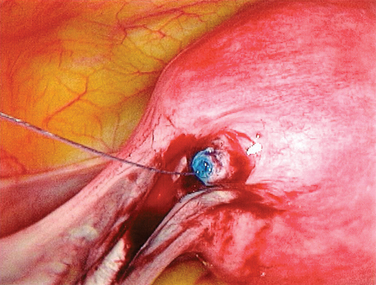
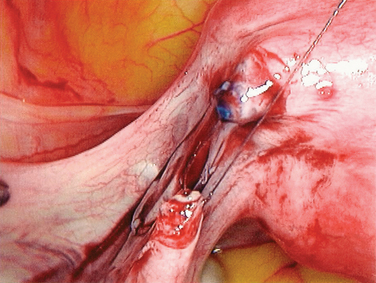
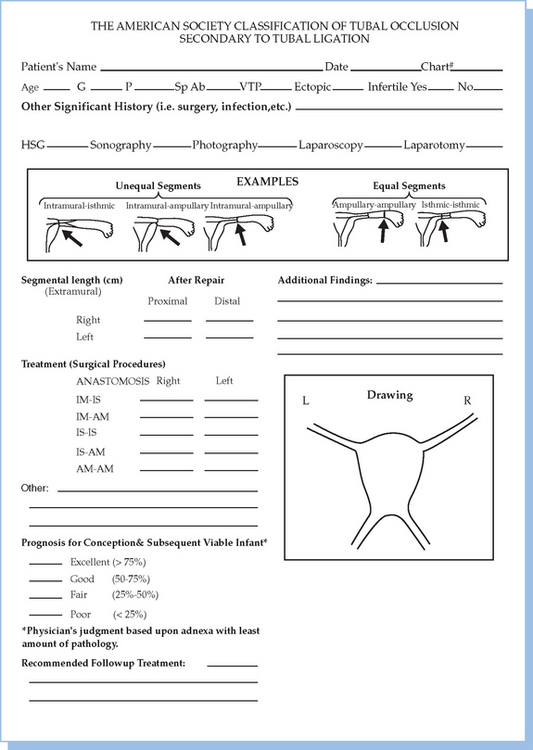
Reversal of tubal sterilization is the most common indication for surgery of midtubal occlusion (Fig. 47-5). The technique is demonstrated in Figures 47-6 to 47-10. At the beginning of the procedure, the type of occlusion should be reported according to the American Society for Reproductive Medicine (ASRM, formerly the American Fertility Society) classification of tubal occlusion secondary to tubal ligation (Fig. 47-11).27
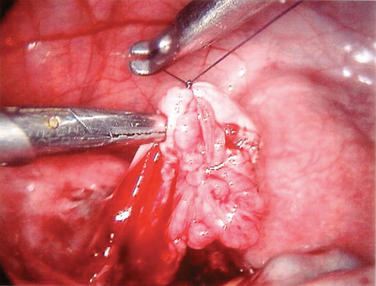
Isthmic–isthmic tubal anastomosis yields the best pregnancy rate (81%). A similarly high pregnancy rate can be obtained by a laparoscopic approach (Table 47-4). Yoon and coworkers reported a pregnancy rate of 82.8%.28 There has been no randomized trial comparing tubal anastomosis by laparoscopy and laparotomy. Using a life table analysis, Hawkins and colleagues reported that there is no difference in cumulative pregnancy rates between tubal anastomosis by laparoscopy and laparotomy.29 The ectopic pregnancy rates at 18-month follow-up were 11.8% and 12% in the laparoscopy and laparotomy groups, respectively.
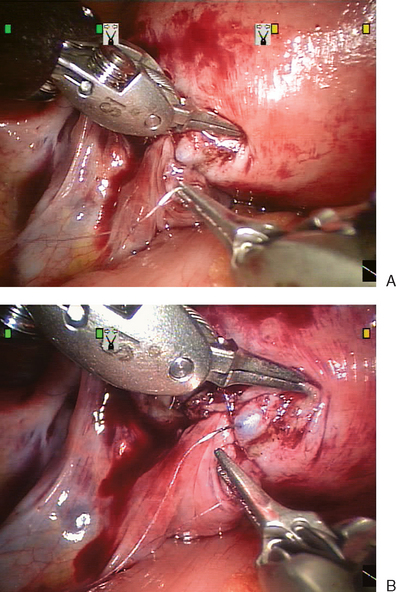
The technical difficulty of accomplishing this task by laparoscopy has led to modified versions of the classical two-layer closure. Using a one-stitch technique after laparoscopic tubal anastomosis, Dubuisson and Chapron reported an 87.5% patency rate, 53.1% intrauterine pregnancy rate, and an ectopic pregnancy rate of 6.25%.35 Falcone and colleagues reported on laparoscopic reversal of sterilization with the assistance of a robotic device.36 The daVinci robotic arms can turn 360 degrees and theoretically facilitate laparoscopic suturing (see Fig. 47-10). Although initial reports showed increased operative time, this may decrease as the technology improves.
Distal Tubal Occlusion
Distal tubal damage represents 85% of all cases of tubal infertility. The causes of distal tubal occlusion are PID, pelvic peritonitis, and previous surgery. A standardized method of operative report should be used. There are different scoring systems for distal tubal disease, but the most widely used is the ASRM classification for distal tubal occlusion (Fig. 47-12).27

Figure 47-12 The American Society for Reproductive Medicine classification of distal tubal occlusion.
Salpingostomy
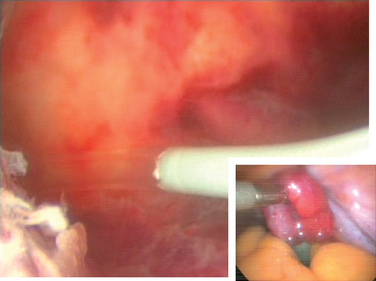
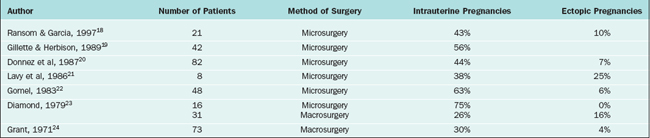
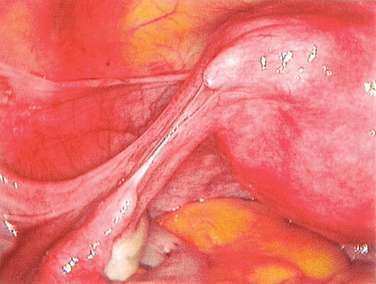
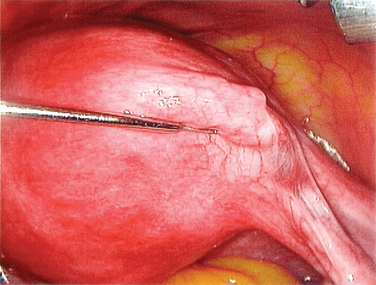
The procedure to open a distally occluded tube is called a terminal salpingostomy or salpingoneostomy. A systematic approach is required. First, any adhesions around the tubes should be liberated. The tube is then distended with dilute solution of contrast medium solution, which is injected through the intrauterine cannula (Figs. 47-13 and 47-14). The distended hydrosalpinx is then inspected. An opening on the hydrosalpinx along the avascular white line of the hydrosalpinx is created using laparoscopic scissors (Fig. 47-15). The tube is immobilized by uterine manipulation and with the help of laparoscopic forceps. Eversion of the mucosal flap can be achieved by suturing them in place with interrupted sutures of 6-0 polyglactin (Fig. 47-16). It can also be achieved by light coagulation of the serosal flap approximately 5 mm from the tubal margin. This leads to retraction of the mucosal flap, creating an eversion (Fig. 47-17).
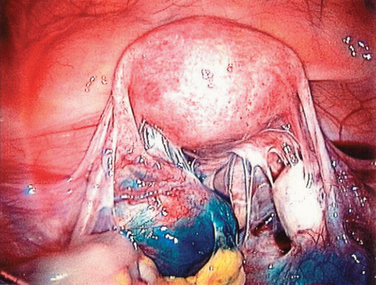
Figure 47-13 Dense adhesions surrounding the tubes and ovaries. Chromopertubation revealed bilateral distal tubal occlusion.
Depending on the degree of tubal damage, the overall pregnancy rate after salpingostomy is between 10% and 35% (Table 47-5). A meta-analysis found that increased term pregnancy rates and decreased ectopic rates were associated with microscopic salpingostomy compared to the macroscopic method.37 They also found that intrauterine pregnancy rates were significantly lower with the laparoscopic approach. However, this conclusion was based on the findings of four nonrandomized studies.
Fimbrioplasty
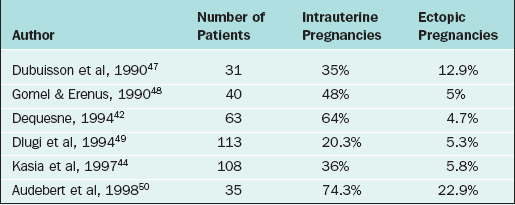
In more severe forms of fimbrial disease, the distal tube may be dilated but patent. The peritoneal adhesive bands are divided by laparoscopic scissors. The procedure can also be done by inserting two graspers into the tubal lumen and then spreading them apart. The procedure is repeated until the fimbria is opened. In severe cases, several interrupted sutures of 6-0 polyglactin to maintain the tubal opening are needed. The results of laparoscopic fimbrioplasty are depicted in Table 47-6.47–50
Salpingectomy
Several studies have shown the deleterious effect of hydrosalpinx on the outcome of IVF–embryo transfer (ET).51–55 Perhaps this is due to toxic agents in hydrosalpinx fluid that enter the uterine cavity and impair implantation. Meta-analysis of large retrospective series revealed that the IVF pregnancy rate in women with hydrosalpinx is half of those with tubal infertility of other causes, and the miscarriage rate is double.56,57 In attempt to improve the live birth rate of IVF treatment, it is recommended that the tubes be removed before IVF.58,59 In particular, hydrosalpinges that are visible on ultrasound should be removed. However, it is less certain if removal is necessary for mild cases seen only on HSG. Interestingly, salpingectomy or proximal tubal occlusion of a unilateral hydrosalpinx has been shown to increase spontaneous pregnancy rates.60 Treatment options for hydrosalpinx-related IVF include drainage, salpingostomy, proximal tubal ligation, and salpingectomy.
Occluding the proximal tube appears to be a reasonable alternative for women with hydrosalpinx undergoing IVF. Pregnancy rates after proximal tubal occlusion are similar to those after salpingectomy.61 In one retrospective study of patients undergoing IVF treatment, it was observed that management of hydrosalpinx by laparoscopic salpingectomy or by occluding the proximal tubes yielded statistically similar responses to an IVF–ET cycle.61 Occluding the proximal portion of the tubes is simpler and is a method of choice for women with severe pelvic adhesions. Recently some case reports have suggested a possible role for hysteroscopic tubal occlusion in patients with hydrosalpinx and severe pelvic disease at high risk for complications.
Salpingectomy has been shown to be effective. A randomized trial comparing laparoscopic salpingectomy to no treatment for hydrosalpinx before IVF found the pregnancy and delivery rates (37% and 29%, respectively) in the salpingectomy group were significantly higher than in the nontreated group (24% and 16%, respectively).59 It seems clear that salpingectomy is a worthwhile procedure before IVF.62 The technique of salpingectomy could affect the blood supply to the ovary; caution should be exercised when excising the tube.
Prophylactic antibiotics may be all that is necessary to improve implantation rates in the presence of hydrosalpinx, at least in theory. It has been reported in a retrospective study that doxycycline treatment improved the IVF pregnancy rate.63 However, in general, a tubal occlusion procedure is recommended.
Periadnexal Adhesions
Reproductive surgery can cause adhesion formation. Most surgery on reproductive organs is not performed for infertility but in women who have a disorder such as a myoma or ovarian cyst and want to conceive in the future. Measures such as meticulous handling of the tissue, frequent irrigation with isotonic solution, gentle and minimal tissue handling, and good hemostasis decrease but do not eliminate adhesion formation. Adjunctive therapies, such as prophylactic antibiotics, intraperitoneal corticosteroids, and adhesion-preventing substances also fail to improve the reproductive performance of these women (see Chapter 52).
Peritubal and periovarian adhesions impair fertility by interfering with gamete transfer and the ovum pickup mechanism. Removal of these adhesions is associated with improved fecundity. The ASRM (American Fertility Society) classification of adnexal adhesions is shown in Figure 47-18.27 Tulandi and colleagues evaluated pregnancy occurrence in infertile women with periadnexal adhesions.64 The pregnancy rate in women whose adhesions were lysed by laparotomy was higher than in those untreated. The cumulative probability of conception at 12 and 24 months follow-up were 8% and 13%, respectively, in the nontreated group and 40% and 42%, respectively, in the treated group. The ectopic pregnancy rate between the treated and nontreated group was similar. Intrinsic damage to the fallopian tube is more important in the development of ectopic pregnancy than the adhesions.
Lysis of adhesions can be performed using laparoscopic scissors, electrocautery, or laser. The results are similar.65–68 The vascular type of adhesions should be coagulated first or vaporized with the laser. The procedure is performed by keeping the adhesions under tension with grasping forceps and divided. Filmy adhesions are divided, but dense adhesions should be excised. The overall pregnancy rate after laparoscopic salpingo-ovariolysis is 60% and the ectopic pregnancy rate is 5%.65 However, the pregnancy rate after salpingo-ovariolysis in women with severe and dense adhesions is poor (10% to 15%). These patients are better treated with IVF.
In a retrospective study, there was no statistical difference between the cumulative conception rates of microsurgical and laparoscopic adhesiolysis after being stratified according to the duration of infertility.66 In addition, there was no difference in adhesion score after microsurgical and laparoscopic adhesiolysis. However, the laparoscopic approach has additional benefits, such as reduced postoperative pain, fast recovery, short hospital stay, and reduced risk of infection and thromboembolic accidents.
The benefit of second-look laparoscopy was studied by Tulandi and coworkers in a randomized, prospective study.68 There was no increase in the pregnancy rate or decrease in the incidence of ectopic pregnancy after a second-look laparoscopy done 1 year after reproductive surgery.
CONCLUSIONS
1 Westrom L. Incidence, prevalence, and trends of acute pelvic inflammatory disease and its consequences in industrialized countries. Am J Obstet Gynecol. 1980;138:880-892.
2 DeCherney AH, Cholst I, Naftolin F. Structure and function of the fallopian tubes following exposure to diethylstilbestrol (DES) during gestation. Fertil Steril. 1981;36:741-745.
3 Novy M, Thurmond AS, Patton P, et al. Diagnosis of cornual obstruction by transcervical fallopian tube cannulation. Fertil Steril. 1988;50:434-440.
4 Hurd WW, Wyckoff ET, Reynolds DB, et al. Patient rotation and resolution of unilateral cornual obstruction during hysterosalpingography. Obstet Gynecol. 2003;101:1275-1278.
5 Steiner AZ, Meyer WR, Clark RL, Hartmann KE. Oil- Soluble contrast during hysterosalpingography in women with proven tubal patency. Obstet Gynecol. 2003;101:109-113.
6 Ekerhoved E, Fried G, Granberg S. An ultrasound-based approach to the assessment of infertility, including the evaluation of tubal patency. Best Pract Res Clin Obstet Gynecol. 2004;18:13-28.
7 Holz K, Becker R, Schurmann R. Ultrasound in the investigation of tubal patency. A meta-analysis of three comparative studies of Echovist-200 including 1007 women. Zentralblatt fur Gynekologie. 1997;119:366-373.
8 Gordts S, Watrelot R, Campo R, Brosens I. Risk and outcome of bowel injury during transvaginal pelvic endoscopy. Fertil Steril. 2001;76:1238-1241.
9 Brosens IA, Boeckx W, Delattin P, et al. Salpingoscopy: A new preoperative diagnostic tool in tubal surgery. BJOG. 1987;94:768-773.
10 Marana R, Catalano GF, Muzii L, et al. The prognostic role of salpingoscopy in laparoscopic tubal surgery. Hum Reprod. 1999;14:2991-2995.
11 Marchino GL, Gigante V, Gennarelli G, et al. Salpingoscopic and laparoscopic investigation in relation to fertility outcome. J Am Assoc Gynecol Laparosc. 2001;8:218-221.
12 Gordts S, Campo R, Puttemans P, et al. A one-step outpatient endoscopy based approach. Hum Reprod. 2002;17:1684-1687.
13 Kerin J, Daykhosky L, Segalowitz J, et al. A microendoscopic technique for visual exploration of the human fallopian tube from the uterotubal ostium to the fimbriae using a transvaginal approach. Fertil Steril. 1990;54:390-400.
14 Surrey ES, Adamson GD, Surrey MW, et al. Introduction of new coaxial falloposcopy system: A multicenter feasibility study. J Am Assoc Gynecol Laparosc. 1997;4:473-478.
15 Pearlstone AC, Surrey ES, Kerin F. The linear everting catheter: A nonhysteroscopic transvaginal technique for access and microendoscopy of the fallopian tube. Fertil Steril. 1992;58:854-857.
16 Malik S. Genital tuberculosis and implantation in assisted reproduction. Rev Gynecol Pract. 2003;3:160-164.
17 Sulak PJ, Letterie GS, Coddington CC, et al. Histology of proximal tubal occlusion. Fertil Steril. 1987;48:437-440.
18 Ransom M, Garcia A. Surgical management of cornual–isthmic tubal obstruction. Fertil Steril. 1997;68:887-891.
19 Gillette WR, Herbison GP. Tubocornual anastomosis: Surgical considerations and coexistent infertility factors in determining the prognosis. Fertil Steril. 1989;51:241-246.
20 Donnez J, Casanas-Roux F, Nisolle-Pochet M, et al. Surgical management of tubal obstruction at the uterotubal junction. Acta Eur Fertil. 1987;18:5-9.
21 Lavy G, Diamond MP, DeCherney AH. Pregnancy following tubocornual anastomosis. Fertil Steril. 1986;46:21-25.
22 Gomel V. An odyssey through the oviduct. Fertil Steril. 1983;39:144-156.
23 Diamond E. A comparison of gross and microsurgical techniques for the repair of cornual occlusion in infertility: A retrospective study, 1968–1978. Fertil Steril. 1979;32:370-376.
24 Grant A. Infertility surgery of the oviduct. Fertil Steril. 1971;22:496-503.
25 Honere GM, Holden AE, Schenken RS. Pathophysiology and management of proximal tubal blockage. Fertil Steril. 1999;71:785-795.
26 Platz-Christensen JJ, Tronstad SE, Johansson O, Carlsson SA. Evaluation of regret after tubal sterilization. Int J Gynaecol Obstet. 1992;38:223-226.
27 American Fertility Society. Classification of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988;49:944-955.
28 Yoon TK, Sung HR, Kang HG, et al. Laparoscopic tubal anastomosis: Fertility outcome in 202 cases. Fertil Steril. 1999;72:1121-1126.
29 Hawkins J, Dube D, Kaplow M, Tulandi T. Cost analysis of tubal anastomosis by laparoscopy and by laparotomy. J Am Assoc Gynecol Laparosc. 2002;9:120-124.
30 DeCherney AH, Mezer HC, Naftolin F. Analysis of failure of microsurgical anastomosis after mid-segment, non-coagulation tubal ligation. Fertil Steril. 1983;39:618-622.
31 Paterson PJ. Factors influencing the success of microsurgical tuboplasty for sterilization reversal. Clin Reprod Fertil. 1985;3:57-64.
32 Lu ZY. Tubal anastomosis for reversal of sterilization with microsurgical technique in 246 women. Zhonghua Fu Chan Ke Za Zhi. 1989;24:203-205.
33 Kim SH, Shin CJ, Kim JC, et al. Microsurgical reversal of tubal sterilization: A report on 1118 cases. Fertil Steril. 1997;68:865-870.
34 Bisonette F, Lapensee L, Bouzayen R. Outpatient laparoscopic tubal anastomosis and subsequent fertility. Fertil Steril. 1999;72:549.
35 Dubuisson JB, Chapron CL. Single suture laparoscopic tubal re-anastomosis. Curr Opin Obstet Gynecol. 1998;10:307-313.
36 Falcone T, Goldberg JM, Margossian H, Stevens L. Robotically assisted laparoscopic microsurgical tubal anastomosis: A human pilot study. Fertil Steril. 2000;73:1040-1042.
37 Watson A, Vandekerckhove P, Lilford R. Techniques for pelvic surgery in subfertility. Cochrane Database Syst Rev. 3, 2003. CD000221.
38 Daniell JF, Diamond MP, McLaughlin DS, et al. Clinical results of terminal salpingostomy with the use of the CO2 laser: Report of the intra-abdominal laser study group. Fertil Steril. 1986;45:175-178.
39 Canis M, Mage G, Pouly JL, et al. Laparoscopic distal tuboplasty: Report of 87 cases and a four years experience. Fertil Steril. 1991;56:616-621.
40 Dubuisson JP, Chapron C, Morice P, et al. Laparoscopic salpingostomy: Fertility results according to the tubal mucosal appearance. Hum Reprod. 1994;9:334-339.
41 Sasse V, Karageorgieva E, Keckstein J. Laparoscopic treatment of distal tubal occlusion-reocclusion and pregnancy rate. J Am Assoc Gynecol Laparosc. 1994;1:S32.
42 Dequesne JG. CO2 laser laparoscopy in tubo-ovarian infertility. J Am Assoc Gynecol Laparosc. 1994;1:S10.
43 Oh ST. Tubal patency and conception rates with three methods of laparoscopic terminal salpingostomy. J Am Assoc Gynecol Laparosc. 1996;3:519-523.
44 Kasia J, Raiga J, Doh A, et al. Laparoscopic fimbrioplasty and neosalpingostomy: Experience of the Yaounde General Hospital, Cameroon (report of 194 cases). Eur J Obstet Gynecol Reprod Biol. 1997;73:71-77.
45 Milingos SD, Kallipolitis GK, Loutradis DC, et al. Laparoscopic treatment of hydrosalpinx: Factors affecting pregnancy rate. J Am Assoc Gynecol Laparosc. 2000;7:355-361.
46 Taylor RC, Berkowitz J, McComb PF. Role of laparoscopic salpingostomy in the treatment of hydrosalpinx. Fertil Steril. 2001;75:594-600.
47 Dubuisson PJ, Bouquet De Joliniere J, Aubriot FX, et al. Terminal tuboplasties by laparoscopy: 65 consecutive cases. Fertil Steril. 1990;54:401-403.
48 Gomel V, Erenus M. Prognostic value of the American Fertility Society’s (AFS) classification for distal tubal occlusion (DTO). In: American Fertility Society 46th Annual Meeting program Supplement (p097). Washington, D.C.: American Fertility Society; 1990:S106.
49 Dlugi AM, Reddy S, Saleh WA, et al. Pregnancy rates after operative endoscopic treatment of total (neosalpingostomy) or near total (salpingostomy) distal tubal occlusion. Fertil Steril. 1994;62:913-920.
50 Audebert A, Pouly JL, Von Theobald P. Laparoscopic fimbrioplasty: An evaluation of 35 cases. Hum Reprod. 1998;13:1496-1499.
51 Anderson AN, Yue Z, Meng FJ, Petersen K. Low implantation rate after in vitro fertilization in patients with hydrosalpinges diagnosed by ultrasonography. Hum Reprod. 1994;9:1935-1938.
52 Strandell A, Waldenstrom NL, Hamberger L. Hydrosalpinx reduces in vitro fertilization/embryo transfer pregnancy rates. Hum Reprod. 1994;9:861-863.
53 Vandromme J, Chasse E, Lejeune B, et al. Hydrosalpinges in in vitro fertilization: An unfavorable feature. Hum Reprod. 1995;10:576-579.
54 Bedaiwy MA, Goldberg JM, Falcone T, et al. Relationship between oxidative stress and embryotoxicity of hydrosalpingeal fluid. Hum Reprod. 2002;17:601-604.
55 Bedaiwy MA, Falcone T, Goldberg JM, et al. The relationship between cytokines and the embryotoxicity of hydrosalpingeal fluid. J IVF Genet. 2005;22:171-176.
56 Blazar AS, Hogan JW, Seifer DB, et al. The impact of hydrosalpinx on successful pregnancy in tubal factor infertility treated by in vitro fertilization. Fertil Steril. 1997;67:517-520.
57 Camus E, Poncelet C, Goffinet F, et al. Pregnancy rates after in vitro fertilization in cases of tubal infertility with and without hydrosalpinx: A meta-analysis of published comparative studies. Hum Reprod. 1999;14:1243-1249.
58 Zeyneloglu HB, Arici A, Olive D. Adverse affects of hydrosalpinx on pregnancy rates after in vitro fertilization–embryo transfer. Fertil Steril. 1998;11:208-2084.
59 Strandell A, Lindhard A, Waldenstrom U, Thorburn J. Hydrosalpinx and IVF outcome: Cumulative results after salpingectomy in a randomized controlled trial. Hum Reprod. 2001;16:2403-2410.
60 Sagoskin AW, Lessey BA, Mottla GL, et al. Salpingectomy or proximal tubal occlusion of unilateral hydrosalpinx increases the potential for spontaneous pregnancy. Hum Reprod. 2003;18:2634-2637.
61 Surrey ES, Schoolcraft WB. Laparoscopic management of hydrosalpinges before in vitro fertilization–embryo transfer: Salpingectomy versus proximal tubal occlusion. Fertil Steril. 2001;75:612-617.
62 Johnson N, Mak W, Sowter M. Laparoscopic salpingectomy for women with hydrosalpinges enhances the success of IVF: A Cochrane review. Hum Reprod. 2002;17:543-548.
63 Hurst BS, Tucker KE, Awoniyi CA, Schlaff WD. Hydrosalpinx treated with extended doxycycline does not compromise the success of in vitro fertilization. Fertil Steril. 2001;75:1017-1019.
64 Tulandi T, Collins JA, Burrows E. Treatment-dependent and treatment-independent pregnancy among women with periadenexal adhesions. Am J Obstet Gynecol. 1990;162:354-357.
65 Tulandi T, Bugnah M. Operative laparoscopy: Surgical modalities. Fertil Steril. 1995;63:237-245.
66 Saravelos HG, Li TC, Cooke ID. An analysis of the outcome of microsurgical and laparoscopic adhesiolysis for infertility. Hum Reprod. 1995;10:2887-2894.
67 Tulandi T. Salpingo-ovariolysis: A comparison between laser surgery and electrosurgery. Fertil Steril. 1986;45:489-491.
68 Tulandi T, Falcone T, Kafka I. Second-look operative laparoscopy one year following reproductive surgery. Fertil Steril. 1989;52:421-424.