Chapter 28 Truncus Arteriosus
Truncus arteriosus was recognized in 1798,1 and the clinical and necropsy findings were described in 1864.2 Humphreys summarized the reports up to 1932, and Lev and Saphir3 critically reviewed published accounts during the following decade. The malformation accounts for approximately 1% to 2% of cases of congenital heart disease at necropsy and approximately 0.7% to 1.2% of all congenital cardiac malformations.4
Truncus arteriosus is characterized by a single great artery with a single semilunar valve that leaves the base of the heart and gives rise to the coronary, pulmonary, and systemic circulations.4–7 A second semilunar valve is neither present nor implied. The single arterial trunk receives the output from both ventricles, so a ventricular septal defect is obligatory.4,5,8 Type IV, or pseudotruncus with a biventricular aorta and an atretic pulmonary valve, is now uniformly considered to be a form of pulmonary atresia with ventricular septal defect rather than truncus arteriosus (see Chapter 18).9 Edwards, with disarming candor, stated, “Twenty-eight years after the introduction of the term, we doubt that the condition which Collett and Edwards had called truncus Type IV exists.”5 Similarly, a solitary pulmonary trunk referred to in 1814 by Farrell10 is considered to be a variety of aortic atresia.11,12 Hemitruncus, a term no longer used, referred to a rare anomaly in which one pulmonary artery branch arose from the ascending aorta just above the aortic sinuses and in which the main pulmonary artery with the other branch arose in its normal position.13,14 Rarely, the main pulmonary artery arises anteriorly and proximally at the level of the sinus of the common arterial trunk.15
The truncus is large because it accepts the entire output of both systemic and pulmonary circulations (Figures 28-1 and 28-2A), although inherent medial abnormalities contribute to the dilation.16 Agenesis of the ductus arteriosus occurs in 50% to 75% of cases,4,7,17 which is not surprising because a fetal ductus is not needed to channel pulmonary arterial blood into the aorta.18
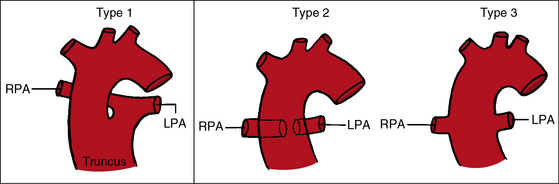
The greatest anatomic variability is in the branching patterns of the common trunk.19,20 In 1949, Collett and Edwards5 classified truncus arteriosus into four types based on the origins of the pulmonary arteries. The first three types were reconsidered by van Praagh and van Praagh7 in 1965 and form the basis of current terminology (Figure 28-3). Type 4 is now regarded as pulmonary atresia with ventricular septal defect (see previous and see Chapter 18). The most common variety is type 1, which is characterized by a short main pulmonary artery that originates from the truncus and gives rise to the right and left pulmonary arteries (Figures 28-2A, 28-4, and 28-5).7 Type 2 and type 3 of Collett and Edwards were originally defined by right and left pulmonary arteries that arose from separate ostia at either the side or the back of the truncus (see Figure 28-3).4,5 These two types are now grouped within a single category referred to as type 2 (see Figures 28-1B, and 28-3).7 In about 15% of cases, the right or left pulmonary artery is absent or hypoplastic (Figure 28-4B). An absent21 or hypoplastic pulmonary artery is usually concordant with the side of the aortic arch,22 which is right-sided in as many as 30% of patients with truncus arteriosus (see Figures 28-1 and 28-5).4,7,19,23 Rarely, there is a double aortic arch24,25 or an interrupted aortic arch.26
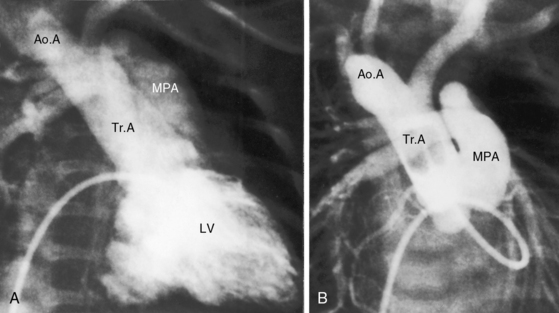
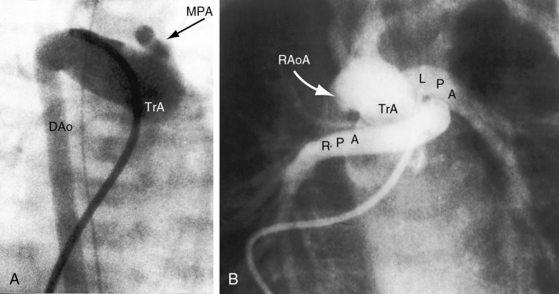
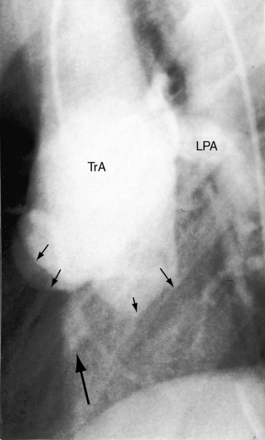
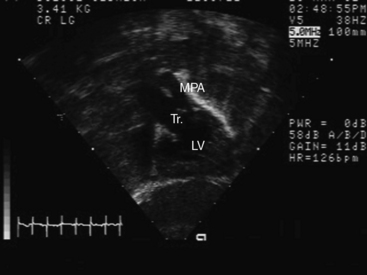
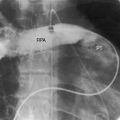
Figure 28-5 Angiocardiograms from a male neonate with truncus arteriosus type 1 and DiGeorge syndrome (see Figure 28-6). A, The left ventriculogram (LV) visualized the truncus (Tr.A) that continued as a right aortic arch (AoA). The main pulmonary artery (MPA) originated directly from the truncus. B, Contrast material into the truncus delineated a main pulmonary artery originating directly from the truncus and continuing as a right aortic arch (AoA).
In approximately two thirds of patients, the truncal valve has three leaflets, and its has either two leaflets (bicuspid) or four leaflets (quadricuspid) in most of the others. Very rarely, the valve is pentacuspid or hexacuspid (see Figures 28-10 and 28-22).19 A normal trileaflet aortic valve differs from a trileaflet truncal valve because of the presence of truncal raphes and cuspal inequality and because trileaflet truncal valves tend to be thickened and focally or diffusely dysplastic.17,27,28 A trileaflet truncal valve with raphes and cusps in excess of three represents a morphogenetic combination of aortic and pulmonary valves.7 Truncal valves are poorly supported therefore and are frequently incompetent.4,29 Stenotic truncal valves are less common,4,27,30,31 may be dysplastic,32 and may calcify in older adults.33
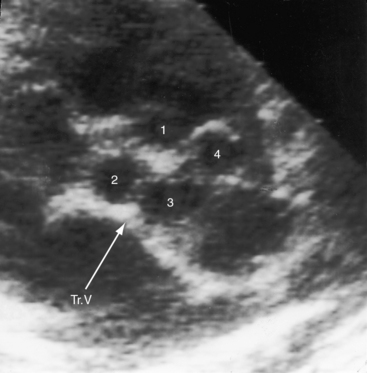
The coronary arteries in truncus arteriosus are defined by their relationship to the truncal sinuses and by their epicardial courses (see Figure 32-12).7,8,34–36 Coronary arterial ostia appear after division of the embryonic truncus is complete during normal morphogenesis.37 There are initially six coronary artery anlagen (primordia), three from the aorta and three from the pulmonary artery.38 Anlagen in the three pulmonary sinuses and in one of the aortic sinus normally undergo involution, leaving two coronary arteries that arise from two persistent anlagen in the right and left aortic sinuses. In truncus arteriosus, the sinus substrates to which coronary arteries are assigned are influenced by failure of septation of the embryonic truncus and by developmental abnormalities of the truncal valve.34,36 The left coronary artery tends to arise from the left posterior aspect of the truncus, and the right coronary artery tends to arise from the right anterior aspect of the truncus, whether the truncal valve is bicuspid, tricuspid, or quadricuspid (see Figure 32-12).8 When the truncal valve is quadricuspid, which is usually the case, coronary artery orifices originate in opposite sinuses rather than in adjacent sinuses, and high ostial origins are frequent.8,36 The right coronary artery is dominant in about 85% of cases; the conus branch of the right coronary artery is large, and the left anterior descending artery is small.8 There is an increased incidence of single coronary artery (see Chapter 32).34,36 An ostial membrane of the left coronary artery has been reported.39
A ventricular septal defect results from absence or deficiency of the infundibular septum and is almost always nonrestrictive and roofed by the truncal valve, setting the stage for inadequate support and truncal valve regurgitation.40 The biventricular truncal valve is assigned equally to the two ventricles, or predominantly to the right ventricle but only infrequently to the left ventricle.4,6,17 Cardiovascular anomalies commonly associated with truncus arteriosus include a right aortic arch (see previous and see Figures 28-1 and 28-5), truncal valve abnormalities (see previous and see Figures 28-10 and 28-22), anomalies of origin and distribution of the coronary arteries (see previous and see Figure 32-12), absence of the right or left pulmonary artery (see Figure 28-4B), and atresia of the ductus arteriosus. Abnormalities that occur sporadically include single ventricle, aberrant subclavian artery, left superior vena cava, and total anomalous pulmonary venous connection.41,42 When truncus arteriosus occurs with complete interruption of the aortic arch, the interruption is distal to the origin of the left carotid artery.17 The left subclavian artery arises from the descending aorta, and a patent ductus provides continuity from truncus to descending aorta.17
A common arterial trunk is a feature of normal early embryogenesis, an understanding of which sheds light on the morphogenesis of persistent truncus arteriosus.43,44 Septation of the arterial pole of the normal heart begins with the appearance of two opposing truncal cushions that rapidly enlarge and fuse to form the truncal septum. The proximal truncal septum normally fuses with the distal infundibular septum, a process that completes the spiral division of the truncus arteriosus and establishes left ventricular origin of the aorta and right ventricular origin of the pulmonary trunk. The aortic and pulmonary valves and their sinuses develop from truncal tissue at the line of fusion of the truncal and infundibular septa. In persistent truncus arteriosus, the truncal septum fails to develop. The infundibular septum is deficient or absent, which is responsible for a nonrestrictive ventricular septal defect roofed by the truncal valve. Vestigial development of the distal truncal septum is responsible for a short main pulmonary artery that arises from the truncus. When the truncal septum is absent altogether with no vestigial remnant, the main pulmonary artery is also absent, so the right and left pulmonary arteries arise directly from the truncus by separate ostia.
Physiologic consequences of truncus arteriosus depend on the size of the pulmonary arteries and on the pulmonary vascular resistance. Right ventricular pressure is identical with systemic because both ventricles communicate directly with the biventricular truncus via the nonrestrictive ventricular septal defect. When a main pulmonary artery arises directly from the truncus, blood flow from the left and right ventricles tends to cross, so oxygen content is higher in the aorta than in the pulmonary artery. Systemic arterial oxygen saturation is high when pulmonary resistance is low and pulmonary blood flow is increased, an advantage purchased at the price of volume overload of the left ventricle and congestive heart failure. Truncal valve regurgitation or truncal valve stenosis adds to the hemodynamic burden of the volume-overloaded left ventricle4,30 and to the hemodynamic derangements that are imposed on the right ventricle because of the biventricular truncus. As pulmonary vascular resistance rises, pulmonary blood flow falls. Volume overload of the left ventricle is curtailed at the price of increased cyanosis. Occasionally, pulmonary blood flow is adequately regulated by mild to moderate hypoplasia of both pulmonary arteries. In patients with truncus arteriosus and an absent right or left pulmonary artery, early vascular disease develops in the contralateral pulmonary artery (see Figure 28-16A).22
History
Truncus arteriosus occurs with equal frequency in males and females.17 Isolated examples have been reported in siblings17,45–47 and in twins,48–50 and a relatively high incidence rate of congenital cardiac malformations is found in siblings of children with truncus arteriosus. Familial recurrence of nonsyndromic truncus with interrupted aortic arch has been described.51 Truncus arteriosis has been reported with chromosome 22q11 deletion,15,52 trisomy 18,53 and trisomy 21.
Truncus arteriosus comes to attention in the first few weeks of life because of tachypnea, diaphoresis, poor feeding, and failure to thrive. As pulmonary resistance falls, pulmonary blood flow increases and neonatal cyanosis diminishes or may virtually vanish. Truncal valve regurgitation is responsible for biventricular failure because regurgitant flow is received by both ventricles. A systolic murmur awaits the fall in neonatal pulmonary vascular resistance analogous to the time course with ventricular septal defect (see Chapter 17).
Infants with truncus arteriosus seldom reach their first birthday.5,17,54 Most die of congestive heart failure in the first few months of life. In van Praagh and van Praagh’s7 necropsy series, mean age at death was 5 weeks. A rise in pulmonary vascular resistance occasionally regulates pulmonary blood flow and relieves the volume-overloaded left ventricle, so symptoms of congestive heart failure improve while cyanosis deepens. A small but not insignificant number of patients reach their third, fourth, or fifth decade (see Figures 28-15 through 28-18),5,54–57 with an occasional survival into the sixth58,59 or seventh decade.60 A patient with truncus arteriosus “type 4” survived to age 54 years.61 Patients with truncus arteriosus and Eisenmenger’s syndrome are subject to the multisystem systemic disorders described in Chapter 17. Rarely, death is from sudden intramural dissection and rupture of the common trunk.62 Morbidity and mortality are also influenced by a host of noncardiac abnormalities that coexist with truncus arteriosus, as described subsequently.
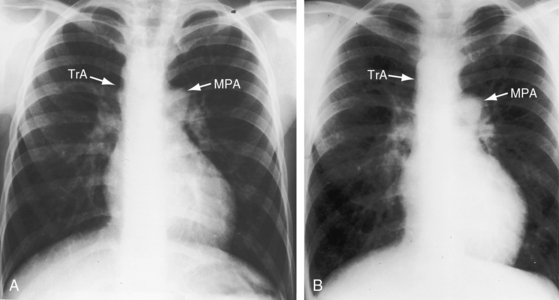
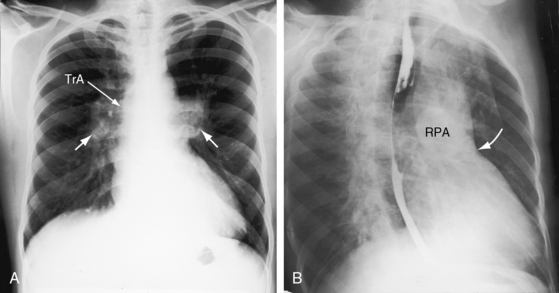
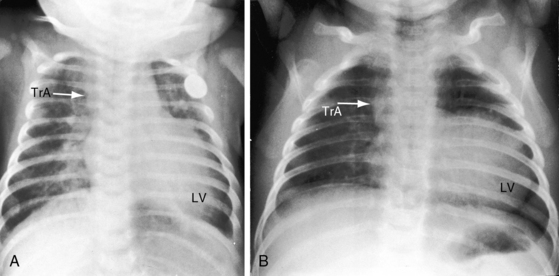
Figure 28-18 X-rays at age 10 years (A) and at age 42 years (B) from the same man with truncus arteriosus type 1 and pulmonary vascular disease. His electrocardiogram is shown in Figure 28-13. The two films are virtually identical except for body size. A dilated main pulmonary artery (MPA) originated from the truncus (TrA) and gave rise to enlarged hypertensive right and left branches. The truncus continued as a right aortic arch, a high transverse aorta, and a right descending aorta. Pulmonary vascularity was reduced because of pulmonary vascular disease. The left ventricle is convex but of normal size.
Physical appearance
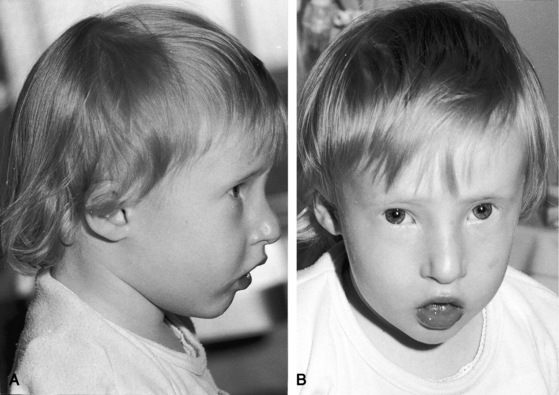
Infants with increased pulmonary blood flow and congestive heart failure are frail and have poor development. Cyanosis is inconspicuous or absent. When truncus arteriosus occurs with hypoplasia of a pulmonary artery, the ipsilateral hemithorax tends to be small (see Figure 28-16A).4 A distinctive appearance in truncus arteriosus is DiGeorge syndrome, which includes hypertelorism, low-set ears, micrognathia, a small fishlike mouth, a short philtrum, short down-slanting palpebral fissures, deformed or absent pinnae, cleft lip, a high-arched or cleft palate, a malformed nose, and bilateral cataracts (Figure 28-6).63–66 Extracardiac congenital abnormalities involve the limbs, kidneys, and intestines.64 Infants experience hypocalcemic seizures and severe infections as a result of deficient cell-mediated immunity and absence of the thymus and parathyroid glands.64 The CHARGE association includes coloboma, heart disease, choanal atresia, retardation of growth and of mental development, genital hypoplasia, and ear anomalies.67 Conotruncal anomalies, especially truncus arteriosus, have been reported in 42% of patients with CHARGE association.67
Arterial pulse
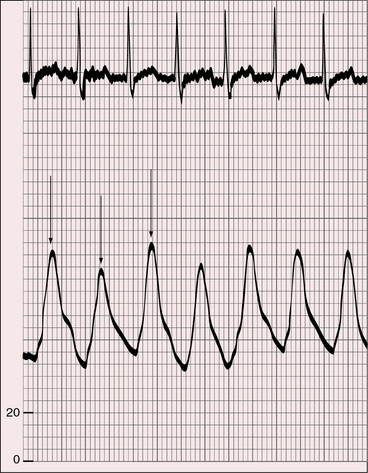
The pulse pressure is wide because low pulmonary vascular resistance permits a diastolic runoff from the truncus into the pulmonary bed (Figure 28-7). The rate of rise of the arterial pulse is brisk because of rapid ejection of a large left ventricular stroke volume. A brisk arterial pulse is especially noteworthy in the context of left ventricular failure and pulsus alternans (see Figure 28-7). Truncal valve regurgitation independently causes a bounding arterial pulse analogous to the water-hammer pulse of aortic regurgitation.4,29
Jugular venous pulse
In infants with congestive heart failure, the jugular veins are distended, the liver is enlarged, and waveform or a liver pulse cannot be identified. In adults with truncus arteriosus and Eisenmenger’s syndrome, the jugular venous pulse is normal or nearly so, analogous to the jugular pulse in Eisenmenger’s syndrome with nonrestrictive ventricular septal defect (see Chapter 17).
Auscultation
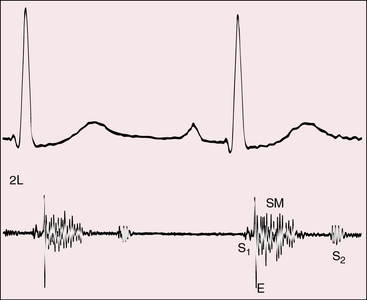
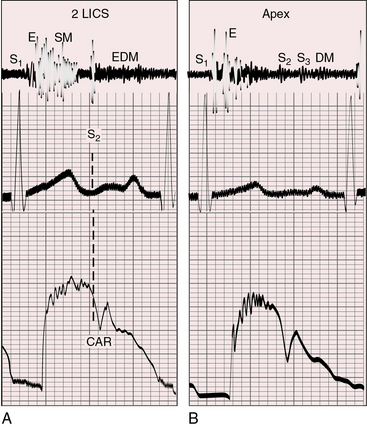
A normal first heart sound is followed by a high-pitched ejection sound generated within the dilated truncus (Figures 28-8 and 28-9).68 The systolic murmur through the ventricular septal defect and into the truncus is decrescendo, beginning with the ejection sound and ending before the second heart sound (see Figures 28-8 and 28-9).68 The murmur is harsh, blowing, and usually grade 3/6 to 4/6, with maximal intensity in the third or fourth left intercostal space. Radiation is upward and to the right because a truncus takes the course of an ascending aorta (see Figure 28-5). The murmur is more prominent when the biventricular truncus arises chiefly above the right ventricle and is less prominent and often midsystolic when the truncus arises chiefly above the left ventricle, or when pulmonary vascular disease minimizes the shunt. Flow is then across the truncal valve rather than across the ventricular septal defect. When the truncal valve is thickened, dysplastic, or stenotic, the murmur is midsystolic, as in aortic stenosis (see Chapter 7).
The truncal valve closure sound is prominent because the enlarged truncus is closer to the chest wall and because the truncal valve closes at systemic pressure (see Figure 28-9). Splitting is not be possible because the truncus is equipped with only one valve. However, the second heart sound arising from a truncal valve that is equipped with three or more well-formed cusps may be impure or reduplicated, but phonocardiograms show that the vibrations merge and do not separate into two distinct sounds separated by an interval. On the other hand, the phonocardiogram occasionally records two components that have been attributed to cuspal inequality with asynchronous closure of tricuspid or quadricuspid truncal valves.68
A high-frequency blowing early diastolic murmur is caused by truncal valve regurgitation (Figures 28-9A and 28-10).4,29 An apical mid-diastolic murmur introduced by a third heart sound (Figure 28-9B) results from increased mitral valve flow in response to increased pulmonary blood flow. Mid-diastolic or presystolic Austin Flint murmurs are thought to be associated with severe truncal valve regurgitation.
Electrocardiogram
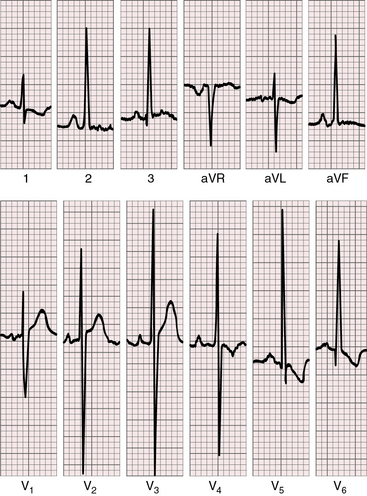
Tall peaked right atrial P waves appear in limb leads and in precordial leads (Figures 28-11 and 28-12) and are often accompanied by notched bifid left atrial P waves.69 Infants may exhibit isolated left atrial P waves generated by increased pulmonary blood flow.
When pulmonary blood flow is reduced, the QRS axis is rightward and depolarization is clockwise because an underfilled left ventricle is coupled with a systemic right ventricle (Figures 28-12 and 28-13). The axis is normal or leftward when increased pulmonary blood flow overloads the left ventricle (see Figure 28-11).4 However, the conduction system and QRS axis in truncus arteriosus are also related to the location of the ventricular septal defect.70
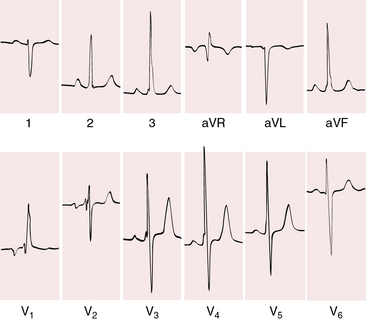
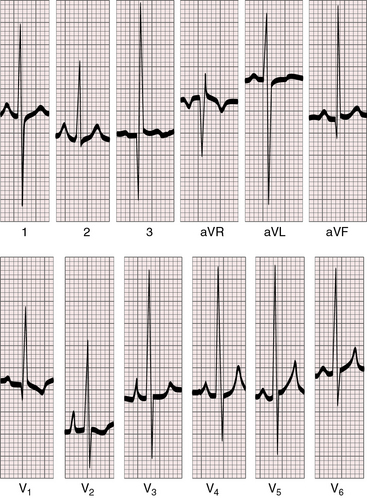
Figure 28-13 Electrocardiogram from a 42-year-old man with truncus arteriosus type 1 and pulmonary vascular disease. His x-rays are shown in Figure 28-18. The P wave in lead 2 is slightly peaked. The negative P terminal force in lead V1 is a right atrial abnormality. There is right axis deviation. Right ventricular hypertrophy is manifested by a tall monophasic R wave in lead V1, by deep S waves in left precordial leads, and by increased amplitude of the R waves in leads 3 and aVF. The R waves in leads V4-5 indicate that the left ventricle is well-developed.
Precordial leads exhibit biventricular hypertrophy (see Figures 28-11 and 28-12). Pure right ventricular hypertrophy is reserved for adults with pulmonary vascular disease (see Figure 28-13).4,59 When pulmonary blood flow is increased, left precordial leads exhibit the deep Q waves, tall R waves, and inverted T waves of left ventricular hypertrophy, and right precordial leads continue to display the tall R waves of right ventricular hypertrophy (see Figures 28-11 and 28-12). Large equidiphasic QRS complexes in central chest leads indicate combined ventricular hypertrophy (see Figure 28-11).
X-Ray
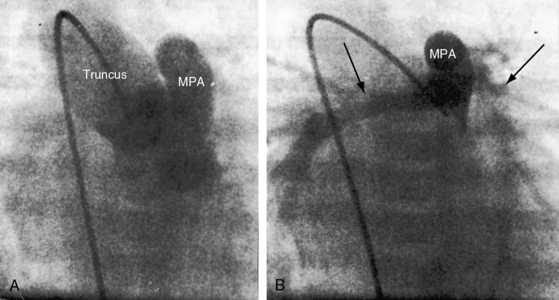
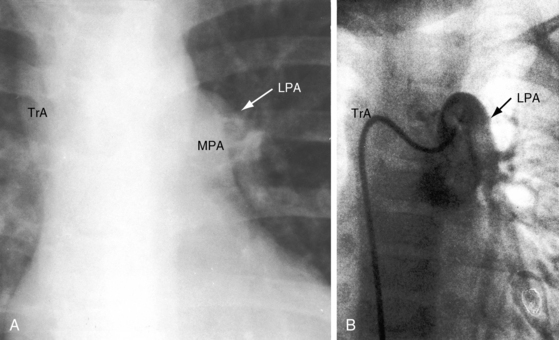
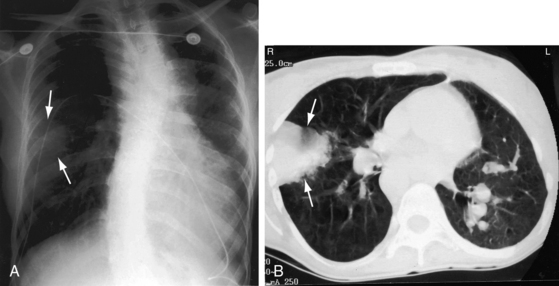
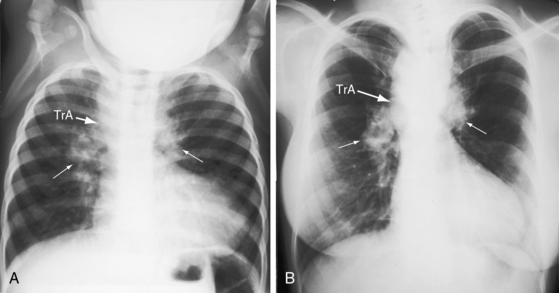
Increased pulmonary vascularity is a combination of both pulmonary arterial blood flow and pulmonary venous congestion (Figure 28-14). The main pulmonary artery segment is absent when right and left pulmonary arteries arise directly from the truncus (see Figure 28-14B). The concave profile stands out in the right anterior oblique projection (see Figure 28-17B). A dilated left pulmonary artery may occupy the concavity, but its shadow can usually be recognized (see Figure 28-17A). A prominent left pulmonary artery may reveal itself as a high shadow that curves upward to form a left hilar comma (see Figure 28-4), which is especially evident when the aortic arch is right-sided.4 The convex main pulmonary artery segment of truncus type 1 tends to arise at a higher level (see Figure 28-18) compared with other forms of pulmonary artery dilation. In older adults with pulmonary vascular disease, the dilated hypertensive main pulmonary artery segment is especially prominent (see Figure 28-18). A large truncus arteriosus resembles a large ascending aorta (see Figure 28-14) that may continue as a right aortic arch (see Figure 28-5) and a high transverse aorta (Figure 28-15).
The left ventricle dilates in response to volume overload–associated increased pulmonary blood flow (see Figures 28-14 and 28-15A). Enlargement of the right ventricle and right atrium accompany congestive heart failure and occur when the right ventricle receives regurgitant flow across an incompetent biventricular truncal valve. Hypoplasia or absence of a pulmonary artery results in reduced pulmonary vascularity and a smaller ipsilateral hemithorax (Figure 28-16A). Absence of a pulmonary artery is usually on the same side as the aortic arch (see Figure 28-4B),71 in contrast to Fallot’s tetralogy (see Chapter 18).
In adults with high pulmonary vascular resistance, the x-ray shows decreased pulmonary vascularity, increased prominence of the main pulmonary artery and its right and left branches, and a relatively normal left ventricle (Figures 28-17 and 28-18).
Echocardiogram
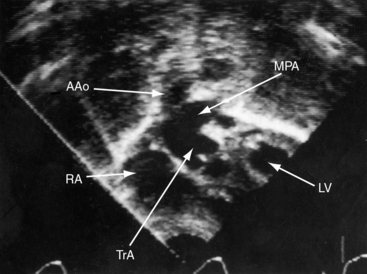
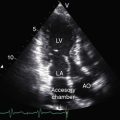
Echocardiography with color flow imaging and Doppler interrogation establish the type of truncus arteriosus, the relationship of the truncus to the left and right ventricles, the morphologic and functional derangements of the truncal valve, and the physiologic consequences of the malformation.19,72,73 A single great arterial trunk overrides a nonrestrictive ventricular septal defect in the infundibular septum (Figures 28-19 and 20-20, and Video 28-1). The truncal valve forms the roof of the defect (see Figure 28-20). Right and left pulmonary arteries either arise from a short main pulmonary artery (see Figures 28-19 and 28-20) or arise directly from the truncus by separate ostia. Biventricular unbalanced right or left ventricular origin of the truncus can be determined (see Figure 28-20), and the morphology of the truncal valve can be established (Figure 28-21). Color flow imaging determines the presence and degree of truncal valve regurgitation, and continuous wave Doppler scan determines the presence and degree of truncal valve stenosis.
1 Wilson J. A description of a very unusual malformation of the human heart. Philosophical Transactions of the Royal Society of London. 1798;88:346-356.
2 Buchanan A. Malformation of heart. Undivided truncus arteriosus. Heart otherwise double. Transactions of the Pathologic Society of London. 1864;15:89.
3 Lev M., Saphir O. Truncus arteriosus communis persistens. J Pediatr. 1942;20:74-88.
4 Calder L., Van Praagh R., Van Praagh S., et al. Truncus arteriosus communis. Clinical, angiocardiographic, and pathologic findings in 100 patients. Am Heart J. 1976;92:23-38.
5 Collett R.W., Edwards J.E. Persistent truncus arteriosus; a classification according to anatomic types. Surg Clin North Am. 1949;29:1245-1270.
6 Van Praagh R. Editorial: classification of truncus arteriosus communis (TAC). Am Heart J. 1976;92:129-132.
7 Van Praagh R., van Praagh S. The anatomy of common aorticopulmonary trunk (truncus arteriosus communis) and its embryologic implications. A study of 57 necropsy cases. Am J Cardiol. 1965;16:406-425.
8 Anderson K.R., McGoon D.C., Lie J.T. Surgical significance of the coronary arterial anatomy in truncus arteriosus communis. Am J Cardiol. 1978;41:76-81.
9 Kapoor A., Gupta N. Adult survival of persistent truncus arteriosus type IV. Int J Cardiol. 2003;88:323. author reply 325
10 Farrell J.R. Pathological researches. Essay 1. On malformation of the human heart. London: Longman; 1814.
11 Allwork S.P., Bentall R.H. Truncus solitarius pulmonalis. Br Heart J. 1973;35:977-980.
12 Sennari E., Nishiguchi T., Okishima T., et al. Truncus solitarius pulmonalis. Pediatr Cardiol. 1990;11:50-53.
13 Fong L.V., Anderson R.H., Siewers R.D., Trento A., Park S.C. Anomalous origin of one pulmonary artery from the ascending aorta: a review of echocardiographic, catheter, and morphological features. Br Heart J. 1989;62:389-395.
14 Fontana G.P., Spach M.S., Effmann E.L., Sabiston D.C.Jr. Origin of the right pulmonary artery from the ascending aorta. Ann Surg. 1987;206:102-113.
15 Phelps C.M., Da Cruz E., Fagan T., Younoszai A.K., Tissot C. Images in cardiovascular medicine. Anterior origin of the main pulmonary artery from the arterial valvar sinus: unusual truncus arteriosus. Circulation. 2009;119:624-627.
16 Niwa K., Perloff J.K., Bhuta S.M., et al. Structural abnormalities of great arterial walls in congenital heart disease: light and electron microscopic analyses. Circulation. 2001;103:393-400.
17 Butto F., Lucas R.V.Jr, Edwards J.E. Persistent truncus arteriosus: pathologic anatomy in 54 cases. Pediatr Cardiol. 1986;7:95-101.
18 Mello D.M., Mcelhinney D.B., Parry A.J., Silverman N.H., Hanley F.L. Truncus arteriosus with patent ductus arteriosus and normal aortic arch. Ann Thorac Surg. 1997;64:1808-1810.
19 Colon M., Anderson R.H., Weinberg P., et al. Anatomy, morphogenesis, diagnosis, management, and outcomes for neonates with common arterial trunk. Cardiol Young. 2008;18(suppl 3):52-62.
20 Jacobs M.L. Congenital Heart Surgery Nomenclature and Database Project: truncus arteriosus. Ann Thorac Surg. 2000;69:S50-S55.
21 Wong M.N.L., Kirk R., Quek S.C. Persistent truncus arteriosus with absence of right pulmonary artery. Heart. 2003;89:549.
22 Fyfe D.A., Driscoll D.J., Di Donato R.M., et al. Truncus arteriosus with single pulmonary artery: influence of pulmonary vascular obstructive disease on early and late operative results. J Am Coll Cardiol. 1985;5:1168-1172.
23 Hastreiter A.R., D’cruz I.A., Cantez T., Namin E.P., Licata R. Right-sided aorta. I. Occurrence of right aortic arch in various types of congenital heart disease. II. Right aortic arch, right descending aorta, and associated anomalies. Br Heart J. 1966;28:722-739.
24 Bhan A., Gupta M., Kumar M.J.S., Kothari S.S., Gulati G.S. Persistent truncus arteriosus with double aortic arch. Pediatr Cardiol. 2006;27:378-380.
25 Huang S.-C., Wang C.-J., Su W.-J., Chu J.-J., Hwang M.-S. The rare association of truncus arteriosus with a cervical double aortic arch presenting with left main bronchial compression. Cardiology. 2008;111:16-20.
26 Verhaert D., Arruda J., Thavendiranathan P., Cook S.C., Raman S.V. Truncus arteriosus with aortic arch interruption: cardiovascular magnetic resonance findings in the unrepaired adult. J Cardiovasc Magn Reson. 2010;12:16.
27 Ledbetter M.K., Tandon R., Titus J.L., Edwards J.E. Stenotic semilunar valve in persistent truncus arteriosus. Chest. 1976;69:182-187.
28 Marino B., Digilio M.C., Dallapiccola B. Severe truncal valve dysplasia: association with DiGeorge syndrome? Ann Thorac Surg. 1998;66:980.
29 Deely W.J., Hagstrom J.W., Engle M.A. Truncus insufficiency: common truncus arteriousus with regurgitant truncus valve. Report of four cases. Am Heart J. 1963;65:542-548.
30 Burnell R.H., Mcenery G., Miller G.A. Truncal valve stenosis. Br Heart J. 1971;33:423-424.
31 Lee M.H., Bellon E.M., Liebman J., Perrin E.V. Truncal valve stenosis. Am Heart J. 1973;85:397-400.
32 Elzein C., Ilbawi M., Kumar S., Ruiz C. Severe truncal valve stenosis: diagnosis and management. J Card Surg. 2005;20:589-593.
33 MacGilpin H.H.Jr. Truncus arteriosus communis persistence. Am Heart J. 1950;39:615-625.
34 De La Cruz M.V., Cayre R., Angelini P., Noriega-Ramos N., Sadowinski S. Coronary arteries in truncus arteriosus. Am J Cardiol. 1990;66:1482-1486.
35 Shrivastava S., Edwards J.E. Coronary arterial origin in persistent truncus arteriosus. Circulation. 1977;55:551-554.
36 Suzuki A., Ho S.Y., Anderson R.H., Deanfield J.E. Coronary arterial and sinusal anatomy in hearts with a common arterial trunk. Ann Thorac Surg. 1989;48:792-797.
37 Heifetz S.A., Robinowitz M., Mueller K.H., Virmani R. Total anomalous origin of the coronary arteries from the pulmonary artery. Pediatr Cardiol. 1986;7:11-18.
38 Hackensellner H.A. Ueber akessorische, von der Arteria Pulmonalis abgehende Herzgefaesse und ihre bei den Tung fuer das Verstaendis der formalen Genese des Ursprunges einer oder beider Coronararterien von der Lungenschlagader. Frankf Z Pathol. 1955;66:263.
39 Van Son J.A., Autschbach R., Hambsch J. Congenital ostial membrane of left coronary artery in truncus arteriosus. J Thorac Cardiovasc Surg. 1999;118:1132-1134.
40 Rosenquist G.C., Bharati S., McAllister H.A., Lev M. Truncus arteriosus communis: truncal valve anomalies associated with small conal or truncal septal defects. Am J Cardiol. 1976;37:410-412.
41 Litovsky S.H., Ostfeld I., Bjornstad P.G., Van Praagh R., Geva T. Truncus arteriosus with anomalous pulmonary venous connection. Am J Cardiol. 1999;83:801-804. A810
42 Paris Y.M., Bhan I., Marx G.R., Rhodes J. Truncus arteriosus with a single left ventricle: case report of a previously unrecognized entity. Am Heart J. 1997;133:377-380.
43 Orts-Llorca F., Puerta Fonolla J., Sobrado J. The formation, septation and fate of the truncus arteriosus in man. J Anat. 1982;134:41-56.
44 Yu I.T., Hutchins G.M. Truncus arteriosus malformation: a developmental arrest at Carnegie stage 14. Teratology. 1996;53:31-37.
45 Brunson S.C., Nudel D.B., Gootman N., Aftalion B. Truncus arteriosus in a family. Am Heart J. 1978;96:419-420.
46 Goodyear J.E. Persistent truncus arteriosus in two siblings. Br Heart J. 1961;23:194-196.
47 Shapiro S.R., Ruckman R.N., Kapur S., et al. Single ventricle with truncus arteriosus in siblings. Am Heart J. 1981;102:456-459.
48 Benesova D., Sikl H. A rare concordant malformation in monochoriate twins; persistent common arterial trunk. J Pathol Bacteriol. 1954;67:367-370.
49 Lang M.J., Aughton D.J., Riggs T.W., Milad M.P., Biesecker L.G. Dizygotic twins concordant for truncus arteriosus. Clin Genet. 1991;39:75-79.
50 Mas C., Delatycki M.B., Weintraub R.G. Persistent truncus arteriosus in monozygotic twins: case report and literature review. Am J Med Genet. 1999;82:146-148.
51 Digilio M.C., Marino B., Musolino A.M., Giannotti A., Dallapiccola B. Familial recurrence of nonsyndromic interrupted aortic arch and truncus arteriosus with atrioventricular canal. Teratology. 2000;61:329-331.
52 Momma K., Ando M., Matsuoka R. Truncus arteriosus communis associated with chromosome 22q11 deletion. J Am Coll Cardiol. 1997;30:1067-1071.
53 Moore J.W., Wight N.E., Jones M.C., Krous H.F. Truncus arteriosus associated with trisomy 18. Pediatr Cardiol. 1994;15:154-156.
54 Guenther F., Frydrychowicz A., Bode C., Geibel A. Cardiovascular flashlight. Persistent truncus arteriosus: a rare finding in adults. Eur Heart J. 2009;30:1154.
55 Carr F.B., Goodale R.H., Rockwell A.E.P. Persistent truncus arteriosus in a man aged thirty-six years. Arch Pathol. 1935;19:833.
56 Hicken P., Evans D., Heath D. Persistent truncus arteriosus with survival to the age of 38 years. Br Heart J. 1966;28:284-286.
57 Silverman J.J., Scheinesson G.P. Persistent truncus arteriosus in a 43 year old man. Am J Cardiol. 1966;17:94-96.
58 Carter J.B., Blieden L.C., Edwards J.E. Persistent truncus arteriosus. Report of survival to age of 52 years. Minn Med. 1973;56:280-282.
59 Niwa K., Perloff J.K., Kaplan S., Child J.S., Miner P.D. Eisenmenger syndrome in adults: ventricular septal defect, truncus arteriosus, univentricular heart. J Am Coll Cardiol. 1999;34:223-232.
60 Menon S.C., Julsrud P.R., Cetta F. Late diagnosis of common arterial trunk. Cardiol Young. 2007;17:333-335.
61 Bodi V., Insa L., Sanchis J., et al. Persistent truncus arteriosus type 4 with survival to the age of 54 years. Int J Cardiol. 2002;82:75-77.
62 Gutierrez P.S., Binotto M.A., Aiello V.D., Mansur A.J. Chest pain in an adult with truncus arteriosus communis. Am J Cardiol. 2004;93:272-273.
63 Freedom R.M., Rosen F.S., Nadas A.S. Congenital cardiovascular disease and anomalies of the third and fourth pharyngeal pouch. Circulation. 1972;46:165-172.
64 Radford D.J., Perkins L., Lachman R., Thong Y.H. Spectrum of Di George syndrome in patients with truncus arteriosus: expanded Di George syndrome. Pediatr Cardiol. 1988;9:95-101.
65 Van Mierop L.H., Kutsche L.M. Cardiovascular anomalies in DiGeorge syndrome and importance of neural crest as a possible pathogenetic factor. Am J Cardiol. 1986;58:133-137.
66 Farrell M.J., Stadt H., Wallis K.T., et al. HIRA, a DiGeorge syndrome candidate gene, is required for cardiac outflow tract septation. Circ Res. 1999;84:127-135.
67 Lin A.E., Chin A.J., Devine W., Park S.C., Zackai E. The pattern of cardiovascular malformation in the CHARGE association. Am J Dis Child. 1987;141:1010-1013.
68 Victorica B.E., Gessner I.H., Schiebler G.L. Phonocardiographic findings in persistent truncus arteriosus. Br Heart J. 1968;30:812-816.
69 Portillo B., Perez Martin R. Truncus arteriosus communis. Electrocardiographic study in 17 cases. Arch Inst Cardiol Mex. 1960;30:609-620.
70 Bharati S., Karp R., Lev M. The conduction system in truncus arteriosus and its surgical significance. A study of five cases. J Thorac Cardiovasc Surg. 1992;104:954-960.
71 Mair D.D., Ritter D.G., Danielson G.K., Wallace R.B., McGoon D.C. Truncus arteriosus with unilateral absence of a pulmonary artery. Criteria for operability and surgical results. Circulation. 1977;55:641-647.
72 Duke C., Sharland G.K., Jones A.M., Simpson J.M. Echocardiographic features and outcome of truncus arteriosus diagnosed during fetal life. Am J Cardiol. 2001;88:1379-1384.
73 Child J.S. Transthoracic and transesophageal echocardiographic imaging: Anatomic and hemodynamic assessment. In: Perloff J.K., Child J.S., editors. Congenital heart disease in adults. 2nd ed. Philadelphia: W.B. Saunders Company; 1998:91.