CHAPTER 45 Truncus Arteriosus
Truncus arteriosus is an uncommon but potentially lethal congenital heart disease that manifests during the neonatal period or early infancy. It is defined by a common origin of the aorta and the pulmonary arteries, resulting from an incomplete embryologic septation and separation of the aorta and the pulmonary trunk. This congenital cardiac anomaly was first described in 1798 by Wilson.1 In 1942, Lev and Saphir2 proposed the anatomic criteria that defined truncus arteriosus. Since then, different classifications had been proposed by Collett and Edwards3 in 1949, by Van Praagh and Van Praagh4 in 1965, and by the Society of Thoracic Surgeons in 2000.5
CLASSIFICATIONS
Historically, two major classification schemes for anatomic variations of the truncus arteriosus were proposed by Collett and Edwards3 and by Van Praagh and Van Praagh.4 The Collett and Edwards classification is strictly defined by the anatomic origins of the pulmonary arteries. It divides truncus arteriosus into four types, I to IV (Fig. 45-1). In Collett and Edwards type I, a common arterial trunk divides into an aorta and a short pulmonary trunk, which divides into the left and right branch pulmonary arteries. This is the most common configuration (48% to 68% of cases). In Collett and Edwards type II (29% to 48% of cases), the left and right branch pulmonary arteries arise close together from the common arterial trunk without a distinct pulmonary trunk. Collett and Edwards type III (6% to 10% of cases) is similar to type II except that the origins of the pulmonary arteries are far apart. Collett and Edwards described a type IV configuration in which the pulmonary arteries arise from the descending aorta. These arteries are now known to be major aorticopulmonary collateral arteries associated with pulmonary atresia with ventricular septal defect (VSD) and not truncus arteriosus.
The Collett and Edwards classification developed in 1949 did not consider the roles of patent ductus arteriosus (PDA) and interrupted aortic arch, which are associated with truncus arteriosus and have prognostic and surgical implications. Subsequently, Van Praagh and Van Praagh4 proposed a different classification with four types, 1 to 4 (Fig. 45-2). Each number may have a prefix letter A if a VSD is present (common) or B if a VSD is absent (rare). Van Praagh and Van Praagh type 1 is identical to Collett and Edwards type I, describing a short pulmonary trunk arising from the common arterial trunk. Van Praagh and Van Praagh type 2 describes separate origins of the left and right branch pulmonary arteries arising from the common arterial trunk, regardless of the distance separating their origins (Fig. 45-3). It corresponds to Collett and Edwards type II and type III. Van Praagh and Van Praagh type 3 describes one branch pulmonary artery arising from the common arterial trunk and the other connected to a ductus arteriosus or an aorticopulmonary collateral artery (Fig. 45-4). Van Praagh and Van Praagh type 4 describes the coexistence of a common arterial trunk and an interrupted or a severely hypoplastic aortic arch. The descending aorta is supplied by a PDA. Van Praagh and Van Praagh type 3 and type 4 have no correspondence in the Collett and Edwards classification.
Although the Van Praagh and Van Praagh classification provides a more refined description of truncus arteriosus, it does not completely serve the needs of surgeons. In 2000, the Society of Thoracic Surgeons proposed a uniform reporting system with modifiers that better describe anatomic features useful for surgical outcome studies.5 In this system, cryptic numerical labels are replaced by descriptive phrases. Three major types are described: truncus arteriosus with confluent or near-confluent pulmonary arteries (corresponding to Van Praagh and Van Praagh type 1 and type 2), truncus arteriosus with absence of one pulmonary artery (corresponding to Van Praagh and Van Praagh type 3), and truncus arteriosus with interrupted aortic arch or coarctation (corresponding to Van Praagh and Van Praagh type 4). Additional modifiers describe the presence or absence of VSD, the number of truncal leaflets, the presence of truncal insufficiency or stenosis or both, coexisting coronary anomalies, any ventricular hypoplasia, the truncal valve position relative to the ventricles, and the presence or absence of thymus.
EPIDEMIOLOGY AND GENETICS
Truncus arteriosus occurs in 1% to 2% of infants born with congenital heart defects, or about 10 cases per 100,000 live births.6 Among conotruncal anomalies, truncus arteriosus has a similar rate of occurrence as congenitally corrected transposition of the great arteries and double outlet right ventricle, but it is three times less common than complete transposition of the great arteries and four times less common than tetralogy of Fallot. There is no predilection by sex. Of patients with truncus arteriosus, 34% to 41% harbor a chromosome 22q11 deletion.7 This deletion is also seen in a large proportion of patients with conotruncal developmental anomalies as found in DiGeorge syndrome, velocardiofacial syndrome, and interrupted aortic arch, suggesting the importance of this gene in the regulation of the embryologic development of the conotruncus. Today, this chromosomal deletion is readily detected with the fluorescence in situ hybridization technique.
Genetic screening is important because 22q11 deletion is inherited in an autosomal dominant fashion from one parent in 6% to 28% of cases.8 This is important information for genetic family counseling. Knowledge of this deletion also heightens clinical suspicions for associated anomalies, including athymia, hypocalcemia, and nasopalatal malformation. Truncus arteriosus is also associated with trisomy 8 and chromosomal 10p deletion.9
ETIOLOGY AND PATHOPHYSIOLOGY
Embryology
In normal embryologic development, beginning in the fifth week of gestation, fusion of the endocardial cushions separates the atrioventricular canal into right and left openings, which are destined to become the tricuspid annulus and the mitral annulus. Through these two openings, blood flow develops into two intertwined, spiraling streams that flow out the conus cordis and the truncus arteriosus, or conotruncus for short. Between the two spiraling streams is a layer of stagnant blood. There is evidence that this layer of stagnant blood promotes the development of two ridges of tissue at opposing margins inside the conotruncus.10 These two ridges grow toward each other until they meet and fuse. The fusion begins at the conotruncal junction. Similar to the closing of a zipper, the fusion proceeds upward through the truncus arteriosus, creating two spiraling lumens that become the ascending aorta and the pulmonary trunk. From this junction, the fusion also travels downward through the conus cordis until it meets the ventricular septum, separating the right and left ventricles. Separation of the systemic and the pulmonary circulations is completed by 9 weeks of gestation. Beginning in the seventh week, specialized tissue swellings from the conotruncal junction evolve into the aortic and the pulmonary semilunar valves; these are also completed by 9 weeks.
Associated Cardiac Anomalies
Because the conus cordis is not partitioned properly below the truncal valve, a VSD usually exists at the infundibular septum beneath the truncal valve (Fig. 45-5). This VSD is often large and nonrestrictive. The right ventricle, subjected to the systemic pressure generated by the left ventricle, becomes hypertrophic. In most instances (68% to 83%), the common arterial trunk and the truncal valve straddle the ventricular septum in a manner resembling the overriding aorta in tetralogy of Fallot or pulmonary atresia with VSD. Uncommonly (11% to 29%), the truncal valve aligns exclusively with the right ventricle. Rarely (4% to 6%), it aligns with the left ventricle. In the two latter situations, the VSD may be small or absent.
The truncal valve may have one to five leaflets. Tricuspid truncal valve (Fig. 45-6A) is the most common (69%), followed by quadricuspid valve (22%) (see Fig. 45-6B) and bicuspid valve (9%). The truncal valve has fibrous continuity with the mitral valve, similar to the normal relationship between the aortic valve and mitral valve. A muscle bridge usually separates the truncal valve from the tricuspid valve. Competency of the truncal valve has important implications to survival and surgical outcome. In most autopsy cases, the truncal valve leaflets are dysplastic and thickened with myxomatous degeneration. Truncal regurgitation is common, occurring in half of the cases. In 20% of cases, the truncal valve may be stenotic.11 Severe regurgitation or stenosis heralds a poor prognosis.
In the most common situation, the truncal valve is tricuspid with a posterior cusp, a right anterior cusp, and a left anterior cusp. The right coronary artery usually arises from the right anterior cusp, and the left main coronary artery arises from the posterior cusp (Fig. 45-7A).12 Variation from this pattern is extremely common, however, because the development of the proximal coronary arteries is closely coordinated with the development of the conotruncus. In a quadricuspid valve, the right and left coronary arteries most commonly originate from the opposing right and left cusps (see Fig. 45-7B). In pathology series, a single coronary artery was seen in 10% to 20% of cases,13 and stenosis at the coronary ostium was found in 7% of cases.14
Compared with a normal ascending aorta, the common arterial trunk usually appears large, but some can expand into an aneurysm. Histologic studies have shown tissue pathology similar to that of Marfan syndrome. The pathophysiology is unknown, but pulmonary hypertension may play a role. Truncus rupture and dissection have been reported.15 The aortic arch is right-sided in one third of truncus arteriosus cases, usually with a mirror-image branching pattern of the cervical arteries. Aortic arch interruption is seen in 11% to 38% of cases. The most common interruption (84%) occurs between the left common carotid artery and the left subclavian artery (type B interrupted aortic arch).16 In this configuration, the left and right carotid arteries are fed by the common arterial trunk, and the descending aorta and left subclavian artery are fed by the PDA. The right subclavian artery may be fed by either of these arteries depending on whether it has a normal or an aberrant origin. Less commonly (16%), the interruption occurs distal to the left subclavian artery (type A interrupted aortic arch). Interruption between the left and right carotid arteries (type C interrupted aortic arch) is rare.
The pulmonary arteries usually arise from the left posterior aspect of the common arterial trunk. When the branch pulmonary arteries arise from the common arterial trunk separately, the left branch pulmonary artery is often superiorly related to the right branch pulmonary artery (see Fig. 45-3). If a PDA is present, its size is inversely related to the aortic arch and the aortic isthmus. In the extreme case of aortic arch interruption, blood flow to the descending aorta is carried entirely by a large ductus arteriosus. Other cardiac anomalies associated with truncus arteriosus are secundum atrial septal defect, aberrant subclavian artery, persistent left superior vena cava, and tricuspid stenosis.
MANIFESTATIONS OF DISEASE
Clinical Presentation
During the first week of life, the pulmonary vascular resistance decreases rapidly below the systemic vascular resistance. The shunt ratio can reach 5 : 1 or greater, which implies a pulmonary flow five times the systemic flow. This torrential pulmonary flow returns through the pulmonary veins into the left ventricle. Assuming that there is no shunting at the atrial level, the left ventricle must handle this amount of flow by stroking five times the volume of the right ventricle. The left ventricle is severely volume overloaded, leading to congestive heart failure. Chest radiography may show evidence for shunt vascularity and pulmonary edema (Fig. 45-8). If the truncal valve is incompetent, the regurgitant flow adds to the volume load, exacerbating the heart failure.
The prognosis of truncus arteriosus without surgical repair is poor. The median age of survival ranges from 2 weeks to 3 months; mortality at 1 year of life is greater than 80%. Adult survivors have been reported, but are extremely rare.17 As expected, early deaths are mostly due to heart failure and later mortality caused by pulmonary hypertension. Other factors that worsen prognosis are coexisting interrupted aortic arch, coarctation, truncal valve regurgitation, and truncal valve stenosis. Late survivors may have benefited from incidental pulmonary artery stenoses that protect the pulmonary vasculature from high flow and high pressure.
TREATMENT OPTIONS
Because of the poor prognosis of truncus arteriosus and the early onset of pulmonary vascular disease, current treatment favors primary surgical repair within the first month of life.18,19 Before the current era, pulmonary banding was performed to reduce pulmonary flow and the left ventricular volume load. Pulmonary banding was a palliative approach that did not require cardiopulmonary bypass, but its long-term outcome was generally unsatisfactory. The first complete repair was performed by McGoon and colleagues20 in 1967 using an aortic homograft for the pulmonary connection.
The basic surgical approach for complete repair is the same today. After establishing cardiopulmonary bypass, the pulmonary arteries from the common arterial trunk or the aorta are identified and excised. Through a right ventriculotomy, the subtruncal VSD is visualized. A patch is sutured along the margin of the VSD and right ventricular side of the truncal annulus, connecting the common arterial trunk and the truncal valve to the left ventricle exclusively (Fig. 45-9). A valved pulmonary conduit is placed at the right ventriculotomy site and connected to the excised pulmonary arteries, establishing the pulmonary flow (Fig. 45-10). After surgery, the truncal valve effectively becomes the aortic valve, and the common arterial trunk becomes the ascending aorta. If the native truncal valve is dysfunctional and cannot be repaired, it is replaced with a prostatic valve. In cases with associated interrupted aortic arch or coarctation (Van Praagh and Van Praagh type 4), the arch is reconstructed to maintain continuity among the common arterial trunk, the cervical arteries, and the descending aorta.
Long-term prognosis after successful surgical repair is very good, with 80% survival rate at 20 years.21 Major factors associated with postoperative complications and mortality are truncal valve abnormalities, interrupted aortic arch, nonconfluence or small pulmonary arteries, and coronary anomalies.22 Of these, interrupted aortic arch seems to be of the greatest concern, with some series reporting early modality near 50%.16,23 Reoperation, even after successful initial repair, is common. Most reoperations are done for the replacement of a malfunctioning pulmonary conduit. Other reasons for reoperation include the repair or replacement of an incompetent truncal valve, arterioplasty of branch pulmonary artery stenoses, and closure of residual VSD.21
IMAGING INDICATIONS AND REPORTING
The goals of imaging are to secure the early diagnosis of truncus arteriosus, to delineate vascular anatomy for surgical planning, to identify factors that adversely affect surgical outcome, and to evaluate complications that require reoperation. Fetal ultrasonography can diagnose truncus arteriosus, and this information may be useful for parental counseling during pregnancy.24 In the neonatal period, echocardiography is the modality of choice for the diagnosis and characterization of truncus arteriosus.25 In some centers, an echocardiographic diagnosis of uncomplicated truncus arteriosus is deemed sufficient for surgery without additional angiographic imaging. Color and gray-scale imaging at multiple imaging planes should be performed by an experienced operator, with special attention in differentiating truncus arteriosus, pulmonary atresia with VSD, and tetralogy of Fallot. Presurgical echocardiography should report (1) the alignment of the common arterial trunk with respect to the ventricles, (2) the size and location of the VSD, (3) the ventricular functions, (4) the morphology and function of the truncal valve, (5) the origins of the coronary arteries, (6) the origins of the pulmonary arteries, and (7) any anomaly of the aortic arch.
Patients who have undergone complete surgical repair for truncus arteriosus are now surviving to adulthood in increasing numbers. Imaging goals for these patients are to detect postoperative complications and to help determine the optimal timing for reoperation. The two most important postoperative structures are the valved pulmonary conduit and the truncal valve. Regurgitation or stenosis of either or both valves has deleterious effects on the right and left ventricles. For these reasons, postoperative patients usually undergo routine surveillance with echocardiography. The problem with the valved pulmonary conduit after truncus arteriosus repair is analogous to the problem after tetralogy of Fallot repair. Surgical replacement of a failing pulmonary conduit is best timed just before the irreversible loss of right ventricular function because the new conduit would also deteriorate over time.26 MRI, by quantifying right ventricular size and function, may be helpful in decision making.27 These results may be applicable to truncus arteriosus. In addition, cardiac MRI can evaluate the truncal valve and the left ventricular function in the same study. The clinical role of MRI in the postoperative management of truncus arteriosus is of ongoing research interest.
Less commonly, new vascular lesions develop after surgery in the forms of pseudoaneurysm from a ruptured pulmonary conduit (Fig. 45-11); coarctation restenosis; branch pulmonary artery stenosis; and compression of the pulmonary arteries, veins, or airways by an aneurysmal common arterial trunk (Fig. 45-12).28 These lesions are readily evaluated noninvasively by CT angiography or MR angiography.
Connelly M. Common arterial trunk. In: Gatzoulis MA, Webb GD, Daubeney PEF, editors. Diagnosis and Management of Adult Congenital Heart Disease. Edinburgh: Churchill Livingstone; 2003:265-271.
Dorfman AL, Geva T. Magnetic resonance imaging evaluation of congenital heart disease: conotruncal anomalies. J Cardiovasc Magn Reson. 2006;8:645-659.
Larsen WJ. Development of the heart. In Human Embryology, 2nd ed. New York: Churchill Livingstone; 1997.
McElhinney DB, Driscoll DA, Emanuel BS, et al. Chromosome 22q11 deletion in patients with truncus arteriosus. Pediatr Cardiol. 2003;24:569-573.
Tsai IC, Chen MC, Jan SL, et al. Neonatal cardiac multidetector row CT: why and how we do it. Pediatr Radiol. 2008;38:438-451.
1 Wilson J. A description of a very unusual formation of the human heart. Phil Trans R Soc Lond (Biol) Part II. 1798:346-356.
2 Lev M, Saphir O. Truncus arteriosus communis persistens. J Pediatr. 1943;20:74.
3 Collett RW, Edwards JE. Persistent truncus arteriosus: a classification according to anatomic types. Surg Clin North Am. 1949;29:1245-1270.
4 Van Praagh R, Van Praagh S. The anatomy of common aorticopulmonary trunk (truncus arteriosus communis) and its embryological implications: a study of 57 necropsy cases. Am J Cardiol. 1965;16:406-425.
5 Jacobs ML. Congenital heart surgery nomenclature and database project: truncus arteriosus. Ann Thorac Surg. 2000;69:S50-S55.
6 Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890-1900.
7 Momma K, Matsuoka R, Takao A. Aortic arch anomalies associated with chromosome 22q11 deletion (CATCH 22). Pediatr Cardiol. 1999;20:97-102.
8 Digilio MC, Angioni A, De SM, et al. Spectrum of clinical variability in familial deletion 22q11.2: from full manifestation to extremely mild clinical anomalies. Clin Genet. 2003;63:308-313.
9 Pierpont ME, Basson CT, Benson DWJr, et al. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:3015-3038.
10 Development of the cardiovascular and lymphatic systems. In Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 39th ed, 2005. Churchill Livingstone. Edinburgh.
11 Elzein C, Ilbawi M, Kumar S, et al. Severe truncal valve stenosis: diagnosis and management. J Card Surg. 2005;20:589-593.
12 Chiu IS, Wu SJ, Chen MR, et al. Anatomic relationship of the coronary orifice and truncal valve in truncus arteriosus and their surgical implication. J Thorac Cardiovasc Surg. 2002;123:350-352.
13 de la Cruz MV, Cayre R, Angelini P, et al. Coronary arteries in truncus arteriosus. Am J Cardiol. 1990;66:1482-1486.
14 Butto F, Lucas RVJr, Edwards JE. Persistent truncus arteriosus: pathologic anatomy in 54 cases. Pediatr Cardiol. 1986;7:95-101.
15 Gutierrez PS, Binotto MA, Aiello VD, et al. Chest pain in an adult with truncus arteriosus communis. Am J Cardiol. 2004;93:272-273.
16 Konstantinov IE, Karamlou T, Blackstone EH, et al. Truncus arteriosus associated with interrupted aortic arch in 50 neonates: a Congenital Heart Surgeons Society study. Ann Thorac Surg. 2006;81:214-222.
17 Silverman JJ, Scheinesson GP. Persistent truncus arteriosus in a 43 year old man. Am J Cardiol. 1966;17:94-96.
18 Rodefeld MD, Hanley FL. Neonatal truncus arteriosus repair: surgical techniques and clinical management. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2002;5:212-217.
19 Thompson LD, McElhinney DB, Reddy M, et al. Neonatal repair of truncus arteriosus: continuing improvement in outcomes. Ann Thorac Surg. 2001;72:391-395.
20 McGoon DC, Rastelli GC, Ongley PA. An operation for the correction of truncus arteriosus. JAMA. 1968;205:69-73.
21 Henaine R, Azarnoush K, Belli E, et al. Fate of the truncal valve in truncus arteriosus. Ann Thorac Surg. 2008;85:172-178.
22 Brown JW, Ruzmetov M, Okada Y, et al. Truncus arteriosus repair: outcomes, risk factors, reoperation and management. Eur J Cardiothorac Surg. 2001;20:221-227.
23 Miyamoto T, Sinzobahamvya N, Kumpikaite D, et al. Repair of truncus arteriosus and aortic arch interruption: outcome analysis. Ann Thorac Surg. 2005;79:2077-2082.
24 Duke C, Sharland GK, Jones AM, et al. Echocardiographic features and outcome of truncus arteriosus diagnosed during fetal life. Am J Cardiol. 2001;88:1379-1384.
25 Sanders SP, Bierman FZ, Williams RG. Conotruncal malformations: diagnosis in infancy using subxiphoid 2-dimensional echocardiography. Am J Cardiol. 1982;50:1361-1367.
26 Ammash NM, Dearani JA, Burkhart HM, et al. Pulmonary regurgitation after tetralogy of Fallot repair: clinical features, sequelae, and timing of pulmonary valve replacement. Congenit Heart Dis. 2007;2:386-403.
27 Geva T. Indications and timing of pulmonary valve replacement after tetralogy of Fallot repair. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006;9:11-22.
28 Murashita T, Hatta E, Imamura M, et al. Giant pseudoaneurysm of the right ventricular outflow tract after repair of truncus arteriosus: evaluation by MR imaging and surgical approach. Eur J Cardiothorac Surg. 2002;22:849-851.

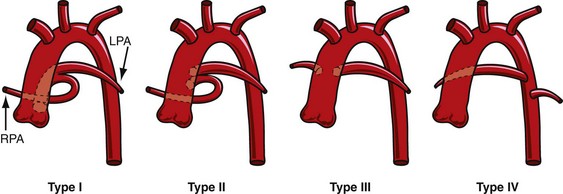
 FIGURE 45-1
FIGURE 45-1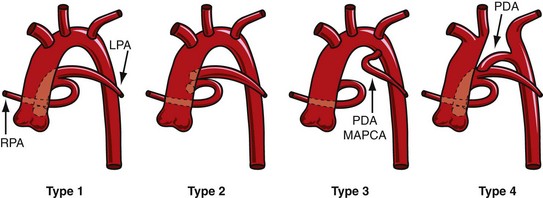
 FIGURE 45-2
FIGURE 45-2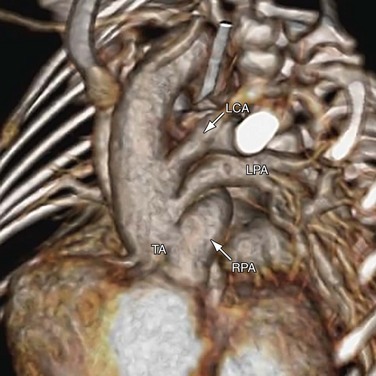
 FIGURE 45-3
FIGURE 45-3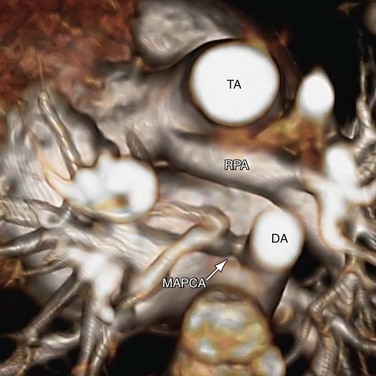
 FIGURE 45-4
FIGURE 45-4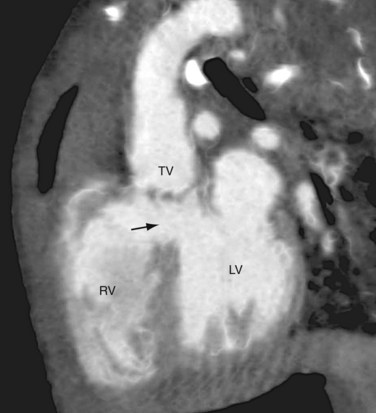
 FIGURE 45-5
FIGURE 45-5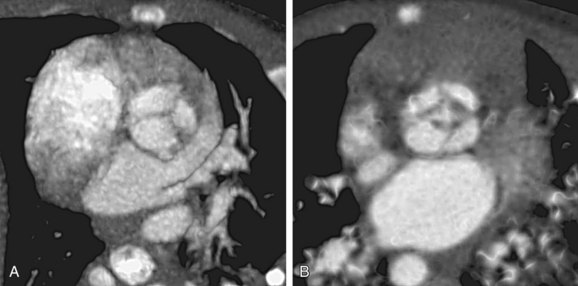
 FIGURE 45-6
FIGURE 45-6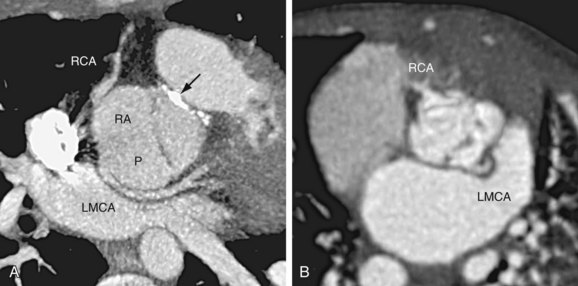
 FIGURE 45-7
FIGURE 45-7
 FIGURE 45-8
FIGURE 45-8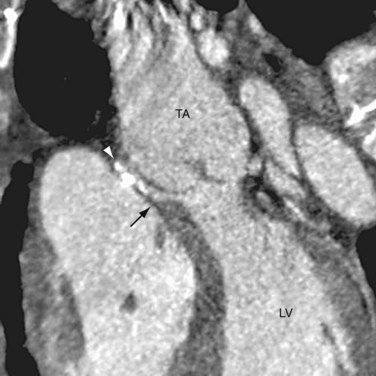
 FIGURE 45-9
FIGURE 45-9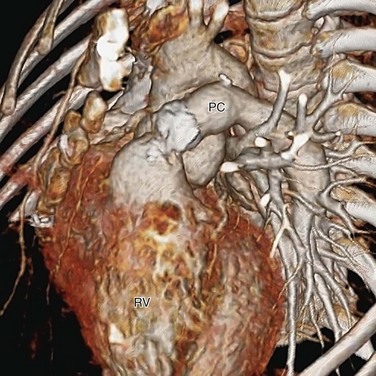
 FIGURE 45-10
FIGURE 45-10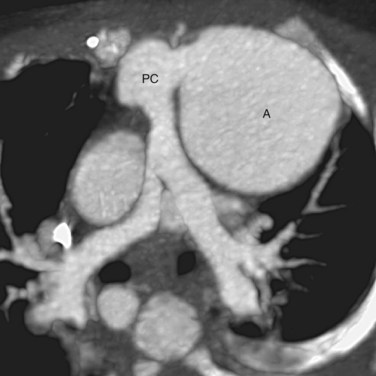
 FIGURE 45-11
FIGURE 45-11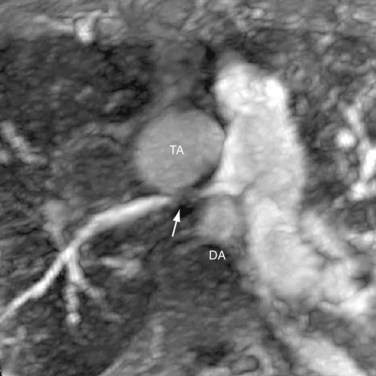
 FIGURE 45-12
FIGURE 45-12





