CHAPTER 59 TRIGEMINAL AUTONOMIC CEPHALALGIAS: CLUSTER HEADACHE AND RELATED CONDITIONS
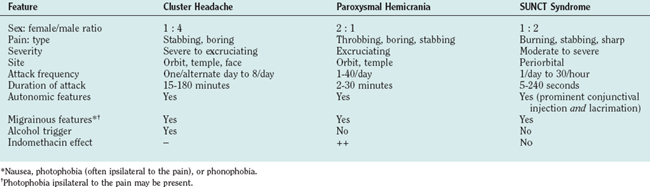
The trigeminal autonomic cephalalgias (TACs) are a grouping of headache syndromes recognized in the second edition of the International Headache Society (IHS) classification.1 The term was coined to reflect the underlying pathophysiology of a prominent part of the phenotype of the acute attacks: namely, the excessive cranial parasympathetic autonomic reflex activation in response to nociceptive input in the ophthalmic division of the trigeminal nerve.2 The TACs are classified in section III of the second edition of the IHS classification1 and include cluster headache,3 paroxysmal hemicrania, and shortlasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT) (Table 59-1).4 In an early draft, hemicrania continua was included5 but this was finally classified in Section IV, which is appropriate, given the availability of newer brain imaging data.6 This chapter covers the shared physiology of these disorders and then the clinical aspects of each one in turn. Agents that are useful for these disorders are discussed. More detail on older clinical trials and agents that are used but are not particularly effective can be found elsewhere.4
TABLE 59-1 Differential Diagnosis of Trigeminal Autonomic Cephalalgias (TACs)
| Primary TACs | Similar Secondary Headaches | Secondary TACs |
|---|---|---|
AVM, arteriovenous malformation; SUNCT, shortlasting unilateral neuralgiform headache attacks with conjunctival injection and tearing.
PATHOPHYSIOLOGY OF TRIGEMINAL AUTONOMIC CEPHALALGIAS
Any explanation of the pathophysiology of TACs must account for the two major shared clinical features that are characteristic of the conditions: trigeminal distribution pain and ipsilateral cranial autonomic features.2 The pain-producing innervation of the cranium projects through branches of the trigeminal and upper cervical nerves7,8 to the trigeminocervical complex,9 from which nociceptive pathways project to higher centers.10 A reflex activation of the cranial parasympathetic outflow provides the efferent loop.
Experimental Studies
In the cat, stimulation of the trigeminal ganglion produces cranial vasodilation and release of neuropeptides, notably calcitonin gene–related peptide and substance P.11 The dilation is mediated by antidromic activation of the trigeminal nerve, which accounts for 20% of the effect, and orthodromic activation through the cranial parasympathetic outflow via the facial cranial nerve (VII), which accounts for the other 80%.12 The afferent pathway of the trigeminal-parasympathetic reflex traverses the trigeminal root,12 synapses in the trigeminal nucleus, and then projects to neurons of the superior salivatory nucleus in the pons.13 There is a glutamatergic excitatory receptor in the pontine synapse14 and projection via the facial nerve15 without synapse in the geniculate ganglion. The greater superficial petrosal nerve supplies classic autonomic preganglionic fibers to the sphenopalatine (pterygopalatine in humans) and otic ganglia.16 The sphenopalatine synapse involves a hexamethonium-sensitive nicotinic ganglion.16 Cranial nerve VII activation is associated with release of vasoactive intestinal polypeptide (VIP)17 and blocked by VIP antibodies.18 Blood flow changes in the brain depend on the frequency of stimulation19,20 and are independent of cerebral metabolism.21 VIP is found in the sphenopalatine ganglion,22 as is nitric oxide synthase, which is also involved in the vasodilator mechanism.23
Human Studies
The basic science implies an integral role for the ipsilateral trigeminal nociceptive pathways in TACs and predicts some degree of cranial parasympathetic autonomic activation. The ipsilateral autonomic features monifest clinically, such as lacrimation, rhinorrhea, nasal congestion, and eyelid edema, are consistent with cranial parasympathetic activation and sympathetic hypofunction (ptosis and miosis). The latter is likely to be a neurapraxic effect of carotid wall swelling24,25 with cranial parasympathetic activation. Cranial autonomic symptoms to some degree are, therefore, normal physiological responses to cranial nociceptive input.26–28 Indeed, other primary headaches, notably migraine,29 or secondary headache, such as trigeminal neuralgia,30 or trigeminal dysesthesias31 would be expected to have cranial autonomic activation, and they do. The distinction between the TACs and other headache syndromes is the degree of cranial autonomic activation, not its presence alone.32 This is why some patients with migraine have minor cranial autonomic activation, which is termed cluster migraine, whereas most such patients have migraine with cranial autonomic activation. This reflex also explains the curious report of a sense of aural fullness that patients with these syndromes may report if asked specifically and that has been reported clearly.33
Permitting Trigeminal-Parasympathetic Activation
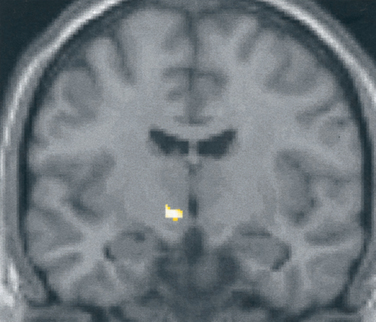
What is the basis for the prominence of cranial autonomic symptoms in the TACs? Is it a result of a central disinhibition of the trigeminal-autonomic reflex?32 Evidence from functional imaging studies—positron emission tomography studies in cluster headache34–36 (Fig. 59-1), and paroxysmal hemicrania36a and functional magnetic resonance imaging (MRI) studies in SUNCT (Fig. 59-2)37,38—have demonstrated ipsilateral posterior hypothalamic activation. Posterior hypothalamic activation seems specific to these syndromes and is not present in episodic39–41 or chronic42 migraine or in experimental ophthalmic trigeminal distribution head pain.43 Of interest is that in hemicrania continua, there is contralateral posterior hypothalamic activation, in contrast to substantially ipsilateral activation in cluster headache, and additional pontine and midbrain activation.6 There are direct hypothalamic-trigeminal connections,44 and the hypothalamus is known to have a modulatory role on the nociceptive and autonomic pathways, specifically trigeminovascular nociceptive pathways.45 Hence, the TACs involve abnormal activation in the region of the hypothalamus with subsequent trigeminovascular and cranial autonomic activation. Cranial autonomic features are not invariably linked with trigeminal pain and may persist after trigeminal nerve lesions.46
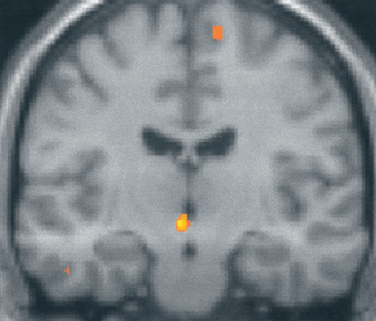
Figure 59-1 Brain imaging of cluster headache. Changes in the posterior hypothalamic gray area are revealed with positron emission tomography in patients with chronic cluster headache34 imaged during an acute attack triggered by nitroglycerin.
(From May A, Bahra A, Buchel C, et al: Hypothalamic activation in cluster headache attacks. Lancet 1998; 352:275-278.)
DIFFERENTIAL DIAGNOSIS OF TRIGEMINAL AUTONOMIC CEPHALALGIAS
The primary TACs need to be differentiated from secondary TAC-producing lesions, from other primary headaches, and from each other (see Table 59-1). The differentiation from secondary causes is not a problem if patients undergo imaging, but it can be extremely difficult if they do not. MRI of the brain with attention to the pituitary fossa and cavernous sinus reveals most secondary causes. In view of the rarity of paroxysmal hemicrania and SUNCT, MRI is a reasonable part of the initial evaluation of such patients. The situation is more complex for cluster headache. There are no studies with clear findings. The impression from a cohort that now exceeds 700 (the National Hospital for Neurology and Neurosurgery, Queen Square, London) is that MRI would detect no more than 1 per 100 cases of lesions in episodic cluster headache (ECH), and so its routine use cannot be recommended. For chronic cluster headache (CCH), MRI seems reasonable, in view of the very difficult nature of the long-term management and developments in neuromodulation as a treatment,47 which then make brain imaging more complex.
Among other primary headaches, migraine is the biggest problem in the differential diagnosis of cluster headache. Migraine can cluster, and despite the best descriptions of the IHS classification committee, short attacks do occur. Cranial autonomic symptoms are well reported,29 and the neuropeptide changes are substantially similar48 to those in cluster headache.49 The occurrence of attacks together does not seem to have the seasonal preponderance that is so typical of cluster headache,50,51 and this fact can be a useful differential diagnostic feature. This author regards the term cluster migraine as unhelpful and has yet to see a convincing case of a distinct biological entity usefully described by this name. Migraine could occur by chance in up to one third of cluster headache sufferers, in view of the peak female migraine prevalence and the generally accepted dominant inheritance pattern of migraine. The criterion for the effect of movement was added to the IHS description of cluster headache to sharpen the difference from migraine. The IHS committee hoped that this would draw attention to the fact that most patients with cluster headache feel restless or agitated,52 whereas most migraine patients are quiescent, as recognized in the first edition of the IHS classification.53 In clinical practice, this symptom and the periodicity are extremely helpful in establishing the differential diagnosis. The other feature of cluster headache, which is a feature of TACs in comparison with migraine, is that patients with TACs more often complain of unilateral, homolateral photophobia. In addition, triggering of headache quickly with alcohol, within 30 minutes, is more typical of cluster headache, whereas alterations in sleep patterns, eating, stress, or menses do not generally affect cluster headache. Warm environments seem to be a trigger in cluster headache, whereas barometric pressure change is a trigger of migraine.54
The TACs themselves (Table 59-2) can often be differentiated by the length of attack. This is certainly true when cluster headache is compared with SUNCT. The IHS criteria for TACs does betray an uncomfortable biological naivety with regard to the timing. The A, C, D, and E criteria are rather similar for each TAC (Tables 59-3 to 59-5). It would be easy to classify the attacks if those in SUNCT were up to 4 minutes long, those in paroxysmal hemicrania were 2 to 30 minutes long, and cluster headaches were 15 minutes and longer. The overlap then seems minimal. This seems wrong in absolute terms, because biology rarely provides such clear-cut rules, but it does provide a useful way to identify cases of sufficient similarity to make biological studies meaningful.
|
B. Severe or very severe unilateral orbital, supraorbital, and/or temporal pain lasting 15 to 180 minutes if untreated
|
TABLE 59-4 Diagnostic Criteria for Paroxysmal Hemicrania
TABLE 59-5 Diagnostic Criteria for Short-Lasting Unilateral Neuralgiform Headache Attacks with Conjunctival Injection and Tearing (3.3)
CLUSTER HEADACHE
Cluster headache is a form of primary headache that is almost always unilateral and occurs in association with cranial autonomic features. Most patients report a striking circannual and circadian periodicity. It is an excruciating syndrome and is probably one of the most painful conditions known to humans; affected women have described the attacks as being worse than childbirth.
Epidemiology
The prevalence of cluster headache is estimated to be 0.4%,55 slightly higher than that of multiple sclerosis in the United Kingdom.56 The male/female ratio is 3.5 : 1 to 7 : 1.52,57 The male/female ratio has changed in case series since the early 1990s, with a trend toward a decreasing male preponderance; this is likely to be an ascertainment issue and not a real shift in female incidence. Cluster headache can begin at any age, although the most common age at onset is the third or fourth decade of life. Children as young as 4 years of age have been affected, but this is unusual.
Clinical Features
The Cluster Attack
With very few exceptions, cluster attacks are strictly unilateral, although the headache may alternate sides. The pain is excruciating. It is located mainly around the orbital and temporal regions, although any part of the head can be affected. The headache usually lasts 45 to 90 minutes, but the duration can range from 15 minutes to 3 hours. The onset and cessation are abrupt. Some patients experience interictal pain or discomfort.58
The signature feature of cluster headache is the association with autonomic symptoms, and it is extremely unusual for these not to be reported. According to the IHS diagnostic criteria,1 the attacks are accompanied by at least one of the following, which are present on the painful side: conjunctival injection, lacrimation, miosis, ptosis, eyelid edema, rhinorrhea, nasal blockage, forehead or facial sweating, or a sense of restlessness or agitation (see Table 59-2). The autonomic features are transient, lasting only for the duration of the attack, with the exception of partial Horner’s syndrome; in rare cases, ptosis or miosis may persist, especially after frequent attacks.
The full range of typical migrainous symptoms are reported in a significant proportion of patients with cluster headache.52,59,60 Premonitory symptoms (tiredness, yawning), associated features (nausea, vomiting, photophobia, phonophobia), and aura symptoms have all been described in relation to cluster attacks. However, in contrast to migraine, cluster headache sufferers are usually restless and irritable, preferring to move about, looking for a movement or posture that may relieve the pain.52
The cluster attack frequency varies between one every alternate day to three daily, although some sufferers have as many as eight daily, and clinical experience suggests that even more are possible. The condition can have a striking circadian rhythmicity; some patients report that the attacks occur at the same time each day. Alcohol, nitroglycerin, exercise, and elevated environmental temperature are recognized precipitants of acute cluster attacks. Alcohol induces acute attacks, usually within an hour of intake, in most sufferers, in contrast with migraine sufferers, who generally have headache some hours after alcohol intake. Alcohol triggers attacks during a cluster bout but not in a remission. Allergies, food sensitivities, reproductive hormonal changes,52 and stress do not appear to have any significant role in precipitating attacks.
Natural History
Although there is a paucity of literature on the long-term prognosis of cluster headache, the available evidence suggests that it is a lifelong disorder in most patients. In one study, in about 10% of patients with ECH, the condition evolved into CCH, whereas in one third of patients with CCH, it transformed into ECH.61 An encouraging piece of information for cluster headache sufferers is that a substantial proportion of them can expect to develop longer remission periods as they age.62
Treatment
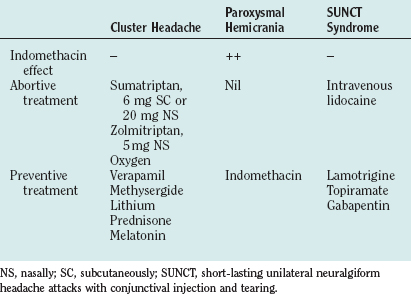
The management of cluster headache includes offering patients advice on general measures and treating them with abortive agents, preventive agents, and, in rare cases, surgery (Table 59-6).
Abortive Agents
Triptans
Sumatriptan
Subcutaneous sumatriptan, 6 mg, is the drug of choice in the abortive treatment of a cluster attack. A randomized, placebo-controlled, double-blind, crossover study of 39 patients63 showed that headache severity was reduced at 15 minutes in 74% of attacks in which sumatriptan was administered, in comparison with 26% who received placebo. Of patients who took sumatriptan, 36% were pain free within 10 minutes, in comparison with 3% who took placebo. Sumatriptan was well tolerated, and there were no serious adverse events. A further study demonstrated no significant advantage to doubling the dose to 12 mg, although there were more side effects.64 Two large clinical trials show that long-term subcutaneous sumatriptan administration is well tolerated by patients who have cluster headache with no evidence of tachyphylaxis.65,66
A randomized, placebo-controlled, double-blind, crossover study of 86 patients67 showed that the severity of the headache at 30 minutes was reduced in 56% of attacks in which sumatriptan nasal spray, 20 mg, was administered, in comparison with 26% for which placebo was given. This formulation offers the prospect of effectively treating up to three attacks in 24 hours, in comparison with the subcutaneous 6-mg injection, for which the indicated dosing schedule is two doses in 24 hours.
Zolmitriptan
A double-blind, placebo-controlled trial compared the efficacy of oral zolmitriptan, 5 mg and 10 mg, to treat acute attacks in ECH and CCH.68 With headache response defined as a 2-point reduction on a 5-point pain intensity scale, 30-minute response rates in ECH were 29%, 40%, and 47% after placebo and 5 mg and 10 mg of zolmitriptan, respectively. The difference reached statistical significance only for 10-mg zolmitriptan in comparison with placebo. The efficacy of oral zolmitriptan in ECH is modest and does not approach the efficacy or speed of subcutaneous sumatriptan or oxygen; therefore, its utility in clinical practice is limited. It may be considered for patients who cannot tolerate subcutaneous or intranasal sumatriptan and oxygen or for those who desire oral medications. The intranasal formulation of zolmitriptan might be more appropriate for acute cluster headache because of its better overall pharmacokinetics,69 and a recent controlled trial has demonstrated its efficacy.
Oxygen
Oxygen inhalation is a safe and effective method for the acute treatment of cluster headache. Its mechanism of action remains unclear. Horton was the first to discover that inhaling 100% oxygen at the onset of attacks alleviates cluster headache pain.70 Kudrow71 noted a significant relief from cluster pain in 75% of 52 randomly selected outpatients treated with 100% oxygen administered through a facial mask at a rate of 7 L/minute for 15 minutes. Oxygen at 6 L/minute for 15 minutes was compared with air inhalation in a double-blind crossover study of 19 sufferers.72 Eleven patients used both gases. Of 16 patients who used oxygen, 9 (56%) perceived complete or substantial relief in 80% or more of their cluster attacks, in comparison with only 1 (7%) of 14 patients who used air.
Inhalation of 100% oxygen at 7 to 12 L/minute is usually rapidly effective in relieving pain. It should be inhaled continuously for 15 to 20 minutes through a non-rebreathing facial mask. Patients need to be informed that they should cover any apertures on the facial mask. When oxygen inhalation is initiated as soon as the attack starts, the attack is often aborted rapidly and entirely,71 although some patients find oxygen completely effective if taken when the pain is at maximal intensity.73 Up to 25% of patients note that oxygen simply delays the attack for minutes to hours rather than completely aborting it.71
Topical local anesthetics: lidocaine (lignocaine)
Kittrelle and colleagues74 first reported that lignocaine solution applied topically to the region of the pterygopalatine fossa alleviated the pain of the cluster attack. In an open-label trial of intranasal lignocaine 4% solution, four of five patients obtained rapid relief of nitroglycerin-induced cluster headache. Lignocaine was also effective in relieving spontaneous attacks. Robbins75 reported on the use of 4 to 6 sprays of lidocaine 4% in the nostril ipsilateral to the painful side in an open-label trial in 30 patients. Lidocaine (10%) applied bilaterally for 5 minutes was reported to be effective in aborting nitroglycerin-induced cluster attacks in a double-blind, placebo-controlled study in nine patients.76
Ergotamine and dihydroergotamine
Oral ergotamine is absorbed too slowly to be useful. It was widely recommended before the advent of the triptans, but it has little practical use in the modern treatment of acute cluster headache. Parenteral dihydroergotamine has been considered an effective abortive agent for cluster headache for some time.77,78 There are no controlled trials of injectable dihydroergotamine; however, clinical experience has demonstrated that intravenous administration provides prompt and effective relief of cluster headache within 15 minutes.79 Because of the frequency and the rapid peak intensity of cluster attacks, intravenous dihydroergotamine is not a feasible long-term solution. The intramuscular and subcutaneous routes of administration provide slower relief, although they have the advantage of being able to be self-administered. Dihydroergotamine nasal spray, 1 mg, has been studied in a double-blind, placebo-controlled, crossover trial in 25 patients.80 There was no difference in headache frequency or duration, but the pain intensity was significantly reduced with dihydroergotamine in comparison with placebo. The dosage used (1 mg) was lower than the recommended dosage for migraine (2 mg) and less than the currently available preparations of dihydroergotamine nasal spray (4 mg).
Somatostatin receptor antagonists
Intravenous somatostatin (25 μg/minute for 20 minutes) was compared with ergotamine (250 μg intramuscularly) or placebo in a double-blind trial comprising 72 attacks in eight patients.81 Infusion of somatostatin significantly reduced the maximal pain intensity and the duration of pain in comparison with placebo and, to a degree, was comparable with intramuscular ergotamine. In another randomized, double-blind study, subcutaneous somatostatin was compared with ergotamine.82 Five patients were treated for three attacks by each of the drugs. Subcutaneous somatostatin and ergotamine were equally beneficial with regard to effects on maximal pain intensity and the pain area, but somatostatin was less effective in reducing the duration of pain. Matharu and associates compared subcutaneous octreotide (100 μg), a somatostatin receptor agonist, with a matching placebo in acute cluster headache.83 The primary endpoint was the headache response, defined as very severe, severe, or moderate pain becoming mild or nil at 30 minutes. Fifty-seven patients were recruited, of whom 46 provided efficacy data on attacks treated with octreotide and 45 with placebo. The headache response rate with subcutaneous octreotide was 52%, whereas the response with placebo was 36%. When the treatment outcome was modeled as a binomial in which response was determined by treatment and period effect, sex, and cluster headache type were other variables of interest, subcutaneous octreotide, 100 μg, was significantly superior to placebo (P < 0.01). More work in this area may produce a novel non-vasoconstrictor approach to the treatment of acute cluster headache.
Preventive Treatments
Verapamil
Verapamil was first reported to be an effective preventive agent for cluster headache by Meyer and Hardenberg.84 In an open-label trial with verapamil at doses of 160 to 720 mg/day in five CCH patients, a reduction in the mean monthly headache frequency was reported in all patients. In another open-label trial with verapamil at doses of 240 to 600 mg/day for ECH and 120 to 1200 mg/day for CCH, an improvement of more than 75% was noted in 33 (69%) of 48 patients.85 A double-blind, crossover trial in which verapamil, 360 mg/day, was compared with the then standard prophylactic drug, lithium, 900 mg/day, each given for 8 weeks, demonstrated equivalent effects in the 24 CCH patients who completed the trial.86 Verapamil and lithium were superior to placebo. Verapamil caused fewer side effects and had a shorter latency period. In a double-blind, placebo-controlled trial, the efficacy of verapamil, 360 mg/day over a 2-week period, was evaluated in 26 patients with ECH.87 A statistically significant reduction in headache frequency and analgesic consumption occurred in the verapamil-treated patients, with a greater reduction in the second week of treatment.
Verapamil is the preventive drug of choice for both CCH and ECH, the latter when the bout is sufficiently long to establish a suitable dose. Clinical experience has clearly demonstrated that higher doses are needed for cluster headache than for cardiological indications. Dosages commonly employed range from 240 to 960 mg/day in divided doses. Verapamil can cause heart block by slowing conduction in the atrioventricular node,88 as demonstrated by prolongation of the A-H interval.89 In observing for P-R interval prolongation on the electrocardiogram, clinicians can monitor potential development of heart block, although it is a coarse measure. No formal guidelines are available. After obtaining a baseline electrocardiogram, the author and colleagues start patients on 80 mg three times daily, and the total daily dose is increased in increments of 80 mg every 10 to 14 days. An electrocardiogram is obtained before each increment and at least 10 days after the last dose change. The dosage is increased until the cluster attacks are suppressed, side effects intervene, or the maximum dose of 960 mg/day is achieved. It is unproved clinical experience that standard preparations of verapamil are more effective than the modified-release formulations.58,90
Constipation is the most common side effect, but dizziness, ankle swelling, nausea, fatigue, hypotension, and bradycardia may also occur. Gingival enlargement is a recognized problem that needs prompt attention from a dentist.91 β Blockers should not be given concurrently.
Lithium
The effectiveness of lithium in psychiatric conditions of a cyclical nature, such as manic-depressive psychosis and seasonal affective disorder, led Ekbom92,93 to try this agent in five patients with cluster headache, three of whom had CCH and clearly benefited from lithium. Open-label trials have been reviewed.94 Collectively, in more than 28 clinical trials involving 468 patients, good to excellent results were found in 236 (78%) of 304 patients with CCH. The response to lithium in patients with ECH was less robust than in those with CCH; good efficacy was obtained in 103 (63%) of a total of 164 patients treated. In most unblinded trials, a lithium dose ranging from 600 to 1200 mg/day was used. Lithium was often effective at serum concentrations of 0.4 to 0.8 mEq/L, less than those usually required for the treatment of bipolar disorder. Some patients eventually become resistant to lithium.95
Lithium has also been evaluated in two randomized, double-blind trials. A double-blind, crossover trial in which verapamil, 360 mg/day, was compared with lithium, 900 mg/day, each given for 8 weeks, demonstrated equivalent effects in the 24 patients with CCH who completed the trial86; verapamil and lithium were superior to placebo. In a double-blind, placebo-controlled, randomized, parallel-group trial of sustained-relief lithium with 27 patients with ECH, 13 received 800 mg/day, and 14 received placebo. Efficacy was assessed 1 week after treatment was begun.96 Cessation of attacks occurred in two patients in each group, and substantial improvement was noted in 6 (43%) of 14 patients taking placebo and 8 (62%) of 13 patients taking lithium. Lithium treatment was associated with a subjective improvement rate, but this was not statistically significant in comparison with placebo. The authors made an assumption at the onset of the trial that the placebo response would be zero. This is clearly incorrect for both acute attack and preventive treatment approaches in cluster headache.97
Lithium is an effective agent for cluster headache prophylaxis, although the response is less robust in ECH than in CCH. Most patients benefit from dosages between 600 and 1200 mg/day. Lithium has the potential for many side effects and has a narrow therapeutic window. Side effects include weakness, nausea, thirst, tremor, slurred speech, and blurred vision. Toxicity is manifested by nausea, vomiting, anorexia, diarrhea, neurological signs of confusion, nystagmus, ataxia, extrapyramidal signs, and seizures. Hypothyroidism and polyuria (nephrogenic diabetes insipidus) can occur with long-term use. Polymorphonuclear leukocytosis may occur and be mistaken for occult infection. Renal and thyroid function tests should be performed before and during treatment. The author and colleagues start patients on 300 mg twice per day and then titrate the dosage up until the cluster headaches are suppressed, side effects intervene, or the serum lithium level is in the upper part of the therapeutic range. The serum concentrations should be measured 12 hours after the last dose and should not exceed the upper level of the therapeutic range. Drug withdrawal at least once annually is advised, to detect the patients in whom CCH has transformed to ECH. The concomitant use of NSAIDs, diuretics, and carbamazepine is contraindicated.
Methysergide
Methysergide is an ergot alkaloid that is an antagonist at 5-hydroxytryptamine 2A (5-HT2A), 5-HT2B, and 5-HT2C receptors and an agonist at 5-HT1B/5-HT1D receptors. Methysergide was first reported to be effective in cluster headache by Sicuteri.98 Several authors subsequently confirmed this observation in open-label trials.99–105 The open-trials were reviewed by Curran and associates,106 who noted that methysergide, 3 to 12 mg/day, was effective in 329 (73%) of 451 patients with episodic cluster headache and chronic cluster headache.
For treatment of cluster headache, methysergide is indicated in dosages up to 12 mg/day, if tolerated. To minimize side effects, patients can start with a low dosage and increase the dosage gradually. The author and colleagues start patients on 1 mg once daily and increase the daily dose by 1 mg every 3 days in a three-times-daily regimen until the daily dose reaches 5 mg; thereafter, the dosage is increased incrementally by 1 mg every 5 days. Common short-term side effects include nausea, vomiting, dizziness, muscle cramps, abdominal pain, and peripheral edema. Uncommon but troublesome side effects are caused by vasoconstriction (coronary or peripheral arterial insufficiency), which usually necessitate cessation of the drug. Prolonged treatment has been associated with retroperitoneal, pulmonary, pleural, and cardiac fibrotic reactions, although these are rare.107 Ideally, the drug should be used only by patients with short cluster bouts, preferably less than 3 to 4 months. If prolonged use is intended, the risk of fibrotic reactions can be minimized by taking the drug for 6 months, followed by a 1-month holiday before starting the drug again. To avoid a sudden increase in headache frequency when methysergide is stopped, the patient should be weaned off over a 1-week period. Some authorities administer methysergide continuously with careful monitoring, which includes auscultation of the heart and yearly echocardiogram, chest radiograph, and abdominal MRI. All patients receiving methysergide should remain under the supervision of the treating physician108 and should be examined regularly for the development of visceral fibrosis or vascular complications.
Contraindications to methysergide use include pregnancy, peripheral vascular disorders, severe arteriosclerosis, coronary artery disease, severe hypertension, thrombophlebitis or cellulitis of the legs, peptic ulcer disease, fibrotic disorders, lung diseases, collagen disease, liver or renal function impairment, and valvular heart disease.109
Ergots, triptans, and methysergide
There is considerable controversy surrounding the use of ergot derivatives and triptans concomitantly with methysergide. This is not at all an easy subject. It is usually recommended that ergotamine or dihydroergotamine not be taken concomitantly with methysergide, and other vasoconstrictive agents should be used with caution. Methysergide is an ergot derivative,110 but it is a weak vasoconstrictor in comparison with ergotamine.111 It is demethylated in vivo to methylergonovine, to which it may owe some of its activity.112 There are no reported prospective drug interaction studies between methysergide and sumatriptan. In some of the early clinical studies with sumatriptan, methysergide continued to be used.113 Eighty patients were taking either methysergide or pizotifen, both serotonin 5-HT2 antagonists. Thirty-eight had used sumatriptan injections, and 42 had used tablets. There was insufficient power to analyze this group, but they had a similar adverse event profile.113 The most worrisome case was that of a 43-year-old woman who experienced a myocardial infarction while taking methysergide and sumatriptan.114 She had a history of migraine without aura. She had controlled hypertension and atypical chest pain attributed to gastroesophageal reflux. Coronary angiography revealed a 50% block of the left anterior descending coronary artery, which was treated by stenting. In retrospect, sumatriptan should have been contraindicated in this patient because of the ischemic heart disease, although it might be argued that this was a difficult diagnostic issue. For cluster headache, the author and colleagues are unaware of any similar case. Thus, the combined use of ergot derivates or sumatriptan with methysergide must remain a clinical decision based on the balance of the very considerable benefit, particularly for sumatriptan and dihydroergotamine, and the concomitant use of methysergide, with each case judged on its merits.
Ergotamine
Ekbom first reported the use of ergotamine tartrate for the prophylactic treatment of cluster headache in 1947.115 Sixteen patients were given ergotamine at a dose of 2 to 3 mg/day for 1 to 4 weeks, and 13 patients experienced considerable improvement. Later it was reported that a rectal suppository of ergotamine (2 mg) and caffeine (100 mg) or intramuscular injections of ergotamine (0.25 to 0.5 mg) at bedtime were effective in preventing nocturnal attacks.116 Ergotamine was widely used as the first-choice prophylaxis until the efficacy of lithium and verapamil became evident. Regular administration of ergotamine, 2 to 4 mg/day for 2 to 3 weeks, may be useful. If the patient has nocturnal attacks, 1 to 2 mg may be given at night in the form of tablets or suppositories.
Dihydroergotamine
Repetitive intravenous dihydroergotamine administered in an inpatient setting over a period of 3 days has been reported to be very useful for some patients with both ECH and CCH. In a study of 54 patients with intractable cluster headache (23 episodic, 31 chronic), open-label use of repetitive intravenous dihydroergotamine rendered all patients headache free.117 At the 12-month follow-up, 83% and 39% of patients with ECH and CCH patients, respectively, remained free of headache.
Prednisolone (Prednisone)
The use of corticosteroids in cluster headache was first reported by Horton,78 who found that cortisone at doses of 100 mg/day was effective in only 4 of 21 patients. The effectiveness of prednisolone (prednisone) in stopping bouts of cluster headache was established in a double-blind trial by Jammes.118 Couch and Ziegler119 reported that prednisolone (prednisone), 10 to 80 mg/day, employed in 19 patients with cluster headache (9 with ECH, 10 with CCH) provided greater than 50% relief in 14 patients (74%) and complete relief in 11 (58%) patients. Seventy-nine percent of patients reported headache recurrence when the prednisolone (prednisone) dosage was tapered.
Corticosteroids are highly efficacious and the most rapid acting of the preventive agents. As in other disorders, the use of corticosteroids is contraindicated by a history of tuberculosis or psychotic disturbance. Caution has to be exercised because of the potential for serious side effects. In this regard, bony problems with steroid use have been reviewed, and the shortest course of prednisolone (prednisone) reported to be associated with osteonecrosis of the femoral head is a 30-day course. Courses of adrenocorticotrophic hormone have produced osteonecrosis after 16 days, and courses of dexamethasone, after 7 days.120 Thus, a tapering course of prednisolone (prednisone) for 21 days is prudent, with an excess risk for bony problems occurring if more than two courses are administered per year.120 The author and colleagues start patients on oral prednisolone (prednisone), 1 mg/kg, increasing the dosage to a maximum of 60 mg every day for 5 days, and thereafter decreasing the dosage by 10 mg every 3 days. Unfortunately, relapse almost invariably occurs as the dose is tapered. For this reason, steroids are used as an initial therapy in conjunction with preventive agents, until the latter are effective.
Topiramate
In an open-label study of two patients with CCH, seven patients with ECH, and one patient with cluster headache–tic syndrome, treatment with topiramate was associated with rapid improvement.121 A topiramate dosage of 50 to 125 mg/day shortened the cluster period duration in all ECH patients and the total treated cluster period duration in four ECH patients. Topiramate induced remission in the two patients with CCH within 1 and 3 weeks, respectively. Only three patients reported mild side effects while taking topiramate in this study. Further open-label studies followed this one.122–126
Melatonin
Melatonin is a sensitive surrogate marker of circadian rhythm in humans and is under the control of the suprachiasmatic nucleus.127 Serum melatonin levels are reduced in patients with cluster headache, particularly during a cluster bout.128,129 On the basis of these observations, the striking circadian periodicity of cluster headache, and the importance of the hypothalamus in the pathogenesis of this disorder,34 the efficacy of melatonin has been evaluated as a prophylactic agent in cluster headache. Leone and coworkers130 performed a double-blind pilot study of melatonin versus placebo in the prophylaxis of cluster headache. Twenty patients with cluster headache (18 with ECH, 2 with CCH) participated in the study. The authors found that, in comparison with the run-in period, there was a reduction in the mean number of daily attacks and a strong trend toward reduced analgesic consumption in the melatonin recipients but not in the placebo recipients. Five melatonin recipients responded to the treatment, with cessation of cluster headaches after 5 days of treatment. No placebo recipient responded. The author and colleagues prescribe a daily dosage of 9 mg and have found it useful for some patients.
Other medicines often discussed
Despite early promise from open-label experience, the results of a double-blind, placebo-controlled, parallel-group study of sodium valproate (1000 to 2000 mg/day) in the prophylaxis of cluster headache were negative.131 There were some issues with the study, although the result is not inconsistent with clinical experience. Gabapentin was first reported to be effective as a prophylactic agent in two case reports of cluster headache.132,133 Subsequently, Leandri and associates134 gave gabapentin, 900 mg/day, in an open-label manner to eight patients with ECH and four with CCH. All patients were rendered pain free within 8 days of initiating therapy. This remarkable effect is not obvious in practice. Baclofen, a γ-amino butyric acid (GABA) β receptor agonist, was evaluated in an open-label study of nine ECH patients.135 The patients received baclofen, 15 mg/day, for 2 days before the dose was increased to 30 mg/day. Six patients were rendered pain free within 1 week; one patient was substantially better at 1 week and became pain free by the end of the second week. The treatment was well tolerated. These results require further investigation with a larger number of patients in a double-blind study.
Intranasal capsaicin was first reported by Sicuteri and coworkers to be beneficial as a prophylactic agent in cluster headache.136 In this open-label preliminary study, capsaicin, 300 μg, was applied once daily for 5 consecutive days into both nostrils of patients with cluster headache. The number of spontaneously occurring attacks was significantly reduced in the 60 days after the end of capsaicin treatment. The group subsequently reported their findings in 45 cluster headache patients (35 with ECH, 10 with CCH).137 A significant reduction in the number of attacks was observed during the 60-day observation period. Intranasal capsaicin produces an intense burning sensation, lacrimation, and rhinorrhea that last for approximately 20 minutes, although these symptoms progressively decrease and disappear after five to eight applications. Consequently, placebo-controlled studies are not easily performed, because patients are readily aware of what they are receiving. In a double-blind, placebo-controlled study of 13 patients, seven were treated with capsaicin 0.025% twice daily for 7 days in the nostril ipsilateral to the pain, and the six control patients received camphor 3% to simulate the painful irritation associated with topical capsaicin.138 The capsaicin-treated group experienced significantly less severe headaches during the 8 days after treatment than during the 7 days of treatment. This improvement was not observed in the placebo-treated group. In an attempted single-blind study designed to verify the difference in efficacy of treatment with nasal capsaicin, depending on the side of application, 26 patients with ECH received capsaicin on the symptomatic side, and 26 patients were treated on the nonsymptomatic side.139 Application of capsaicin on the symptomatic side had a temporary beneficial effect in most patients, but attacks occurred within 40 days in all patients. No benefit was observed with contralateral application. In a multicenter, vehicle-controlled study, patients with ECH were treated for 7 days; 18 received civamide 0.025% (25 μg), and 10 received placebo in a volume of 100 μL.140 They were evaluated in a 20-day post-treatment period. Although the number of headaches was reduced in the first seven days (-60% versus −26%), the overall effect at day 20 was not significant. Nasal burning sensation was common in the civamide recipients.
Greater occipital nerve blockade
Anthony141 described the use of local anesthetic and corticosteroid injections around the greater occipital nerve homolateral to the pain. This procedure has been widely used but not subjected to systematic evaluation. It was reported that of 14 patients treated with greater occipital nerve injection, 4 had a good response, 5 had a moderate response, and 5 had no response.142 The author and colleagues find it a variable but sometimes effective strategy that, when performed by experienced clinicians, has almost no morbidity except about a 1% incidence of localized alopecia caused by fat atrophy at the injection site.143
Surgery
Only patients whose headaches are exclusively unilateral should be considered for destructive surgery, because patients whose attacks have alternated sides are at risk of a contralateral recurrence after surgery. A number of procedures that interrupt either the trigeminal sensory or autonomic (cranial parasympathetic) pathways can be performed, although few are associated with long-lasting results, and the side effects can be devastating. The procedures that have been reported to show some success include trigeminal sensory rhizotomy via a posterior fossa approach,144,145 radiofrequency trigeminal gangliorhizolysis,146 and microvascular decompression of the trigeminal nerve with or without microvascular decompression of the nervus intermedius.147 Gamma knife treatment seems ineffective in view of its morbidity.148 Complete trigeminal analgesia may be required for the best results. Complications include diplopia, hyperacusis, jaw deviation, corneal anesthesia, and anesthesia dolorosa. Aggressive long-term ophthalmic follow-up is essential.
Leone and colleagues149 reported the use of posterior hypothalamic neurostimulation in one patient and subsequently in a cohort of patients with CCH.47 The target was derived from brain imaging work in cluster headache,34 and this procedure has proved effective in those patients. Unfortunately, there is a risk of mortality associated with this procedure,150 which has led to some caution in its adoption. On the basis of a promising report of greater occipital nerve stimulation151 in other headache forms, and effects particularly in migraine,42 trials of suboccipital nerve stimulation in cluster headache are being performed, again with promising early results. These nondestructive procedures need careful evaluation so that the best candidates are selected for their application in practice.
PAROXYSMAL HEMICRANIA
Paroxysmal hemicrania, like cluster headache, is characterized by strictly unilateral, brief, excruciating headaches that occur in association with cranial autonomic features. Paroxysmal hemicrania differs from cluster headache mainly in the high frequency and shorter duration of individual attacks, although there is certainly overlap in these characteristics. However, paroxysmal hemicrania responds in a dramatic and absolute manner to indomethacin, which underlines the importance of distinguishing it from cluster headache, which is not responsive to indomethacin (see Table 59-4). Chronic paroxysmal hemicrania was first described by Sjaastad and Dale,152 when they reported a case they rather aptly named “a new treatable headache entity.” They consequently coined the term chronic paroxysmal hemicrania to describe this disorder.153 The initial cases described were characterized by daily headaches for years without remission. It subsequently became apparent that not all patients experienced a chronic, unremitting course; some patients had discrete headache bouts separated by prolonged pain-free remissions.2,154–156 This remitting pattern was named episodic paroxysmal hemicrania.154
Epidemiology
Paroxysmal hemicrania is a rare syndrome. The prevalence of paroxysmal hemicrania is not known, but the relationship in comparison with cluster headache is reported to be approximately 1% to 3%.157 Cluster headache occurs in approximately 1 per 1000; the estimated prevalence of paroxysmal hemicrania is 1 per 50,000. The disorder has been reported in various parts of the world158,159 and affects different races.160 In contrast to cluster headache, paroxysmal hemicrania predominates in females by a sex ratio of 2.13 : 1 to 2.36 : 1.157,161 The condition usually begins in adulthood at the mean age of 34 years and in the range of 6 to 81 years.161
Clinical Features
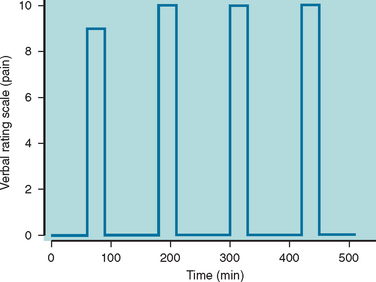
The attack profile of paroxysmal hemicrania is highly characteristic.2,157,161,162 The headache is strictly unilateral and without side shift in the majority of affected patients. However, the headache demonstrated side shift in four reported cases,163–166 and the pain was bilateral in one patient.167 The maximum pain is most often centered on the ocular, temporal, maxillary, and frontal regions; less often, the pain involves the neck, occiput, and retro-orbital regions. The pain occasionally radiates into the ipsilateral shoulder and arm. The pain is typically excruciating in severity and is described as a “clawlike,” throbbing, aching, or boring sensation. The headache usually lasts 2 to 30 minutes, although it can go on for as long as 2 hours (Fig. 59-3). Interictal discomfort or pain is present in as many as one third of patients.157 Attacks of paroxysmal hemicrania occur in association with ipsilateral cranial autonomic features. Lacrimation, conjunctival injection, nasal congestion, or rhinorrhea frequently accompany the headache; eyelid edema, ptosis, miosis, and facial sweating are less frequently reported. Photophobia and nausea may accompany some attacks, although vomiting and phonophobia are rare. There exists one case report of a typical migrainous aura occurring in association with paroxysmal hemicrania attacks.168 During episodes of pain, approximately half the sufferers prefer to sit or lie still; the other half assume the pacing activity usually observed with cluster headache.157 The frequency of attacks ranges from 2 to 40 daily. The attacks occur regularly throughout the 24-hour period without the preponderance of nocturnal attacks that occur in cluster headache.
Although the majority of attacks are spontaneous, approximately 10% of attacks are precipitated mechanically, either by bending or by rotating the head. Attacks may also be provoked by external pressure against the transverse processes of C4 to C5, the C2 root, or the greater occipital nerve. Alcohol ingestion triggers headaches in only 7% of patients.157
About 20% of patients have episodic paroxysmal hemicrania, which is diagnosed when there are clear remission periods that may last from a few weeks to years between bouts of attacks. The remaining 80% of patients have chronic paroxysmal hemicrania, which is diagnosed when the patients have daily attacks without any clear remission periods (see Table 59-4). Notably, in paroxysmal hemicrania, the chronic form dominates the clinical presentation, in contrast to cluster headache, in which the episodic form prevails.
Symptomatic Paroxysmal Hemicrania and Associations
Secondary paroxysmal hemicrania is relatively common and can be caused by diverse pathological processes at various sites (Table 59-7).
TABLE 59-7 Lesions Reported to Produce a Paroxysmal Hemicrania–like Picture
| Collagen Vascular Disease246 |
Paroxysmal hemicrania has been reported to occur in association with migraine,169 cluster headache,159,170–172 trigeminal neuralgia,173–176 and cough headache.177 Similar to cluster headache–tic syndrome, the paroxysmal hemicrania–tic syndrome is recognized, and treatment of both conditions is required.
Diagnostic Workup
A good clinical history, a detailed neurological examination, and a therapeutic trial of indomethacin are all that are necessary to make a diagnosis of paroxysmal hemicrania. Because a relatively high number of symptomatic cases have been reported (see Table 59-7), MRI of the brain should be routinely performed in all patients with paroxysmal hemicrania.
The therapeutic trial of oral indomethacin should be initiated at 25 mg three times daily; if there is no response or only a partial response after 5 days, the dosage should be increased to 50 mg three times daily for 5 days; if the index of suspicion is high and there is no or only a partial response, then the dosage should be further increased to 75 mg three times daily for 14 days. Complete resolution of the headache is prompt, usually occurring within 1 to 2 days of initiating the effective dose. Intramuscular injectable indomethacin, 50 to 100 mg (“indotest”) has been proposed as a diagnostic test for paroxysmal hemicrania.178 The indotest has the advantage that the diagnosis can be rapidly established, and although it needs further validation at this stage, it is, in the absence of a biological marker, likely to become the test of choice in TACs.
Management
The treatment of paroxysmal hemicrania is prophylactic. Indomethacin is the treatment of choice. Complete resolution of the headache is prompt, usually occurring within 1 to 2 days of initiating the effective dose. The typical maintenance dosage ranges from 25 to 100 mg/day, but doses up to 300 mg/day are occasionally required. Dosage adjustments may be necessary to address the clinical fluctuations that occur in paroxysmal hemicrania. During active headache cycles, skipping or even delaying doses may result in the prompt recurrence of the headache. Gastrointestinal side effects secondary to indomethacin may be treated with antacids, misoprostol, histamine H2 receptor antagonists, or proton pump inhibitors and should always be considered for patients who require long-term treatment. The mechanism behind the absolute responsiveness to indomethacin is unknown. It appears to be independent of indomethacin’s effect on prostaglandin synthesis, inasmuch as other NSAIDs have little or no effect on paroxysmal hemicrania.
The clinician faces a difficult challenge with patients who cannot tolerate indomethacin. No other drug is consistently effective in paroxysmal hemicrania. Drugs other than indomethacin reported to be partially or completely effective, mainly in isolated cases, include other NSAIDs such as aspirin,157,179 naproxen,180 and piroxicam β-cyclodextrin181; the cyclooxygenase-2 inhibitor celecoxib182; the calcium channel antagonists verapamil183,184 and flunarizine185; acetazolamide186; and corticosteroids.187 The author and colleagues have tried cyclooxygenase-2 inhibitors and verapamil with limited success. They have found greater occipital nerve block with depo-corticosteroids and local anesthetic to be useful.
SHORTLASTING UNILATERAL NEURALGIFORM HEADACHE ATTACKS WITH CONJUNCTIVAL INJECTION AND TEARING
SUNCT syndrome, like the other TACs, manifests as a unilateral headache that occurs in association with cranial autonomic features. The features that distinguish it from the other TACs are (1) the presence of prominent conjunctival injection and lacrimation and (2) very brief-duration attacks that can occur very frequently. Both these features are present in the majority of patients. Because some patients have clinically the same problem, but either conjunctival injection or tearing is absent, the author and colleagues believe that the syndrome should be renamed shortlasting unilateral neuralgiform headache attacks (SUNA) with cranial autonomic features (Table 59-8) or that both forms should be recognized. SUNCT syndrome was described in 1978188 and more fully characterized in 1989.189
TABLE 59-8 Short-Lasting Unilateral Neuralgiform Headache Attacks (SUNA) with Cranial Autonomic Symptoms
|
B. Attacks of unilateral orbital, supraorbital or temporal stabbing pain lasting from 2 seconds to 10 minutes
|
Epidemiology
The prevalence of SUNCT syndrome is not known, although the extremely low number of reported cases suggests that it is rare. The disorder has a male predominance (36 male patients, 16 female patients) with a sex ratio of 2.1 : 1. The typical age at onset is between 40 and 70 years, although it ranges from 10 to 77 years.190
Clinical Features
The pain of SUNCT is usually maximal in the ophthalmic distribution of the trigeminal nerve, especially the orbital or periorbital regions, forehead, and temple, although it may radiate to the other ipsilateral trigeminal divisions. Attacks are typically unilateral; however, three patients experienced the pain simultaneously on the opposite side.191 The pain is generally moderate to severe and described as stabbing, burning, pricking, or electric shock–like in character. The individual attacks are very brief, lasting between 5 and 250 seconds,192 although attacks lasting up to 2 hours each have been described.193–195 The paroxysms begin abruptly, reaching maximal intensity within 2 to 3 seconds; the pain is maintained at the maximal intensity before abating rapidly.191 Most patients are completely pain free between attacks, although some report a persistent, dull, interictal discomfort.195
The temporal pattern is quite variable, with symptomatic periods alternating with remissions in an erratic manner. Symptomatic periods generally last from a few days to several months and occur once or twice annually. Remissions typically last a few months, although they can range from 1 week to 7 years. Symptomatic periods appear to increase in frequency and duration over time.191 The attack frequency during the symptomatic phase varies greatly between sufferers and within an individual sufferer. Attacks may be as infrequent as once a day or less or as often as 30 attacks an hour.
Acute headache episodes in SUNCT syndrome are accompanied by a variety of associated symptoms. The attacks are virtually always accompanied by both ipsilateral conjunctival injection and lacrimation. Ipsilateral nasal congestion, rhinorrhea, eyelid edema, ptosis, miosis, and facial redness or sweating are less commonly reported. These cranial autonomic symptoms, particularly conjunctival injection and lacrimation, are typically very prominent in SUNCT syndrome. The associated conjunctival injection and tearing usually begin 1 to 2 seconds after the onset of pain and may outlast the pain by a few seconds. Nausea, vomiting, photophobia, and phonophobia are not ordinarily associated with SUNCT syndrome. Unlike cluster headache, restlessness is not a feature of SUNCT syndrome.191
The majority of patients can precipitate attacks by touching certain trigger zones within the trigeminal innervated distribution and, occasionally, even from an extratrigeminal territory. Precipitants include touching the face or scalp, washing, shaving, eating, chewing, brushing the teeth, talking, and coughing.191 Unlike patients with trigeminal neuralgia, most patients with SUNCT have no refractory period.
SECONDARY SUNCT
Secondary SUNCT has been reported in eight patients, seven of whom have had posterior fossa abnormalities. The secondary causes include homolateral cerebellopontine angle arteriovenous malformations in two patients,196,197 a brainstem cavernous hemangioma,198 a posterior fossa lesion in a patient with human immunodeficiency virus infection/acquired immunodeficiency syndrome,2 severe basilar impression causing pontomedullary compression in a patient with osteogenesis imperfecta,199 craniosynostosis resulting in a foreshortened posterior fossa,200 and ischemic brainstem201 or hemispheric202 stroke. The posterior fossa abnormalities emphasize the absolute need for cranial MRI in any suspected case of SUNCT. The author’s experience203 and other reported cases204,205 suggest that a SUNCT-like picture may be present with pituitary adenomas and that this is not related to tumor size.206
Differential Diagnosis
Differentiating SUNCT from trigeminal neuralgia can be challenging, because there is considerable overlap in the clinical phenotypes of the two syndromes. With both headaches, duration is short, attacks can have a high frequency, and clustering of attacks occurs. Both are principally unilateral headaches, and the trigger zones behave similarly. The usual onset is during middle or old age for both. However, there are a number of striking differences between these two syndromes (Table 59-9), awareness of which can aid in their differentiation.32,207
TABLE 59-9 Differentiating Features of Typical SUNCT and Trigeminal Neuralgia
| Feature | SUNCT | Trigeminal Neuralgia |
|---|---|---|
| Gender ratio (male/female) | 2.1 : 1 | 1 : 2 |
| Site of pain | V1 | V2/V3 |
| Severity of pain | Moderate to severe | Very severe |
| Duration (seconds) | 5 to 250 | <5 |
| Autonomic features | Prominent | Sparse or none |
| Refractory period | Absent | Present |
| Response to carbamazepine | Partial | Complete |
SUNCT, shortlasting unilateral neuralgiform headache attacks with conjunctival injection and tearing.
Primary (idiopathic) stabbing headache refers to brief sharp or jabbing pains in the head that occur either as a single episode or in brief repeated volleys. The pain is usually over the ophthalmic trigeminal distribution, whereas the face is generally spared. The pain usually lasts a fraction of a second but can persist for up to 1 minute, thereby overlapping with the phenotype of SUNCT, and recurs at irregular intervals (hours to days). In general, these headaches are easily distinguishable clinically because they differ in several respects: in primary stabbing headache, there is a female preponderance; the site and radiation of pain often vary between attacks; the majority of the attacks tend to be spontaneous; cranial autonomic features are absent; and the attacks commonly subside with indomethacin administration.208,209
Treatment
Traditionally, SUNCT was believed to be highly refractory to treatment.210 Several categories of drugs used in other headache syndromes—namely, NSAIDs (including indomethacin), paracetamol (acetaminophen), 5-HT agonists (triptans, ergotamine, dihydroergotamine), β blockers, tricyclic antidepressants, calcium channel antagonists (verapamil, nifedipine), methysergide, lithium, prednisolone (prednisone), phenytoin, baclofen, and intravenous lignocaine—were reported to be ineffective.210 The author and colleagues have found intravenous lidocaine very effective in the acute suppression of SUNCT,211 although they are cautious of the neuropsychiatric side effects that are very common in these patients.212 Partial improvement with carbamazepine has been observed in several patients.193,194,210,213,214
Lamotrigine has been reported to be highly efficacious in a number of patients.215–219 Lamotrigine, given in an open-label manner at 100 to 300 mg/day, induced a complete remission in seven patients and produced about an 80% improvement in the other two patients. Although ultimate confirmation of lamotrigine’s utility in the treatment of this debilitating syndrome should come from a randomized, double-blind, placebo-controlled clinical trial, it is for now the treatment of choice.
There are a number of reported cases of patients with SUNCT who responded completely to gabapentin,220–222 typically 900 to 2700 mg/day. Matharu and associates reported a patient who responded completely to topiramate, 50 mg/day.194 These observations clearly need to be confirmed in other cases. Nonetheless, given the debilitating nature of this headache, gabapentin and topiramate are reasonable second-line agents in patients in whom a trial of lamotrigine fails.
Several surgical approaches have been tried for SUNCT syndrome. Anesthetic blockades of pericranial nerves have been reported to be ineffective.210 Black and Dodick reported two SUNCT cases that were refractory to various surgical procedures.223 The first patient underwent glycerol rhizotomy, Gamma knife radiosurgery, and microvascular decompression of the trigeminal nerve; the second patient underwent Gamma knife radiosurgery of the trigeminal root exit zone and two microvascular decompressions of the trigeminal nerve. Neither patient benefited from these procedures. In addition, the first patient suffered from anesthesia dolorosa and the second patient from unilateral deafness, chronic vertigo, and disequilibrium as a result of surgery. The author and colleagues have seen two patients who had failed to demonstrate a persistent response after trigeminal thermocoagulation and microvascular decompression (unpublished observations). Although there are some reports of successful procedures, none has had greater than 18 months’ follow-up, and the author and colleagues currently do not recommend destructive procedures at all. The most exciting developments in this area may mirror those in cluster headache, in view of a report that deep brain stimulation in the region of the posterior hypothalamic is useful in SUNCT.224
Lance JW, Goadsby PJ. Mechanism and Management of Headache, 7th ed. New York: Elsevier, 2005.
Matharu MS, Boes CJ, Goadsby PJ. Management of trigeminal autonomic cephalalgias and hemicrania continua. Drugs. 2003;63:1637-1677.
Matharu MS, Cohen AS, Boes CJ, et al. SUNCT syndrome: a review. Curr Pain Headache Rep. 2003;7:308-318.
Pareja JA, Sjaastad O. SUNCT syndrome. A clinical review. Headache. 1997;37:195-202.
Silberstein SD, Lipton RB, Goadsby PJ. Headache in Clinical Practice, 2nd ed. London: Martin Dunitz, 2002.
1 Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 2nd ed. Cephalalgia. 2004;24(Suppl 1):1-160.
2 Goadsby PJ, Lipton RB. A review of paroxysmal hemicranias, SUNCT syndrome and other shortlasting headaches with autonomic features, including new cases. Brain. 1997;120:193-209.
3 Goadsby PJ. Pathophysiology of cluster headache: a trigeminal autonomic cephalgia. Lancet Neurol. 2002;1:37-43.
4 Matharu MS, Boes CJ, Goadsby PJ. Management of trigeminal autonomic cephalalgias and hemicrania continua. Drugs. 2003;63:1637-1677.
5 Olesen J. Revision of the International Headache Classification. An interim report. Cephalalgia. 2001;21:261.
6 Matharu MS, Cohen AS, McGonigle DJ, et al. Posterior hypothalamic and brainstem activation in hemicrania continua. Headache. 2004;44:747-761.
7 McNaughton FL, Feindel WH. Innervation of intracranial structures: a reappraisal. In: Rose FC, editor. Physiological Aspects of Clinical Neurology. Oxford, UK: Blackwell Scientific; 1977:279-293.
8 Feindel W, Penfield W, McNaughton F. The tentorial nerves and localization of intracranial pain in man. Neurology. 1960;10:555-563.
9 Goadsby PJ, Hoskin KL. The distribution of trigeminovascular afferents in the nonhuman primate brain Macaca nemestrina: a c-fos immunocytochemical study. J Anat. 1997;190:367-375.
10 May A, Goadsby PJ. The trigeminovascular system in humans: pathophysiological implications for primary headache syndromes of the neural influences on the cerebral circulation. J Cereb Blood Flow Metab. 1999;19:115-127.
11 Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of man and the cat during activation of the trigeminovascular system. Ann Neurol. 1988;23:193-196.
12 Lambert GA, Bogduk N, Goadsby PJ, et al. Decreased carotid arterial resistance in cats in response to trigeminal stimulation. J Neurosurg. 1984;61:307-315.
13 Spencer SE, Sawyer WB, Wada H, et al. CNS projections to the pterygopalatine parasympathetic preganglionic neurons in the rat: a retrograde transneuronal viral cell body labeling study. Brain Res. 1990;534:149-169.
14 Nakai M, Tamaki K, Ogata J, et al. Parasympathetic cerebrovascular center of the facial nerve. Circ Res. 1993;72:470-475.
15 Goadsby PJ, Lambert GA, Lance JW. Effects of locus coeruleus stimulation on carotid vascular resistance in the cat. Brain Res. 1983;278:175-183.
16 Goadsby PJ, Lambert GA, Lance JW. The peripheral pathway for extracranial vasodilatation in the cat. J Auton Nerv Syst. 1984;10:145-155.
17 Goadsby PJ, Shelley S. High frequency stimulation of the facial nerve results in local cortical release of vasoactive intestinal polypeptide in the anesthetised cat. Neurosci Lett. 1990;112:282-289.
18 Goadsby PJ, Macdonald GJ. Extracranial vasodilatation mediated by VIP (vasoactive intestinal polypeptide). Brain Res. 1985;329:285-288.
19 Goadsby PJ. Characteristics of facial nerve elicited cerebral vasodilatation determined with laser Doppler flowmetry. Am J Physiol. 1991;260:R255-R262.
20 Seylaz J, Hara H, Pinard E, et al. Effect of stimulation of the sphenopalatine ganglion on cortical blood flow in the rat. J Cereb Blood Flow Metab. 1988;8:875-878.
21 Goadsby PJ. Effect of stimulation of the facial nerve on regional cerebral blood flow and glucose utilization in cats. Am J Physiol. 1989;257:R517-R521.
22 Uddman R, Tajti J, Moller S, et al. Neuronal messengers and peptide receptors in the human sphenopalatine and otic ganglia. Brain Res. 1999;826:193-199.
23 Goadsby PJ, Uddman R, Edvinsson L. Cerebral vasodilatation in the cat involves nitric oxide from parasympathetic nerves. Brain Res. 1996;707:110-118.
24 Ekbom K, Greitz T. Carotid angiography in cluster headache. Acta Radiol. 1970;10:177-186.
25 May A, Buchel C, Bahra A, et al. Intracranial vessels in trigeminal transmitted pain: a PET study. Neuroimage. 1999;9:453-460.
26 Drummond PD, Lance JW. Pathological sweating and flushing accompanying the trigeminal lacrimation reflex in patients with cluster headache and in patients with a confirmed site of cervical sympathetic deficit. Evidence for parasympathetic cross-innervation. Brain. 1992;115:1429-1445.
27 Drummond PD. Autonomic disturbance in cluster headache. Brain. 1988;111:1199-1209.
28 May A, Buchel C, Turner R, et al. MR-angiography in facial and other pain: neurovascular mechanisms of trigeminal sensation. J Cereb Blood Flow Metab. 2001;21:1171-1176.
29 Barbanti P, Fabbrini G, Pesare M, et al. Unilateral cranial autonomic symptoms in migraine. Cephalalgia. 2002;22:256-259.
30 Benoliel R, Sharav Y. Trigeminal neuralgia with lacrimation or SUNCT syndrome? Cephalalgia. 1998;18:85-90.
31 Goadsby PJ, Edvinsson L, Ekman R. Cutaneous stimulation leading to facial flushing and release of calcitonin gene-related peptide. Cephalalgia. 1992;12:53-56.
32 Goadsby PJ, Matharu MS, Boes CJ. SUNCT syndrome or trigeminal neuralgia with lacrimation. Cephalalgia. 2001;21:82-83.
33 Boes CJ, Swanson JW, Dodick DW. Chronic paroxysmal hemicrania presenting as otalgia with a sensation of external acoustic meatus obstruction: two cases and a pathophysiologic hypothesis. Headache. 1998;38:787-791.
34 May A, Bahra A, Buchel C, et al. Hypothalamic activation in cluster headache attacks. Lancet. 1998;352:275-278.
35 May A, Bahra A, Buchel C, et al. PET and MRA findings in cluster headache and MRA in experimental pain. Neurology. 2000;55:1328-1335.
36 Sprenger T, Boecker H, Tolle TR, et al. Specific hypothalamic activation during a spontaneous cluster headache attack. Neurology. 2004;62:516-517.
36a Mathara MS, Cohen AS, Frackowiak RS, Goadsby PJ. Posterior hypothalamic activation in paroxysmal hemicrania. Ann Neurol. 2006;59:535-545.
37 May A, Bahra A, Buchel C, et al. Functional MRI in spontaneous attacks of SUNCT: shortlasting neuralgiform headache with conjunctival injection and tearing. Ann Neurol. 1999;46:791-793.
38 Cohen AS, Matharu MS, Kalisch R, et al. Functional MRI in SUNCT shows differential hypothalamic activation with increasing pain. Cephalalgia. 2004;24:1098-1099.
39 Weiller C, May A, Limmroth V, et al. Brain stem activation in spontaneous human migraine attacks. Nat Med. 1995;1:658-660.
40 Bahra A, Matharu MS, Buchel C, et al. Brainstem activation specific to migraine headache. Lancet. 2001;357:1016-1017.
41 Afridi S, Giffin NJ, Kaube H, et al: A PET study in spontaneous migraine. Arch Neurol 2005:in press.
42 Matharu MS, Bartsch T, Ward N, et al. Central neuromodulation in chronic migraine patients with suboccipital stimulators: a PET study. Brain. 2004;127:220-230.
43 May A, Kaube H, Buechel C, et al. Experimental cranial pain elicited by capsaicin: a PET-study. Pain. 1998;74:61-66.
44 Malick A, Burstein R. Cells of origin of the trigeminohy-pothalamic tract in the rat. J Comp Neurol. 1998;400:125-144.
45 Bartsch T, Levy MJ, Knight YE, et al. Differential modulation of nociceptive dural input to [hypocretin] orexin A and B receptor activation in the posterior hypothalamic area. Pain. 2004;109:367-378.
46 Matharu MS, Goadsby PJ. Persistence of attacks of cluster headache after trigeminal nerve root section. Brain. 2002;175:976-984.
47 Franzini A, Ferroli P, Leone M, et al. Stimulation of the posterior hypothalamus for treatment of chronic intractable cluster headaches. The first reported series. Neurosurgery. 2003;52:1095-1101.
48 Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183-187.
49 Goadsby PJ, Edvinsson L. Human in vivo evidence for trigeminovascular activation in cluster headache. Brain. 1994;117:427-434.
50 Kudrow L. Cluster Headache: Mechanisms and Management. Oxford, UK: Oxford University Press, 1980.
51 Sjaastad O. Cluster Headache Syndrome. London: WB Saunders, 1992.
52 Bahra A, May A, Goadsby PJ. Cluster headache: a prospective clinical study in 230 patients with diagnostic implications. Neurology. 2002;58:354-361.
53 Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8(Suppl 7):1-96.
54 Cooke LJ, Rose MS, Becker WJ. Chinook winds and migraine headache. Neurology. 2000;54:302-307.
55 Sjaastad O, Bakketeig LS. Cluster headache prevalence. Vaga study of headache epidemiology. Cephalalgia. 2003;23:528-533.
56 Ford HL, Gerry E, Johnson M, et al. A prospective study of the incidence, prevalence and mortality of multiple sclerosis in Leeds. J Neurol. 2002;249:260-265.
57 Manzoni GC. Gender ratio of cluster headache over the years: a possible role of changes in lifestyle. Cephalalgia. 1998;18:138-142.
58 Lance JW, Goadsby PJ. Mechanism and Management of Headache, 7th ed. New York: Elsevier, 2005.
59 Silberstein SD, Niknam R, Rozen TD, et al. Cluster headache with aura. Neurology. 2000;54:219-221.
60 Siow HC, Young WB, Peres MF, et al. Hemiplegic cluster. Headache. 2002;42:136-139.
61 Manzoni GC, Micieli G, Granella F, et al. Cluster headache—course over ten years in 189 patients. Cephalalgia. 1991;11:169-174.
62 Igarashi H, Sakai F. Natural history of cluster headache. Cephalalgia. 1996;16:390-391.
63 Treatment of acute cluster headache with sumatriptan. The Sumatriptan Cluster Headache Study Group. N Engl J Med. 1991;325:322-326.
64 Ekbom K, Monstad I, Prusinski A, et al. Subcutaneous sumatriptan in the acute treatment of cluster headache: a dose comparison study. Acta Neurol Scand. 1993;88:63-69.
65 Ekbom K, Krabbe A, Micelli G, et al. Cluster headache attacks treated for up to three months with subcutaneous sumatriptan (6 mg). Cephalalgia. 1995;15:230-236.
66 Gobel H, Lindner A, Heinze A, et al. Acute therapy for cluster headache with sumatriptan: findings of a one year long-term study. Neurology. 1998;51:908-911.
67 van Vliet JA, Bahra A, Martin V, et al. Intranasal sumatriptan is effective in the treatment of acute cluster headache—a double-blind placebo-controlled crossover study. Cephalalgia. 2001;21:270-271.
68 Bahra A, Gawel MJ, Hardebo J-E, et al. Oral zolmitriptan is effective in the acute treatment of cluster headache. Neurology. 2000;54:1832-1839.
69 Yates RA, Tateno M, Nairn K, et al. The pharmacokinetics of the antimigraine compound zolmitriptan in Japanese and Caucasian subjects. Eur J Clin Pharmacol. 2002;58:247-252.
70 Horton BT. Histaminic cephalalgia: differential diagnosis and treatment. Proc Mayo Clin. 1956;31:325-333.
71 Kudrow L. Response of cluster headache attacks to oxygen inhalation. Headache. 1981;21:1-4.
72 Fogan L. Treatment of cluster headache: a double blind comparison of oxygen vs air inhalation. Arch Neurol. 1985;42:362-363.
73 Igarashi H, Sakai F, Tazaki Y. The mechanism by which oxygen interrupts cluster headache. Cephalalgia. 1991;11(Suppl 11):238-239.
74 Kittrelle JP, Grouse DS, Seybold ME. Cluster headache: local anesthetic abortive agents. Arch Neurol. 1985;42:496-498.
75 Robbins L. Intranasal lidocaine for cluster headache. Headache. 1995;35:83-84.
76 Costa A, Pucci E, Antonaci F, et al. The effect of intranasal cocaine and lidocaine on nitroglycerin-induced attacks in cluster headache. Cephalalgia. 2000;20:85-91.
77 Friedman AP, Mikropoulos HE. Cluster headache. Neurology (Minneapolis). 1958;8:653-663.
78 Horton BT. Histaminic cephalgia. Lancet. 1952;72:92-98.
79 Dodick DW, Rozen TD, Goadsby PJ, et al. Cluster headache. Cephalalgia. 2000;20:787-803.
80 Andersson PG, Jespersen LT. Dihydroergotamine nasal spray in the treatment of attacks of cluster headache. Cephalalgia. 1986;6:51-54.
81 Sicuteri F, Geppetti P, Marabini S, et al. Pain relief by somatostatin in attacks of cluster headache. Pain. 1984;18:359-365.
82 Geppetti P, Brocchi A, Caleri D, et al. Somatostatin for cluster headache attack. In: Pfaffenrath V, Lundberg PO, Sjaastad O, editors. Updating in Headache. Berlin: Spring-Verlag; 1985:302-305.
83 Matharu MS, Levy MJ, Meeran K, et al. Subcutaneous octreotide in cluster headache—randomized placebo-controlled double-blind crossover study. Ann Neurol. 2004;56:488-494.
84 Meyer JS, Hardenberg J. Clinical effectiveness of calcium entry blockers in prophylactic treatment of migraine and cluster headache. Headache. 1983;23:266-277.
85 Gabai IJ, Spierings ELH. Prophylactic treatment of cluster headache with verapamil. Headache. 1989;29:167-168.
86 Bussone G, Leone M, Peccarisi C, et al. Double blind comparison of lithium and verapamil in cluster headache prophylaxis. Headache. 1990;30:411-417.
87 Leone M, D’Amico D, Attanasio A. Verapamil is an effective prophylactic for cluster headache: results of a double-blind multicentre study versus placebo. In: Olesen J, Goadsby PJ, editors. Cluster Headache & Related Conditions. Oxford, UK: Oxford University Press; 1999:296-299.
88 Singh BN, Nademanee K. Use of calcium antagonists for cardiac arrhythmias. Am J Cardiol. 1987;59:153B-162B.
89 Naylor WG. Calcium Antagonists. London: Academic Press, 1988.
90 Krabbe A, Steiner TJ. Prophylactic treatment of cluster headache. In: Sjaastad O, Nappi G, editors. Cluster Headache Syndrome in General Practice: Basic Concepts. London: Smith-Gordon; 2000:91-96.
91 Matharu MS, van Vliet JA, Ferrari MD, et al. Verapamil-induced gingival enlargement in cluster headache. J Neurol Neurosurg Psychiatry. 2005;76:124-127.
92 Ekbom K. Litium rid kroniska symptom av cluster headache. Opusc Med. 1974;19:148-156.
93 Ekbom K. Lithium in the treatment of chronic cluster headache. Headache. 1977;17:39-40.
94 Ekbom K, Solomon S. Management of cluster headache. In: Olesen J, Tfelt-Hansen P, Welch KMA, editors. The Headaches. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2000:731-740.
95 Ekbom K. Lithium for cluster headache: review of the literature and preliminary results of long-term treatment. Headache. 1981;21:132-139.
96 Steiner TJ, Hering R, Couturier EGM, et al. Double-blind placebo controlled trial of lithium in episodic cluster headache. Cephalalgia. 1997;17:673-675.
97 Nilsson Remahl AIM, Laudon Meyer E, Cordonnier CS, et al. Placebo response rates in cluster headache trials. A review. Cephalalgia. 2003;23:504-510.
98 Sicuteri F. Prophylactic and therapeutic properties of 1-methyl-lysergic acid butanolamide in migraine: preliminary report. Int Arch Allergy Appl Immunol. 1959;15:300-307.
99 Dalsgaard-Neilsen T. Uber die prophylaktische Behandlung der Migraine mit Deseril [On the prophylactic treatment of migraine with deseril]. Praxis. 1960;49:867-868.
100 Friedman AP. Clinical observations with 1-methyl-lysergic acid butanolamide (UML-491) in vascular headache. Angiology. 1960;11:364-366.
101 Graham JR. Use of a new compound, UML-491 (1-methyl-D-lysergic acid butanolamide), in the prevention of various types of headache. N Engl J Med. 1960;263:1273-1277.
102 Heyck H. Serotoninantagonisten in der behandlung der migraine und der erythroprosopalgic Bings oder des Horton-syndroms [Serotonin antagonists in the therapy of migraine and Bing’s erythroprosopalgia or the Horton syndrome.]. Schweiz Med Wochenschr. 1960;90:203-209.
103 Friedman AP, Losin S. Evaluation of UML-491 in the treatment of vascular headache. Arch Neurol. 1961;4:241-245.
104 Harris MC. Prophylactic treatment of migraine and histamine cephalgia with a serotonin antagonist (methysergide). Ann Allergy. 1961;19:500-504.
105 Ekbom KA. Treatment of migraine, Horton’s syndrome and restless legs with deseril (UML-491). Acta Neurol Scand. 1962;38:313-318.
106 Curran DA, Hinterberger H, Lance JW. Methysergide. Res Clin Stud Headache. 1967;1:74-122.
107 Graham JR, Suby HI, LeCompte PR, et al. Fibrotic disorders associated with methysergide therapy for headache. N Engl J Med. 1966;274:360-368.
108 Raskin NH. Headache. New York: Churchill-Livingstone, 1988.
109 Silberstein SD. Methysergide. Cephalalgia. 1998;18:421-435.
110 Berde B, Schild HO. Ergot alkaloids and related compounds. Born GVR, Eichler O, Farah A, et al, editors. Handbook of Experimental Pharmacology, vol 49. Berlin: Springer-Verlag, 1978.
111 Garrison JC. Histamine, bradykinin, 5-hydroxytryptamine and their antagonists. In: Gilman AG, Rall TW, Niles AS, et al, editors. The Pharmacological Basis of Therapeutics. New York: Pergamon Press; 1990:595.
112 Saxena PR, De Boer MO. Pharmacology of antimigraine drugs. J Neurol. 1991;238:S28-S35.
113 Blakeborough P, Fowler PA, Ashford EA. The use of sumatriptan in patients taking migraine prophylactic agents. Cephalalgia. 1993;13(Suppl):163.
114 Liston H, Bennett L, Usher B, et al. The association of the combination of sumatriptan and methysergide in myocardial infarction in a premenopausal woman. Arch Intern Med. 1999;159:511-513.
115 Ekbom K. Ergotamine tartrate orally in Horton’s “histaminic cephalalgia” (also called Harris’s ciliary neuralgia). Acta Psychiatr Scand. 1947;Suppl 46:106-113.
116 Symonds CP. A particular variety of headache. Brain. 1956;79:217-232.
117 Mather P, Silberstein SD, Schulman E, et al. The treatment of cluster headache with repetitive intravenous dihydroergotamine. Headache. 1991;31:525-532.
118 Jammes JL. The treatment of cluster headaches with prednisone. Dis Nerv Syst. 1975;36:375-376.
119 Couch JR, Ziegler DK. Prednisone therapy for cluster headache. Headache. 1978;18:219-221.
120 Mirzai R, Chang C, Greenspan A, et al. The pathogenesis of osteonecrosis and the relationships to corticosteroids. J Asthma. 1999;36:77-95.
121 Wheeler SD, Carrazana EJ. Topiramate-treated cluster headache. Neurology. 1999;53:234-236.
122 Forderreuther S, Mayer M, Straube A. Treatment of cluster headache with topiramate: effects and side-effects in five patients. Cepahalalgia. 2002;22:186-189.
123 Mathew NT, Kailasam J, Meadors L. Prophylaxis of migraine, transformed migraine, and cluster headache with topiramate. Headache. 2002;42:796-803.
124 Leone M, Dodick D, Rigamonti A, et al. Topiramate in cluster headache prophylaxis: an open trial. Cephalalgia. 2003;23:1001-1002.
125 Lainez MJ, Pascual J, Pascual AM, et al. Topiramate in the prophylactic treatment of cluster headache. Headache. 2003;43:749-784.
126 Rapoport AM, Bigal ME, Tepper SJ, et al. Treatment of cluster headache with topiramate: effects and side-effects in five patients. Cephalalgia. 2003;23:69-70.
127 Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336:186-195.
128 Waldenlind E, Gustafsson SA, Ekbom K, et al. Circadian secretion of cortisol and melatonin in cluster headache during active cluster periods and remission. J Neurol Neurosurg Psychiatry. 1987;50:207-213.
129 Leone M, Lucini V, D’Amico D, et al. Twenty-four-hour melatonin and cortisol plasma levels in relation to timing of cluster headache. Cephalalgia. 1995;15:224-229.
130 Leone M, D’Amico D, Moschiano F, et al. Melatonin versus placebo in the prophylaxis of cluster headache: a double-blind pilot study with parallel groups. Cephalalgia. 1996;16:494-496.
131 El Amrani M, Massiou H, Bousser M-G. A negative trial of sodium valproate in cluster headache: methodological issues. Cephalalgia. 2002;22:205-208.
132 Ahmed F. Chronic cluster headache responding to gabapentin: a case report. Cephalalgia. 2000;20:252-253.
133 Tay BA, Ngan Kee WD, Chung DC. Gabapentin for the treatment and prophylaxis of cluster headache. Reg Anesth Pain Med. 2001;26:373-375.
134 Leandri M, Luzzani M, Cruccu G, et al. Drug-resistant cluster headache responding to gabapentin: a pilot study. Cephalalgia. 2001;21:744-746.
135 Hering-Hanit R, Gadoth N. Baclofen in cluster headache. Headache. 2000;40:48-51.
136 Sicuteri F, Fusco BM, Marabini S. Beneficial effect of capsaicin application to the nasal mucosa in cluster headache. Clin J Pain. 1989;5:49-53.
137 Sicuteri F, Faneiullaeci M, Nicolodi M, et al. Substance P theory: a unique focus on the painful and painless phenomena of cluster headache. Headache. 1990;30:69-79.
138 Marks DR, Rapoport A, Padla D. A double-blind placebo-controlled trial of intranasal capsaicin for cluster headache. Cephalalgia. 1993;13:114-116.
139 Fusco BM, Marabini S, Maggi CA, et al. Preventative effect of repeated nasal applications of capsaicin in cluster headache. Pain. 1994;59:321-325.
140 Saper JR, Klapper J, Mathew NT, et al. Intranasal civamide for the treatment of episodic cluster headaches. Arch Neurol. 2002;59:990-994.
141 Anthony M. Arrest of attacks of cluster headache by local steroid injection of the occipital nerve. In: Rose FC, editor. Migraine: Clinical and Research Advances. London: Karger; 1985:169-173.
142 Peres MFP, Stiles MA, Siow HC, et al. Greater occipital nerve blockade for cluster headache. Cephalalgia. 2002;22:520-522.
143 Shields KG, Levy MJ, Goadsby PJ. Alopecia and cutaneous atrophy following greater occipital nerve infiltration. Neurology. 2004;63:2193-2194.
144 Kirkpatrick PJ, O’Brien M, MacCabe JJ. Trigeminal nerve section for chronic migrainous neuralgia. Br J Neurosurg. 1993;7:483-490.
145 Jarrar RG, Black DF, Dodick DW, et al. Outcome of trigeminal nerve section in the treatment of chronic cluster headache. Neurology. 2003;60:1360-1362.
146 Mathew NT, Hurt W. Percutaneous radiofrequency trigeminal gangliorhizolysis in intractable cluster headache. Headache. 1988;28:328-331.
147 Lovely TJ, Kotsiakis X, Jannetta PJ. The surgical management of chronic cluster headache. Headache. 1998;38:590-594.
148 Donnet A, Valade D, Regis J. Gamma knife treatment for refractory cluster headache: prospective open trial. J Neurol Neurosurg Psychiatry. 2005;76:218-221.
149 Leone M, Franzini A, Bussone G. Stereotatic stimulation of the posterior hypothalamic gray matter in a patient with intractable cluster headache. N Engl J Med. 2001;345:1428-1429.
150 Vandenheede M, Maertens de Noordhout AS, Remacle JM, et al. Deep brain stimulation of posterior hypothalamus in chronic cluster headache. Neurology. 2004;62(Suppl 5):A356.
151 Weiner RL, Reed KL. Peripheral neurostimulation for control of intractable occipital neuralgia. Neuromodulation. 1999;2:217-222.
152 Sjaastad O, Dale I. Evidence for a new (?), treatable headache entity. Headache. 1974;14:105-108.
153 Sjaastad O, Dale I. A new (?) clinical headache entity “chronic paroxysmal hemicrania” 2. Acta Neurol Scand. 1976;54:140-159.
154 Kudrow L, Esperanca P, Vijayan N. Episodic paroxysmal hemicrania? Cephalalgia. 1987;7:197-201.
155 Blau JN, Engel H. Episodic paroxysmal hemicrania: a further case and review of the literature. J Neurol Neurosurg Psychiatry. 1990;53:343-344.
156 Newman LC, Gordon ML, Lipton RB, et al. Episodic paroxysmal hemicrania: two new cases and a literature review. Neurology. 1992;42:964-966.
157 Antonaci F, Sjaastad O. Chronic paroxysmal hemicrania (CPH): a review of the clinical manifestations. Headache. 1989;29:648-656.
158 Rapoport AM, Sheftell FD, Baskin SM. Chronic paroxysmal hemicrania: case report of the second known definite occurrence in a male. Cephalalgia. 1981;1:67-70.
159 Tehindrazanarivelo AD, Visy JM, Bousser MG. Ipsilateral cluster headache and chronic paroxysmal hemicrania: two case reports. Cephalalgia. 1992;12:318-320.
160 Joubert J, Powell D, Djikowski J. Chronic paroxysmal hemicrania in a South African black. A case report. Cephalalgia. 1987;7:193-196.
161 Newman LC, Goadsby PJ. The paroxysmal hemicranias, SUNCT syndrome, and hypnic headache. In: Silberstein SD, Lipton RB, Dalessio DJ, editors. Wolff’s Headache and Other Head Pain. Oxford, UK: Oxford University Press; 2001:310-324.
162 Russell D. Paroxysmal hemicrania. In: Olesen J, Goadsby PJ, editors. Cluster Headache & Related Conditions. Oxford, UK: Oxford University Press; 1999:27-36.
163 Pelz M, Merskey H. A case of pre-chronic paroxysmal hemicrania. Cephalalgia. 1982;2:47-50.
164 Bogucki A, Szymanska R, Braciak W. Chronic paroxysmal hemicrania: lack of a pre-chronic stage. Cephalalgia. 1984;4:187-189.
165 Pradalier A, Dry J. [Chronic paroxysmal hemicrania. Treatment with indomethacin and diclofenac]. Therapie. 1984;39:185-188.
166 Pareja JA. Chronic paroxysmal hemicrania: dissociation of the pain and autonomic features. Headache. 1995;35:111-113.
167 Pollmann W, Pfaffenrath V. Chronic paroxysmal hemicrania: the first possible bilateral case. Cephalalgia. 1986;6:55-57.
168 Matharu MS, Goadsby PJ. Post-traumatic chronic paroxysmal hemicrania (CPH) with aura. Neurology. 2001;56:273-275.
169 Pareja J, Pareja J. Chronic paroxysmal hemicrania coexisting with migraine. Differential response to pharmacological treatment. Headache. 1992;32:77-78.
170 Jotkowitz S. Chronic paroxysmal hemicrania and cluster. Ann Neurol. 1978;4:389.
171 Pearce SHS, Cox JGC, Pearce JMS. Chronic paroxysmal hemicrania, episodic cluster headache and classic migraine in one patient. J Neurol Neurosurg Psychiatry. 1987;50:1599.
172 Centonze V, Bassi A, Causarano V, et al. Simultaneous occurrence of ipsilateral cluster headache and chronic paroxysmal hemicrania: a case report. Headache. 2000;40:54-56.
173 Hannerz J. Trigeminal neuralgia with chronic paroxysmal hemicrania: the CPH-tic syndrome. Cephalalgia. 1993;13:361-364.
174 Caminero AB, Pareja JA, Dobato JL. Chronic paroxysmal hemicrania–tic syndrome. Cephalalgia. 1998;18:159-161.
175 Zukerman E, Peres MFP, Kaup AO, et al. Chronic paroxysmal hemicrania–tic syndrome. Neurology. 2000;54:1524-1526.
176 Martinez-Salio A, Porta-Etessam J, Perez-Martinez D, et al. Chronic paroxysmal hemicrania–tic syndrome. Headache. 2000;40:682-685.
177 Mateo I, Pascual J. Coexistence of chronic paroxysmal hemicrania and benign cough headache. Headache. 1999;39:437-438.
178 Antonaci F, Pareja JA, Caminero AB, et al. Chronic paroxysmal hemicrania and hemicrania continua. Parenteral indomethacin: the “Indotest.”. Headache. 1998;38:122-128.
179 Kudrow DB, Kudrow L. Successful aspirin prophylaxis in a child with chronic paroxysmal hemicrania. Headache. 1989;29:280-281.
180 Durko A, Klimek A. Naproxen in the treatment of chronic paroxysmal hemicrania. Cephalalgia. 1987;7:361-362.
181 Sjaastad O, Antonaci F. A piroxicam derivative partly effective in chronic paroxysmal hemicrania and hemicrania continua. Headache. 1995;35:549-550.
182 Mathew NT, Kailasam J, Fischer A. Responsiveness to celecoxib in chronic paroxysmal hemicrania. Neurology. 2000;55:316.
183 Shabbir N, McAbee G. Adolescent chronic paroxysmal hemicrania responsive to verapamil monotherapy. Headache. 1994;34:209-210.
184 Evers S, Husstedt I-W. Alternatives in drug treatment of chronic paroxysmal hemicrania. Headache. 1996;36:429-432.
185 Coria F, Claveria LE, Jimenez-Jimenez FJ, et al. Episodic paroxysmal hemicrania responsive to calcium channel blockers. J Neurol Neurosurg Psychiatry. 1992;55:166.
186 Warner JS, Wamil AW, McLean MJ. Acetazolamide for the treatment of chronic paroxysmal hemicrania. Headache. 1994;34:597-599.
187 Hannerz J, Ericson K, Bergstrand G. Chronic paroxysmal hemicrania: orbital phlebography and steroid treatment. A case report. Cephalalgia. 1987;7:189-192.
188 Sjaastad O, Russell D, Horven I, et al. Multiple neuralgiform unilateral headache attacks associated with conjunctival injection and appearing in clusters. A nosological problem. Proceedings of the Scandinavian Migraine Society. Arhus. 1978:31.
189 Sjaastad O, Saunte C, Salvesen R, et al. Shortlasting unilateral neuralgiform headache attacks with conjunctival injection, tearing, sweating, and rhinorrhea. Cephalalgia. 1989;9:147-156.
190 Matharu MS, Cohen AS, Boes CJ, et al. SUNCT syndrome: a review. Curr Pain Headache Rep. 2003;7:308-318.
191 Pareja JA, Sjaastad O. SUNCT syndrome. A clinical review. Headache. 1997;37:195-202.
192 Pareja JA, Ming JM, Kruszewski P, et al. SUNCT syndrome: duration, frequency and temporal distribution of attacks. Headache. 1996;36:161-165.
193 Raimondi E, Gardella L. SUNCT syndrome. Two cases in Argentina. Headache. 1998;38:369-371.
194 Matharu MS, Boes CJ, Goadsby PJ. SUNCT syndrome: prolonged attacks, refractoriness and response to topiramate. Neurology. 2002;58:1307.
195 Pareja JA, Joubert J, Sjaastad O. SUNCT syndrome. Atypical temporal patterns. Headache. 1996;36:108-110.
196 Bussone G, Leone M, Volta GD, et al. Shortlasting unilateral neuralgiform headache attacks with tearing and conjunctival injection: the first symptomatic case. Cephalalgia. 1991;11:123-127.
197 Morales F, Mostacero E, Marta J, et al. Vascular malformation of the cerebellopontine angle associated with SUNCT syndrome. Cephalalgia. 1994;14:301-302.
198 De Benedittis G. SUNCT syndrome associated with cavernous angioma of the brain stem. Cephalalgia. 1996;16:503-506.
199 ter Berg HWM, Goadsby PJ. Significance of atypical presentation of symptomatic SUNCT: a case report. J Neurol Neurosurg Psychiatry. 2001;70:244-246.
200 Moris G, Ribacoba R, Solar DN, et al. SUNCT syndrome and seborrheic dermatitis associated with craniosynostosis. Cephalalgia. 2001;21:157-159.
201 Penart A, Firth M, Bowen JRC. Shortlasting unilateral neuralgiform headache with conjunctival injection and tearing (SUNCT) following presumed dorsolateral brainstem infarction. Cephalalgia. 2001;21:236-239.
202 van Vliet JA, Ferrari MD, Haan J. SUNCT syndrome resolving after contralateral hemispheric ischaemic stroke. Cephalalgia. 2003;23:235-237.
203 Levy MJ, Matharu MS, Goadsby PJ. Prolactinomas, dopamine agonist and headache: two case reports. Eur J Neurol. 2003;10:169-174.
204 Ferrari MD, Haan J, van Seters AP. Bromocriptine-induced trigeminal neuralgia attacks in a patient with pituitary tumor. Neurology. 1988;38:1482-1484.
205 Massiou H, Launay JM, Levy C, et al. SUNCT syndrome in two patients with prolactinomas and bromocriptine-induced attacks. Neurology. 2002;58:1698-1699.
206 Levy M, Jager HR, Powell MP, et al. Pituitary volume and headache: size is not everything. Arch Neurol. 2004;61:721-725.
207 Sjaastad O, Kruszewski P. Trigeminal neuralgia and “SUNCT” syndrome: similarities and differences in the clinical picture. An overview. Functional Neurology. 1992;7:103-107.
208 Pareja JA, Ruiz J, Deisla C, et al. Idiopathic stabbing headache (jabs and jolts syndrome). Cephalalgia. 1996;16:93-96.
209 Pareja JA, Kruszewski P, Caminero AB. SUNCT syndrome versus idiopathic stabbing headache (jabs and jolts syndrome). Cephalalgia. 1999;19(Suppl 25):46-48.
210 Pareja JA, Kruszewski P, Sjaastad O. SUNCT syndrome: trials of drugs and anesthetic blockades. Headache. 1995;35:138-142.
211 Matharu MS, Cohen AS, Goadsby PJ. SUNCT syndrome responsive to intravenous lidocaine. Cephalalgia. 2004;24:985-992.
212 Gil-Gouveia R, Goadsby PJ: Neuropsychiatric side effects of lidocaine: examples from the treatment of headache and a review. Cephalalgia. In press.
213 Ertsey C, Bozsik G, Afra J, et al. A case of SUNCT syndrome with neurovascular compression. Cephalalgia. 2000;20:325.
214 Peatfield R, Bahra A, Goadsby PJ. Trigeminal-autonomic cephalgias (TACs). Cephalalgia. 1998;18:358-361.
215 Leone M, Rigamonti A, Usai S, et al. Two new SUNCT cases responsive to lamotrigine. Cephalalgia. 2000;20:845-847.
216 D’Andrea G, Granella F, Ghiotto N, et al. Lamotrigine in the treatment of SUNCT syndrome. Neurology. 2001;57:1723-1725.
217 Gutierrez-Garcia JM. SUNCT syndrome responsive to lamotrigine. Headache. 2002;42:823-825.
218 Chakravarty A, Mukherjee A. SUNCT syndrome responsive to lamotrigine: documentation of the first Indian case. Cephalalgia. 2003;23:474-475.
219 Piovesan EJ, Siow C, Kowacs PA, et al. Influence of lamotrigine over the SUNCT syndrome: one patient follow-up for two years. Arq Neuropsiquiatr. 2003;61:691-694.
220 Graff-Radford SB. SUNCT syndrome responsive to gabapentin. Cephalalgia. 2000;20:515-517.
221 Porta-Etessam J, Martinez-Salio A, Berbel A, et al. Gabapentin (Neurontin) in the treatment of SUNCT syndrome. Cephalalgia. 2002;22:249.
222 Hunt CH, Dodick DW, Bosch P. SUNCT responsive to gabapentin. Headache. 2002;42:525-526.
223 Black DF, Dodick DW. Two cases of medically and surgically intractable SUNCT: a reason for caution and an argument for a central mechanism. Cephalalgia. 2002;22:201-204.
224 Leone M, Franzini A, D’Amico D, et al. Hypothalamic deep brain stimulation to relieve intractable chronic SUNCT: the first case. Neurology. 2004;62(Suppl 5):A356.
225 Goadsby PJ. Raeders Syndrome: “Paratrigeminal” paralysis of oculo-pupillary sympathetic. J Neurol Neurosurg Psychiatry. 2002;72:297-299.
226 Cremer P, Halmagyi GM, Goadsby PJ. Secondary cluster headache responsive to sumatriptan. J Neurol Neurosurg Psychiatry. 1995;59:633-634.
227 Leira EC, Cruz-Flores S, Leacock RO, et al. Sumatriptan can alleviate headaches due to carotid artery dissection. Headache. 2001;41:590-591.
228 Mainardi F, Maggioni F, Dainese F, et al. Spontaneous carotid artery dissection with clusterlike headache. Cephalalgia. 2002;22:557-559.
229 West P, Todman D. Chronic cluster headache associated with a vertebral artery aneurysm. Headache. 1991;31:210-212.
230 Koenigsberg AD, Solomon GD, Kosmorsky DO. Pseudoaneurysm within the cavernous sinus presenting as cluster headache. Headache. 1994;34:111-113.
231 Greve E, Mai J. Cluster headache-like headaches: a symptomatic feature? A report of three patients with intracranial pathologic findings. Cephalalgia. 1988;8:79-82.
232 Giffin N, Goadsby PJ. Basilar artery aneurysm with autonomic features: an interesting pathophysiological problem. J Neurol Neurosurg Psychiatry. 2001;71:805-808.
233 Mani S, Deeter J. Arteriovenous malformation of the brain presenting as a cluster headache—a case report. Headache. 1982;22:184-185.
234 Munoz C, Diez-Tejedor E, Frank A, et al. Cluster headache syndrome associated with middle cerebral artery arteriovenous malformation. Cephalalgia. 1996;16:202-205.
235 Kuritzky A. Cluster headache-like pain caused by an upper cervical meningioma. Cephalalgia. 1984;4:185-186.
236 de la Sayette V, Schaeffer S, Coskun O, et al. Cluster headache-like attack as an opening symptom of a unilateral infarction of the cervical cord: persistent anaesthesia and dysaesthesia to cold stimuli. J Neurol Neurosurg Psychiatry. 1999;66:397-400.
237 Cid C, Berciano J, Pascual J. Retro-ocular headache with autonomic features resembling “continuous” cluster headache in a lateral medullary infarction. J Neurol Neurosurg Psychiatry. 2000;69:134-141.
238 Tfelt-Hansen P, Paulson OB, Krabbe AE. Invasive adenoma of the pituitary gland and chronic migrainous neuralgia. A rare coincidence or a causal relationship? Cephalalgia. 1982;2:25-28.
239 Porta-Etessam J, Ramos-Carrasco A, Berbel-Garcia A, et al. Clusterlike headache as first manifestation of a prolactinoma. Headache. 2001;41:723-725.
240 Hannerz J. A case of parasellar meningioma mimicking cluster headache. Cephalalgia. 1989;9:265-269.
241 Scorticati MC, Raina G, Federico M. Clusterlike headache associated to a foreign body in the maxillary sinus. Neurology. 2002;59:643-644.
242 Lance JW. Mechanism and Management of Headache, 5th ed. London: Butterworth Scientific, 1993.
243 Heidegger S, Mattfeldt T, Rieber A, et al. Orbitosphenoidal aspergillus infection mimicking cluster headache: a case report. Cephalalgia. 1997;17:676-679.
244 Lee MS, Lessell S. Orbital myositis posing as cluster headache. Arch Neurol. 2002;59:635-636.
245 Hunter CR, Mayfield FH. Role of the upper cervical roots in the production of pain in the head. Am J Surg. 1949;78:743-749.
246 Medina JL. Organic headaches mimicking chronic paroxysmal hemicrania. Headache. 1992;32:73-74.
247 Newman LC, Herskovitz S, Lipton R, et al. Chronic paroxysmal headache: two cases with cerebrovascular disease. Headache. 1992;32:75-76.
248 Broeske D, Lenn NJ, Cantos E. Chronic paroxysmal hemicrania in a young child: possible relation to ipsilateral occipital infarction. J Child Neurol. 1993;8:235-236.
249 Vijayan N. Symptomatic chronic paroxysmal hemicrania. Cephalalgia. 1992;12:111-113.
250 Sjaastad O, Stovner LJ, Stolt-Nielsen A, et al. CPH and hemicrania continua: requirements of high dose indomethacin dosages—an ominous sign? Headache. 1995;35:363-367.
251 Gatzonis S, Mitsikostas DD, Ilias A, et al. Two more secondary headaches mimicking chronic paroxysmal hemicrania. Is this the exception or the rule? Headache. 1996;36:511-513.
252 Mariano HS, Bigal ME, Bordini CA, et al. Chronic paroxysmal hemicrania (CPH)–like syndrome as a first manifestation of cerebral metastasis of parotid epidermoid carcinoma: a case report. Cephalalgia. 1999;19:442.
253 Delreux V, Kevers L, Callewaert A. Hemicranie paroxystique inaugurant un syndrome de Pancoast [Paroxysmal hemicrania preceding Pancoast’s syndrome]. Rev Neurol. 1989;145:151-152.
254 Hannerz J, Jogestrand T. Intracranial hypertension and sumatriptan efficacy in a case of chronic paroxysmal hemicrania which became bilateral (the mechanism of indomethacin in CPH). Headache. 1993;33:320-323.
255 MacMillan JC, Nukada H. Chronic paroxysmal hemicrania. N Z Med J. 1989;102:251-252.
256 Silberstein SD, Lipton RB, Goadsby PJ. Headache in Clinical Practice, 2nd ed. London: Martin Dunitz, 2002.