Chapter 8 Treatment of Perforating Veins
Historical Background
Although the role of perforator veins (PVs) in the development of signs and symptoms remains unclear, the number of incompetent PVs and the size of both competent and incompetent PVs have been shown to increase with worsening chronic venous disease (CVD).1–4 Furthermore, it was recently reported that the duration of outward flow in these veins was longer in patients with ulcers compared with those in lower classes of CVD.2
Etiology and Natural History of Disease
Reflux in PVs is defined as outward flow from the deep to the superficial veins. It has been suggested that high flow from the deep veins during muscular contraction eventually renders the PVs incompetent.5 The etiology of venous reflux in superficial veins and PVs is unknown. The most predominant theory is that the weakening of the venous wall eventually leads to valve failure.6 During early stages of the disease, reflux is most prevalent in the superficial veins.7–9 Others have also suggested that reflux in the PVs is caused by volume overload at the reentry points of incompetent superficial veins.10,11 However, direct evidence for both of these theories is lacking because most investigations have been cross-sectional population studies without sufficient longitudinal study regarding disease progression.
Labropoulos et al.11 identified two other patterns by which previously competent PVs become incompetent—these were ascending development and new sites becoming incompetent. The ascending development of reflux into PVs from previously competent segments of superficial veins was more prevalent. A smaller number of incompetent PVs were detected in new locations that previously did not have reflux in any system PV reflux was always associated with reflux in an adjacent superficial vein and underscores the important role of superficial vein reflux in the development of PV incompetence. Because most limbs in the early stages of CVD exhibit reflux in the superficial veins only, it can be assumed that one of the mechanisms for development of PV insufficiency involves the presence of reflux in an adjacent superficial vein segment that acts as a capacitor for the refluxing PV. As local hemodynamic conditions change and as intravenous pressure increases, the diameter of the PV increases, and the PV valve becomes incompetent. This may be in combination with or separate from primary venous wall disease.
Deep vein reflux is not required for development of PV incompetence in primary venous disease. Rather, deep vein reflux can develop as a result of increased flow from the incompetent superficial veins through the PV, the diameter of which has increased. Labropoulos et al.11 showed that only five new incompetent PVs were seen in association with juxtaposed reflux in the deep vein. At all five sites, deep vein reflux was not present at the time of the initial duplex study, when the adjacent PV was still competent; the deep venous incompetence developed simultaneously with PV incompetence. Superficial vein reflux was present at all sites.
Finally, this study also suggested that the development of reflux in previously normal PVs was seen in association with worsening of the clinical stage of CVD in 40% of limbs. Although the worsening of the clinical stage cannot be attributed to extension of reflux in the PVs alone, one can assume that the natural history of long-standing reflux in the superficial veins is that of progressive deterioration, with extension of reflux to other previously competent segments of the superficial veins and their associated PVs.12
Patient Selection
Endovascular Instrumentation
Operative Steps
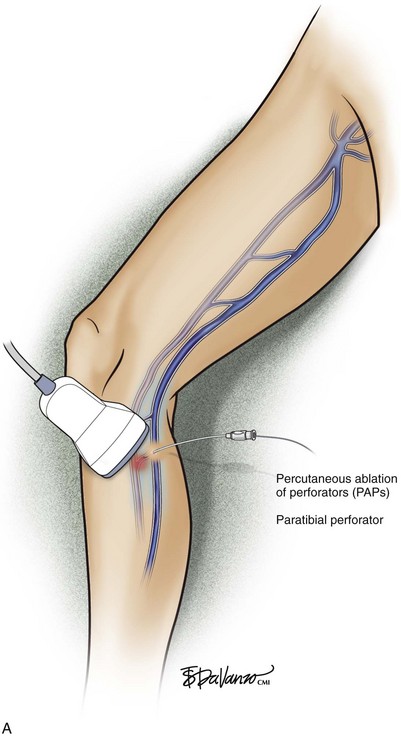
Percutaneous ablation of perforators (PAPs) was coined by Elias and Peden.12 The basic method involves (1) ultrasound-guided intraluminal access; (2) introduction of some ablative element (chemical or thermal); (3) confirmation of initial treatment success; and (4) follow-up of treatment success. Thus far, the techniques used have been either chemical (sodium tetradecyl sulfate [STS], aethoxysclerol, or sodium morrhuate)13,14 or thermal (radiofrequency [RF] or laser).15–17
PAPs Technique: Chemical Ablation
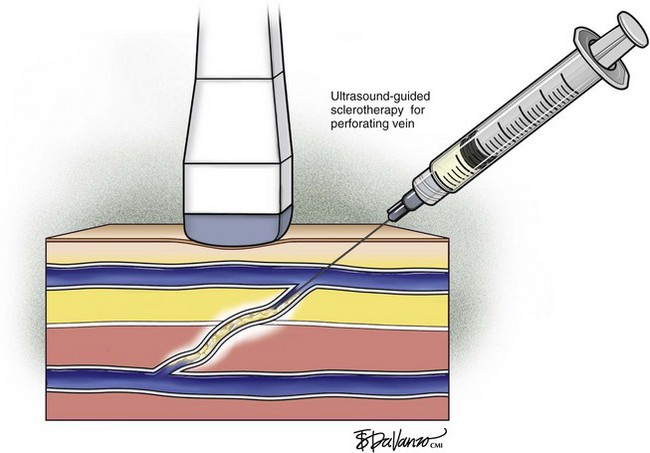
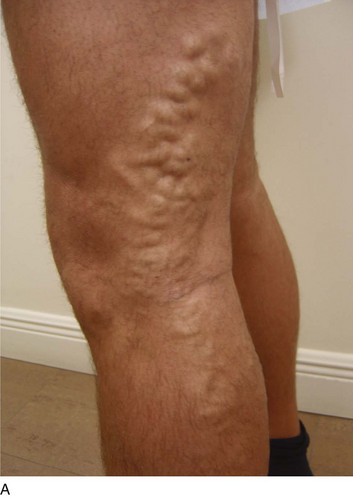
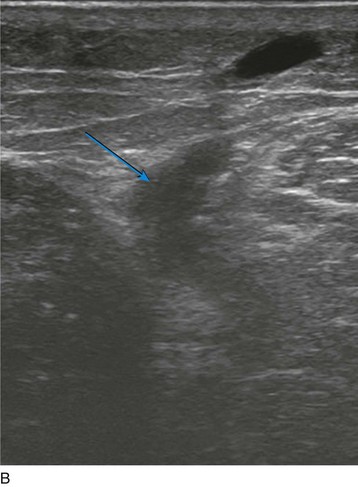
Ultrasound-guided sclerotherapy (UGS) (Fig. 8-1) is an effective and durable method of eliminating incompetent PVs and results in significant reduction of symptoms and signs as determined by venous clinical scores. As an alternative to open interruption or SEPS, UGS may lead to fewer skin and wound-healing complications. Little has been published regarding the outcomes following UGS for PVs.
In a series by Masuda et al.,13 patients primarily had isolated perforator disease (83%) without concomitant axial reflux from the thigh to the calf in the saphenous or deep systems. Clinical improvement following UGS was suggested by improvement of the Venous Clinical Severity Score (VCSS) and Venous Disease Severity (VDS) and lack of perforator recurrence with a mean follow-up of 20 months. In this study, successful obliteration of PVs with no recurrent symptoms was 75%. Perforator recurrence occurs particularly in those with ulcerations, and therefore surveillance duplex scanning after UGS and repeat injections may be needed. This study suggests that patients with perforator disease without axial reflux appear to benefit from injection sclerotherapy.
In 1992, Thibault and Lewis17 reported their early experience with injection of incompetent PVs by using ultrasound guidance and showed that PVs remained successfully obliterated in 84% at 6 months after treatment. In 2000 at the Pacific Vascular Symposium in Hawaii, Jerome Guex18 from France reported his experience with direct perforator treatment with ultrasound guidance. The sclerosing agent used was sodium tetradecyl sulphate (Sotradecol) (3%) (Bioniche Life Sciences Inc., Belleville, Ontario, Canada) or polidocanol (3%) for veins larger than 4 mm, and a more dilute solution was used for veins smaller than 4 mm. He estimated that 90% of PVs could be eliminated after one to three sessions of injections.
This method also involves ultrasound-guided access and confirmation with aspiration of blood. Many types of sclerosants have been used: sodium morrhuate, STS, and aethoxysclerol. Some advocates use the sclerosant in a liquid state. More recently, foam sclerotherapy has been advocated as being more efficacious. Most studies use STS 3% in the liquid form, injecting 0.5 to 1 ml of sclerosant or sodium morrhuate 5% in a similar manner, with care being taken not to inject the accompanying artery.14,19 After infusion of the sclerosant, compression is applied with wraps or stockings and direct pressure over the treated PV.
PAPs Technique Using the Laser System
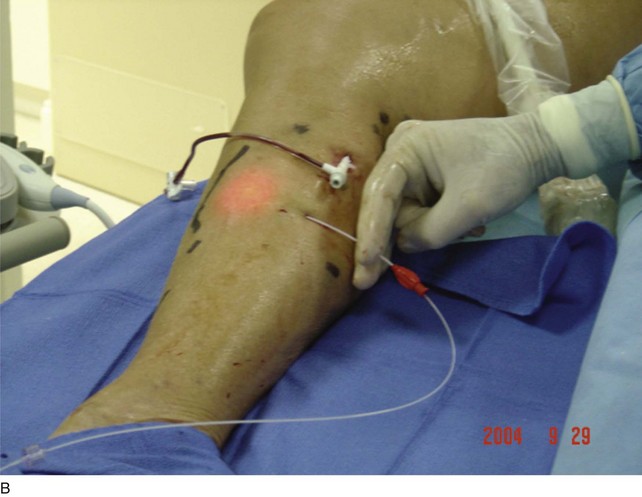
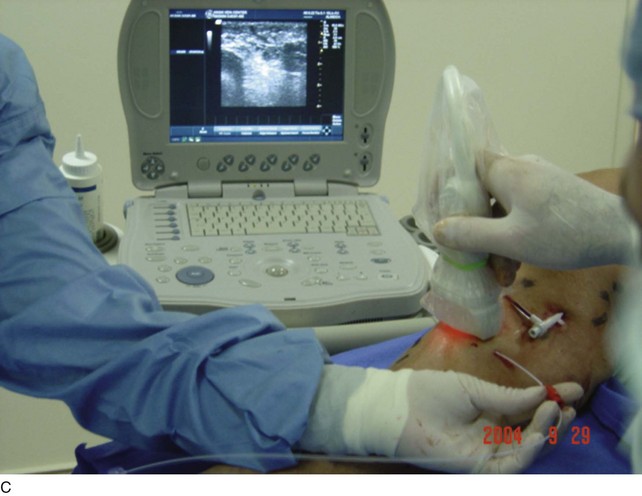
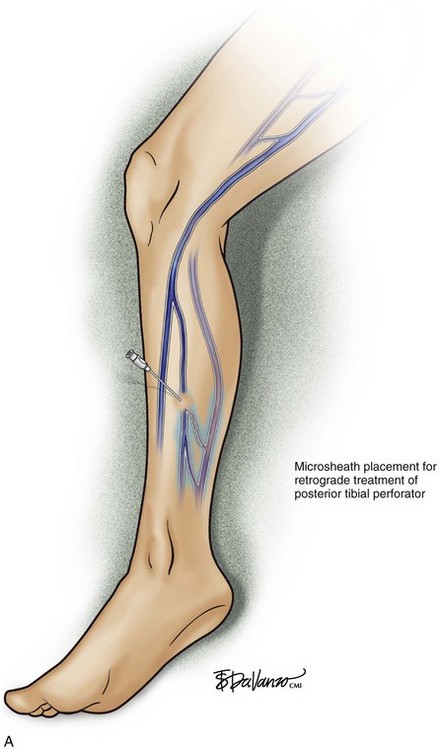
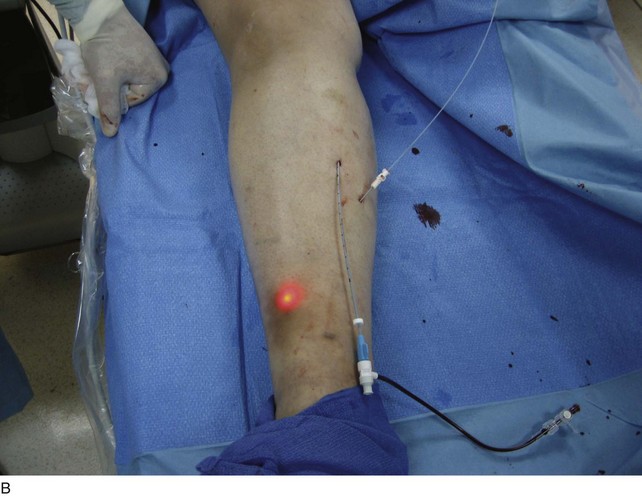
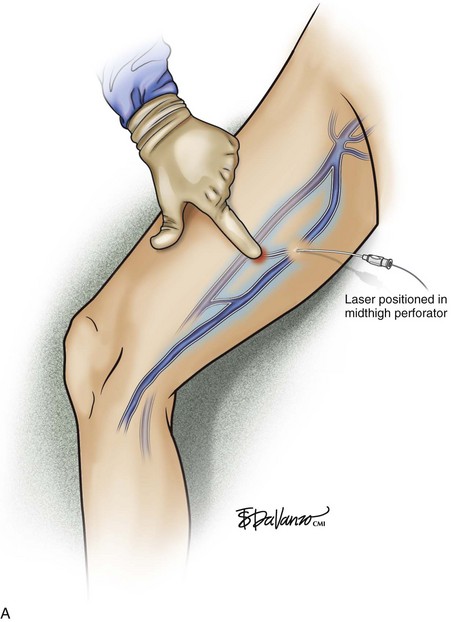
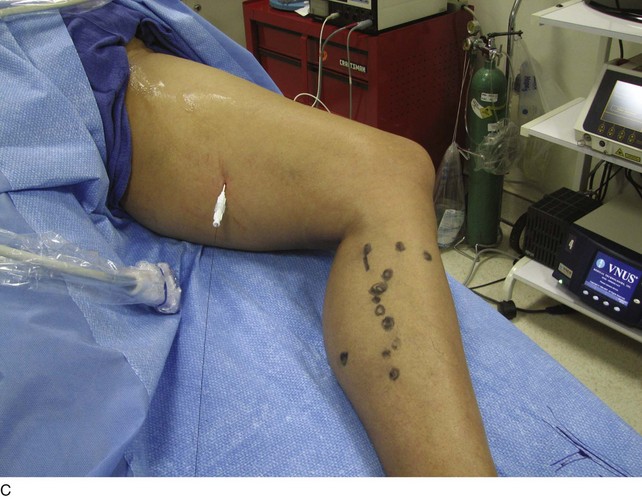
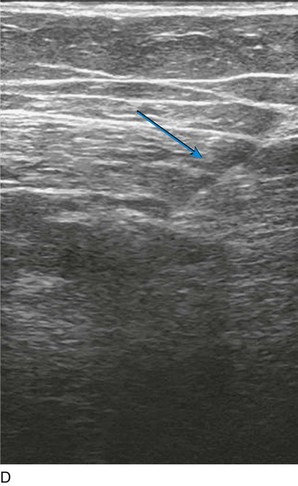
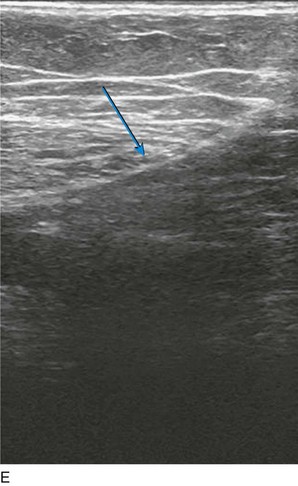
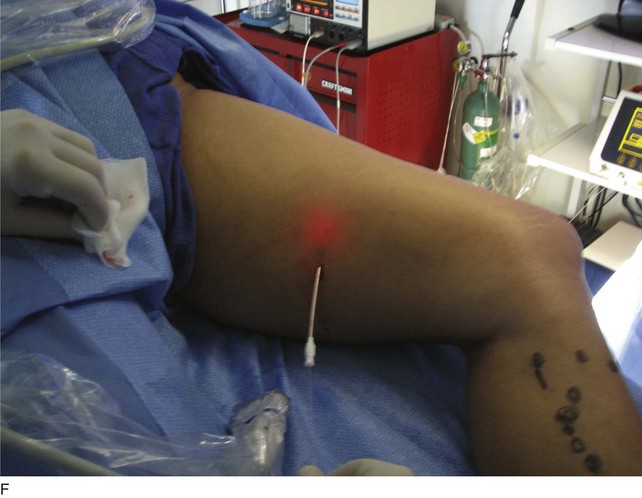
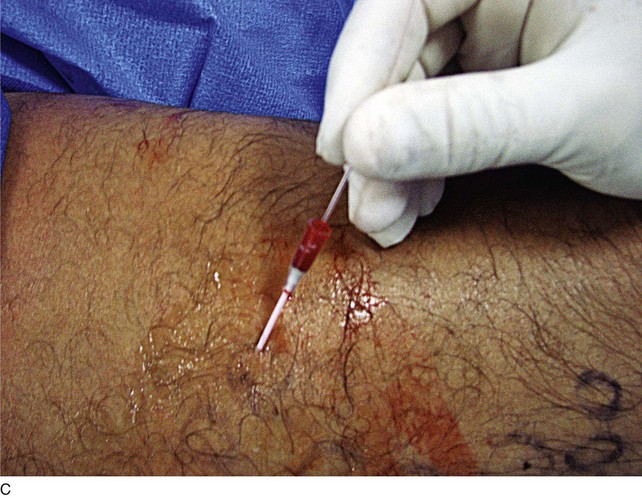
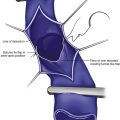
As with RF, intraluminal access into the target PV is the key step. There are two methods of laser access: a 21-gauge micropuncture needle or a 16-gauge angiocath. A direct puncture is made using ultrasound guidance with the patient in the reverse Trendelenburg position. Intraluminal placement is confirmed by ultrasonography and aspiration of blood. If a micropuncture needle is used (21 gauge), a 400-µm fiber can be passed directly through the needle into the PV with ultrasound visualization and positioned at or just below the fascial level; this is similar to RF. If a 600-µm fiber is used, then a 16-gauge angiocath or the sheath from the micropuncture access kit is used (Figs. 8-2 to 8-5). A 600-µm fiber diameter is too large to traverse the 21-gauge micropuncture needle.
Pulsed or continuous delivery methods of energy can be used. If pulsed, the laser is set for 15 W with a 4-second pulse interval during laser pullback. Each segment of vein is treated twice, thus administering 120 J to each segment; three segments are usually treated. At Miami Vein Center we use 10 W in the continuous mode and deliver 60 to 80 J/cm (see Figs. 8-2 to 8-5). Often we approach an ankle PV in a retrograde fashion (Fig. 8-3).
Proebstle and Herdemann16 also attempt to treat three locations within the PV (just below the fascia, at the fascia level, and just above). Each treated segment receives between 60 and 100 J. After energy delivery, an eccentric pressure dressing is applied for 1 minute over the PV, as is done with RF. During wrapping, a pressure bandage is applied with direct pressure over each PV with a cotton ball or something similar. They reported on a total of 67 PVs treated with 1320 nm at 10 W (median of 250 J) or with 940 nm at 30 W (median of 290 J). With the exception of one vein, all others were occluded on postoperative day 1. Side effects were moderate. They concluded that ultrasound-guided endovenous laser ablation of incompetent PVs is safe and feasible.16
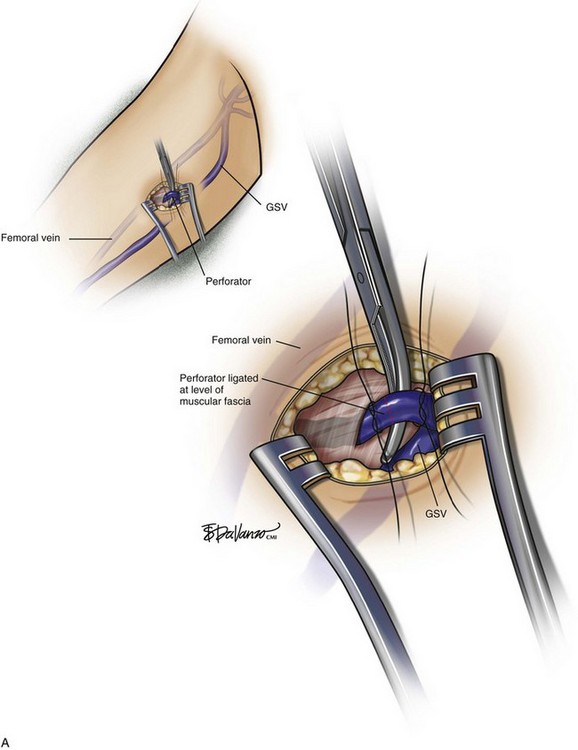
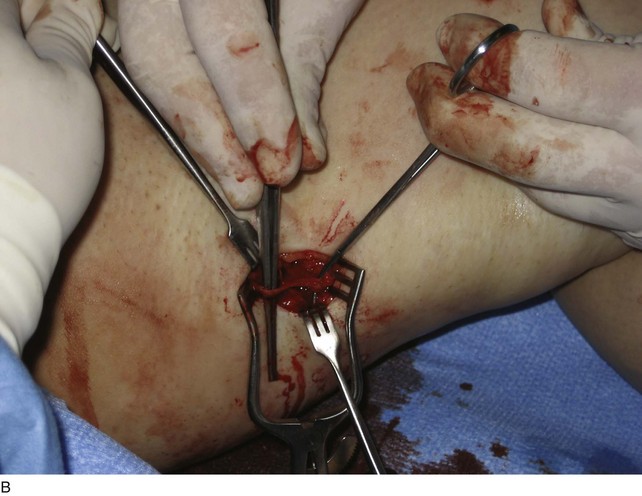
Rarely, a small incision is made with the patient under local anesthesia to access larger-diameter PVs to ligate them (Fig. 8-6). Confirmation of occlusion is documented with duplex imaging, and patency of the deep vessels is also confirmed. The results have been quite good.
Pearls and Pitfalls
The question still exists as to which PVs need treatment. Incompetent PVs are usually associated with superficial venous reflux, and therefore incompetent PVs may not require specific surgical interventions after saphenous truncal ablation. Ninety percent of the patients with C5-C6 disease have incompetent PVs, while less than half of the patients with C2 disease have incompetent PVs. Therefore, incompetent PVs are clearly associated with venous ulcers, and the question is whether these incompetent PVs have any hemodynamic significance and whether they really contribute to the inflammatory changes in these limbs.20
Comparative Effectiveness of Existing Treatments
Referable to the controversial topic of the role of PV incompetence causing nonhealing venous stasis ulcers, the ESCHAR trial20 establishes a Grade 1A recommendation that ligation and stripping of the GSV are associated with the prevention of ulcer recurrence. More traditional methods such as the Linton procedure and its modifications of open perforator ligation have a relatively high incidence of wound complication ranging from 20% to 40%.21–23 At present, there is no compelling Level 1 evidence to provide a Grade A recommendation that the treatment of incompetent PVs alone affects venous ulcer healing or recurrence.
Unfortunately, the treatment of incompetent PVs is blurred by concomitant treatment of GSV incompetence in the two RCTs that putatively explored the role of incompetent PV treatment.24,25 Moreover, surrogate hemodynamic outcomes, which assess the treatment of incompetent PVs alone as well as the effect of GSV treatment alone on perforator competence, also argue against the importance of treating incompetent PVs. This observation emphasizes the need for a properly designed trial in CEAP C5/C6 patients. Based on the RCTs cited and the results of studies with surrogate endpoints of restoring perforator competence, the current role of incompetent PV treatment can be considered a Grade 2B recommendation.
The effectiveness of treating PVs with isolated reflux was suggested by the randomized trial reported in 1974 by Hobbs26 comparing compression sclerotherapy with surgery that included saphenous vein stripping and subfascial ligation of PVs. He found that in the face of great or small saphenous vein reflux, surgery proved to have better 6-year results than sclerotherapy alone. On the other hand, when incompetent PVs were present without concomitant saphenous vein disease, sclerotherapy of calf PVs proved to be better than surgery.
1 Pascarella L, Schmid Schonbein GW. Causes of telangiectasias, reticular veins and varicose veins. Semin Vasc Surg. 2005;18:2-4.
2 Labropoulos N, Mansour MA, Kang SS, et al. New insights into perforator vein incompetence. Eur J Vasc Endovasc Surg. 1999;18:228-234.
3 Lees TA, Lambert D. Patterns of venous reflux in limbs with skin changes associated with chronic venous insufficiency. Br J Surg. 1993;80:725-728.
4 Pierik EG, Wittens CH, van Urk H. Subfascial endoscopic ligation in the treatment of incompetent perforating veins. Eur J Vasc Endovasc Surg. 1995;9:38-41.
5 Cockett FB, Elgan-Jones DE. The ankle blow-out syndrome. Lancet. 1953;1:17-23.
6 Kistner RL, Eklof B, Masuda EM. Diagnosis of chronic venous disease of the lower extremities: the “CEAP” classification. Mayo Clin Proc. 1996;71:338-345.
7 Labropoulos N. CEAP in clinical practice. Vasc Surg. 1997;31:224-225.
8 Labropoulos N, Delis K, Nicolaides AN, et al. The role of the distribution and anatomic extent of reflux in the development of signs and symptoms in chronic venous insufficiency. J Vasc Surg. 1996;23:504-510.
9 Delis K. Leg perforator vein incompetence: functional anatomy. Radiology. 2005;235:327-334.
10 Zamboni P. Pathophysiology of perforators in primary chronic venous insufficiency. World J Surg. 2005;29(Suppl 1):S115-S118.
11 Labropoulos N, Tassiopoulos AK, Bhatti AF, et al. Development of reflux in the perforator veins in limbs with primary venous disease. J Vasc Surg. 2006;43:558-562.
12 Elias S, Peden E. Ultrasound-guided percutaneous ablation for the treatment of perforating vein incompetence. Vascular. 2007;15:281-289.
13 Masuda EM, Kessler DM, Lurie F, et al. The effect of ultrasound-guided sclerotherapy of incompetent perforator veins on venous clinical severity and disability scores. J Vasc Surg. 2006;43:551-557.
14 Guex JJ. Ultrasound guided sclerotherapy (UGS) for perforating veins (PV). Hawaii Med J. 2000;59:261-262.
15 Peden E, Lumsden A. Radiofrequency ablation of incompetent perforator veins. Perspect Vasc Surg Endovasc Ther. 2007;19:73-77.
16 Proebstle TM, Herdemann S. Early results and feasibility of incompetent perforator vein ablation by endovenous laser treatment. Dermatol Surg. 2007;33:162-168.
17 Thibault PK, Lewis WA. Recurrent varicose veins. Part 2: injection of incompetent perforating veins using ultrasound guidance. J Dermatol Surg Oncol. 1992;18:895-900.
18 Guex JJ. Ultrasound guided sclerotherapy (USGS) for perforating veins (PV). J Vasc Surg. 2000;31:1307-1312.
19 Stuart WP, Lee AJ, Allan PL, et al. Most incompetent calf perforating veins are found in association with superficial venous reflux. J Vasc Surg. 2001;34:774-778.
20 Barwell JR, Davies CE, Deacon J, et al. Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study): randomized controlled trial. Lancet. 2004;363:1854-1859.
21 Sato DT, Goff CD, Gregory RT, et al. Subfascial perforator vein ablation: comparison of open versus endoscopic techniques. J Endovasc Surg. 1999;6:147-154.
22 Pierik EG, Van Urk H, Hop WC, et al. Endoscopic versus open subfascial division of incompetent perforating veins in the treatment of leg ulceration: a randomized trial. J Vasc Surg. 1997;26:1049-1054.
23 Stacey MC, Burnand KG, Layer GT, et al. Calf pump function in patients with healed venous ulcers is not improved by surgery to the communicating veins or by elastic stockings. Br J Surg. 1988;75:436-439.
24 van Gent WB, Hop WC, van Pragg MC, et al. Conservative versus surgical treatment of venous leg ulcers: a prospective, randomized, multicenter trial. J Vasc Surg. 2006;44:563-571.
25 O’Donnell TF. The present status of surgery of the superficial venous system in the management of venous ulcer and the evidence for the role of perforator interruption. J Vasc Surg. 2008;48:1044-1052.
26 Hobbs JT. Surgery and sclerotherapy in the treatment of varicose veins, a random trial. Arch Surg. 1974;109:793-796.