CHAPTER 78 TREATMENT OF MULTIPLE SCLEROSIS
The ultimate goals of multiple sclerosis therapy are to stop ongoing inflammatory and degenerative processes that lead to central nervous system damage and to repair the existing damage responsible for impairment, disability, and handicap. Unfortunately, there do not yet exist treatments that fully address these goals. In their absence, however, there are treatments that can reduce disease activity, delay the progression of disability, and ameliorate symptoms that affect quality of life. This chapter describes the benefits of the currently available course-modifying therapies (Table 78-1) and symptomatic treatments as demonstrated by rigorous clinical trials.
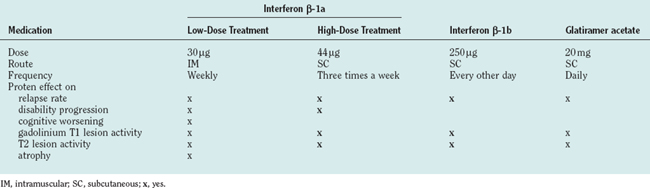
TABLE 78-1 U.S. Food and Drug Administration–Approved Course-Modifying Therapy for Multiple Sclerosis
| Disease Type | Drug |
|---|---|
| Relapsing | Interferon β-1a (Avonex, Rebif) |
| Interferon β-1b (Betaseron) | |
| Glatiramer acetate (Copaxone) | |
| Secondary progressive | Mitoxantrone (Novantrone) |
| Primary progressive | None approved |
TREATMENT FOR ACUTE RELAPSES
Since the early 1950s, neurologists have used corticosteroids to treat acute exacerbations.1 Placebocontrolled clinical trials2–5 have demonstrated that short-term corticosteroid treatment hastens recovery from a relapse, but the optimal treatment regimen remains unclear. Results of head-to-head comparisons of intravenous methylprednisolone and subcutaneous adrenocorticotrophic hormone have been mixed. One study of 25 patients with clinically definite multiple sclerosis6 revealed that patients receiving intravenous methylprednisolone had more rapid improvement than patients receiving intramuscular adrenocorticotrophic hormone. But this difference, seen at days 3 and 28, was no longer apparent after 3 months. Other studies were not able to demonstrate any significant difference between the agents.7–9 Typically, patients begin experiencing improvement between the first and third days of steroid treatment, and improvement continues for 15 to 45 days before symptoms stabilize clinically.4
The Optic Neuritis Treatment Trial, assessing recovery of vision in patients with their first episode of acute optic neuritis, provided further insight into the short- and long-term effects of corticosteroids on demyelination. Study patients (N = 457) received (1) intravenous methylprednisolone plus a prednisone taper, (2) prednisone alone, or (3) oral placebo. As in the earlier studies in patients with multiple sclerosis exacerbations, patients receiving steroids recovered faster, especially those receiving intravenous treatment.10 But the differences in visual acuity between the treatment and placebo recipients seen at days 4 and 15 were no longer apparent by 6 months,10 which, again, indicates that steroids affect the timing of recovery from an attack but not the extent of the recovery.11
In general, for treatment of an acute relapse, higher doses of steroids are more effective. For example, in a study of 32 patients with relapse who were randomly assigned to receive 1000 mg of intravenous methylprednisolone per day for 1 day or 5 days, disability scores at 1 month improved more after the extended treatment.12 In another study, 2g/day of intravenous methylprednisolone was compared with 0.5g/day, both for 5 days, in a randomized, doubleblind study of 31 patients with relapsingremitting multiple sclerosis. Although the average improvement in disability scores was comparable between the groups, the higher dose recipients had fewer contrast-enhancing lesions on magnetic resonance imaging (MRI) at 30 and 60 days, respectively, which indicates more thorough reduction in ongoing inflammation.13 The rate of occurrence of side effects was similar in the two groups. The Optic Neuritis Treatment Trial showed that high-dose, intravenous steroid administration is more beneficial than low-dose prednisone.10 The effects of high doses of steroids given orally, however, may be more comparable. A small (N = 35) randomized, doubleblind trial in which 500 mg of oral methylprednisolone was compared with 500 mg given intravenously, both for 5 days, revealed no difference in disability score at 5 and 28 days.14 The safety of oral prednisone, 1250 mg/day, has been demonstrated15; the efficacy of this approach is being studied.
In rare cases, serious side effects have been reported with high-dose methylprednisolone therapy, including fatal arrhythmias,16,17 anaphylaxis,18 anaphylactoid reactions,19 and seizures.20 Although pulse-administered steroids have also been shown to transiently depress some markers of bone growth,21 a prospective study of the effect of sporadic methylprednisolone pulse treatment on bone density did not demonstrate bone loss 6 months after treatment.22 As with any intervention, these risks must be weighed against the potential benefits of treatment.
Despite treatment with corticosteroids, relapses frequently leave patients with permanent deficits,23 a fact that has prompted researchers to seek additional therapies. Intravenous immunoglobulin (IVIg) infusions were shown to improve functional outcome in a small study of patients with acute disseminated encephalomyelitis refractory to intravenous steroids.24 However, in a randomized, doubleblind, placebocontrolled pilot study of 19 patients with multiple sclerosis relapse, in which IVIg infusions were added to treatment with intravenous methylprednisolone, there was no difference in disability after 4 weeks; this suggests that the benefit of adding IVIg infusions to intravenous methylprednisolone is minimal at best.
In two randomized, blind, controlled studies, researchers have examined the value of plasma exchange for acute exacerbations. In the first, 116 patients with an acute multiple sclerosis relapse were treated with intramuscular adrenocorticotrophic hormone plus oral cyclophosphamide, and then randomly assigned to receive 11 plasma exchange treatments or 11 sham treatments, administered over 8 weeks.25 Although patients receiving plasma exchange experienced moderately enhanced improvement after 2 weeks in comparison with the sham-treated patients, and although the median time to recovery of disability status before attack was significantly shorter in the plasma exchange recipients, there was no difference between groups at 12 months.
The second controlled plasma exchange study included only patients with severe deficits from a recent demyelinating event that did not improve with intravenous corticosteroids.26 Of the 22 patients studied, 12 had multiple sclerosis; the others had an acute demyelinating syndrome such as acute disseminated encephalomyelitis, neuromyelitis optica, or transverse myelitis. Patients receiving plasma exchange were more likely to have a “moderate or greater” improvement in their primary disability score than were those receiving sham treatment (42% versus 66%), which supports the use of plasma exchange for severe multiple sclerosis exacerbations refractory to steroids.
COURSE-MODIFYING TREATMENT (Table 78-2)
Relapsing-Remitting Multiple Sclerosis
Interferon β
Interferons are cytokines normally produced by the immune system in response to viral infection. In 1993, the IFNB Study Group reported the first multicenter, randomized, placebocontrolled, doubleblind study to clearly demonstrate that interferon β-1b (IFN β-1b) can modify the course of multiple sclerosis.27 Patients with relapsingremitting multiple sclerosis (N = 372) received 1.6MIU or 8MIU of IFN β-1b (Betaseron) or placebo, by subcutaneous injection, every other day for up to 5 years. The primary outcome measure, annual relapse rate, was reduced by 31% in the high-dose recipients in comparison with the placebo recipients, and there was a consistent dose response for all endpoints. MRI, used innovatively, demonstrated a pathological basis for the observed clinical benefits, confirming that they reflected a persistent, profound effect on the inflammatory process.28 Reduction in the rate of accumulation of disability, however, could not be demonstrated in the post hoc analysis.
In 1996, another large (N = 301), randomized, doubleblind, multicenter study confirmed and extended these observations by demonstrating that IFN β—in this case, 30μg of weekly intramuscular IFN β-1a (Avonex)—delayed the time to sustained progression of disability, defined as a one-point worsening of the Expanded Disability Status Scale (EDSS) score.29 Over 2 years, 21.9% of patients receiving interferon experienced progression of disability, in comparison with 34.9% of placebo recipients. Post hoc analysis showed that over the course of 2 years, fewer treated patients progressed to EDSS scores of 4 (moderate disability) or 6 (requiring a cane).30
The largest placebocontrolled study of IFN β in relapsing multiple sclerosis included 560 patients randomly assigned to receive 22 or 44μg of subcutaneous IFN β-1a (Rebif) or matching placebo three times per week for 2 years. The study demonstrated that IFN β decreased the annual relapse rate by 27% to 33%, in comparison with placebo31; delayed progression of disability, as measured by the EDSS31; and prevented the accumulation of new and enlarging lesions, as demonstrated on T2-weighted MRI.32 After the 2-year placebocontrolled phase of the study, 79% of the participants continued in a 2-year extension phase, in which patients originally randomly assigned to receive active treatment continued with their assigned interferon dose, whereas patients originally assigned to receive placebo were randomly reassigned to receive either 22 or 44μg of IFN β-1a three times weekly.33 The change to active drug decreased the relapse rate and the MRI parameters of disease of those who had initially received placebo, but they continued to have more rapid progression of disability and more MRI lesions over the entire study period than did those who had received IFN β from the start. These observations, combined with studies demonstrating significant benefits in patients treated with low doses of IFN β at the time of their first clinically apparent demyelinating event,34,35 suggest that early treatment is appropriate for preventing as much central nervous system damage and resulting disability as possible.
To determine the relative benefits of different interferon formulations and dosing regimens, head-to-head studies are needed. In the largest and most rigorous of these studies to date, 677 patients with relapsingremitting multiple sclerosis were randomly assigned to receive either subcutaneous IFN β-1a (Rebif), 44μg three times weekly, or intramuscular IFN β-1a (Avonex), 30μg once weekly for a year. The relapse rate in the high-dose, high-frequency recipients was 21% lower than that in the low-dose, weekly recipients.36 Participants on the high-dose high-frequency interferon regimen also had better control of MRI disease activity. The increase in efficacy in the high-dose recipients was associated with more frequent injection site reactions and (asymptomatic) elevations in liver enzyme levels. At the end of this comparative phase of the study, most participants continued into a crossover phase in which all patients received the high-dose, high-frequency IFN β-1a regimen. Patients who had crossed over from low-dose, weekly IFN β-1a experienced significant reductions in relapse rates and MRI activity, in comparison with those who had continued the high-dose treatment from the start of the study.37 A smaller study in which patients were randomly assigned to receive high-dose, high-frequency IFN β-1b (Betaseron) or low-dose weekly IFNβ-1a (Avonex) yielded similar results.38
Several side effects can occur with interferon therapy. Most patients experience flulike symptoms, including myalgia, arthralgia, headache, malaise, and fever for 12 to 24 hours after each injection. This reaction is most common when treatment is initiated and tends to decrease in intensity with continued therapy. It can be minimized with dose titration and concomitant acetaminophen, nonsteroidal antiinflammatory medications, or low doses of prednisone.39 Transient injection site reactions, with focal erythema and skin induration, are also common with subcutaneous interferon injections. Pretreatment of the injection site with a topical anesthetic or ice can ameliorate this symptom. In rare cases, injection site reactions can persist or progress into focal areas of necrosis, necessitating surgical treatment.40 There has been concern that interferons may worsen depression,41 which is common in patients with multiple sclerosis. However, a post hoc analysis of data from the large Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis (PRISMS) trial demonstrated no increase in the frequency of depression in patients treated with interferon in comparison with those receiving placebo.42
Most patients develop antibodies against IFN β during treatment, especially those receiving high-dose, high-frequency treatment by subcutaneous injection. Antibodies generally arise in the first year of treatment and have variable persistence thereafter.43 A subset of these antibodies interferes with the effects of IFN β in in vitro assays; these have been called neutralizing antibodies. Although the results of clinical studies conflict somewhat, most studies suggest that patients with persistent high titers of neutralizing antibodies have increased signs of disease activity, although this may take years to become clinically apparent. For example, in the initial 2-year PRISMS study, antibodies to IFN β-1a did not affect clinical outcome measures,31 but in the subsequent 2-year extension phase of the study, patients with neutralizing antibodies to IFN β-1a had more relapses and MRI activity than did those without antibodies.33 Because the extension phase had no placebo condition, it was not possible to quantify any partial benefit from treatment despite the antibodies.
Glatiramer Acetate
Glatiramer acetate is a mixture of synthetic polypeptides with amino acids in a ratio similar to that in myelin basic protein, a major component of the myelin sheath. In the pivotal trial, 251 patients were treated with glatiramer acetate or placebo for 2 years.44 The primary outcome, relapse rate, was reduced by 29% in those receiving active drug, in comparison to placebo. With regard to disability, those receiving placebo were more likely to experience worsening (by one EDSS point or more) over the course of the study, but the percentages of patients with “sustained disability” (lasting at least 90 days) were comparable in the two groups, as was their ability to ambulate. Most of the initially randomly assigned patients (83%) continued to be monitored in the open-label extension phase of the study,45 in which patients who initially received placebo were offered glatiramer acetate. At 6 years, those remaining in the study had substantially fewer relapses and progression of disability46 than would be predicted by natural history studies.47 At 8 years, 142 (56.6%) of the original patients remained in the study. Those receiving glatiramer acetate from the time of random assignment had a very low rate of relapse, about one every 5 years, and were also about 20% less likely to have worsening of their disability than were those who had initially received placebo.48
A separate MRI study of 239 patients with multiple sclerosis demonstrated that the mean total number of enhancing lesions was 29% lower in patients receiving glatiramer acetate than in those receiving placebo during 9 months of treatment.49 Secondary analysis showed that in patients treated with glatiramer acetate, fewer gadolinium-enhancing lesions became hypointense on T1-weighted MRI, which would indicate the most severe tissue damage.50 Most of these patients were also evaluated for changes in brain volume by MRI, but no difference could be detected between patients receiving glatiramer acetate and those receiving placebo.51
Natalizumab
Natalizumab is a humanized monoclonal antibody to α4β1 integrin, an adhesion molecule important for the transmigration of leukocytes across the blood-brain barrier. The medication was approved for prescription use in the United States in November 2004 on the basis of favorable preliminary results from two large randomized trials. In February 2005, the medication was withdrawn from the market because of the occurrence of progressive multifocal leukoencephalopathy in two patients receiving natalizumab in combination with IFN β for more than 2 years. It is hoped that additional studies will provide more information about the safe use of this medication.
Intravenous Immunoglobulin
After it was demonstrated to have efficacy in the treatment of peripheral demyelinating disease,52,53 IVIg treatment was studied for the treatment of relapsingremitting multiple sclerosis in four randomized, doubleblind studies.54–59 Although the dosages of IVIg differed substantially among the studies (0.2 to 2.0g/kg body weight/month), all four studies revealed significant benefits in the patients who received IVIg, in comparison with those receiving placebo. The largest of these studies (N = 150), the Austrian Immunoglobulin in Multiple Sclerosis Study Group,56 compared disability scores and the proportions of patients whose disability had improved, stabilized, or worsened with IVIg, 0.15 to 0.2g/kg/month for 2 years, as opposed to placebo. Disability scores improved more among patients receiving active treatment than among placebo recipients (31% versus 14%) and worsened among fewer treated patients (16% versus 23%).56 The other three studies, which were smaller, also demonstrated differences between treatment and placebo with regard to relapse rate55,57 and number of enhancing lesions on brain MRI.59 One study, in which two dosage conditions (0.2g/kg body weight and 0.4g/kg body weight monthly) were used, demonstrated no differences in relapse rates between low and high doses, but both yielded better results than did placebo.55 The incidence of side effects was comparable with that of placebo in the studies that included the lower doses. High-dose (2.0g/kg body weight/month) IVIg infusions led to adverse events more frequently than did placebo (headache, 26% versus 6%; nausea, 9% versus 0%; and urticaria, 7% versus 1%).59
Corticosteroids
Corticosteroid pulse treatment clearly shortens the duration of acute relapses, but its long-term effect on disease course is less certain. Treatment of multiple sclerosis with long-term, low-dose corticosteroids has been studied for several decades and has not proved effective.60 In the Optic Neuritis Treatment Trial, patients experiencing their first demyelinating event were less likely to have another attack over the next 2 years if they were treated with intravenous methylprednisolone treatment (7.5%) than if they received oral prednisone (14.7%) or placebo (16.7%).61 The validity of this observation is unclear, however, because group differences were not sustained beyond 2 years,11 and the results have not been replicated in similar studies.62 The benefits of periodic corticosteroid pulse treatment were addressed more directly in a study of 88 patients with relapsingremitting multiple sclerosis randomly assigned to receive a 5-day course of high-dose intravenous methylprednisolone either every 4 months or only at the time of a relapse. After 5 years, the group receiving scheduled treatments had less progression in brain atrophy and lower hypointense lesion volume on T1-weighted MRI.63 The clinical significance of these observations remains to be determined.
3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase Inhibitors
3-Hydroxy-3-methylglutaryl-coenzyme A (HMGCoA) reductase inhibitors (statins) have been known to have antiinflammatory effects for many years64 and have shown benefit in animal models of multiple sclerosis65,66 and in vitro studies.67 Two preliminary, uncontrolled studies of statins have been published to date. The first study (N = 7) showed that 20 mg of lovastatin per day was well tolerated, although no change in multiple sclerosis parameters could be documented. Another study (N = 30) revealed that simvastatin, 80 mg/day, decreased the average frequency of new gadolinium-enhancing lesions in comparison with patients’ pretreatment baselines.68 These preliminary study results suggest that larger controlled studies are warranted.
Clinically Isolated Syndromes Suggestive of Multiple Sclerosis
Patients with a single episode of neurological dysfunction that is probably the result of demyelination, such as optic neuritis, transverse myelitis, or a brainstem syndrome, are at substantial risk for future demyelinating events. If the initial brain MRI shows a clinically silent lesion, the 10-year risk of developing multiple sclerosis is 83%, in comparison with 11% when no such lesion is demonstrated.69 Because of the preventive nature of multiple sclerosis immunotherapy, it is logical to consider treatment in patients as soon as the disease process has been identified. In two randomized, controlled studies, researchers have examined the effect of IFN β-1a on preventing progression to definite multiple sclerosis. Both studies included only patients with one or more asymptomatic brain lesion. The first, a large (N = 383) randomized, doubleblind, multicenter study, showed that patients with a first-ever demyelinating event could decrease the 3-year risk of developing a second demyelinating event by 44% by using weekly intramuscular IFN β-1b (Avonex) injections.34 In addition, treated patients demonstrated improvement in several MRI parameters of disease at 18 months, including a substantial decrease in the median growth (in total volume) of T2-weighted lesions (1% versus 16%) and mean number of enhancing T1-weighted lesions (0.4 versus 1.4).34 In the second study (N = 308), a lower dosage of IFN β-1a (Rebif, 22μg) was administered subcutaneously once a week. At 2 years, this treatment decreased the risk of developing a second exacerbation by 48%.70 Treated patients also had significantly less brain atrophy than did those receiving placebo.35 IVIg has also been studied for treatment of a clinically isolated event. A randomized, doubleblind study (N = 91) of high-dose IVIg (2.0g/kg loading dose, with a 0.4g/kg booster dose every 6 weeks) found that over 1 year, treated patients had a 64% reduction in the risk of progressing to definite multiple sclerosis.71
Secondary Progressive Multiple Sclerosis
Most cases of relapsing multiple sclerosis eventually transition from a course with relapses intermixed with stable deficits to one with progressive deficits and less prominent relapses.72 The pathophysiology underlying this transition is not completely understood, but the transition is believed to be the result of a change in the multiple sclerosis disease process from primarily inflammatory to primarily neurodegenerative.73 Because the disease-modifying therapies for relapsingremitting multiple sclerosis are immune modulators, it might be reasonable to expect less benefit from these treatments in the secondary progressive phase.
Interferon β
In four large, randomized, doubleblind, multicenter trials, investigators assessed the safety and efficacy of IFN β in patients with secondary progressive multiple sclerosis.74–77 All four studies revealed that interferons decrease the annual relapse rate and improve MRI measures of disease activity and cumulative disease burden. Effects on disability progression, on the other hand, were found in the earliest European study74 but not in the other two trials, in which change in EDSS scores was the primary endpoint.75–77 The disparate results may reflect subtle differences between patients enrolled in these studies. Of most importance, more patients in the trial demonstrating IFN β effects still had a relapsing quality to their course; that subgroup appeared to be most likely to respond to treatment.78 Furthermore, the EDSS may not be responsive to change adequately to demonstrate benefits over 2 to 3 years. To address this possibility, the Improving Mood: Promoting Access to Collaborative Treatment (IMPACT) investigators used the Multiple Sclerosis Functional Composite (MSFC)79 as the primary measure of disability progression.76 In that large (N = 436) randomized, doubleblind, multicenter study, patients receiving high-dose (60μg) weekly IFN β (Avonex) had less worsening in MSFC scores at 2 years than did subjects receiving placebo. Neither group showed significant changes in EDSS scores, which confirms that the EDSS was more sensitive to change and therapeutic effects.
Intravenous Immunoglobulin
IVIg, which had demonstrated enhanced remyelination in an animal model of multiple sclerosis,80 was of particular interest for patients with long-standing deficits from multiple sclerosis. Unfortunately, in two small studies of patients with secondary progressive multiple sclerosis, IVIg was not able to appreciably change either central motor conduction time81 or stable neurological deficits,82 in comparison with placebo. In the large (N = 318) randomized, placebocontrolled, multicenter European Study on Intravenous Immunoglobulin in Multiple Sclerosis (ESIMS),83 IVIg treatment (1g/kg body weight/month) did not affect the time to progression of disability (by one EDSS point) over 2 years, its primary outcome measure. The rate of relapse was also no different between the treated patients and the placebo recipients.83 Furthermore, the improvement in MRI parameters of disease observed in earlier studies, which included patients with both relapsingremitting and secondary progressive multiple sclerosis,59 were not seen in this study of only patients with secondary progressive multiple sclerosis. The ESIMS patients were then studied with the more sensitive magnetization transfer MRI analysis, which is believed to detect multiple sclerosis pathology in areas of white matter that appear normal on standard MRI sequences.84 No significant difference between groups was seen with this more sensitive method.85
Mitoxantrone
Chemotherapeutic agents, including azathioprine, methotrexate, and cyclophosphamide, have been used in patients in whom multiple sclerosis progresses despite other medications. One such agent, mitoxantrone (Novantrone), was approved by the U.S. Food and Drug Administration in 2000 for worsening multiple sclerosis, on the basis of the results of a large (N = 194) randomized, multicenter study in patients with worsening relapsingremitting or secondary progressive multiple sclerosis.86 In that study, treatment with mitoxantrone infusions (12 mg/m2) every three months for 2 years, in comparison with placebo, led to improvement in a composite endpoint that incorporated change in EDSS, change in ambulation index, number of relapses requiring steroids, length of time to first treated relapse, and change in a standardized neurological rating scale.86 Although mitoxantrone is generally well tolerated, the potential for serious side effects, including cardiotoxicity and leukemia, limits its use.
Primary Progressive Multiple Sclerosis
It remains unclear whether primary progressive multiple sclerosis should be considered within the spectrum of inflammatory and degenerative changes seen in relapsing multiple sclerosis, or as a distinct disease.87 Regardless, MRI and pathological studies88 indicate that primary progressive multiple sclerosis has less inflammatory activity, which suggests that immunomodulatory treatments may be less effective than in relapsing multiple sclerosis. Furthermore, the lack of relapses, often the primary endpoint in studies of patients experiencing relapse, makes it difficult to study patients with primary progressive and relapsing disease in the same trial. As a result, few studies have focused on course modification in patients with primary progressive multiple sclerosis. The only treatment subjected to rigorous trials in this setting is IFN β; 50 patients were randomly assigned to receive 30μg, 60μg, or placebo once weekly. No effect on length of time to sustained progression of disability was demonstrated, although the study was powered only to detect large effects. MRI evidence of treatment effect was mixed: Subjects receiving 30μg weekly had a lower rate of accumulation of T2-weighted lesion load than did subjects receiving placebo, but subjects receiving 60μg weekly had a greater rate of ventricular enlargement than did controls.89 To date, there is no well-established treatment for these patients, although immunomodulators and chemotherapeutic agents with demonstrated efficacy in relapsing and secondary progressive multiple sclerosis are often used when advancing disability forces action.
SYMPTOMATIC THERAPY (Table 78-3)
Spasticity
Spasticity causes stiffness, cramps, spasms, and clonus. Stretching exercises can often reduce mild spasticity, but more significant symptoms necessitate medication. Both baclofen and tizanidine have well-established effects on spasticity.90 They have similar antispasticity effects and side effects, although tizanidine may be less likely to cause muscle weakness.91 Gabapentin and benzodiazepines may be useful adjunctive medications.92,93 Use of all of these medications tends to be limited by their tendency to cause sedation. Dantrolene, which acts directly on muscles, does not cause sedation but is more likely to cause weakness.94 In rare cases, it can cause severe liver toxicity.94 Patients with refractory spasticity often respond to intrathecal baclofen, which is infused through an implantable subcutaneous pump.95 Botulinum toxin is helpful for focal spasticity.96
Tremor
Tremor can be one of the most disabling symptoms of multiple sclerosis and is generally difficult to treat satisfactorily. Occupational therapy can sometimes help patients partially compensate for tremor, with specialized utensils and positioning training.97 A wide variety of treatments, including benzodiazepines,98 primidone,99 propranolol,100 isoniazid,101 trazodone,102 5-hydroxytryptamine-3 antagonists,103 and cannabinoids,104 as well as stereotactic surgery,105 have been tried but frequently prove to be intolerable and/or are only partially effective. None of these treatments has demonstrated reproducible benefits in randomized controlled trials; thus, tremor is one of the most disabling and untreatable symptoms of multiple sclerosis.
Weakness
Physical therapy can help strengthen muscles that are weak as a result of disuse and can help patients learn techniques for moving more safely and efficiently. Assistive devices such as ankle-foot orthoses, canes, and walkers can help maintain mobility despite substantial leg weakness. Individualized comprehensive rehabilitation programs have been demonstrated to improve functional abilities,106 but the duration of this benefit is unknown. Several investigators have examined the effect of 4-aminopyridine and 3,4-diaminopyridine on motor function in patients with multiple sclerosis.107,108 Modest benefits in strength, spasticity, and walking speed have been consistently demonstrated, although the optimal use of this medication remains uncertain because of a dose-dependent risk of seizures.109
Fatigue
In general, management of fatigue begins with identification and amelioration of other factors contributing to it, such as depression, pain, sleep disorders, and comorbid medical conditions. Nonpharmacological treatments, including graded exercise training,110 “energy management” strategies,111 and cooling therapy,112–114 have been tried, but evidence supporting their effectiveness is limited. The main treatments demonstrated to have an effect on fatigue in placebocontrolled clinical trials are amantadine, pemoline, and modafinil, but all these studies had limitations. Of five randomized, placebocontrolled trials of amantadine,115–119 all had relatively small sample sizes (10 to 32 patients treated with amantadine) and brief treatment periods (1 to 6 weeks); in four, a crossover design was used. The investigators used self-report measures of fatigue severity as primary endpoints and generally demonstrated modest but significant benefits of amantadine versus placebo.
Pemoline is a central nervous system stimulant with dopaminergic rather than sympathomimetic effects.120 Two randomized, placebocontrolled trials of pemoline for the treatment of fatigue in multiple sclerosis have been published.119,121 Limitations of these studies are similar to those of the amantadine trials, and results conflicted. Moreover, potential liver toxicity122,123 has limited further pemoline use.
Modafinil has been studied in one controlled trial, in which patients with multiple sclerosis crossed over from placebo to modafinil and back to placebo over 9 weeks.124 During treatment, scores on two commonly used self-report questionnaires improved significantly. However, the design of this study does not adequately rule out the possibility that temporary placebo effects confounded the results. In a more rigorous randomized, placebocontrolled, doubleblind, parallel-group study, modafiniland placebo-treated patients had equally dramatic improvements in questionnaire scores, which raises serious questions about its true benefits.125
Sensory Dysfunction
Multiple sclerosis causes both positive symptoms (dysesthesia/allodynia) and negative symptoms (hypesthesia). Negative symptoms cannot be corrected, but they are not usually major contributors to disability. Positive sensory symptoms, on the other hand, can cause significant distress and can often be minimized with the same medications used for other forms of neuropathic pain, including antidepressants and anticonvulsants.126 Paroxysmal dysesthesias such as trigeminal neuralgia are especially responsive to anticonvulsants.127 Surgical ablation can be helpful for medically refractory cases.128
Bladder Dysfunction
Bladder dysfunction in patients with multiple sclerosis often causes a mixture of symptoms, including urgency, retention, and dyssynergia. Scheduled voiding may be an adequate treatment for milder symptoms. Anticholinergics are helpful when urgency, related to uncontrolled detrusor activity, is the dominant problem. α-Adrenergic antagonists may be helpful when retention is the dominant problem. Significant retention necessitates intermittent catheterization, both for extending the time between voiding episodes and for reducing the incidence of urinary tract infections. More complicated cases necessitate urodynamic monitoring and a combined approach.129
Sexual Dysfunction
The most common symptoms of sexual dysfunction in women with multiple sclerosis are decreased desire, anorgasmia, and decreased lubrication. Counseling and foreplay may be helpful for all of these symptoms130; synthetic lubricants are also beneficial. Men with multiple sclerosis have erectile dysfunction, anorgasmia, and decreased desire. Again, counseling and foreplay may help all of these symptoms. Sildenafil (Viagra) has been shown to be effective for erectile dysfunction in a randomized, placebocontrolled study in patients with multiple sclerosis.131
Affective Symptoms
Depression and anxiety are extremely common in patients with multiple sclerosis, partly because of situational factors associated with chronic disease and disability and probably also as a result of the underlying brain disease. In general, the same treatments that are helpful for depression and anxiety in the general population are equally successful in patients with multiple sclerosis. As in the general population, combined approaches with psychotherapy and medications are most effective.132 Pathological laughter and crying (pseudobulbar affect) occur in 10% of patients with multiple sclerosis and can be socially debilitating symptoms.133 They can often be treated successfully with low doses of antidepressants.134
Cognitive Impairment
Pharmacotherapy may also be helpful in ameliorating symptoms. The effect of immunotherapies (interferon, glatiramer acetate) on cognitive function has been assessed in several studies,135,136 but the effects probably represent course modification rather than symptomatic benefit. A small single-center study showed that donepezil can improve verbal memory in mildly impaired patients with multiple sclerosis137; larger studies are under way to examine the effects of cholinesterase inhibitors in a broader group of patients.
CONCLUSIONS
Beck RW, Cleary PA, Anderson MMJr, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med. 1992;326:581-588.
Jacobs LD, Beck RW, Simon JH, et al. Intramuscular interferon β-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med. 2000;343:898-904.
Kappos L, Weinshenker B, Pozzilli C, et al. Interferon β-1b in secondary progressive MS: a combined analysis of the two trials. Neurology. 2004;63:1779-1787.
Randomised doubleblind placebocontrolled study of interferon β-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet. 1998;352:1498-1504.
Weinshenker BG, O’Brien PC, Petterson TM, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol. 1999;46:878-886.
1 Fog T. [ACTH therapy of disseminated sclerosis.]. Nord Med. 1951;46:1742-1748.
2 Miller H, Newell DJ, Ridley A. Multiple sclerosis. Treatment of acute exacerbations with corticotrophin (A.C.T.H.). Lancet. 1961;2:1120-1122.
3 Milligan NM, Newcombe R, Compston DA. A doubleblind controlled trial of high dose methylprednisolone in patients with multiple sclerosis: 1. Clinical effects. J Neurol Neurosurg Psychiatry. 1987;50:511-516.
4 Durelli L, Cocito D, Riccio A, et al. High-dose intravenous methylprednisolone in the treatment of multiple sclerosis: clinical-immunologic correlations. Neurology. 1986;36:238-243.
5 Rose AS, Kuzma JW, Kurtzke JF, et al. Cooperative study in the evaluation of therapy in multiple sclerosis. ACTH vs. placebo—final report. Neurology. 1970;20(5):1-59.
6 Barnes MP, Bateman DE, Cleland PG, et al. Intravenous methylprednisolone for multiple sclerosis in relapse. J Neurol Neurosurg Psychiatry. 1985;48:157-159.
7 Abbruzzese G, Gandolfo C, Loeb C. “Bolus” methylprednisolone versus ACTH in the treatment of multiple sclerosis. Ital J Neurol Sci. 1983;4:169-172.
8 Milanese C, La Mantia L, Salmaggi A, et al. Doubleblind randomized trial of ACTH versus dexamethasone versus methylprednisolone in multiple sclerosis bouts. Clinical, cerebrospinal fluid and neurophysiological results. Eur Neurol. 1989;29:10-14.
9 Thompson AJ, Kennard C, Swash M, et al. Relative efficacy of intravenous methylprednisolone and ACTH in the treatment of acute relapse in MS. Neurology. 1989;39:969-971.
10 Beck RW, Cleary PA, Anderson MMJr, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med. 1992;326:581-588.
11 Beck RW. The optic neuritis treatment trial: three-year follow-up results. Arch Ophthalmol. 1995;113:136-137.
12 Bindoff L, Lyons PR, Newman PK, et al. Methylprednisolone in multiple sclerosis: a comparative dose study. J Neurol Neurosurg Psychiatry. 1988;51:1108-1109.
13 Oliveri RL, Valentino P, Russo C, et al. Randomized trial comparing two different high doses of methylprednisolone in MS: a clinical and MRI study. Neurology. 1998;50:1833-1836.
14 Alam SM, Kyriakides T, Lawden M, et al. Methylprednisolone in multiple sclerosis: a comparison of oral with intravenous therapy at equivalent high dose. J Neurol Neurosurg Psychiatry. 1993;56:1219-1220.
15 Metz LM, Sabuda D, Hilsden RJ, et al. Gastric tolerance of high-dose pulse oral prednisone in multiple sclerosis. Neurology. 1999;53:2093-2096.
16 Bocanegra TS, Castaneda MO, Espinoza LR, et al. Sudden death after methylprednisolone pulse therapy. Ann Intern Med. 1981;95:122.
17 Moses RE, McCormick A, Nickey W. Fatal arrhythmia after pulse methylprednisolone therapy. Ann Intern Med. 1981;95:781-782.
18 Burgdorff T, Venemalm L, Vogt T, et al. IgE-mediated anaphylactic reaction induced by succinate ester of methylprednisolone. Ann Allergy Asthma Immunol. 2002;89:425-428.
19 Pryse-Phillips WE, Chandra RK, Rose B. Anaphylactoid reaction to methylprednisolone pulsed therapy for multiple sclerosis. Neurology. 1984;34:1119-1121.
20 Suchman AL, Condemi JJ, Leddy JP. Seizure after pulse therapy with methyl prednisolone. Arthritis Rheum. 1983;26:117.
21 Cosman F, Nieves J, Herbert J, et al. High-dose glucocorticoids in multiple sclerosis patients exert direct effects on the kidney and skeleton. J Bone Miner Res. 1994;9:1097-1105.
22 Schwid SR, Goodman AD, Puzas JE, et al. Sporadic corticosteroid pulses and osteoporosis in multiple sclerosis. Arch Neurol. 1996;53:753-757.
23 Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology. 2003;61:1528-1532.
24 Marchioni E, Marinou-Aktipi K, Uggetti C, et al. Effectiveness of intravenous immunoglobulin treatment in adult patients with steroid-resistant monophasic or recurrent acute disseminated encephalomyelitis. J Neurol. 2002;249:100-104.
25 Weiner HL, Dau PC, Khatri BO, et al. Doubleblind study of true vs. sham plasma exchange in patients treated with immunosuppression for acute attacks of multiple sclerosis. Neurology. 1989;39:1143-1149.
26 Weinshenker BG, O’Brien PC, Petterson TM, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol. 1999;46:878-886.
27 Interferon β-1b is effective in relapsingremitting multiple sclerosis. I. Clinical results of a multicenter, randomized, doubleblind, placebocontrolled trial. The IFNB Multiple Sclerosis Study Group. Neurology. 1993;43:655-661.
28 Paty DW, Li DK. Interferon β-1b is effective in relapsingremitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, doubleblind, placebocontrolled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Neurology. 1993;43:662-667.
29 Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon β-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol. 1996;39:285-294.
30 Rudick RA, Goodkin DE, Jacobs LD, et al. Impact of interferon β-1a on neurologic disability in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Neurology. 1997;49:358-363.
31 Randomised doubleblind placebocontrolled study of interferon β-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet. 1998;352:1498-1504.
32 Li DK, Paty DW. Magnetic resonance imaging results of the PRISMS trial: a randomized, doubleblind, placebocontrolled study of interferon-β1a in relapsingremitting multiple sclerosis. Prevention of Relapses and Disability by Interferon-β1a Subcutaneously in Multiple Sclerosis. Ann Neurol. 1999;46:197-206.
33 PRISMS-4: Long-term efficacy of interferon-β-1a in relapsing MS. Neurology. 2001;56:1628-1636.
34 Jacobs LD, Beck RW, Simon JH, et al. Intramuscular interferon β-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med. 2000;343:898-904.
35 Filippi M, Rovaris M, Inglese M, et al. Interferon β-1a for brain tissue loss in patients at presentation with syndromes suggestive of multiple sclerosis: a randomised, doubleblind, placebocontrolled trial. Lancet. 2004;364:1489-1496.
36 Panitch H, Goodin DS, Francis G, et al. Randomized, comparative study of interferon β-1a treatment regimens in MS: The EVIDENCE Trial. Neurology. 2002;59:1496-1506.
37 Schwid SR, Thorpe J, Sharief M, et al. Enhanced benefit of increasing interferon β-1a dose and frequency in relapsing multiple sclerosis: the EVIDENCE study. Arch Neurol. 2005;62:785-792.
38 Durelli L, Verdun E, Barbero P, et al. Every-other-day interferon β-1b versus once-weekly interferon β-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN). Lancet. 2002;359:1453-1460.
39 Rio J, Nos C, Bonaventura I, et al. Corticosteroids, ibuprofen, and acetaminophen for IFNβ-1a flu symptoms in MS: a randomized trial. Neurology. 2004;63:525-528.
40 Frohman EM, Brannon K, Alexander S, et al. Disease modifying agent related skin reactions in multiple sclerosis: prevention, assessment, and management. Mult Scler. 2004;10:302-307.
41 Mohr DC, Likosky W, Dwyer P, et al. Course of depression during the initiation of interferon β-1a treatment for multiple sclerosis. Arch Neurol. 1999;56:1263-1265.
42 Patten SB, Metz LM. Interferon β-1a and depression in relapsingremitting multiple sclerosis: an analysis of depression data from the PRISMS clinical trial. Mult Scler. 2001;7:243-248.
43 Vartanian TK, Zamvil SS, Fox E, et al. Neutralizing antibodies to disease-modifying agents in the treatment of multiple sclerosis. Neurology. 2004;63(11, Suppl 5):S42-S49.
44 Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsingremitting multiple sclerosis: results of a phase III multicenter, doubleblind placebocontrolled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45:1268-1276.
45 Johnson KP, Brooks BR, Cohen JA, et al. Extended use of glatiramer acetate (Copaxone) is well tolerated and maintains its clinical effect on multiple sclerosis relapse rate and degree of disability. Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1998;50:701-708.
46 Johnson KP, Brooks BR, Ford CC, et al. Sustained clinical benefits of glatiramer acetate in relapsing multiple sclerosis patients observed for 6 years. Copolymer 1 Multiple Sclerosis Study Group. Mult Scler. 2000;6:255-266.
47 Weinshenker BG, Rice GP, Noseworthy JH, et al. The natural history of multiple sclerosis: a geographically based study. 3. Multivariate analysis of predictive factors and models of outcome. Brain. 1991;114(Pt 2):1045-1056.
48 Johnson KP, Ford CC, Lisak RP, et al. Neurologic consequence of delaying glatiramer acetate therapy for multiple sclerosis: 8-year data. Acta Neurol Scand. 2005;111:42-47.
49 Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, doubleblind, randomized, placebocontrolled study of the effects of glatiramer acetate on magnetic resonance imaging—measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Glatiramer Acetate Study Group. Ann Neurol. 2001;49:290-297.
50 Filippi M, Rovaris M, Rocca MA, et al. Glatiramer acetate reduces the proportion of new MS lesions evolving into “black holes.”. Neurology. 2001;57:731-733.
51 Rovaris M, Comi G, Rocca MA, et al. Short-term brain volume change in relapsingremitting multiple sclerosis: effect of glatiramer acetate and implications. Brain. 2001;124(Pt 9):1803-1812.
52 van der Meche FG, Schmitz PI. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain-Barré syndrome. Dutch Guillain-Barré Study Group. N Engl J Med. 1992;326:1123-1129.
53 Dyck PJ, Litchy WJ, Kratz KM, et al. A plasma exchange versus immune globulin infusion trial in chronic inflammatory demyelinating polyradiculoneuropathy. Ann Neurol. 1994;36:838-845.
54 Sorensen PS, Wanscher B, Schreiber K, et al. A doubleblind, crossover trial of intravenous immunoglobulin G in multiple sclerosis: preliminary results. Mult Scler. 1997;3:145-148.
55 Lewanska M, Siger-Zajdel M, Selmaj K. No difference in efficacy of two different doses of intravenous immunoglobulins in MS: clinical and MRI assessment. Eur J Neurol. 2002;9:565-572.
56 Fazekas F, Deisenhammer F, Strasser-Fuchs S, et al. Randomised placebocontrolled trial of monthly intravenous immunoglobulin therapy in relapsingremitting multiple sclerosis. Austrian Immunoglobulin in Multiple Sclerosis Study Group. Lancet. 1997;349:589-593.
57 Achiron A, Gabbay U, Gilad R, et al. Intravenous immunoglobulin treatment in multiple sclerosis. Effect on relapses. Neurology. 1998;50:398-402.
58 Strasser-Fuchs S, Fazekas F, Deisenhammer F, et al. The Austrian Immunoglobulin in MS (AIMS) study: final analysis. Mult Scler. 2000;6(Suppl 2):S9-S13.
59 Sorensen PS, Wanscher B, Jensen CV, et al. Intravenous immunoglobulin G reduces MRI activity in relapsing multiple sclerosis. Neurology. 1998;50:1273-1281.
60 Troiano R, Cook SD, Dowling PC. Steroid therapy in multiple sclerosis. Point of view. Arch Neurol. 1987;44:803-807.
61 Beck RW, Cleary PA, Trobe JD, et al. The effect of corticosteroids for acute optic neuritis on the subsequent development of multiple sclerosis. The Optic Neuritis Study Group. N Engl J Med. 1993;329:1764-1769.
62 Sharrack B, Hughes RA, Morris RW, et al. The effect of oral and intravenous methylprednisolone treatment on subsequent relapse rate in multiple sclerosis. J Neurol Sci. 2000;173:73-77.
63 Zivadinov R, Rudick RA, De Masi R, et al. Effects of IV methylprednisolone on brain atrophy in relapsingremitting MS. Neurology. 2001;57:1239-1247.
64 Kurakata S, Kada M, Shimada Y, et al. Effects of different inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMGCoA) reductase, pravastatin sodium and simvastatin, on sterol synthesis and immunological functions in human lymphocytes in vitro. Immunopharmacology. 1996;34:51-61.
65 Youssef S, Stuve O, Patarroyo JC, et al. The HMGCoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78-84.
66 Nath N, Giri S, Prasad R, et al. Potential targets of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor for multiple sclerosis therapy. J Immunol. 2004;172:1273-1286.
67 Kieseier BC, Archelos JJ, Hartung HP. Different effects of simvastatin and interferon β on the proteolytic activity of matrix metalloproteinases. Arch Neurol. 2004;61:929-932.
68 Vollmer T, Key L, Durkalski V, et al. Oral simvastatin treatment in relapsingremitting multiple sclerosis. Lancet. 2004;363:1607-1608.
69 O’Riordan JI, Thompson AJ, Kingsley DP, et al. The prognostic value of brain MRI in clinically isolated syndromes of the CNS. A 10-year follow-up. Brain. 1998;121(Pt 3):495-503.
70 Comi G, Filippi M, Barkhof F, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet. 2001;357:1576-1582.
71 Achiron A, Kishner I, Sarova-Pinhas I, et al. Intravenous immunoglobulin treatment following the first demyelinating event suggestive of multiple sclerosis: a randomized, doubleblind, placebocontrolled trial. Arch Neurol. 2004;61:1515-1520.
72 Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain. 1989;112(Pt 1):133-146.
73 Trapp BD, Ransohoff R, Rudick R. Axonal pathology in multiple sclerosis: relationship to neurologic disability. Curr Opin Neurol. 1999;12:295-302.
74 Placebocontrolled multicentre randomised trial of interferon β-1b in treatment of secondary progressive multiple sclerosis. European Study Group on interferon β-1b in secondary progressive MS. Lancet. 1998;352:1491-1497.
75 Randomized controlled trial of interferon-β-1a in secondary progressive MS: Clinical results. Neurology. 2001;56:1496-1504.
76 Cohen JA, Cutter GR, Fischer JS, et al. Benefit of interferon β-1a on MSFC progression in secondary progressive MS. Neurology. 2002;59:679-687.
77 Panitch H, Miller A, Paty D, et al. Interferon β-1b in secondary progressive MS: results from a 3-year controlled study. Neurology. 2004;63:1788-1795.
78 Kappos L, Weinshenker B, Pozzilli C, et al. Interferon β-1b in secondary progressive MS: a combined analysis of the two trials. Neurology. 2004;63:1779-1787.
79 Cutter GR, Baier ML, Rudick RA, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999;122(Pt 5):871-882.
80 Rodriguez M, Lennon VA. Immunoglobulins promote remyelination in the central nervous system. Ann Neurol. 1990;27:12-17.
81 Stangel M, Boegner F, Klatt CH, et al. Placebo controlled pilot trial to study the remyelinating potential of intravenous immunoglobulins in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2000;68:89-92.
82 Noseworthy JH, O’Brien PC, Weinshenker BG, et al. IV immunoglobulin does not reverse established weakness in MS. Neurology. 2000;55:1135-1143.
83 Hommes OR, Sorensen PS, Fazekas F, et al. Intravenous immunoglobulin in secondary progressive multiple sclerosis: randomised placebocontrolled trial. Lancet. 2004;364:1149-1156.
84 van Waesberghe JH, van Walderveen MA, Castelijns JA, et al. Patterns of lesion development in multiple sclerosis: longitudinal observations with T1-weighted spin-echo and magnetization transfer MR. AJNR Am J Neuroradiol. 1998;19:675-683.
85 Filippi M, Rocca MA, Pagani E, et al. European study on intravenous immunoglobulin in multiple sclerosis: results of magnetization transfer magnetic resonance imaging analysis. Arch Neurol. 2004;61:1409-1412.
86 Hartung HP, Gonsette R, Konig N, et al. Mitoxantrone in progressive multiple sclerosis: a placebocontrolled, doubleblind, randomised, multicentre trial. Lancet. 2002;360:2018-2025.
87 Bjartmar C, Wujek JR, Trapp BD. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci. 2003;206:165-171.
88 Katz D, Taubenberger JK, Cannella B, et al. Correlation between magnetic resonance imaging findings and lesion development in chronic, active multiple sclerosis. Ann Neurol. 1993;34:661-669.
89 Leary SM, Miller DH, Stevenson VL, et al. Interferon β-1a in primary progressive MS: an exploratory, randomized, controlled trial. Neurology. 2003;60:44-51.
90 Nance PW, Sheremata WA, Lynch SG, et al. Relationship of the antispasticity effect of tizanidine to plasma concentration in patients with multiple sclerosis. Arch Neurol. 1997;54:731-736.
91 Bass B, Weinshenker B, Rice GP, et al. Tizanidine versus baclofen in the treatment of spasticity in patients with multiple sclerosis. Can J Neurol Sci. 1988;15:15-19.
92 Cutter NC, Scott DD, Johnson JC, et al. Gabapentin effect on spasticity in multiple sclerosis: a placebocontrolled, randomized trial. Arch Phys Med Rehabil. 2000;81:164-169.
93 From A, Heltberg A. A doubleblind trial with baclofen (Lioresal) and diazepam in spasticity due to multiple sclerosis. Acta Neurol Scand. 1975;51:158-166.
94 Pinder RM, Brogden RN, Speight TM, et al. Dantrolene sodium: a review of its pharmacological properties and therapeutic efficacy in spasticity. Drugs. 1977;13:3-23.
95 Zahavi A, Geertzen JH, Middel B, et al. Long term effect (more than five years) of intrathecal baclofen on impairment, disability, and quality of life in patients with severe spasticity of spinal origin. J Neurol Neurosurg Psychiatry. 2004;75:1553-1557.
96 Hyman N, Barnes M, Bhakta B, et al. Botulinum toxin (Dysport) treatment of hip adductor spasticity in multiple sclerosis: a prospective, randomised, double blind, placebo controlled, dose ranging study. J Neurol Neurosurg Psychiatry. 2000;68:707-712.
97 Gillen G. Improving mobility and community access in an adult with ataxia. Am J Occup Ther. 2002;56:462-466.
98 Orsnes GB, Sorensen PS. Evaluation of electronic equipment for quantitative registration of tremor. Acta Neurol Scand. 1998;97:36-40.
99 Henkin Y, Herishanu YO. Primidone as a treatment for cerebellar tremor in multiple sclerosis—two case reports. Isr J Med Sci. 1989;25:720-721.
100 Koller WC, Biary N. Effect of alcohol on tremors: comparison with propranolol. Neurology. 1984;34:221-222.
101 Bozek CB, Kastrukoff LF, Wright JM, et al. A controlled trial of isoniazid therapy for action tremor in multiple sclerosis. J Neurol. 1987;234:36-39.
102 Sanson F, Schergna E, Semenzato D, et al. [Therapeutic effects of trazodone in the treatment of tremor. Multicentric doubleblind study]. Riv Neurol. 1986;56:358-364.
103 Gbadamosi J, Buhmann C, Moench A, et al. Failure of ondansetron in treating cerebellar tremor in MS patients–an open-label pilot study. Acta Neurol Scand. 2001;104:308-311.
104 Fox P, Bain PG, Glickman S, et al. The effect of cannabis on tremor in patients with multiple sclerosis. Neurology. 2004;62:1105-1109.
105 Schuurman PR, Bosch DA, Bossuyt PM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. 2000;342:461-468.
106 Petajan JH, Gappmaier E, White AT, et al. Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann Neurol. 1996;39:432-441.
107 Bever CTJr, Anderson PA, Leslie J, et al. Treatment with oral 3,4 diaminopyridine improves leg strength in multiple sclerosis patients: results of a randomized, doubleblind, placebocontrolled, crossover trial. Neurology. 1996;47:1457-1462.
108 Schwid SR, Petrie MD, McDermott MP, et al. Quantitative assessment of sustained-release 4-aminopyridine for symptomatic treatment of multiple sclerosis. Neurology. 1997;48:817-821.
109 Bever CTJr, Young D, Anderson PA, et al. The effects of 4-aminopyridine in multiple sclerosis patients: results of a randomized, placebocontrolled, doubleblind, concentration-controlled, crossover trial. Neurology. 1994;44:1054-1059.
110 Di Fabio RP, Soderberg J, Choi T, et al. Extended outpatient rehabilitation: its influence on symptom frequency, fatigue, and functional status for persons with progressive multiple sclerosis. Arch Phys Med Rehabil. 1998;79:141-146.
111 Stuifbergen AK, Rogers S. The experience of fatigue and strategies of self-care among persons with multiple sclerosis. Appl Nurs Res. 1997;10:2-10.
112 Schwid SR, Petrie MD, Murray R, et al. A randomized controlled study of the acute and chronic effects of cooling therapy for MS. Neurology. 2003;60:1955-1960.
113 Beenakker EA, Oparina TI, Hartgring A, et al. Cooling garment treatment in MS: clinical improvement and decrease in leukocyte NO production. Neurology. 2001;57:892-894.
114 Flensner G, Lindencrona C. The cooling-suit: case studies of its influence on fatigue among eight individuals with multiple sclerosis. J Adv Nurs. 2002;37:541-550.
115 Murray TJ. Amantadine therapy for fatigue in multiple sclerosis. Can J Neurol Sci. 1985;12:251-254.
116 A randomized controlled trial of amantadine in fatigue associated with multiple sclerosis. The Canadian MS Research Group. Can J Neurol Sci. 1987;14:273-278.
117 Rosenberg GA, Appenzeller O. Amantadine, fatigue, and multiple sclerosis. Arch Neurol. 1988;45:1104-1106.
118 Cohen RA, Fisher M. Amantadine treatment of fatigue associated with multiple sclerosis. Arch Neurol. 1989;46:676-680.
119 Krupp LB, Coyle PK, Doscher C, et al. Fatigue therapy in multiple sclerosis: results of a doubleblind, randomized, parallel trial of amantadine, pemoline, and placebo. Neurology. 1995;45:1956-1961.
120 Montuori E, Gonzalez HA, Cenal EE. [Pharmacologic study of magnesium pemoline. Mechanism of action]. C R Seances Soc Biol Fil. 1974;168:1152.
121 Weinshenker BG, Penman M, Bass B, et al. A doubleblind, randomized, crossover trial of pemoline in fatigue associated with multiple sclerosis. Neurology. 1992;42:1468-1471.
122 Berkovitch M, Pope E, Phillips J, et al. Pemoline-associated fulminant liver failure: testing the evidence for causation. Clin Pharmacol Ther. 1995;57:696-698.
123 Adcock KG, MacElroy DE, Wolford ET, et al. Pemoline therapy resulting in liver transplantation. Ann Pharmacother. 1998;32:422-425.
124 Rammohan KW, Rosenberg JH, Lynn DJ, et al. Efficacy and safety of modafinil (Provigil) for the treatment of fatigue in multiple sclerosis: a two centre phase 2 study. J Neurol Neurosurg Psychiatry. 2002;72:179-183.
125 Stankoff B, Waubant E, Confavreux C, et al. Modafinil for fatigue in MS: a randomized placebocontrolled double blind study. Neurology. 2005;64:1139-1143.
126 Ross EL. The evolving role of antiepileptic drugs in treating neuropathic pain.Neurology. 2000;55(5 Suppl 1):S41-S46. discussion, Neurology. 2000;55(5 Suppl 1):S48-S54.
127 Khan OA. Gabapentin relieves trigeminal neuralgia in multiple sclerosis patients. Neurology. 1998;51:611-614.
128 Tenser RB. Trigeminal neuralgia: mechanisms of treatment. Neurology. 1998;51:17-19.
129 Andrews KL, Husmann DA. Bladder dysfunction and management in multiple sclerosis. Mayo Clin Proc. 1997;72:1176-1183.
130 Fowler CJ. The cause and management of bladder, sexual and bowel symptoms in multiple sclerosis. Baillieres Clin Neurol. 1997;6:447-466.
131 Miller CJF, Sharief M. Effect of sildenafil citrate (Viagra) on quality of life in men with erectile dysfunction and multiple sclerosis. Ann Neurol. 1999;46:496-497.
132 Mohr DC, Boudewyn AC, Goodkin DE, et al. Comparative out-comes for individual cognitive-behavior therapy, supportive-expressive group psychotherapy, and sertraline for the treatment of depression in multiple sclerosis. J Consult Clin Psychol. 2001;69:942-949.
133 Smith RA, Berg JE, Pope LE, et al. Validation of the CNS emotional lability scale for pseudobulbar affect (pathological laughing and crying) in multiple sclerosis patients. Mult Scler. 2004;10:679-685.
134 Schiffer RB, Herndon RM, Rudick RA. Treatment of pathologic laughing and weeping with amitriptyline. N Engl J Med. 1985;312:1480-1482.
135 Fischer JS, Priore RL, Jacobs LD, et al. Neuropsychological effects of interferon β-1a in relapsing multiple sclerosis. Multiple Sclerosis Collaborative Research Group. Ann Neurol. 2000;48:885-892.
136 Weinstein A, Schwid SR, Schiffer RB, et al. Neuropsychologic status in multiple sclerosis after treatment with glatiramer. Arch Neurol. 1999;56:319-324.
137 Krupp LB, Christodoulou C, Melville P, et al. Donepezil improved memory in multiple sclerosis in a randomized clinical trial. Neurology. 2004;63:1579-1585.