CHAPTER 66 Treatment of Gallstone Disease
MEDICAL TREATMENT
Medical treatment of gallstone disease was first proposed by Schiff in Italy in 1873.1 Dabney of Virginia first reported the effective treatment of gallstones with bile acids in 1876, an observation later confirmed by Rewbridge of Minnesota in 1937.2,3 Despite these initial reports, the use of medical dissolution treatment did not gain acceptance until large clinical series were reported in the 1970s. Contact dissolution of gallstones with solvents and percutaneous cholecystolithotomy techniques also have been reported, but these modalities have not proved superior to oral dissolution, shock-wave lithotripsy, or laparoscopic cholecystectomy and have been abandoned. The mainstay of current nonsurgical treatment of gallstone disease is oral dissolution with ursodeoxycholic acid, with or without extracorporeal shock-wave lithotripsy.
Although nonsurgical treatment of gallstones has proved effective in carefully selected patients, only a limited number of patients are candidates for this treatment option. Nonsurgical treatments are effective only in patients with small, cholesterol gallstones. Significant admixtures of pigment or calcium salts make stones indissoluble. In addition, long-term success with medical treatment of gallstones occurs only in patients in whom the lithogenic disturbance that led to gallstone formation is transient. For most patients, gallstone formation represents an imbalance in biliary lipid excretion, gallbladder stasis, or infection of the bile (see Chapter 65). In these patients, successful dissolution is usually followed by recurrence of gallstones in 30% to 50% of patients within five years.4–7 Therefore, the proper choice of treatment must take into account the type and severity of symptoms, the physical characteristics of the stones, gallbladder function, and the characteristics and preference of the patient.
DISSOLUTION THERAPY
The rationale for oral dissolution therapy is the reversal of the condition that led to formation of cholesterol gallstones, namely, the supersaturation of bile with cholesterol (see Chapter 65). Cholesterol stones dissolve if the surrounding medium is capable of solubilizing the cholesterol in the stones. Both chenodeoxycholic acid and ursodeoxycholic acid dissolve gallstones by decreasing biliary cholesterol secretion and desaturating bile. These agents encourage the removal of cholesterol from stones via micellar solubilization, formation of a liquid crystalline phase, or both. Chenodeoxycholic acid was the first bile acid used for gallstone dissolution but has been abandoned because of side effects, including diarrhea and increased serum aminotransferase and cholesterol levels. Ursodeoxycholic acid is well tolerated and is currently used in oral dissolution regimens. In randomized comparisons, ursodeoxycholic acid was just as effective as chenodeoxycholic acid alone or in combination with ursodeoxycholic acid.8–10
The rate of stone dissolution is a function of (1) thermodynamic forces, including the degree of bile desaturation and the concentration of ursodeoxycholic acid in bile; (2) kinetic forces, including stirring of bile; and (3) the surface-to-volume ratio of the stones. Oral dissolution targets the thermodynamic forces.11 Because small stones have a smaller surface-to-volume ratio, they respond more quickly and reliably to oral dissolution therapy.
The use of oral dissolution therapy does not address the problem of gallbladder stasis.12 Although prokinetic agents, including alpha-adrenergic antagonists and clarithromycin, have been shown to increase gallbladder motility, their use in preventing and treating gallstones has not been studied.13,14
Patient Selection
Selection of patients for oral dissolution therapy is a function of the stage of gallstone disease, gallbladder function, and the characteristics of the stones. Selection criteria are summarized in Table 66-1. Oral dissolution therapy should be considered for patients with uncomplicated gallstone disease, including those with mild, infrequent biliary pain. Patients with severe or frequent biliary pain and patients with complications of gallstones, including cholecystitis, pancreatitis, and cholangitis, should not be treated with oral dissolution therapy; these patients should be referred for surgery. In addition, the gallbladder must function and the cystic duct must be patent to allow unsaturated bile and stones to clear from the gallbladder. The patency of the cystic duct is usually evaluated by oral cholecystography. More recently, stimulated cholescintigraphy and functional ultrasonography have been used. These latter modalities assess cystic duct patency as well as gallbladder function.
Table 66-1 Selection Criteria for Oral Bile Acid Dissolution Therapy
| Stage of gallstone disease | Symptomatic (biliary pain) without complications |
| Gallbladder function* |
* Either stimulated cholescintigraphy or functional ultrasonography may be done.
The characteristics of the stones play an important role in determining the efficacy of dissolution treatment. Oral dissolution therapy works only on cholesterol stones. Although verifying the composition of gallstones can be difficult, the appearance of stones on plain films or computed tomography (CT) images can be useful. Cholesterol stones are radiolucent on conventional radiographs, and they are hypodense or isodense to bile and lack stone calcification on CT images.15 During oral cholecystography, the specific gravity of cholesterol stones is less than or equal to that of contrast-enriched bile, thereby resulting in stone buoyancy.16 The number of stones does not influence the success of oral dissolution therapy; however, only patients with stones that occupy less than one half of the gallbladder volume should be considered for treatment. Although oral dissolution therapy has been effective in stones up to 10 mm in diameter, results are best in stones less than 5 mm in size.17,18 The ideal stones for oral dissolution treatment are shown in Figure 66-1.
Therapeutic Regimens
Ursodeoxycholic acid (ursodiol) is the preferred drug for oral dissolution treatment. It is taken in a dose of 10 to 15 mg/kg of body weight per day. Nighttime dosing is more effective and is associated with better patient compliance than mealtime dosing.19 Unlike chenodeoxycholic acid (chenodiol), ursodeoxycholic acid is well tolerated and has no important side effects. Treatment should continue until stone dissolution is documented by two consecutive negative ultrasonograms at least one month apart. Treatment should be stopped if the patient does not tolerate the drug or experiences a complication of gallstones during therapy or if the stones fail to dissolve after six months or dissolve only partially after six months with lack of progression to complete dissolution by two years.
Efficacy
With ursodeoxycholic acid, complete dissolution is achieved in 20% to 70% of patients. The variability in the reported response rates is a function of differences in patient selection, doses of bile acid, treatment times, and diagnostic techniques used to document stone dissolution.9,10,20,21 A meta-analysis of all randomized trials of dissolution treatment showed stone dissolution in 37% of patients.9 The frequency of stone dissolution was 29% for stones larger than 10 mm, 49% for stones smaller than 10 mm, and 70% for stones less than 5 mm. The time to resolution varies among patients, with a median rate of 0.7 mm per month.11 Improvement in symptoms occurs before stones have dissolved completely. In addition, long-term treatment has been reported to decrease the risk of biliary pain and acute cholecystitis, independent of gallstone dissolution.18 Despite initial dissolution of stones in properly selected patients, the rate of gallstone recurrence after oral dissolution therapy is 50% after five years, with most recurrences within the first two years.5,7 The risk is lower in patients with a solitary stone than in those with multiple stones.
EXTRACORPOREAL SHOCK-WAVE LITHOTRIPSY
The application of extracorporeal shock-wave lithotripsy to the treatment of gallstones was first applied to patients by Sauerbruch in 1985.22 The rationale for shock-wave lithotripsy is to diminish the surface-to-volume ratio of a stone, thereby increasing the efficacy of oral dissolution and decreasing stone size to allow small stones and debris to pass directly from the gallbladder into the intestine without causing symptoms. The technique involves the delivery of focused high-pressure sound waves to gallstones. Three types of lithotripters have been developed: underwater spark-gap, piezoelectric crystals, and electromagnetic membrane lithotriptors.23 Regardless of the energy source, the shock waves from the lithotriptor are delivered from an underwater source to the soft tissue. Passage of the shock wave through the soft tissue does not diminish the energy wave significantly. Passage of the shock wave through the anterior and posterior walls of the stone liberates compressive and tensile forces and causes cavitation at the anterior surface of the stone, thereby leading to stone fragmentation. Factors that influence fragmentation include the size, microcrystalline structure, and architecture of the stone. Although the composition of the stone does not influence successful lithotripsy, only cholesterol stone fragments are dissolved effectively by bile acid therapy.
Patient Selection
Because shock-wave lithotripsy is usually combined with oral dissolution therapy, patient selection criteria for shock-wave lithotripsy are similar to those for oral dissolution treatment and are summarized in Table 66-2. Gallbladder function and cystic duct patency are required and are demonstrated by oral cholecystography, functional ultrasonography, or stimulated cholescintigraphy (see Chapter 65). Lithotripsy should be considered only for patients with mild, uncomplicated biliary pain. Pregnant patients and patients on anticoagulants should not undergo lithotripsy. Shock-wave lithotripsy is reserved for patients with a solitary stone, measuring less than 2 cm in size.6,24 Because only cholesterol stones are reliably cleared by the addition of oral dissolution therapy, stones should have radiographic features, such as radiolucency, suggestive of cholesterol stones.
Table 66-2 Selection Criteria for Extracorporeal Shock-Wave Lithotripsy
| Stage of gallstone disease | Symptomatic (biliary pain) without complications |
| Gallbladder function* |
* Either stimulated cholescintigraphy or functional ultrasonography may be done.
Therapeutic Approach
Patients usually are given sedatives and analgesics or are anesthetized and placed in the prone position to minimize the distance between the energy source and the stones and to eliminate interference from intestinal gas and the costal margin. Targeting and monitoring for fragmentation are accomplished with ultrasonography. Multiple treatment sessions are often required to achieve sufficient pulverization. Factors that predict the success of lithotripsy include the degree of fragmentation and gallbladder emptying.25–27 Fragmentation depends on stone characteristics and the dose of the shock wave. Important stone characteristics include the size and number of stones as well as their structure and the presence of calcification.25,28,29 The energy of shock waves, number of shock waves per session, and number of sessions also influence the success rate.30,31 Ursodeoxycholic acid, 10 to 15 mg/kg of body weight per day, is administered orally to dissolve stone fragments, especially when residual stone fragments are larger than 2 mm in size, gallbladder function is poor, or the gallbladder has not cleared small fragments within three to six months of lithotripsy.32 An example of successful combined treatment is shown in Figure 66-2.
Efficacy
The percentage of patients who are free of stones after 6 and 12 months is 47% to 77% and 68% to 84%, respectively.24,25,33–37 Follow-up data reveal 27%, 41%, and 54% cumulative recurrence rates at 3, 5, and 10 years, respectively.38 Recurrence is most often related to the presence of lithogenic bile and gallbladder dysmotility, rather than patient variables such as gender, age, and weight. Factors that predict higher rates of treatment failure include stones larger than 16 mm in size, multiple stones, and stones with a CT density of greater than 84 Hounsfield units.39 Recurrent stones are usually small and multiple and cause recurrent biliary pain. Maintenance therapy with ursodeoxycholic acid after lithotripsy is not cost effective and has not been shown to be effective.7,40
Side effects of lithotripsy include petechiae of the skin at the site of shock-wave delivery (8%), hematuria (4%), and liver hematomas (<1%). No long-term liver biochemical abnormalities have been noted. Biliary pain develops in approximately one third of patients; cystic duct obstruction develops in 5%; and complications of stone passage, such as biliary pancreatitis, develop in less than 2%.25
Lithotripsy is more cost-effective in the elderly than in the young and less cost-effective in patients with multiple stones than in those with a single stone. When combined with ursodeoxycholic acid, lithotripsy is at least as cost-effective as open cholecystectomy for patients with small stones and less cost-effective for those with large stones.41,42 When lithotripsy is compared with laparoscopic cholecystectomy, patients who undergo laparoscopic cholecystectomy experience a greater incremental improvement in quality of life at six months, whereas those who undergo lithotripsy have higher rates of recurrent stones and biliary symptoms.43
Bile Duct Stones
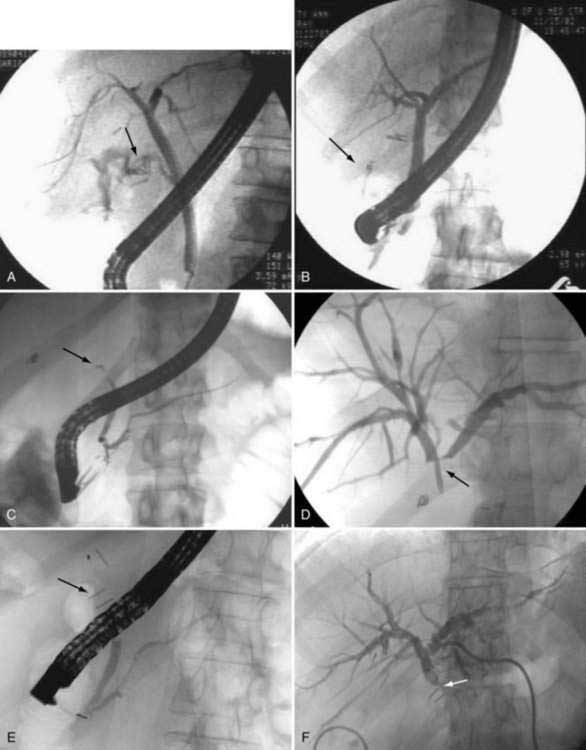
Extracorporeal shock-wave lithotripsy has also been used in the management of choledocholithiasis. Intracorporeal electrohydraulic lithotripsy has been shown to be effective in this setting as well. These treatment options are reserved for patients who fail conventional endoscopic measures (see Chapter 70), mechanical lithotripsy, or surgical treatment of choledocholithiasis. Appropriate indications for shock-wave lithotripsy are large stones impacted in the bile duct that are not amenable to endoscopic extraction, intrahepatic stones, stones above a biliary stricture, cystic duct remnant stones, and bile duct stones associated with Mirizzi’s syndrome (compression of the common hepatic duct) (see Chapter 65). Selection of patients for shock-wave treatment of bile duct stones is similar to that for treatment of uncomplicated gallbladder gallstones.
The success rate for treatment of bile duct stones is 70% to 90%.44–49 Most patients require endoscopic extraction of large stone fragments following treatment. Mild, transient hemobilia occurs in 10% of patients, and biliary sepsis develops in 4% following the procedure. Other complications are similar to those seen after lithotripsy for gallbladder stones. Because of the potential for septic complications, preprocedure endoscopic, nasobiliary, or percutaneous biliary drainage is performed. Antibiotics are given to minimize the risk of biliary sepsis.
SURGICAL TREATMENT
Approximately 700,000 cholecystectomies are performed for gallstone disease in the United States each year. Currently, the vast majority of these operations are performed using laparoscopic techniques. For example, 7888 cholecystectomies were performed in Utah in 2005; 96% of these operations were laparoscopic cholecystectomies, and 4% were open procedures. A review of the National Hospital Discharge Database from 1998 to 2001 showed that 25% of cholecystectomies were performed by an open approach. Of the remaining 75% done laparoscopically, the rate of conversion to open cholecystectomy was 5% to 10%.50 Patients with complicated gallstone disease, including acute cholecystitis, gallstone pancreatitis, and choledocholithiasis, are more likely than those with uncomplicated disease to require an open cholecystectomy or conversion from a laparoscopic to an open approach.50 Despite the increased reliance on minimally invasive techniques in the care of these patients, open cholecystectomy continues to play an important role in the management of complications of gallstones.
OPEN CHOLECYSTECTOMY
Karl Langenbuch, a surgeon in Berlin, is credited with performing the first cholecystectomy in 1882. Since then, cholecystectomy has remained the main therapeutic option for the management of patients with gallstones, largely because of its remarkable success in relieving symptoms and its low morbidity. In prospective studies, 90% to 95% of patients who undergo cholecystectomy experience substantial or complete relief of their symptoms.51,52 Cholecystectomy is more effective in relieving biliary pain than in relieving dyspepsia and flatulence, which are less strongly correlated with gallstone disease.
Results
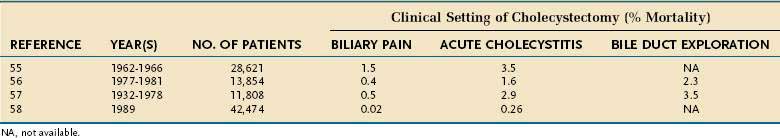
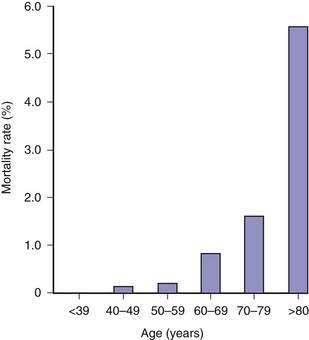
The risk of open cholecystectomy has declined over the years. The overall mortality rate of cholecystectomy in 35,373 patients operated on before 1932 was 6.6%.53 This rate decreased to 1.8% by 1952.54 Since then, the overall mortality rate for cholecystectomy has averaged about 1.5%. The mortality rate is considerably lower in patients operated on electively for biliary pain, with an average of less than 0.5% (Table 66-3).55–57 The risk of death is several-fold higher when cholecystectomy is performed as an emergency for acute cholecystitis and when bile duct exploration is required (see Table 66-3). Additionally, the mortality rate is directly proportional to the patient’s age (Fig. 66-3). In a report of the entire Danish experience with cholecystectomy from 1977 to 1981, patients under the age of 50 years had a risk of death of 0.028% from elective cholecystectomy56; the rate rose to 5.56% in patients older than 80 years of age. The experience in the United States has been similar. Of 11,808 patients who underwent cholecystectomy at the New York Hospital–Cornell Medical Center between 1932 and 1978, the risk of death from elective cholecystectomy for chronic cholecystitis was 0.1% in patients less than age 50 years and 0.8% in those 50 years of age or older.57 In a later series, the overall mortality rate of 42,474 patients who underwent cholecystectomy in 1989 in California and Maryland was 0.17%.58 In this series, the mortality rate in patients less than 65 years of age was 0.03%, compared with more than 0.5% in patients 65 years of age and older. Likewise, the morbidity rate, mean length of hospital stay, and average hospital charges were significantly higher in the older patients than in the younger group. Most mortality following cholecystectomy is related to cardiac disease, particularly myocardial infarction.
Major complications after open cholecystectomy are rare. In a large survey of 28,621 patients who underwent cholecystectomy in the 1960s, complications occurred in 4.0%.55 Subsequent studies have confirmed a 4.0% to 5.0% rate of perioperative morbidity.59–61 Most complications are relatively minor, such as wound infections or seromas, urinary retention or infection, and atelectasis. Complications related specifically to cholecystectomy include bile leaks, bile duct injury, and acute pancreatitis. Of these complications, bile duct injury is the most serious and often requires endoscopic therapy and, in some cases, complicated and technically difficult surgical repair (see Chapter 70). Alternatively, bile duct injury can lead to benign biliary stricture formation and bile duct obstruction with secondary biliary cirrhosis and liver failure. The rate of bile duct injury during open cholecystectomy is not known precisely but has been estimated to be 1 in 200 to 600 cases.62,63 Two ductal injuries occurred in 1200 cholecystectomies at the Cedars-Sinai Medical Center in Los Angeles from 1982 to 1988,64 and no bile duct injuries occurred in 1252 patients who underwent elective cholecystectomy at two large North American and European centers.65 In general, bile duct injuries are preventable complications and are commonly the result of inadequate surgical training, unrecognized variations in bile duct anatomy, or misidentification of normal anatomy. Unusual amounts of bleeding, severe inflammation, and emergency operations do not play as great a role in these injuries as might be supposed.
LAPAROSCOPIC CHOLECYSTECTOMY
Technique
The operative team for laparoscopic cholecystectomy generally consists of a surgeon, assistant surgeon, scrub nurse, circulating nurse, and anesthesiologist. Patients are asked to void immediately prior to entering the operating room. Prophylactic antibiotics are not routinely administered to patients with uncomplicated gallstone disease, including biliary pain.66 Patients with potential infectious complications of gallstones, including acute cholecystitis and cholangitis, and patients with long-standing symptoms or advanced age should receive antibiotics if these agents were not already started before surgery.67 Sequential compression stockings are used to reduce the risk of lower extremity thromboembolism.
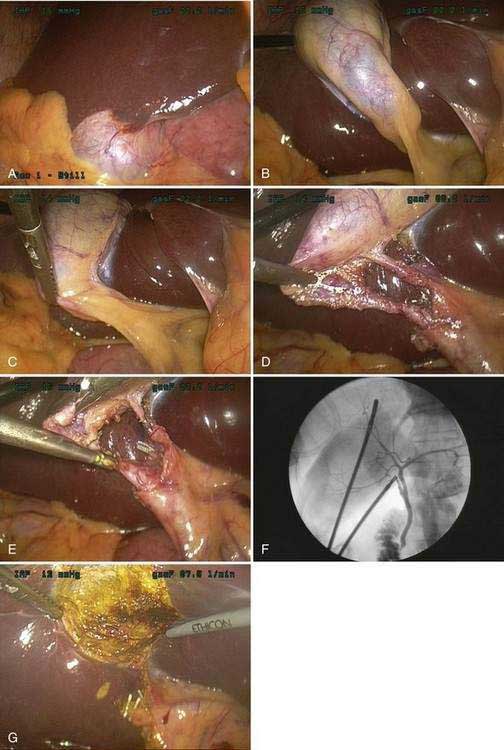
The current technique of laparoscopic cholecystectomy is best described as “the critical view” approach,68 as summarized in Figure 66-4. In this approach, the entire hepatocystic triangle is dissected, exposing the cystic duct and artery, infundibulum of the gallbladder, and junction of the gallbladder and cystic duct before a cholangiogram is performed or the cystic duct and artery are divided. The assistant retracts the gallbladder fundus cephalad, anterior to the liver, and the infundibulum laterally. The surgeon, operating through the epigastric port, identifies and dissects the cystic duct and artery circumferentially. Special care must be taken to identify the junction of the cystic duct and gallbladder, to ensure that the bile duct has not been isolated inadvertently. Cholangiography is performed via cannulation of the cystic duct. If the cholangiogram shows normal anatomy and no evidence of choledocholithiasis, the cholangiocatheter is removed and the cystic duct and artery are divided between small metal clips. The gallbladder is then dissected out of the liver bed and delivered through the umbilical incision. Care is taken to avoid perforation of the gallbladder during its dissection from the liver because the spillage of gallstones and bile has been shown to increase the risk of postoperative fever and intra-abdominal abscess formation.69 The operation concludes with evacuation of the pneumoperitoneum and closure of the incisions.
Rationale for Cholangiography
Cholangiography during laparoscopic cholecystectomy has two main purposes. First, the cholangiogram may detect unsuspected bile duct stones. Second, the cholangiogram confirms the surgeon’s impression of the anatomy of the bile ducts. In the era before laparoscopic cholecystectomy, the value of routine cholangiography during cholecystectomy was debated, with some surgeons arguing in favor of its selective use.70 Routine cholangiography has been criticized because of its relatively low yield, failure to identify all retained stones, occasional false-positive results, cost, and risk. Nevertheless, 8% to 16% of all patients with cholelithiasis harbor bile duct stones. Routine use of operative cholangiography detects unsuspected bile duct stones in about 5% of patients who undergo cholecystectomy and detects anatomic ductal abnormalities in 12%.71 During laparoscopic cholecystectomy, the two-dimensional video image and inability to palpate structures of the porta hepatitis make identification of the cystic duct–bile duct junction problematic. Cholangiography plays an especially important role in delineating bile duct anatomy prior to division of any important structures. Large population studies from Australia and Sweden have demonstrated the importance of routine intraoperative cholangiography in decreasing the frequency of major bile duct injuries.72,73 The rate of bile duct injury during laparoscopic cholecystectomy when routine cholangiography is performed is 0.2% to 0.4%, compared with 0.4% to 0.6% when cholangiography is not performed routinely.72–74 Routine cholangiography is cost effective when the cost associated with bile duct injuries is considered.75 In addition, routine cholangiography permits earlier identification of intraoperative bile duct injuries, if they occur,76 and thereby improves the rate of repair.
Results
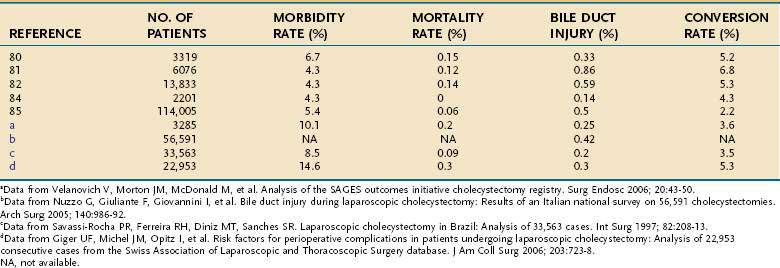
Several large series have now described the experience with laparoscopic cholecystectomy (Table 66-4).76–87 A review of the experience with laparoscopic cholecystectomy in the United States showed an operative mortality rate of 0.06%. Internationally, operative mortality rates have ranged from 0% to 0.15%. Conversion to an open procedure was required in 2.2% in the United States and 3.6% to 8.2% of patients internationally, generally because of inflammation that precludes safe dissection of the porta hepatis. Major morbidity occurred in approximately 5% of patients, and bile duct injuries occurred in 0.14% to 0.5% of patients. Operating time ranged from one to two hours. Initially, most patients were observed overnight after laparoscopic cholecystectomy, but same-day discharge has become standard for elective cases. Most patients return to full activities, including work, within one week.
No randomized, prospective trials have compared the results of laparoscopic cholecystectomy with those of open cholecystectomy in the United States, nor are any likely. Patient enthusiasm for the laparoscopic approach and the rapid acceptance of the procedure by surgeons have made direct, controlled comparison of the two procedures difficult. Nonrandomized data from the United States and small, randomized trials from other countries support the contention that the laparoscopic approach is superior to the open approach.59,88–92 In these analyses, the main benefits of the laparoscopic approach have included a shortened hospital stay, decreased pain, reduced disability, and reduced costs. Population studies have shown a substantial decline in cholecystectomy-related mortality rates following the introduction of the laparoscopic technique (Table 66-5).93
Table 66-5 Cholecystectomy-Related Mortality in Maryland before (1989) and after (1992) the Introduction of Laparoscopic Cholecystectomy
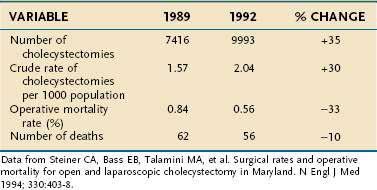
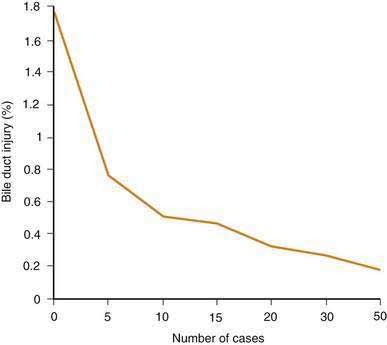
Against the perceived benefits of laparoscopic cholecystectomy over the open approach is concern about unacceptably high complication rates, especially for bile duct injury. Although the exact frequency of bile duct injury around the world is not known, two lines of evidence suggest that the rate has declined. First, regional studies have demonstrated a decrease in the rate of bile duct injury as overall experience with laparoscopic cholecystectomy has increased (Fig. 66-5).94 Curiously, however, the frequency of bile duct injury does not continue to fall with increasing experience of the individual surgeon, but rather plateaus.95–97 Although duct injuries are more common early in an individual surgeon’s experience, they still occur in the hands of seasoned surgeons, albeit at a lower rate. As overall experience has increased, the rate of bile duct injury for laparoscopic cholecystectomy has approximated that seen with open cholecystectomy. Second, the number of patients with bile duct injury treated at tertiary referral medical centers has declined since the early days of laparoscopic cholecystectomy.98 Introduction of laparoscopic cholecystectomy in the United States was rapid and may have exceeded the capability of the medical educational system to train all practitioners adequately. The initial relatively high rates of bile duct injury have been ascribed to a “learning curve.” This experience is a cautionary example for other new technologies that may be introduced into medical practice.
Concern has been raised about the increased use of laparoscopic cholecystectomy for gallstone disease when compared with historical rates for open cholecystectomy. In a defined health maintenance organization population in Pennsylvania, the rate of cholecystectomy increased from 1.35 per 1000 enrollees in 1988, just before the introduction of the laparoscopic approach, to 2.15 per 1000 enrollees in 1992, just after its introduction.99 No significant changes in the rates of herniorrhaphy or appendectomy were observed. Similarly, statewide data from Maryland have shown that the rate of cholecystectomy rose from 1.69 per 1000 residents in 1987 to 1989 to 2.17 per 1000 residents in 1992,93 and Scottish nationwide data have shown a 20% increase in the age-standardized rate of cholecystectomy.100 The reasons for the increases in use are not yet clear. The consensus of experts in the field is that selection of patients for cholecystectomy should not be altered by the availability of the laparoscopic approach.
INDICATIONS FOR TREATMENT
ASYMPTOMATIC GALLSTONES
Decisions regarding the management of the patient with asymptomatic gallstones must be predicated on knowledge of the natural history of the condition, as discussed in Chapter 65. In general, patients with asymptomatic gallstones should be reassured that life-threatening complications are uncommon and that symptoms related to the stones develop in only a minority of patients.101–105 In the event that an asymptomatic patient becomes symptomatic, the initial presentation is most often with uncomplicated biliary pain. In fact, most patients in whom complications of gallstones develop have antecedent biliary pain.106 Decision analysis calculations suggest that the risks of cholecystectomy approximate the potential benefit in preventing future serious sequelae of gallstones.107 These calculations were based on historical data regarding the outcome of open cholecystectomy; the rate of serious sequelae of gallstones was determined from long-term follow-up of a group of male faculty members at a major mid-western university. Whether these data are applicable to the more common female patient considering laparoscopic cholecystectomy today is not known. Nevertheless, the strategy of prophylactic cholecystectomy in all asymptomatic patients probably has no major advantage over the recommendation that cholecystectomy be limited to symptomatic patients.108,109 In addition, studies that have analyzed health-related quality of life do not support cholecystectomy for asymptomatic patients.110
In certain subgroups, the benefits of prophylactic cholecystectomy for asymptomatic gallstones may outweigh the risks. American Indians, for example, appear to have a rate of gallstone-associated gallbladder cancer high enough to justify prophylactic cholecystectomy.110 In morbidly obese persons as well as recipients of heart and lung transplants, complications of gallstone disease carry high morbidity rate, and prophylactic cholecystectomy may be indicated.111–113 Curiously, renal transplant patients with asymptomatic gallstones have a low risk of complications related to gallstone disease and, therefore, should not be considered for prophylactic cholecystectomy (see later).114,115 The risks of complications of gallstone disease in children may outweigh the risk of cholecystectomy (see later).
Diabetic persons have been thought to be particularly prone to gallstone formation and to complications from the stones. Morbidity and mortality rates for diabetic patients who undergo emergency operations for complications of gallstone disease have also been thought to be excessive. These perceptions have not been borne out when confounding variables, such as hyperlipidemia, obesity, cardiovascular disease, and chronic kidney disease, are taken into account.116 Therefore, prophylactic cholecystectomy in an asymptomatic diabetic patient with gallstones does not appear to be warranted.117 Data do support, however, early intervention in diabetic patients in whom symptoms develop because these patients are at an increased risk of developing gangrenous cholecystitis.118 Therefore, the severity of complications may be higher when complications of gallstones arise in these patients.
BILIARY PAIN
Patient Selection
The majority of operations for biliary tract disorders are performed to relieve symptoms related to intermittent obstruction of the cystic duct by gallstones. This constellation of symptoms, including intermittent epigastric or right upper quadrant pain, nausea, and vomiting has been termed biliary pain (“biliary colic” in the past) (see Chapter 65). Histologically, gallbladders from patients suffering from repeated attacks of biliary pain usually, but not always, show fibrosis and mononuclear cell infiltration that are characteristic of chronic cholecystitis. Furthermore, patients with biliary pain are more likely than patients with asymptomatic stones to experience complications of gallstones. Cholecystectomy is indicated in these symptomatic patients. As with any operation, the potential benefits in terms of relief of symptoms and prevention of future complications must be weighed against the risk of surgery. Fortunately, the physiologic stress of cholecystectomy is minimal, and the operation may be undertaken safely even in the elderly and infirm. In the poorly compensated cirrhotic patient, the risk of cholecystectomy is substantially higher.118–120 Operation in this setting is justified only if the symptoms are severe, complications arise, or the cirrhosis is well compensated.121,122 When cholecystectomy is performed for biliary pain, routine perioperative antibiotics are seldom indicated.123
Diagnostic Evaluation
The diagnosis of biliary pain is generally suspected from the clinical history (see Chapter 65). Few important findings specific to gallstone disease are elicited on physical examination. Symptoms and signs of heart disease, especially congestive heart failure, should be detected during the preoperative evaluation because heart disease adversely affects the risk of surgery. Few preoperative laboratory tests are routinely necessary; liver biochemical tests should be obtained to screen for unsuspected choledocholithiasis. Imaging evaluation may be limited to ultrasonography in most patients with biliary pain. Ultrasonography has a high sensitivity (95%) and specificity (98%) in this setting and is also useful for detecting gallbladder inflammation—which is suggested by thickening of the gallbladder wall and pericholecystic fluid—and dilatation of the bile ducts. Ancillary tests, including oral cholecystography, magnetic resonance cholangiopancreatography (MRCP), endoscopic retrograde cholangiography (ERCP), or cholecystokinin scintigraphy, are useful for confirming the diagnosis in the unusual patient in whom gallstones are suspected but ultrasonography is negative. In patients with atypical symptoms, endoscopy, upper gastrointestinal barium radiography, or both, may be performed to exclude other disorders such as esophagitis or peptic ulcer disease.
ACUTE CHOLECYSTITIS
Management of the patient with acute cholecystitis begins with intravenous hydration and restoration of tissue perfusion and electrolyte balance. Vomiting is prominent in most patients, and nasogastric suction may be required. Intravenous antibiotics are indicated because bile or gallbladder wall cultures are positive for bacteria in more than 40% of patients.124 A cephalosporin such as cefoxitin is satisfactory for mildly ill patients, but in more severe cases, broad-spectrum antibiotics, such as piperacillin-tazobactam or a third-generation cephalosporin with metronidazole, should be given. If gangrenous or emphysematous cholecystitis is suspected, an agent effective against anaerobic organisms should be included.
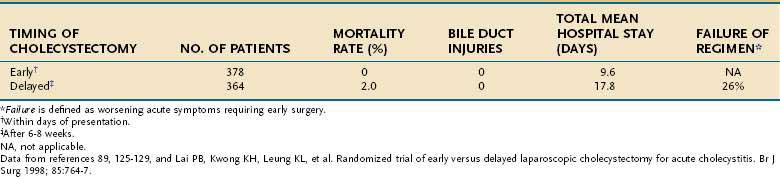
In the past, the timing of cholecystectomy for the typical patient with acute cholecystitis was controversial. Multiple prospective, randomized, controlled clinical trials have compared the strategies of early (within days of presentation) and delayed (after six to eight weeks) surgery for acute cholecystitis (Table 66-6).89,125–131 A meta-analysis of these trials has shown that, for the average patient, early operation is preferable because the total length of hospitalization and costs are reduced, morbidity is less, and deaths related to progressive acute cholecystitis are prevented.130 Early operation does not appear to increase the major risks of cholecystectomy, such as bile duct injury, substantially.
Table 66-6 Early versus Delayed Cholecystectomy for Acute Cholecystitis: Combined Results from Seven Randomized Trials
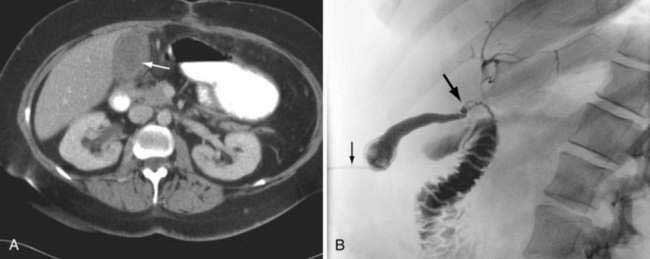
For the high-risk patient with severe concurrent illnesses, such as liver, pulmonary, or heart failure, cholecystostomy (gallbladder drainage) is preferable to cholecystectomy. Operative cholecystostomy has been superseded by a percutaneous approach in the majority of patients. After the patient has recovered from the attack of acute cholecystitis, laparoscopic cholecystectomy should be performed if the patient’s overall condition permits. Alternatively, residual stones can be removed via the cholecystostomy tube, and the patient may be managed expectantly. Recurrent biliary symptoms develop in approximately one half of all patients treated with a cholecystostomy. An example of a patient best managed by percutaneous cholecystostomy is shown in Figure 66-6. The indication for a percutaneous cholecystostomy is the patient’s high surgical risk rather than the severity of the acute cholecystitis or appearance of the gallbladder on an imaging study. Prompt laparoscopic cholecystectomy is the preferred treatment for acute cholecystitis.
Acute cholecystitis in diabetic patients is associated with a significantly higher frequency of infectious complications such as sepsis, compared with nondiabetic patients.132,133 Cholecystectomy should be performed expeditiously in this group of patients. Similarly, acute cholecystitis in the elderly patients population may have a deceptively benign clinical presentation but is associated with a high rate of empyema, gangrene, and perforation.134–136 Factors associated with gangrenous or emphysematous cholecystitis include male gender, diabetes mellitus, cardiovascular disease, and an initial white blood cell count in excess of 15,000/mm3. As with diabetic patients, early cholecystectomy is warranted in elderly patients to ensure prompt control of infection. The routine use of surgical drainage catheters after laparoscopic cholecystectomy for acute cholecystitis is not warranted and may be deleterious.137
Acalculous Cholecystitis
Acute cholecystitis that occurs in the absence of gallstones is termed acalculous cholecystitis (see Chapter 67). Most commonly, acalculous cholecystitis occurs in a patient hospitalized for other serious illnesses, such as trauma, burns, or major surgery. It may develop in outpatients, among whom elderly male patients with peripheral vascular disease appear to be at highest risk.138 Acalculous cholecystitis may also complicate the treatment of patients with the acquired immunodeficiency syndrome.139
The pathophysiology of acalculous cholecystitis is unclear, but biliary stasis caused by fasting, alterations in gallbladder blood flow, activation of factor XII, prostaglandins, and endotoxin all may play roles (see Chapter 67).140–144 Sludge is generally present in the gallbladder and may obstruct the cystic duct. Gangrene, empyema, and perforation of the gallbladder complicate the course of acalculous cholecystitis more commonly than they complicate the course of acute cholecystitis caused by gallstones. In some series, the frequency of these complications approaches 75%.145
Emphysematous Cholecystitis
Emphysematous cholecystitis is an uncommon condition characterized by infection of the gallbladder wall by gas-forming bacteria, particularly anaerobes (see Chapter 65). Diabetes mellitus has been cited as a risk factor.118,146 Gangrene and perforation commonly complicate the course of emphysematous cholecystitis. The treatment of emphysematous cholecystitis is prompt laparoscopic cholecystectomy after restoration of fluid and electrolyte balance. Antibiotics are indicated, with coverage directed against gram-negative rods and anaerobic bacteria.
GALLSTONE PANCREATITIS
The pathophysiology and clinical presentation of patients with gallstone pancreatitis are discussed in Chapters 58 and 65. Initial management of patients with gallstone pancreatitis includes fluid resuscitation, bowel rest, and monitoring for complications. The majority of patients have a relatively mild illness that resolves clinically within one week with conservative management.
The presence of cholelithiasis should be determined by ultrasonography early in the course of the treatment of a patient with acute pancreatitis. If cholelithiasis is present, laparoscopic cholecystectomy generally should be performed prior to the patient’s discharge. In the past, cholecystectomy early in the course of gallstone pancreatitis carried significant risk. For that reason, the timing of cholecystectomy was delayed for one to two months to allow resolution of the inflammatory process. A major disadvantage of this delayed approach was that up to one half of patients had further attacks of pancreatitis during the observation period. It is now recognized that cholecystectomy may be performed safely during the same hospitalization when the clinical signs of pancreatitis have resolved.147–149 This approach shortens the total duration of illness and hospitalization.150 Additionally, it prevents recurrent pancreatitis. Cholangiography should be performed during the cholecystectomy to exclude residual bile duct stones, as recommended by the International Association of Pancreatology.151
In patients with severe or necrotizing pancreatitis, surgery is delayed for several weeks to allow (1) patients to recover from the sequelae of pancreatitis; (2) inflammation of the hepatoduodenal ligament to decrease, thereby permitting safe dissection; and (3) identification of the small subset of patients in whom pancreatic pseudocysts develop and may require additional surgical treatment. A small subset of patients with necrotizing gallstone pancreatitis appear to benefit from early endoscopic sphincterotomy and clearance of the bile duct (see Chapter 58).152–154 Bile duct stones are found in a substantial proportion of patients with severe gallstone pancreatitis when sphincterotomy is performed within the first 24 to 48 hours of hospitalization. The morbidity of this approach is less than that for early surgery with bile duct exploration. Most patients who undergo endoscopic sphincterotomy for gallstone pancreatitis should have elective cholecystectomy when the pancreatitis has subsided. In elderly patients or those at high risk for surgery, cholecystectomy may be deferred, but further symptoms of gallstone disease may be expected in up to 31% on short-term follow-up.150 The risk of symptomatic gallstone disease is even higher in patients with cystic duct obstruction on cholangiography.155
SPECIAL PROBLEMS
Gallstone Disease during Pregnancy
Therapeutic options for gallstone disease are limited in pregnancy. In the past, cholecystectomy during pregnancy was discouraged because of the fear of fetal loss. Complications such as spontaneous abortion and preterm labor were common in operated women in the first and third trimesters of gestation, respectively. In addition, pregnancy was formerly considered an absolute contraindication to laparoscopic surgery because of concern about potential trocar injury to the uterus and the unknown effects of pneumoperitoneum on the fetal circulation. Improvements in anesthesia and tocolytic agents appear to have made abdominal surgery safer during pregnancy. Several large case series have suggested that cholecystectomy may be undertaken during pregnancy with minimal fetal and maternal morbidity.156,157 Laparoscopic cholecystectomy is performed during pregnancy only when necessary. Indications include complicated gallstone disease, including acute cholecystitis and pancreatitis, when the underlying disease poses a threat to the pregnancy, and severe biliary pain, when the mother is unable to maintain adequate nutrition. Surgery is probably safest during the second trimester, when the risk of fetal loss and teratogenicity that may occur in the first trimester and the risk of preterm labor that may occur in the third trimester are both low.156,157
Gallstone Disease during Childhood
Gallstone disease in the pediatric population appears to be increasing in frequency. Chronic hemolysis leading to pigment gallstones is the cause in about 20% of patients.158 A history of prolonged fasting with total parenteral nutritional support is an increasingly important risk factor. Ileal disorders or previous bowel resection increase the risk of gallstone development.
Mirizzi’s Syndrome
Mirizzi’s syndrome refers to common hepatic duct obstruction resulting from compression by a gallstone impacted in the cystic duct. Two types of Mirizzi’s syndrome have been described.159 In type I, the hepatic duct is compressed by a large stone impacted in the cystic duct or Hartman’s pouch. Associated inflammation may contribute to the obstruction and formation of a stricture in the central section of the extrahepatic bile duct. In type II, the calculus has eroded into the hepatic duct to produce a cholecystocholedochal fistula. Mirizzi’s syndrome is rare, occurring in about 1% of all patients who undergo cholecystectomy. Most patients present with repeated bouts of pain, fever, and jaundice. Ultrasonography generally reveals gallstones with a contracted gallbladder and moderate intrahepatic ductal dilatation with normal extrahepatic biliary anatomy. ERCP is useful in delineating the hepatic duct anatomy. The appearance of the obstruction and surrounding inflammation may be confused with a Klatskin tumor (see Chapter 69).
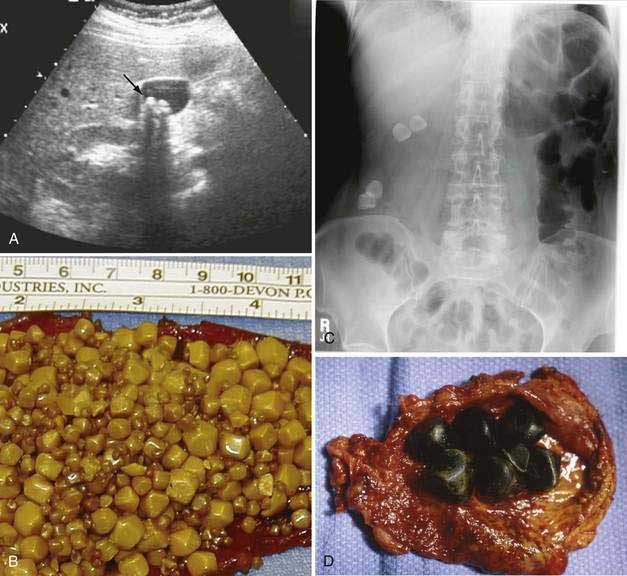
Gallstone Ileus
Gallstone ileus is an uncommon form of bowel obstruction caused by impaction of a large gallstone in the intestinal lumen. Bouveret’s syndrome refers to impaction of a gallstone in the distal duodenum or at the pylorus with resulting symptoms of gastric outlet obstruction. Gallstone ileus represents a true mechanical obstruction rather than a defect in motility as the name “ileus” would suggest. The median age of affected patients is more than 70 years. Most are women. Gallstone ileus is the cause of intestinal obstruction in less than 1% of patients younger than 70 years in age but nearly 5% of those 70 years of age or older.159 Symptoms are typical of mechanical intestinal obstruction and include cramping abdominal pain, vomiting, and abdominal distention. Only a minority of patients have symptoms suggestive of acute cholecystitis, but one half are known to have a history of gallstones.160 Liver biochemical test levels are elevated in 40% of patients, but overt jaundice is rare. Plain abdominal films reveal an intestinal gas pattern compatible with intestinal obstruction in most patients. Pneumobilia is present in about one half of all patients, and the aberrant gallstone is visible in a minority. Upper or lower gastrointestinal barium studies may occasionally identify the site of obstruction or the fistula, but these tests are unnecessary in most cases. Ultrasonography is useful for confirming the presence of cholelithiasis and may allow visualization of the fistula.
Management should be directed initially at restoration of fluid and electrolyte balance, followed by exploratory laparotomy. A laparoscopic approach is technically feasible and effective. Removing the stone via a small enterotomy relieves the intestinal obstruction. A search should be made for additional stones. Bowel resection is necessary only when perforation or intestinal ischemia has occurred. Treatment of the cholecystoenteric fistula is not necessary at the initial operation because many fistulas close spontaneously.161 Elective cholecystectomy and closure of the fistula are indicated if symptoms of chronic cholecystitis persist. Mortality rates in this high-risk patient population are high, averaging 15% to 18%. Gallstone ileus recurs in about 5% of patients.
Incidental Cholecystectomy
The typical patient with gallstones tends to remain asymptomatic. On long-term follow-up, symptoms develop in 18% to 35% of these initially asymptomatic persons.101,107,108 Certain groups, however, are at higher risk. Gallstones in morbidly obese patients, for example, tend to have a more aggressive natural history, making incidental cholecystectomy at the time of gastric bypass surgery appealing.162 Patients with large (>2.5 cm) gallstones and those with calcification of the gallbladder wall (porcelain gallbladder) have an increased risk for the development of acute cholecystitis and gallbladder cancer, and in these patients, incidental cholecystectomy is warranted.163–165 In patients with sickle cell disease, who are at risk for the development of pigment gallstones because of chronic hemolysis, distinguishing the clinical presentation of a sickle cell crisis from acute cholecystitis may be difficult, and incidental cholecystectomy is indicated in these patients.166,167 Similarly, patients with other hemolytic anemias, such as β-thalassemia, are at high risk for the development of gallstones, and a high percentage of them become symptomatic.168 Cholecystectomy appears warranted for asymptomatic patients with stones if splenectomy is undertaken for the hemolytic anemia. Finally, laparotomy for reasons other than cholecystectomy is associated with a high frequency of postoperative biliary symptoms if the gallbladder containing stones is left in situ. Of 68 asymptomatic patients with stones who underwent laparotomy in one study, 54% became symptomatic postoperatively, and 22% required cholecystectomy within 30 days.169,170
The risk of adding incidental cholecystectomy to another abdominal procedure appears to be low.171 If the patient is in otherwise reasonable health, the primary operation has proceeded smoothly, and operative exposure is adequate, incidental cholecystectomy can be done safely at the time of another operation, including colectomy. The risk does not appear to be increased in the elderly. The risk of postoperative wound infections, however, may be increased in some cases by the addition of an incidental cholecystectomy.172
CHOLEDOCHOLITHIASIS
Choledocholithiasis may be detected at the same time that gallbladder stones are discovered during an evaluation for biliary tract symptoms, during cholecystectomy, or after a cholecystectomy. Several management options are available, including oral dissolution therapy, interventional radiologic and endoscopic techniques, and surgery (see Chapter 70). Which management strategy is most appropriate for a given patient depends on the clinical situation in which the stones have been identified (jaundice, cholangitis, pancreatitis, or absence of symptoms), status of the gallbladder, and age and general condition of the patient. Additional factors to consider are the expertise of the available surgical, endoscopic, and radiologic specialists.
CHOLEDOCHOLITHIASIS KNOWN PREOPERATIVELY
When choledocholithiasis is known to be present preoperatively, an acceptable approach is to clear the bile duct by endoscopic sphincterotomy and then proceed with laparoscopic cholecystectomy. An alternative approach is an open or laparoscopic cholecystectomy with bile duct exploration. In the era of laparoscopic cholecystectomy and endoscopic stone retrieval, laparoscopic bile duct exploration has been performed less commonly than open bile duct exploration was performed during the open cholecystectomy era.173 Laparoscopic bile duct exploration via either a transcystic duct approach or direct incision of the bile duct is a technically demanding procedure. When performed by an experienced surgeon, however, the success rate for clearing the duct of stones ranges from 83% to 97%.174–176 Decision analyses and randomized trials have shown that laparoscopic cholecystectomy with bile duct exploration via either a transcystic or transcholedochal approach results in lower rates of morbidity and mortality as well as a shorter hospital stay, quicker return to health, and lower number of procedures than preoperative endoscopic retrieval followed by laparoscopic cholecystectomy.174,175 The decision to proceed with the surgery-only approach depends greatly on the experience and technical skill of the surgeon and his or her team. If a surgeon lacks adequate training or adequately trained staff and equipment, a two-stage approach is preferable.
CHOLEDOCHOLITHIASIS IDENTIFIED DURING CHOLECYSTECTOMY
If unsuspected choledocholithiasis is identified by cholangiography during laparoscopic cholecystectomy, the following three options are available: (1) conversion to an open operation with bile duct exploration; (2) laparoscopic bile duct exploration; and (3) completion of the laparoscopic cholecystectomy with postoperative endoscopic sphincterotomy and stone extraction. An algorithm of these options is shown in Figure 66-7. Factors that influence this decision include the number and location of bile duct stones, the presence of associated ductal pathology, and the skill and experience of the surgeon and endoscopist. Completion of the laparoscopic cholecystectomy with postoperative endoscopic sphincterotomy is satisfactory for most patients and has the advantage of preserving the minimally invasive approach. Endoscopic sphincterotomy may be technically unsuccessful, however, in 5% to 10% of patients—even in the hands of a skilled endoscopist—and complete clearance of stones from the bile duct is possible in only 70% to 80% of patients.177 In such patients, a second attempt may be required. Increasing experience has shown that laparoscopic bile duct exploration is safe and effective. Stone clearance rates average 95%, with an operative mortality rate of 0.5%.174,177–179 Laparoscopic bile duct exploration compares favorably with endoscopic sphincterotomy in terms of efficacy, cost, and safety.180
BILE DUCT STRICTURE
The majority of benign bile duct strictures are the result of iatrogenic injury during cholecystectomy. A minority are the sequelae of chronic pancreatitis, primary sclerosing cholangitis, trauma, liver transplantation, and choledocholithiasis. Operative injury to the bile duct during cholecystectomy may occur because of misinterpretation of the biliary ductal anatomy; inaccurate placement of clips, sutures, or cautery to control hemorrhage; tenting of the bile duct during control of the cystic duct; and ineffective retraction and exposure.181–183 These injuries commonly occur during an otherwise uneventful cholecystectomy and may be unnoticed by the surgeon.
Bile duct injury presents in one of three patterns. If the bile duct has been completely occluded, jaundice develops rapidly in the early postoperative period after cholecystectomy. In the second pattern, the injury is manifested by the development of bile ascites that results from transection of an extrahepatic bile duct, ineffective placement or dislodgement of cystic duct ligatures, or a bile leak from the gallbladder fossa as a result of a divided cystohepatic duct or duct of Luschka. A bile leak is often associated with an infected bile collection in the subhepatic space. In the third pattern, partial bile duct obstruction leads to intermittent episodes of pain, jaundice, or cholangitis, usually within two years of the cholecystectomy. The three patterns of injury are illustrated in Figure 66-8.
The imaging evaluation of a patient with suspected bile duct stricture usually begins with ultrasonography to identify dilated ducts or a subhepatic fluid collection. In the early postoperative period, a technetium-labeled radionuclide scan may expeditiously and noninvasively demonstrate patency of the biliary tree and exclude a bile leak. If these studies suggest bile duct injury, ERCP is indicated to define the lesion. The initial goals of management include control of subhepatic infection, usually via percutaneous drainage of any fluid collection, and biliary drainage, either via an endoscopic or transhepatic route (see Chapter 70). Following control of infection and biliary drainage, complete cholangiography, either endoscopic or radiologic, is necessary to define the anatomy and plan reconstruction.
Most patients with a benign postoperative biliary stricture are best managed with surgical repair. Although numerous operations have been described, the best results are obtained with resection of the stricture and an end-to-side Roux-en-Y choledochojejunostomy or hepaticojejunostomy. The principles of a successful repair include complete dissection of the strictured segment, creation of a tension-free anastomosis, accurate mucosa-to-mucosa approximation of the anastomosis with fine absorbable suture material and unscarred proximal ductal tissue, and preservation of the ductal blood supply. The mortality rate of operations to correct benign biliary strictures averages 0% to 2% in modern series.184–186 The risk of surgery is related directly to the presence of risk factors such as cirrhosis, renal failure, uncontrolled cholangitis, age, and malnutrition. The long-term results of biliary reconstruction for a benign bile duct stricture are good, with cure achieved in 85% to 98% of patients.184–187 Results are worse in patients with high strictures or cirrhosis. In those with high strictures, special techniques may be necessary to obtain healthy ductal tissue that is uninvolved in the inflammatory process for anastomosis; liver resection may be required. Recurrent strictures pose technical difficulties, but satisfactory results are still achieved in about 75% of patients.184–186188
Postoperative strictures may be treated with endoscopic or percutaneous balloon dilation with or without stent placement as long as the remaining duct has not been disrupted. Benign postoperative strictures often can be managed endoscopically with placement of plastic, removable stents. Although several endoscopic procedures are often required, good results can be achieved in appropriately selected patients (see Chapter 70). No randomized, prospective trials have compared surgical, endoscopic, and radiologic approaches. In one nonrandomized trial, long-term bile duct patency was achieved in 88% of patients treated with hepaticojejunostomy compared with 55% of those who underwent balloon dilation.189 No procedure-related mortality was observed. In view of the excellent long-term results and low mortality rate of hepaticojejunstomy in experienced hands, surgery should be offered as the initial treatment to all fit patients with bile duct stricture. Nonoperative management is best reserved for patients with biliary cirrhosis, significant comorbid illness, or high recurrent strictures.
POSTCHOLECYSTECTOMY SYNDROME
Postcholecystectomy syndrome refers to the occurrence of abdominal symptoms that often resemble biliary pain following cholecystectomy (see also Chapters 63 and 65). The term is misleading in that it encompasses a wide spectrum of biliary and non-biliary disorders that are rarely related to the operation itself. The frequency of such symptoms following cholecystectomy ranges from 5% to 40%.51,190–193 The most common postoperative symptoms are dyspepsia, flatulence, and bloating, which usually antedate the cholecystectomy. Other patients have persistence of right upper quadrant or epigastric abdominal pain. A small percentage of patients with postcholecystectomy symptoms present with severe abdominal pain, jaundice, or emesis; investigation is much more likely to reveal a distinct, treatable cause in this group of patients than in those with mild or nonspecific symptoms. If the symptoms arise early in the postoperative period, bile peritonitis secondary to iatrogenic biliary injury must be suspected.
The differential diagnosis of symptoms after cholecystectomy includes extraintestinal disorders such as cardiac ischemia, nonbiliary gastrointestinal conditions such as peptic ulcer disease, biliary disorders such as choledocholithiasis, functional illnesses such as irritable bowel syndrome, and psychiatric diseases. The spectrum of possibilities is listed in Table 66-7. The clinician must carefully consider the possibility of nonbiliary causes of pain and direct the evaluation appropriately. The most common biliary causes are described in the sections that follow.
| Biliary causes |
CHOLEDOCHOLITHIASIS
Bile duct stones are the most common cause of postcholecystectomy symptoms. They may be residual stones overlooked at the time of cholecystectomy or, less frequently, stones that have formed primarily in the duct. The natural history of choledocholithiasis is not known, but in some patients, these stones clearly can cause biliary-type pain, jaundice, pancreatitis, or cholangitis. The diagnosis of choledocholithiasis is suggested by the clinical picture. Liver biochemical test values, particularly the alkaline phosphatase level, may be elevated. Ultrasonography may show a dilated bile duct, but visualization of the stone is uncommon. MRCP and ERCP may confirm the presence of ductal stones and exclude other possibilities such as a bile duct stricture or tumor. Endoscopic sphincterotomy with stone extraction is curative in most patients.194
CYSTIC DUCT REMNANT
In some patients, the cause of postcholecystectomy symptoms has been attributed to pathology in the cystic duct remnant195,196 Abnormalities that have been described include cystic duct stones, fistulas, granulomas, and neuromas. Associated bile duct stones are common. Although the existence of such a syndrome has been controversial, in one randomized trial, complete excision of the cystic duct during cholecystectomy was associated with fewer postoperative sequelae than a standard operative technique in which a portion of the cystic duct was left in situ.197 In the era of laparoscopic cholecystectomy, the cystic duct is divided closer to its origin from the gallbladder to minimize the risk of bile duct and right hepatic artery injury that may arise from dissection at the insertion of the cystic duct into the common hepatic duct; as a result, the frequency of cystic duct stump syndrome may be higher.198 MRCP and ERCP are useful for delineating the biliary anatomy in patients with suspected cystic duct remnant pathology. Treatment is with surgical excision of the cystic duct remnant.
SPHINCTER OF ODDI DYSFUNCTION
Up to 10% of patients with postcholecystectomy pain are found to have a structural or functional abnormality of the sphincter of Oddi (see Chapter 63).199,200 Structural problems have been referred to as papillary stenosis, which is characterized by a fixed narrowing of the sphincter in association with an elevated basal sphincter pressure. The stenosis may occur as a result of trauma such as passage of gallstones, instrumentation, pancreatitis, or infection. Functional or motility disorders have been referred to as biliary or sphincter of Oddi dyskinesia and ampullary spasm. Biliary manometry in these patients reveals elevated sphincter pressure resulting from abnormal tonic or phasic smooth muscle contractions. Because the cause of this disorder is unknown, and in many cases a distinction between a structural or functional process cannot be made, the generic term sphincter of Oddi dysfunction is now preferred.
Clinical manifestations of sphincter of Oddi dysfunction include biliary-type pain, jaundice, and pancreatitis. ERCP findings of a dilated bile duct and delayed (>45 minutes) drainage of contrast medium from the bile duct are typical. The combination of biliary-type pain, abnormal liver biochemical test levels, and a dilated bile duct is highly predictive of a response to endoscopic sphincterotomy.201 In patients in whom the diagnosis is not as clear, biliary manometry is indicated. Treatment is with endoscopic sphincterotomy. Selected patients may require transduodenal sphincteroplasty and septoplasty.202 The pathophysiology, classification, and treatment of this disorder are discussed more fully in Chapter 63.
GALLSTONES, CHOLECYSTECTOMY, AND CANCER
A number of reports have demonstrated associations between either gallstones or cholecystectomy and the development of cancers in organs as diverse as the gallbladder, bile ducts, stomach, colon, breast, and uterus. Whether a causal relationship exists between gallbladder disease or its treatment and the development of these malignancies is unclear. Common environmental factors, perhaps dietary, may influence the rates of all these diseases. On the other hand, alterations in the composition of bile in patients with gallstones could influence the development of carcinoma. Moreover, cholecystectomy increases the enterohepatic circulation of bile acids, which increases mucosal exposure to potentially carcinogenic secondary bile acids such as deoxycholate (see Chapter 64).203,204
BILIARY TRACT CANCER
The strongest association between gallstones and cancer is with cancers of the biliary tree itself, particularly gallbladder carcinoma (see Chapter 69). Most patients with gallbladder cancer have gallstones, and epidemiologic data show a strong relationship between the two diseases. The risk of gallbladder cancer is greater in patients with large gallstones than in those with small gallstones, in those with multiple gallstones than a single gallstone, and in Native Americans.205–207 A weaker statistical association exists between gallstones and cholangiocarcinoma, and a causal relationship is suggested by the finding that the risk is lower in patients who undergo cholecystectomy than in those whose gallstones are untreated.208,209
COLORECTAL CANCER
Studies from the early 1980s identified a statistical association between cholecystectomy and the subsequent development of colorectal cancer, particularly in the right colon.204,210–212 The magnitude of the risk of colorectal cancer, although statistically significant, was low (relative risk 1.5 to 2.0). Subsequent studies have disputed the association or attributed it to the gallstones rather than the cholecystectomy (see Chapter 123). These findings should not represent a deterrent to cholecystectomy in a patient with a clear indication for the procedure.
Cameron DR, Goodman AJ. Delayed cholecystectomy for gallstone pancreatitis: Re-admissions and outcomes. Ann R Coll Surg Engl. 2004;86:358-62. (Ref 147.)
Choudhary A, Bechtold ML, Puli SR, et al. Role of prophylactic antibiotics in laparoscopic cholecystectomy: A meta-analysis. J Gastrointest Surg. 2008;12:1847-53. (Ref 123.)
Flum DR, Dellinger EP, Cheadle A, et al. Intraoperative cholangiography and risk of common bile duct injury during cholecystectomy. JAMA. 2003;289:1639-44. (Ref 74.)
Flum DR, Flowers C, Veenstra DL. A cost-effectiveness analysis of intraoperative cholangiography in the prevention of bile duct injury during laparoscopic cholecystectomy. J Am Coll Surg. 2003;196:385-93. (Ref 75.)
Ito K, Ito H, Whang EE. Timing of cholecystectomy for biliary pancreatitis: Do the data support current guidelines? J Gastrointest Surg. 2008;12:2164-70. (Ref 150.)
Keus F, de Jong JA, Gooszen HG, van Laarhoven CJ. Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev 2006:CD006231. (Ref 59.)
Kharbutli B, Velanovich V. Management of preoperatively suspected choledocholithiasis: A decision analysis. J Gastrointest Surg. 2008;12:1973-80. (Ref 175.)
Papi C, Catarci M, D’Ambrosio L, et al. Timing of cholecystectomy for acute calculous cholecystitis: A meta-analysis. Am J Gastroenterol. 2004;99:147-55. (Ref 130.)
Petroni ML, Jazrawi RP, Pazzi P, et al. Ursodeoxycholic acid alone or with chenodeoxycholic acid for dissolution of cholesterol gallstones: A randomized multicentre trial. The British-Italian Gallstone Study group. Aliment Pharmacol Ther. 2001;15:123-8. (Ref 10.)
Shao T, Yang YX. Cholecystectomy and the risk of colorectal cancer. Am J Gastroenterol. 2005;100:1813-20. (Ref 204.)
Siddiqui T, MacDonald A, Chong PS, Jenkins JT. Early versus delayed laparoscopic cholecystectomy for acute cholecystitis: A meta-analysis of randomized clinical trials. Am J Surg. 2008;195:40-7. (Ref 131.)
Strasberg SM, Eagon CJ, Drebin JA. The “hidden cystic duct” syndrome and the infundibular technique of laparoscopic cholecystectomy—the danger of the false infundibulum. J Am Coll Surg. 2000;191:661-7. (Ref 68.)
Uhl W, Warshaw A, Imrie C, et al. IAP guidelines for the surgical management of acute pancreatitis. Pancreatology. 2002;2:565-73. (Ref 151.)
Williams EJ, Green J, Beckingham I, et al. Guidelines on the management of common bile duct stones (CBDS). Gut. 2008;57:1004-21. (Ref 194.)
Zaliekas J, Munson JL. Complications of gallstones: The Mirizzi syndrome, gallstone ileus, gallstone pancreatitis, complications of “lost” gallstones. Surg Clin North Am. 2008;88:1345-68. (Ref 159.)
1. Schiff M. Il coleinato di soda nella cura dei calcoli biliari. L’Imparziale. 1873;13:97.
2. Dabney WC. The use of choleate of soda to prevent the formation of gallstones. Am J Med Sci. 1876;71:410-15.
3. Rewbridge AG. The disappearance of gallstone disease shadows following the prolonged administration of bile salts. Surgery. 1937;1:395-8.
4. Carrilho-Ribeiro L, Pinto-Correia A, Velosa J, de Moura MC. Long-term gallbladder stone recurrence and risk factors after successful lithotripsy. Eur J Gastroenterol Hepatol. 2000;12:209-15.
5. Petroni ML, Jazrawi RP, Pazzi P, et al. Risk factors for the development of gallstone recurrence following medical dissolution. The British-Italian Gallstone Study Group. Eur J Gastroenterol Hepatol. 2000;12:695-700.
6. Sackmann M, Niller H, Klueppelberg U, et al. Gallstone recurrence after shock-wave therapy. Gastroenterology. 1994;106:225-30.
7. Villanova N, Bazzoli F, Taroni F, et al. Gallstone recurrence after successful oral bile acid treatment. A 12-year follow-up study and evaluation of long-term postdissolution treatment. Gastroenterology. 1989;97:726-31.
8. Fromm H, Roat JW, Gonzalez V, et al. Comparative efficacy and side effects of ursodeoxycholic and chenodeoxycholic acids in dissolving gallstones. A double-blind controlled study. Gastroenterology. 1983;85:1257-64.
9. May GR, Sutherland LR, Shaffer EA. Efficacy of bile acid therapy for gallstone dissolution: A meta-analysis of randomized trials. Aliment Pharmacol Ther. 1993;7:139-48.
10. Petroni ML, Jazrawi RP, Pazzi P, et al. Ursodeoxycholic acid alone or with chenodeoxycholic acid for dissolution of cholesterol gallstones: A randomized multicentre trial. The British-Italian Gallstone Study group. Aliment Pharmacol Ther. 2001;15:123-8.
11. Senior JR, Johnson MF, DeTurck DM, et al. In vivo kinetics of radiolucent gallstone dissolution by oral dihydroxy bile acids. Gastroenterology. 1990;99:243-51.
12. Portincasa P, Di Ciaula A, vanBerge-Henegouwen GP. Smooth muscle function and dysfunction in gallbladder disease. Curr Gastroenterol Rep. 2004;6:151-62.
13. Sengupta S, Modak P, McCauley N, O’Donnell LJ. Effect of oral clarithromycin on gall-bladder motility in normal subjects and those with gall-stones. Aliment Pharmacol Ther. 2006;24:95-9.
14. Sengupta S, Modak P, McCauley N, O’Donnell LJ. Prokinetic effect of alpha-adrenergic antagonist, and beta-adrenergic antagonist on gall-bladder motility in humans with gall-stone disease. Eur J Gastroenterol Hepatol. 2007;19:581-3.
15. Petroni ML, Jazrawi RP, Grundy A, et al. Prospective, multicenter study on value of computerized tomography (CT) in gallstone disease in predicting response to bile acid therapy. Dig Dis Sci. 1995;40:1956-62.
16. Dolgin SM, Schwartz JS, Kressel HY, et al. Identification of patients with cholesterol or pigment gallstones by discriminant analysis of radiographic features. N Engl J Med. 1981;304:808-11.
17. Strasberg SM, Clavien PA. Cholecystolithiasis: Lithotherapy for the 1990s. Hepatology. 1992;16:820-39.
18. Tomida S, Abei M, Yamaguchi T, et al. Long-term ursodeoxycholic acid therapy is associated with reduced risk of biliary pain and acute cholecystitis in patients with gallbladder stones: A cohort analysis. Hepatology. 1999;30:6-13.
19. Inoi J, Shimizu I, Tsuji Y, et al. Effect of administration of ursodeoxycholic acid at bedtime on cholesterol saturation of hepatic bile in Japanese patients with gallstone. J Med Invest. 1998;45:115-22.
20. Erlinger S, Le Go A, Husson JM, Fevery J. Franco-Belgian cooperative study of ursodeoxycholic acid in the medical dissolution of gallstones: A double-blind, randomized, dose-response study, and comparison with chenodeoxycholic acid. Hepatology. 1984;4:308-14.
21. Podda M, Zuin M, Battezzati PM, et al. Efficacy and safety of a combination of chenodeoxycholic acid and ursodeoxycholic acid for gallstone dissolution: A comparison with ursodeoxycholic acid alone. Gastroenterology. 1989;96:222-9.
22. Sauerbruch T, Delius M, Paumgartner G, et al. Fragmentation of gallstones by extracorporeal shock waves. N Engl J Med. 1986;314:818-22.
23. Greiner L, Munks C, Heil W, Jakobeit C. Gallbladder stone fragments in feces after biliary extracorporeal shock-wave lithotripsy. Gastroenterology. 1990;98:1620-4.
24. Elewaut A, Crape A, Afschrift M, et al. Results of extracorporeal shock wave lithotripsy of gall bladder stones in 693 patients: A plea for restriction to solitary radiolucent stones. Gut. 1993;34:274-8.
25. Sackmann M, Pauletzki J, Sauerbruch T, et al. The Munich Gallbladder Lithotripsy Study. Results of the first 5 years with 711 patients. Ann Intern Med. 1991;114:290-6.
26. Ochi H, Tazuma S, Kajihara T, et al. Factors affecting gallstone recurrence after successful extracorporeal shock wave lithotripsy. J Clin Gastroenterol. 2000;31:230-2.
27. Pauletzki J, Sailer C, Kluppelberg U, et al. Gallbladder emptying determines early gallstone clearance after shock-wave lithotripsy. Gastroenterology. 1994;107:1496-502.
28. Sackmann M, Pauletzki J, Delius M, et al. Noninvasive therapy of gallbladder calculi with a radiopaque rim. Gastroenterology. 1992;102:988-93.
29. Tsuchiya Y, Saito H, Saito N, et al. Sonographic patterns of radiolucent gall-bladder stones for predicting successful shock-wave lithotripsy. J Gastroenterol Hepatol. 1995;10:426-31.
30. Boscaini M, Piccinni-Leopardi M, Andreotti F, Montori A. Gall stone pulverisation strategy in patients treated with extracorporeal lithotripsy and follow up results of maintenance treatment with ursodeoxycholic acid. Gut. 1994;35:117-21.
31. Soehendra N, Nam VC, Binmoeller KF, et al. Pulverisation of calcified and non-calcified gall bladder stones: Extracorporeal shock wave lithotripsy used alone. Gut. 1994;35:417-22.
32. Schoenfield LJ, Berci G, Carnovale RL, et al. The effect of ursodiol on the efficacy and safety of extracorporeal shock-wave lithotripsy of gallstones. The Dornier National Biliary Lithotripsy Study. N Engl J Med. 1990;323:1239-45.
33. McSherry CK. The results of the EDAP multicenter trial of biliary lithotripsy in the United States. The EDAP Investigators Group. Surg Gynecol Obstet. 1991;173:461-4.
34. Pelletier G, Delmont J, Capdeville R, et al. Treatment of gallstones with piezoelectric lithotripsy and oral bile acids. A multicenter study. J Hepatol. 1991;12:327-31.
35. Sauter G, Kullak-Ublick GA, Schumacher R, et al. Safety and efficacy of repeated shockwave lithotripsy of gallstones with and without adjuvant bile acid therapy. Gastroenterology. 1997;112:1603-9.
36. Tsuchiya Y, Ishihara F, Kajiyama G, et al. Repeated piezoelectric lithotripsy for gallstones with and without ursodeoxycholic acid dissolution: A multicenter study. J Gastroenterol. 1995;30:768-74.
37. Wehrmann T, Hurst A, Lembcke B, et al. Biliary lithotripsy with a new electromagnetic shock wave source. A 2-year clinical experience. Dig Dis Sci. 1993;38:2113-20.
38. Carrilho-Ribeiro L, Pinto-Correia A, Velosa J, Carneiro De Moura M. A ten-year prospective study on gallbladder stone recurrence after successful extracorporeal shock-wave lithotripsy. Scand J Gastroenterol. 2006;41:338-42.
39. Rabenstein T, Radespiel-Troger M, Hopfner L, et al. Ten years experience with piezoelectric extracorporeal shockwave lithotripsy of gallbladder stones. Eur J Gastroenterol Hepatol. 2005;17:629-39.
40. Hood KA, Gleeson D, Ruppin DC, Dowling RH. Gall stone recurrence and its prevention: The British/Belgian Gall Stone Study Group’s post-dissolution trial. Gut. 1993;34:1277-88.
41. Bass EB, Steinberg EP, Pitt HA, et al. Cost-effectiveness of extracorporeal shock-wave lithotripsy versus cholecystectomy for symptomatic gallstones. Gastroenterology. 1991;101:189-99.
42. Nicholl JP, Brazier JE, Milner PC, et al. Randomised controlled trial of cost-effectiveness of lithotripsy and open cholecystectomy as treatments for gallbladder stones. Lancet. 1992;340:801-7.
43. Barkun AN, Barkun JS, Sampalis JS, et al. Costs and effectiveness of extracorporeal gallbladder stone shock wave lithotripsy versus laparoscopic cholecystectomy. A randomized clinical trial. McGill Gallstone Treatment Group. Int J Technol Assess Health Care. 1997;13:589-601.
44. Adamek HE, Buttmann A, Wessbecher R, et al. Clinical comparison of extracorporeal piezoelectric lithotripsy (EPL) and intracorporeal electrohydraulic lithotripsy (EHL) in difficult bile duct stones. A prospective randomized trial. Dig Dis Sci. 1995;40:1185-92.
45. Amplatz S, Piazzi L, Felder M, et al. Extracorporeal shock wave lithotripsy for clearance of refractory bile duct stones. Dig Liver Dis. 2007;39:267-72.
46. Arya N, Nelles SE, Haber GB, et al. Electrohydraulic lithotripsy in 111 patients: A safe and effective therapy for difficult bile duct stones. Am J Gastroenterol. 2004;99:2330-4.
47. Chang WH, Chu CH, Wang TE, et al. Outcome of simple use of mechanical lithotripsy of difficult common bile duct stones. World J Gastroenterol. 2005;11:593-6.
48. Garg PK, Tandon RK, Ahuja V, et al. Predictors of unsuccessful mechanical lithotripsy and endoscopic clearance of large bile duct stones. Gastrointest Endosc. 2004;59:601-5.
49. Moon JH, Cha SW, Ryu CB, et al. Endoscopic treatment of retained bile-duct stones by using a balloon catheter for electrohydraulic lithotripsy without cholangioscopy. Gastrointest Endosc. 2004;60:562-6.
50. Livingston EH, Rege RV. A nationwide study of conversion from laparoscopic to open cholecystectomy. Am J Surg. 2004;188:205-11.
51. Berger MY, Olde Hartman TC, Bohnen AM. Abdominal symptoms: Do they disappear after cholecystectomy? Surg Endosc. 2003;17:1723-8.
52. Gilliland TM, Traverso LW. Modern standards for comparison of cholecystectomy with alternative treatments for symptomatic cholelithiasis with emphasis on long term relief of symptoms. Surg Gynecol Obstet. 1990;170:39-44.
53. Heuer GJ. The factors leading to death in operations upon the gallbladder and bile ducts. Ann Surg. 1934;99:881-92.
54. Glenn F, Hayes DM. The causes of death following biliary tract surgery for nonmalignant disease. Surg Gynecol Obstet. 1952;94:282-96.
55. Arnold DJ. 28,621 cholecystectomies in Ohio. Results of a survey in Ohio hospitals by the Gallbladder Survey Committee, Ohio Chapter, American College of Surgeons. Am J Surg. 1970;119:714-17.
56. Bredesen J, Jorgensen T, Andersen TF, et al. Early postoperative mortality following cholecystectomy in the entire female population of Denmark, 1977-1981. World J Surg. 1992;16:530-5.
57. McSherry CK, Glenn F. The incidence and causes of death following surgery for nonmalignant biliary tract disease. Ann Surg. 1980;191:271-5.
58. Roslyn JJ, Binns GS, Hughes EF, et al. Open cholecystectomy: A contemporary analysis of 42,474 patients. Ann Surg. 1993;218:129-37.
59. Keus F, de Jong JA, Gooszen HG, van Laarhoven CJ. Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev 2006:CD006231.
60. Keus F, Gooszen HG, Van Laarhoven CJ. Systematic review: Open, small-incision or laparoscopic cholecystectomy for symptomatic cholecystolithiasis. Aliment Pharmacol Ther. 2009;29:359-78.
61. Wolf AS, Nijsse BA, Sokal SM, et al. Surgical outcomes of open cholecystectomy in the laparoscopic era. Am J Surg. 2008. Epub ahead of print
62. Hermann RE. A plea for a safer technique of cholecystectomy. Surgery. 1976;79:609-11.
63. Kune GA. Bile duct injury during cholecystectomy: Causes, prevention and surgical repair in 1979. Aust N Z J Surg. 1979;49:35-40.
64. Morgenstern L, Wong L, Berci G. Twelve hundred open cholecystectomies before the laparoscopic era. A standard for comparison. Arch Surgery. 1992;127:400-3.
65. Clavien PA, Sanabria JR, Mentha G, et al. Recent results of elective open cholecystectomy in a North American and a European center. Comparison of complications and risk factors. Ann Surg. 1992;216:618-26.
66. Catarci M, Mancini S, Gentileschi P, et al. Antibiotic prophylaxis in elective laparoscopic cholecystectomy. Lack of need or lack of evidence? Surg Endosc. 2004;18:638-41.
67. Landau O, Kott I, Deutsch AA, et al. Multifactorial analysis of septic bile and septic complications in biliary surgery. World J Surg. 1992;16:962-4.
68. Strasberg SM, Eagon CJ, Drebin JA. The “hidden cystic duct” syndrome and the infundibular technique of laparoscopic cholecystectomy—the danger of the false infundibulum. J Am Coll Surg. 2000;191:661-7.
69. Zehetner J, Shamiyeh A, Wayand W. Lost gallstones in laparoscopic cholecystectomy: All possible complications. Am J Surg. 2007;193:73-8.
70. Gregg RO. The case for selective cholangiography. Am J Surg. 1988;155:540-5.
71. Kakos GS, Tompkins RK, Turnipseed W, Zollinger RM. Operative cholangiography during routine cholecystectomy: A review of 3,012 cases. Arch Surg. 1972;104:484-8.
72. Fletcher DR, Hobbs MS, Tan P, et al. Complications of cholecystectomy: Risks of the laparoscopic approach and protective effects of operative cholangiography: A population-based study. Ann Surg. 1999;229:449-57.
73. Waage A, Nilsson M. Iatrogenic bile duct injury: A population-based study of 152,776 cholecystectomies in the Swedish Inpatient Registry. Arch Surg. 2006;141:1207-13.
74. Flum DR, Dellinger EP, Cheadle A, et al. Intraoperative cholangiography and risk of common bile duct injury during cholecystectomy. JAMA. 2003;289:1639-44.
75. Flum DR, Flowers C, Veenstra DL. A cost-effectiveness analysis of intraoperative cholangiography in the prevention of bile duct injury during laparoscopic cholecystectomy. J Am Coll Surg. 2003;196:385-93.
76. Ludwig K, Bernhardt J, Steffen H, Lorenz D. Contribution of intraoperative cholangiography to incidence and outcome of common bile duct injuries during laparoscopic cholecystectomy. Surg Endosc. 2002;16:1098-104.
77. Anonymous. A prospective analysis of 1518 laparoscopic cholecystectomies. N Eng J Med. 1991;324:1073-8.
78. Berci G, Sackier JM. SAGES laparoscopic cholecystectomy study. Surg Endosc. 1992;6:977.
79. Cuschieri A, Dubois F, Mouiel J, et al. The European experience with laparoscopic cholecystectomy. Am J Surg. 1991;161:385-7.
80. Dunn D, Nair R, Fowler S, McCloy R. Laparoscopic cholecystectomy in England and Wales: Results of an audit by the Royal College of Surgeons in England. Ann R Coll Surg Engl. 1994;76:269-75.
81. Go PM, Schol F, Gouma DJ. Laparoscopic cholecystectomy in The Netherlands. Br J Surg. 1993;80:1180-3.
82. Ihasz M, Hung CM, Regoly-Merei J, et al. Complications of laparoscopic cholecystectomy in Hungary: A multicentre study of 13,833 patients. Eur J Surg. 1997;163:267-74.
83. Larson GM, Vitale GC, Casey J, et al. Multipractice analysis of laparoscopic cholecystectomy in 1,983 patients. Am J Surg. 1992;163:221-6.
84. Litwin DE, Girotti MJ, Poulin EC, et al. Laparoscopic cholecystectomy: Trans-Canada experience with 2201 cases. Can J Surg. 1992;35:291-6.
85. MacFadyen BVJr, Vecchio R, Ricardo AE, Mathis CR. Bile duct injury after laparoscopic cholecystectomy. The United States experience. Surg Endosc. 1998;12:315-21.
86. Orlando Rd, Russell JC, Lynch J, Mattie A. Laparoscopic cholecystectomy. A statewide experience. The Connecticut Laparoscopic Cholecystectomy Registry. Arch Surg. 1993;128:494-8.
87. Z’Graggen K, Wehrli H, Metzger A, et al. Complications of laparoscopic cholecystectomy in Switzerland. A prospective 3-year study of 10,174 patients. Swiss Association of Laparoscopic and Thoracoscopic Surgery. Surg Endosc. 1998;12:1303-10.
88. Barkun JS, Barkun AN, Sampalis JS, et al. Randomised controlled trial of laparoscopic versus mini cholecystectomy. The McGill Gallstone Treatment Group. Lancet. 1992;340:1116-19.
89. Johansson M, Thune A, Nelvin L, et al. Randomized clinical trial of open versus laparoscopic cholecystectomy in the treatment of acute cholecystitis. Br J Surg. 2004;92:44-9.
90. McMahon AJ, Russell IT, Ramsay G, et al. Laparoscopic and minilaparotomy cholecystectomy: A randomized trial comparing postoperative pain and pulmonary function. Surgery. 1994;115:533-9.
91. Soper NJ, Barteau JA, Clayman RV, et al. Comparison of early postoperative results for laparoscopic versus standard open cholecystectomy. Surg Gynecol Obstet. 1992;174:114-18.
92. Trondsen E, Reiertsen O, Andersen OK, Kjaersgaard P. Laparoscopic and open cholecystectomy. A prospective, randomized study. Eur J Surg. 1993;159:217-21.
93. Steiner CA, Bass EB, Talamini MA, et al. Surgical rates and operative mortality for open and laparoscopic cholecystectomy in Maryland. N Engl J Med. 1994;330:403-8.
94. Moore MJ, Bennett CL. The learning curve for laparoscopic cholecystectomy. The Southern Surgeons Club. Am J Surg. 1995;170:55-9.
95. Jones-Monahan K, Gruenberg JC. Bile duct injuries during laparoscopic cholecystectomy: A community’s experience. Am Surg. 1998;64:638-42.
96. Richardson MC, Bell G, Fullarton GM. Incidence and nature of bile duct injuries following laparoscopic cholecystectomy: An audit of 5913 cases. West of Scotland Laparoscopic Cholecystectomy Audit Group. Br J Surg. 1996;83:1356-60.
97. Windsor JA, Pong J. Laparoscopic biliary injury: More than a learning curve problem. Aust N Z J Surg. 1998;68:186-9.
98. Woods MS, Traverso LW, Kozarek RA, et al. Characteristics of biliary tract complications during laparoscopic cholecystectomy: A multi-institutional study. Am J Surg. 1994;167:27-33.
99. Legorreta AP, Silber JH, Costantino GN, et al. Increased cholecystectomy rate after the introduction of laparoscopic cholecystectomy. JAMA. 1993;270:1429-32.
100. McMahon AJ, Fischbacher CM, Frame SH, MacLeod MC. Impact of laparoscopic cholecystectomy: A population-based study. Lancet. 2000;356:1632-7.
101. Attili AF, De Santis A, Capri R, et al. The natural history of gallstones: The GREPCO experience. The GREPCO Group. Hepatology. 1995;21:655-60.
102. Friedman GD. Natural history of asymptomatic and symptomatic gallstones. Am J Surg. 1993;165:399-404.
103. Gracie WA, Ransohoff DF. The natural history of silent gallstones: The innocent gallstone is not a myth. N Eng J Med. 1982;307:798-800.
104. McSherry CK, Ferstenberg H, Calhoun WF, et al. The natural history of diagnosed gallstone disease in symptomatic and asymptomatic patients. Ann Surg. 1985;202:59-63.
105. Williams CI, Shaffer EA. Gallstone disease: Current therapeutic practice. Curr Treat Options Gastroenterol. 2008;11:71-7.
106. Glasgow RE, Cho M, Hutter MM, Mulvihill SJ. The spectrum and cost of complicated gallstone disease in California. Arch Surg. 2000;135:1021-7.
107. Ransohoff DF, Gracie WA, Wolfenson LB, Neuhauser D. Prophylactic cholecystectomy or expectant management for silent gallstones. A decision analysis to assess survival. Ann Intern Med. 1983;99:199-204.
108. Gibney EJ. Asymptomatic gallstones. Br J Surg. 1990;77:368-72.
109. Ransohoff DF, Gracie WA. Treatment of gallstones. Ann Intern Med. 1993;119:606-19.
110. Quintana JM, Cabriada J, Arostegui I, et al. Health-related quality of life and appropriateness of cholecystectomy. Ann Surg. 2005;241:110-18.
111. Gupta D, Sakorafas GH, McGregor CG, et al. Management of biliary tract disease in heart and lung transplant patients. Surgery. 2000;128:641-9.
112. Miller K, Hell E, Lang B, Lengauer E. Gallstone formation prophylaxis after gastric restrictive procedures for weight loss: A randomized double-blind placebo-controlled trial. Ann Surg. 2003;238:697-702.
113. Richardson WS, Surowiec WJ, Carter KM, et al. Gallstone disease in heart transplant recipients. Ann Surg. 2003;237:273-6.
114. Greenstein SM, Katz S, Sun S, et al. Prevalence of asymptomatic cholelithiasis and risk of acute cholecystitis after kidney transplantation. Transplantation. 1997;63:1030-2.
115. Melvin WS, Meier DJ, Elkhammas EA, et al. Prophylactic cholecystectomy is not indicated following renal transplantation. Am J Surg. 1998;175:317-19.
116. Sandler RS, Maule WF, Baltus ME. Factors associated with postoperative complications in diabetics after biliary tract surgery. Gastroenterology. 1986;91:157-62.
117. Friedman LS, Roberts MS, Brett AS, Marton KI. Management of asymptomatic gallstones in the diabetic patient. A decision analysis. Ann Intern Med. 1988;109:913-19.
118. Fagan SP, Awad SS, Rahwan K, et al. Prognostic factors for the development of gangrenous cholecystitis. Am J Surg. 2003;186:481-5.
119. Aranha GV, Kruss D, Greenlee HB. Therapeutic options for biliary tract disease in advanced cirrhosis. Am J Surg. 1988;155:374-7.
120. Puggioni A, Wong LL. A metaanalysis of laparoscopic cholecystectomy in patients with cirrhosis. J Am Coll Surg. 2003;197:921-6.
121. Poggio JL, Rowland CM, Gores GJ, et al. A comparison of laparoscopic and open cholecystectomy in patients with compensated cirrhosis and symptomatic gallstone disease. Surgery. 2000;127:405-11.
122. Sleeman D, Namias N, Levi D, et al. Laparoscopic cholecystectomy in cirrhotic patients. J Am Coll Surg. 1998;187:400-3.
123. Choudhary A, Bechtold ML, Puli SR, et al. Role of prophylactic antibiotics in laparoscopic cholecystectomy: A meta-analysis. J Gastrointest Surg. 2008;12:1847-53.
124. Thompson JJr, Bennion RS, Doty JE, et al. Predictive factors for bactibilia in acute cholecystitis. Arch Surg. 1990;125:261-4.
125. Jarvinen HJ, Hastbacka J. Early cholecystectomy for acute cholecystitis: A prospective randomized study. Ann Surg. 1980;191:501-5.
126. Lahtinen J, Alhava EM, Aukee S. Acute cholecystitis treated by early and delayed surgery. A controlled clinical trial. Scand J Gastroenterol. 1978;13:673-8.
127. Linden Wvd, Sunzel H. Early versus delayed operation for acute cholecystitis. A controlled clinical trial. Am J Surg. 1970;120:7-13.
128. Lo CM, Liu CL, Fan ST, et al. Prospective randomized study of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Ann Surg. 1998;227:461-7.
129. McArthur P, Cuschieri A, Sells RA, Shields R. Controlled clinical trial comparing early with interval cholecystectomy for acute cholecystitis. Br J Surg. 1975;62:850-2.
130. Papi C, Catarci M, D’Ambrosio L, et al. Timing of cholecystectomy for acute calculous cholecystitis: A meta-analysis. Am J Gastroenterol. 2004;99:147-55.
131. Siddiqui T, MacDonald A, Chong PS, Jenkins JT. Early versus delayed laparoscopic cholecystectomy for acute cholecystitis: A meta-analysis of randomized clinical trials. Am J Surg. 2008;195:40-7.
132. Aucott JN, Cooper GS, Bloom AD, Aron DC. Management of gallstones in diabetic patients. Arch Intern Med. 1993;153:1053-8.
133. Ikard RW. Gallstones, cholecystitis and diabetes. Surg Gynecol Obstet. 1990;171:528-32.
134. Laycock WS, Siewers AE, Birkmeyer CM, et al. Variation in the use of laparoscopic cholecystectomy for elderly patients with acute cholecystitis. Arch Surgery. 2000;135:457-62.
135. Lo CM, Lai EC, Fan ST, et al. Laparoscopic cholecystectomy for acute cholecystitis in the elderly. World J Surg. 1996;20:983-6.
136. Morrow DJ, Thompson J, Wilson SE. Acute cholecystitis in the elderly: A surgical emergency. Arch Surg. 1978;113:1149-52.
137. Gurusamy KS, Samraj K, Mullerat P, Davidson BR. Routine abdominal drainage for uncomplicated laparoscopic cholecystectomy. Cochrane Database Syst Rev 2007:CD006004.
138. Owen CC, Jain R. Acute acalculous cholecystitis. Curr Treat Options Gastroenterol. 2005;8:99-104.
139. Bonacini M. Hepatobiliary complications in patients with human immunodeficiency virus infection. Am J Med. 1992;92:404-11.
140. Babb RR. Acute acalculous cholecystitis. A review. J Clin Gastroenterol. 1992;15:238-41.
141. Kaminski DL, Andrus CH, German D, Deshpande YG. The role of prostanoids in the production of acute acalculous cholecystitis by platelet-activating factor. Ann Surg. 1990;212:455-61.
142. Kaminski DL, Feinstein WK, Deshpande YG. The production of experimental cholecystitis by endotoxin. Prostaglandins. 1994;47:233-45.
143. Owen CC, Bilhartz LE. Gallbladder polyps, cholesterolosis, adenomyomatosis, and acute acalculous cholecystitis. Semin Gastrointest Dis. 2003;14:178-88.
144. Warren BL. Small vessel occlusion in acute acalculous cholecystitis. Surgery. 1992;111:163-8.
145. Johnson LB. The importance of early diagnosis of acute acalculus cholecystitis. Surg Gynecol Obstet. 1987;164:197-203.
146. Hunt DR, Chu FC. Gangrenous cholecystitis in the laparoscopic era. Aust N Z J Surg. 2000;70:428-30.
147. Cameron DR, Goodman AJ. Delayed cholecystectomy for gallstone pancreatitis: Re-admissions and outcomes. Ann R Coll Surg Engl. 2004;86:358-62.
148. Kelly TR, Wagner DS. Gallstone pancreatitis: A prospective randomized trial of the timing of surgery. Surgery. 1988;104:600-5.
149. Tang E, Stain SC, Tang G, et al. Timing of laparoscopic surgery in gallstone pancreatitis. Arch Surg. 1995;130:496-9.
150. Ito K, Ito H, Whang EE. Timing of cholecystectomy for biliary pancreatitis: Do the data support current guidelines? J Gastrointest Surg. 2008;12:2164-70.
151. Uhl W, Warshaw A, Imrie C, et al. IAP guidelines for the surgical management of acute pancreatitis. Pancreatology. 2002;2:565-73.
152. Carr-Locke DL. Role of endoscopy in gallstone pancreatitis. Am J Surg. 1993;165:519-21.
153. Fan ST, Lai EC, Mok FP, et al. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Eng J Med. 1993;328:228-32.
154. Moretti A, Papi C, Aratari A, et al. Is early endoscopic retrograde cholangiopancreatography useful in the management of acute biliary pancreatitis? A meta-analysis of randomized controlled trials. Dig Liver Dis. 2008;40:379-85.
155. Worthley CS, Toouli J. Gallbladder non-filling: An indication for cholecystectomy after endoscopic sphincterotomy. Br J Surg. 1988;75:796-8.
156. Glasgow RE, Visser BC, Harris HW, et al. Changing management of gallstone disease during pregnancy. Surg Endosc. 1998;12:241-6.
157. Rollins MD, Chan KJ, Price RR. Laparoscopy for appendicitis and cholelithiasis during pregnancy: A new standard of care. Surg Endosc. 2004;18:237-41.
158. Lobe TE. Cholelithiasis and cholecystitis in children. Semin Pediatr Surg. 2000;9:170-6.
159. Zaliekas J, Munson JL. Complications of gallstones: The Mirizzi syndrome, gallstone ileus, gallstone pancreatitis, complications of “lost” gallstones. Surg Clin North Am. 2008;88:1345-68.
160. Deitz DM, Standage BA, Pinson CW, et al. Improving the outcome in gallstone ileus. Am J Surg. 1986;151:572-6.
161. Reisner RM, Cohen JR. Gallstone ileus: A review of 1001 reported cases. Am Surg. 1994;60:441-6.
162. Wudel LJJr, Wright JK, Debelak JP, et al. Prevention of gallstone formation in morbidly obese patients undergoing rapid weight loss: Results of a randomized controlled pilot study. J Surg Res. 2002;102:50-6.
163. Diehl AK. Gallstone size and the risk of gallbladder cancer. JAMA. 1983;250:2323-6.
164. Reid KM, Ramos-De la Medina A, Donohue JH. Diagnosis and surgical management of gallbladder cancer: A review. J Gastrointest Surg. 2007;11:671-81.
165. Stephen AE, Berger DL. Carcinoma in the porcelain gallbladder: A relationship revisited. Surgery. 2001;129:699-703.
166. Curro G, Meo A, Ippolito D, et al. Asymptomatic cholelithiasis in children with sickle cell disease: Early or delayed cholecystectomy? Ann Surg. 2007;245:126-9.
167. Serafini AN, Spoliansky G, Sfakianakis GN, et al. Diagnostic studies in patients with sickle cell anemia and acute abdominal pain. Arch Intern Med. 1987;147:1061-2.
168. Goldfarb A, Grisaru D, Gimmon Z, et al. High incidence of cholelithiasis in older patients with homozygous beta-thalassemia. Acta Haematologica. 1990;83:120-2.
169. Bragg LE, Thompson JS. Concomitant cholecystectomy for asymptomatic cholelithiasis. Arch Surg. 1989;124:460-2.
170. McSherry CK, Glenn F. Biliary tract surgery concomitant with other intra-abdominal operations. Ann Surg. 1981;193:169-75.
171. Juhasz ES, Wolff BG, Meagher AP, et al. Incidental cholecystectomy during colorectal surgery. Ann Surg. 1994;219:467-72.
172. Green JD, Birkhead G, Hebert J, et al. Increased morbidity in surgical patients undergoing secondary (incidental) cholecystectomy. Ann Surg. 1990;211:50-4.
173. Schulman CI, Levi J, Sleeman D, et al. Are we training our residents to perform open gall bladder and common bile duct operations? J Surg Res. 2007;142:246-9.
174. Cuschieri A, Lezoche E, Morino M, et al. E.A.E.S. multicenter prospective randomized trial comparing two-stage vs. single-stage management of patients with gallstone disease and ductal calculi. Surg Endosc. 1999;13:952-7.
175. Kharbutli B, Velanovich V. Management of preoperatively suspected choledocholithiasis: A decision analysis. J Gastrointest Surg. 2008;12:1973-80.
176. Tinoco R, Tinoco A, El-Kadre L, et al. Laparoscopic common bile duct exploration. Ann Surg. 2008;247:674-9.
177. Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: An attempt at consensus. Gastrointest Endosc. 1991;37:383-93.
178. Rhodes M, Sussman L, Cohen L, Lewis MP. Randomised trial of laparoscopic exploration of common bile duct versus postoperative endoscopic retrograde cholangiography for common bile duct stones. Lancet. 1998;351:159-61.
179. Rojas-Ortega S, Arizpe-Bravo D, Marin Lopez ER, et al. Transcystic common bile duct exploration in the management of patients with choledocholithiasis. J Gastrointest Surg. 2003;7:492-6.
180. Urbach DR, Khajanchee YS, Jobe BA, et al. Cost-effective management of common bile duct stones: A decision analysis of the use of endoscopic retrograde cholangiopancreatography (ERCP), intraoperative cholangiography, and laparoscopic bile duct exploration. Surg Endosc. 2001;15:4-13.
181. Davidoff AM, Pappas TN, Murray EA, et al. Mechanisms of major biliary injury during laparoscopic cholecystectomy. Ann Surg. 1992;215:196-202.
182. Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 1995;180:101-25.
183. Way LW, Stewart L, Gantert W, et al. Causes and prevention of laparoscopic bile duct injuries: Analysis of 252 cases from a human factors and cognitive psychology perspective. Ann Surg. 2003;237:460-9.
184. Chapman WC, Halevy A, Blumgart LH, Benjamin IS. Postcholecystectomy bile duct strictures. Management and outcome in 130 patients. Arch Surg. 1995;130:597-602.
185. Lillemoe KD, Melton GB, Cameron JL, et al. Postoperative bile duct strictures: Management and outcome in the 1990s. Ann Surg. 2000;232:430-41.
186. Sicklick JK, Camp MS, Lillemoe KD, et al. Surgical management of bile duct injuries sustained during laparoscopic cholecystectomy: Perioperative results in 200 patients. Ann Surg. 2005;241:786-92.
187. Walsh RM, Henderson JM, Vogt DP, Brown N. Long-term outcome of biliary reconstruction for bile duct injuries from laparoscopic cholecystectomies. Surgery. 2007;142:450-6.
188. Stewart L, Way LW. Bile duct injuries during laparoscopic cholecystectomy. Factors that influence the results of treatment. Arch Surg. 1995;130:1123-8.
189. Pitt HA, Kaufman SL, Coleman J, et al. Benign postoperative biliary strictures. Operate or dilate? Arch Surg. 1989;210:417-25.
190. Bates T, Ebbs SR, Harrison M, A’Hern RP. Influence of cholecystectomy on symptoms. Br J Surg. 1991;78:964-7.
191. Fenster LF, Lonborg R, Thirlby RC, Traverso LW. What symptoms does cholecystectomy cure? Insights from an outcomes measurement project and review of the literature. Am J Surg. 1995;169:533-8.
192. Luman W, Adams WH, Nixon SN, et al. Incidence of persistent symptoms after laparoscopic cholecystectomy: A prospective study. Gut. 1996;39:863-6.
193. Weinert CR, Arnett D, Jacobs DJr, Kane RL. Relationship between persistence of abdominal symptoms and successful outcome after cholecystectomy. Arch Intern Med. 2000;160:989-95.
194. Williams EJ, Green J, Beckingham I, et al. Guidelines on the management of common bile duct stones (CBDS). Gut. 2008;57:1004-21.
195. Shaw C, O’Hanlon DM, Fenlon HM, McEntee GP. Cystic duct remnant and the “post-cholecystectomy syndrome.”. Hepatogastroenterology. 2004;51:36-8.
196. Walsh RM, Ponsky JL, Dumot J. Retained gallbladder/cystic duct remnant calculi as a cause of postcholecystectomy pain. Surg Endosc. 2002;16:981-4.
197. Jonson G, Nilsson DM, Nilsson T. Cystic duct remnants and biliary symptoms after cholecystectomy. A randomised comparison of two operative techniques. Eur J Surg. 1991;157:583-6.
198. Palanivelu C, Rangarajan M, Jategaonkar PA, et al. Laparoscopic management of remnant cystic duct calculi: A retrospective study. Ann R Coll Surg Engl. 2009;91:25-9.
199. Madura JA2nd, Madura JA. Diagnosis and management of sphincter of Oddi dysfunction and pancreas divisum. Surg Clin North Am. 2007;87:1417-29.
200. Piccinni G, Angrisano A, Testini M, Bonomo GM. Diagnosing and treating sphincter of Oddi dysfunction: A critical literature review and reevaluation. J Clin Gastroenterol. 2004;38:350-9.
201. Geenen JE, Hogan WJ, Dodds WJ, et al. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter-of-Oddi dysfunction. N Eng J Med. 1989;320:82-7.
202. Morgan KA, Romagnuolo J, Adams DB. Transduodenal sphincteroplasty in the management of sphincter of Oddi dysfunction and pancreas divisum in the modern era. J Am Coll Surg. 2008;206:908-14.
203. Schottenfeld D, Winawer SJ. Cholecystectomy and colorectal cancer. Gastroenterology. 1983;85:966-7.
204. Shao T, Yang YX. Cholecystectomy and the risk of colorectal cancer. Am J Gastroenterol. 2005;100:1813-20.
205. Lowenfels AB, Domellof L, Lindstrom CG, et al. Cholelithiasis, cholecystectomy, and cancer: A case-control study in Sweden. Gastroenterology. 1982;83:672-6.
206. Lowenfels AB, Lindstrom CG, Conway MJ, Hastings PR. Gallstones and risk of gallbladder cancer. J Natl Cancer Inst. 1985;75:77-80.
207. Lowenfels AB, Walker AM, Althaus DP, et al. Gallstone growth, size, and risk of gallbladder cancer: an interracial study. Int J Epidemiol. 1989;18:50-4.
208. Ekbom A, Hsieh CC, Yuen J, et al. Risk of extrahepatic bile duct cancer after cholecystectomy. Lancet. 1993;342:1262-5.
209. Walden DT, Soloway RD, Crowther RS. Cholecystectomy protects against extrahepatic bile duct cancer: Is this a result of the removal of gallstones? Hepatology. 1994;19:1533-4.
210. Linos D, Beard CM, O’Fallon WM, et al. Cholecystectomy and carcinoma of the colon. Lancet. 1981;2:379-81.
211. Turunen MJ, Kivilaakso EO. Increased risk of colorectal cancer after cholecystectomy. Ann Surg. 1981;194(5):639-41.
212. Vernick LJ, Kuller LH. Cholecystectomy and right-sided colon cancer: An epidemiological study. Lancet. 1981;2:381-3.