Chapter 22 Traumatic Skull and Facial Fractures
• Linear skull fractures do not require stabilization or treatment when the scalp is closed. Depressed skull fractures may need exploration depending on the extent of the injury to the underlying brain, frontal sinus, or facial bones. Closed depressed fractures are usually repaired for cosmetic reasons. Compound depressed skull fractures with brain involvement are often neurosurgical emergencies. It becomes an emergency to treat the underlying brain injury, perform a watertight closure of the dura, and débride the devitalized scalp.
• Growing skull fractures, although rare, occur in children under 2 to 3 years of age and require surgical repair. If there is a tear in the dura accompanying a skull fracture after trauma, the pressure of the brain pulsations in a growing brain may enlarge the fracture and dural opening. The brain can herniate through this skull defect, causing a pulsatile mass under the scalp.
• Basilar skull fractures may present with periorbital ecchymoses, hemotympanum, or ecchymosis over the mastoid. The management of these fractures is usually conservative unless a cerebrospinal fluid (CSF) leak is present. Many traumatic CSF leaks will spontaneously resolve in a week. Those that do not may be managed by CSF drainage, or in some cases surgical repair to avoid infection, once the location of the leak is identified.
• Frontal sinus fractures can be diagnosed on computed tomography (CT) and are managed differently depending on whether the frontal or posterior wall is disrupted. Orbital fractures are managed based on the extent of the injury to the globe, optic nerve, and orbital contents. Patients with a fluctuating or worsening visual acuity will require decompression of their optic nerve and relief of globe pressure. There are two indications for surgery of orbital “blow-out” fractures: (1) muscle or ligament entrapment with diplopia or (2) enophthalmus (backward dislocation of the globe) caused by prolapse of the orbital contents through the fracture. Le Fort fractures are often injuries to the entire midface region. Their surgical management depends on the extent and stability of the maxillary fracture.
Skull Fractures
The second factor is the ratio of the impact force to the impact area. If the impact, even one of high energy, is dispersed over a large area, as in a blunt head injury to an individual wearing a motorcycle helmet, it often produces no skull fracture, even though the brain may be severely injured. Parenthetically, it should be noted that some helmets, by the efficiency of their very force-transferring protection, have created basal skull fractures by transferred energy absorbed from protection of the vault and face and then transmitted through the mandible via the chin strap to the skull base. However, if the impact, even one of low energy, is concentrated in a small area, such as from a hammer blow, it often produces a small depressed fracture with multiple linear skull fractures radiating from the site of impact.
Linear Skull Fractures
Management
Linear skull fractures require no stabilization or exploration when the scalp is closed, and when there is no evidence of epidural hematoma or underlying dural or cortical injury. Even when a scalp laceration is present, very seldom is surgical exploration with bone removal necessary. Exceptions would include a machete injury to the skull producing a linear skull fracture with underlying dural laceration and brain damage. The skull fracture does, however, show that significant head trauma has occurred, and a careful assessment of the brain, facial structures, and cervical spine is required. Open linear fractures are débride of foreign material, devitalized soft tissue, and bone fragments; preferably, the damaged soft tissue at the edges of the laceration is excised to healthy, noncontused bleeding tissue (if the tissue excision can be tolerated and will permit primary closure) and the laceration is closed after thorough cleansing. If there is insufficient vascularized soft tissue present to permit excision of the contused devitalized tissue, a rotation flap and skin graft to the donor area may have to be considered (Fig. 22.1).
Growing Skull Fractures in Children
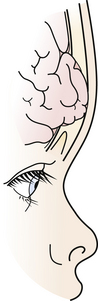
A rare complication after linear skull fracture in young children (usually younger than 2 or 3 years) is a “growing” skull defect at the fracture site. In these cases, the dura is torn under the linear skull fracture. The pathogenesis is thought to be an expanding pouch of arachnoid passing through the torn dura and skull fracture, acting as a one-way valve that traps cerebrospinal fluid (CSF) and causes progressive pressure erosion of the fractured edges to enlarge the fracture. Alternatively, the growth of the brain, which produces pulsating, spreading tensile pressure forces on the edges of an unrepaired dural laceration, may also cause a skull defect to enlarge. These vectors of force by the brain may sometimes cause herniation through the skull defect, causing a new neurological deficit (Fig. 22.2). These lesions are surgically repaired with closure of the dura or with a dural patch and replacement or repair of the bone defect. Some surgeons routinely take a skull film at 1 year after linear skull fracture treated nonoperatively to detect such growing skull fractures. For this reason it is worthwhile for the primary care doctor to examine the scalp and skull of any child with a known skull fracture under the age of 2 or 3.
Comminuted Fractures
Management
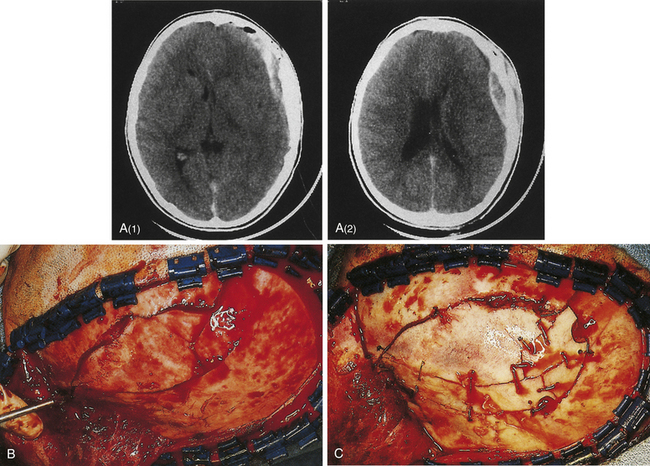
If the skin is closed, and no depression of bone fragments greater than the thickness of the skull is demonstrated on CT, management is as that for linear skull fractures. However, in many of these cases, surgery is performed for the underlying intracranial injury, such as an epidural hematoma (Fig. 22.3). After the intracranial injury has been corrected, the bone fragments are primarily replaced as a bone cranioplasty after cleaning.
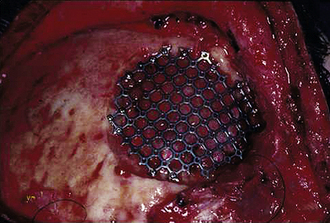
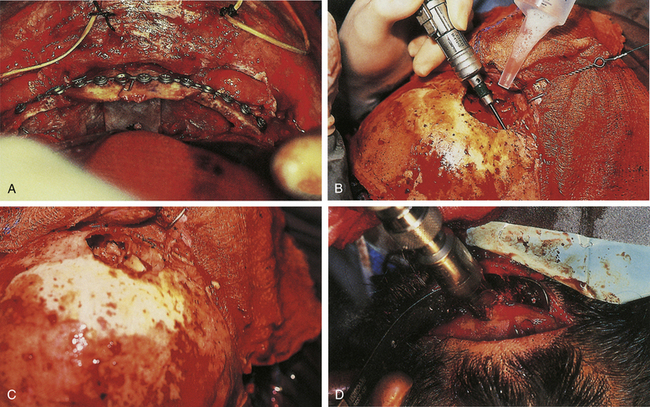
Missing bone can be replaced with a titanium mesh screen. If the skin is open, and free bone fragments are present, cleansing or débridement of the contaminated fragments is performed, before dural and scalp closure (Fig. 22.4). Bone too contaminated may be discarded, and a titanium screen is then used to span the bone defect (Fig. 22.5).
Depressed Skull Fractures
Management
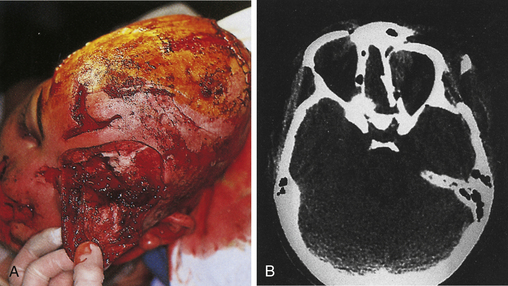
Combined therapy of depressed fractures of the cranial vault extending to involve the frontal sinus or facial bones is covered in the sections on facial fractures. When a depressed skull fracture on the convexity also includes facial fractures, the intracranial injury is typically repaired first with removal of intracerebral hematoma and repair of dural laceration if present (Figs. 22.6 and 22.7).
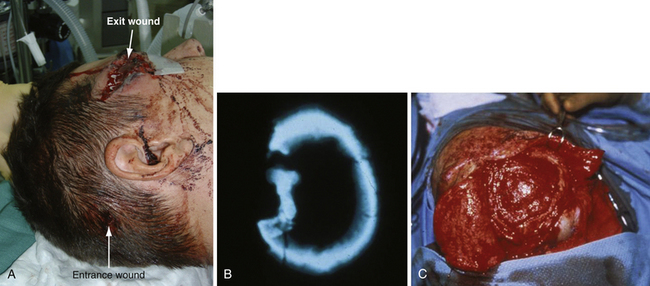
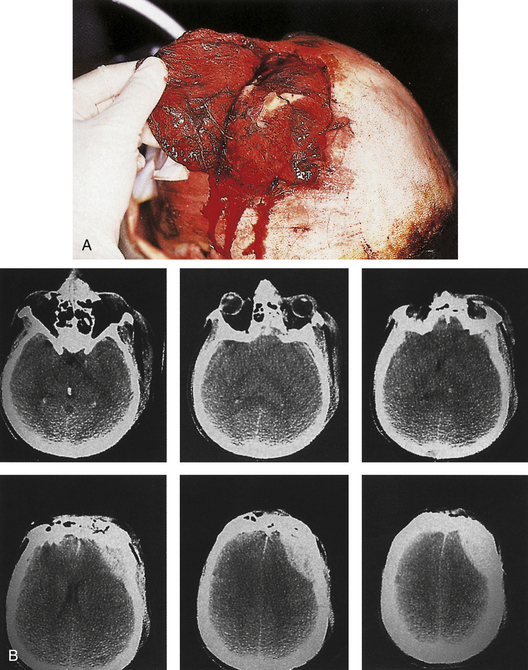
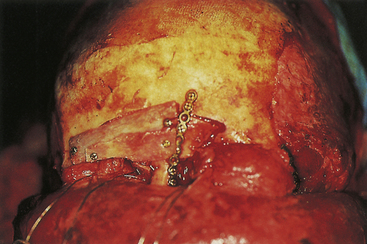
A compound depressed fracture is a neurosurgical emergency because of the risk of bacterial infection of the cranial cavity. The initial surgery is performed within 24 hours and usually within the first 12 hours. The major objectives are removal of contaminated bone fragment and foreign material; débridement of devitalized scalp, dura, and brain; and provision of a watertight closure of the dura. Often, foreign material or hair wedged between bone fragments cannot be seen through the overlying scalp incision, so simple irrigation and closure may be inadequate for débridement of foreign material. Dural closure is essential to prevent CSF leaks from the wound and brain herniation into the fracture area. Dural closure is essential to prevent CSF leaks from the wound and brain herniation into the fracture area. Dural closure also presents intracranial spread of infection from a scalp wound. Reconstruction of the calvarium is performed during the initial surgery if considered safe: otherwise a cranial defect is left and the cosmetic repair is performed later. The major reasons to consider deferred calvarial reconstruction are to shorten additional anesthesia and blood loss by major head injury or multitrauma, especially with hemorrhage; gross contamination of wounds where the bone fragments cannot be adequately cleaned; and a delay of more than 24 hours for the initial surgery.
The scalp laceration associated with a compound depressed skull fracture is usually stellate and may contain areas of contused/devitalized tissue. These areas require débridement to normal vascularized scalp to allow prompt healing and prevent breakdown of the partially viable scalp covering the fracture site (see Fig. 22.1). Scalp breakdown can many times be treated locally but occasionally will require early flap coverage. If early flap coverage is not successful, the replaced cranial bone may require débridement of any dead or nonviable necrotic bone or portion of the skin flap; a subsequent flap rotation and delayed cranioplasty will be required in stages.
Basilar Skull Fractures
Fractures of the base of the skull occur in 3.5% to 24% of head-injured patients. This wide variation results from differences in study populations and the difficulty in obtaining radiographic verification of the fractures. Linear fractures in the skull base carry a risk of meningitis, whereas this risk is extremely low in fractures of the convexity unless the scalp, bone, and dura are all violated. The dura is easily torn in a basal skull fracture; this places the subarachnoid space in direct contact with the paranasal sinuses or middle ear structures, providing a pathway for infection. For example, a persistent fistula allows a continuous CSF leak, and bacterial colonization of the meninges will eventually develop.
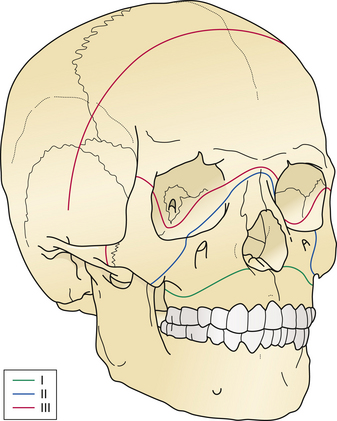
Petrous bone fractures can either be either longitudinal or transverse, relative to the long axis of the petrous pyramid. Longitudinal fractures are more common and usually involve the tympanic membrane or external ear canal, thereby producing otorrhea. Transverse fractures result from higher-energy impacts and can damage middle ear ossicles or the facial nerve. These fractures occur with or in continuity with linear, comminuted, or depressed skull fractures and not infrequently are large linear extensions of vault fractures, crossing the base of the anterior and middle cranial fossae (Fig. 22.8).
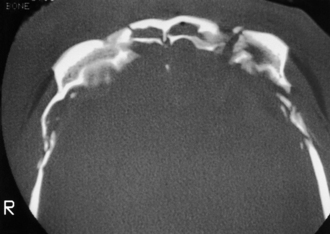
FIGURE 22.8 Type III frontobasilar fracture involves both the lateral and the central segments of the anterior skull.
Diagnosis
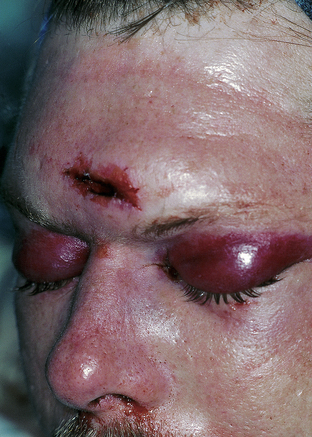
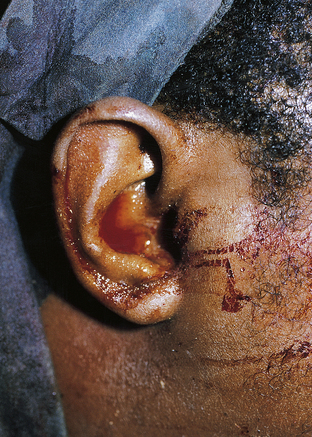
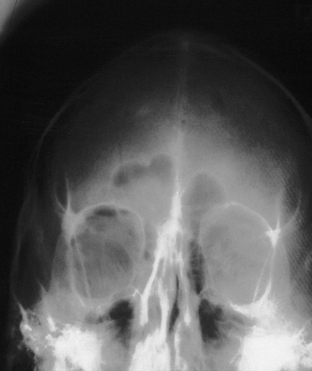
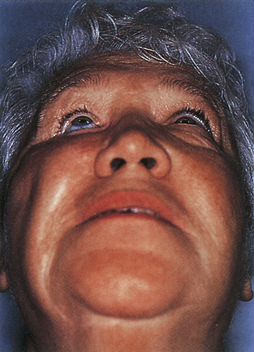
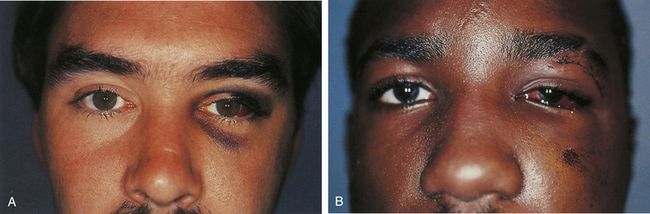
Clinical signs of basal skull fractures include bilateral periorbital ecchymoses (spectacle hematoma) (Fig. 22.9), anosmia, or CSF rhinorrhea for anterior skull base fractures, as well as hemotympanum, blood in the external auditory canal, seventh or eighth cranial nerve palsies, ecchymoses over the mastoids (Battle’s sign), or CSF otorrhea for temporal bone fractures (Fig. 22.10). Frequently, the CSF leak is first detected several days or weeks after the trauma. This delay often occurs because the CSF leak was hidden in bloody nasal discharge from facial fractures, or less frequently, it is the result of delayed development of hydrocephalus with rupture of the arachnoid at the fracture site. A larger clear ring surrounding a central blood-tinged clot when a few drops of bloody discharge are placed on a paper towel indicates that CSF is probably mixed with the blood. This sign (the “double ring”) can also be noted on the patient’s pillow during rounds.
Basal skull fracture with CSF rhinorrhea is common after head injury and has an estimated incidence in the United States of 150,000 cases per year. A clear, watery nasal discharge containing glucose indicates CSF rhinorrhea. An intermittent CSF leak from the paranasal sinuses can often be demonstrated by having the patient sit on the edge of the bed with the head close to the knees for 2 minutes and watching for clear fluid to drip from the nose. CSF mixed with blood may form a halo on a piece of gauze it touches. Testing for beta-2 transferrin presence in the fluid confirms the protein found almost uniquely in CSF.
Maxillofacial Injuries
Assessment
Facial sensation is noted in the supraorbital, supratrochlear, infratrochlear, infraorbital, and mental nerve regions of the trigeminal nerve distribution for both pinprick and light touch sensation. Diminished sensation in the distribution of a specific sensory nerve indicates injury from transaction, impact, or continued compression of the nerve as the result of a fracture. The facial nerve is tested by comparing facial expression bilaterally. Extraocular movements and pupil response are compared, evaluating symmetry, pupil size, and the speed of pupil reaction bilaterally to both direct and consensual responses to light.
The early management of maxillofacial injuries is based entirely on a good clinical examination and facial CT scans (Fig. 22.11). Soft tissue windows are necessary on the CT scan to evaluate the brain and orbital soft tissue fully. Axial (Fig. 22.12) and coronal CT bone windows (direct or reformatted) are crucial to reveal details of fractures of the upper face and orbit. Coronal sections (Fig. 22.13) begin with the nasal pyramid and continue posteriorly through the orbital apex. Axial scans begin at the superior aspect of the skull and progress through the brain with standard axial brain imaging. The size and spacing of the cuts at the level of the frontal sinus are reduced to 5 mm or less to obtain the required detail. When a mandible fracture is suspected, the axial CT scanning is continued through the entire mandible and temporomandibular joints, visualizing both the horizontal and vertical portions of the mandible and the temporomandibular joints. Although three-dimensional reconstruction with shading (Fig. 22.14) adds spatial information, it does not provide the detail of two-dimensional axial and coronal images. In some cases, special reconstructions, as one performed in the longitudinal axis of the optic nerve in orbital injury, provide additional information.
Associated Conditions
Profuse Hemorrhage
Cutaneous bleeding that accompanies facial lacerations is usually controlled with digital pressure, which allows precise identification of the bleeding vessel for control or ligature. Blind probing in facial tissue or unselective cautery or ligature placement can damage branches of the facial nerve and should be avoided.
Coma and Brain Injury
Coma or unconsciousness should not prevent or delay the treatment of facial fractures; many patients with facial fractures are in a coma for several weeks before waking up. In patients with maxillofacial fractures, neurological deficits from frontal lobe symptoms may be subtle or absent despite contusions imaged on brain CT scans. Confusion, somnolence, personality change, irritability, and difficulty in thinking are some of the milder symptoms of frontal brain contusion. In patients with Glasgow Coma Scale scores of 14 or less, and especially when traumatic brain abnormality is visualized on CT scan, an intracranial pressure monitoring device may be employed in those patients who require anesthesia. A fiberoptic intracranial pressure (ICP) monitor or intracranial ventricular pressure monitor is used in the operating room during the facial repair, thus allowing optimal modification of the anesthesia and if necessary CSF drainage in patients in whom multiple injuries require early surgical intervention.
Facial Fracture Classification by Anatomical Region
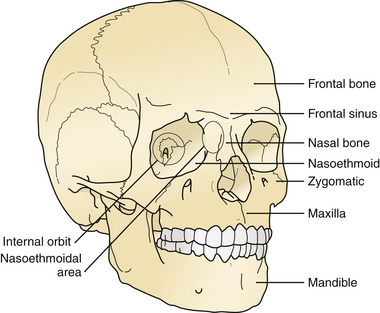
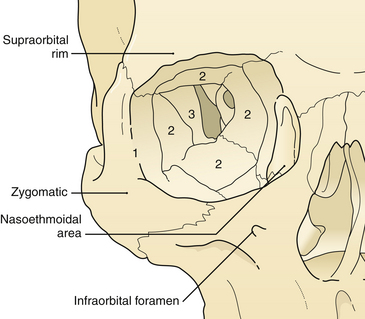
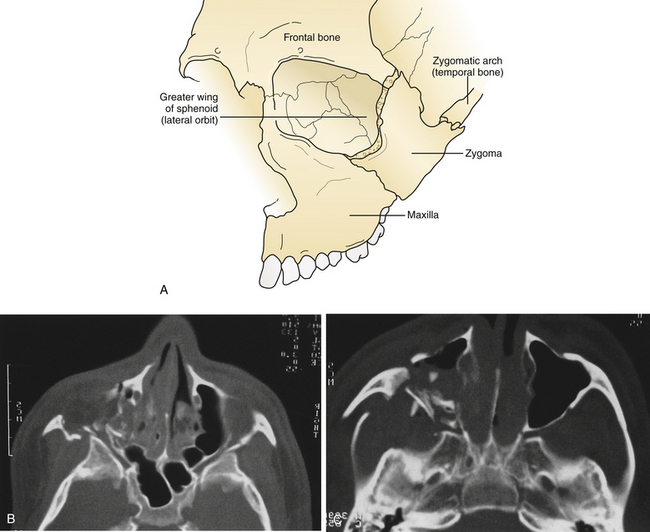
The treatment of maxillofacial fractures is organized by anatomical region (Fig. 22.15). The frontal bone region includes the frontal bone, the supraorbital rims bilaterally, and the frontal sinus (Fig. 22.16). The upper midface region includes the zygoma laterally, the internal orbital area, and the nasoethmoidal area centrally. The lower midface consists of the maxillary alveolus. The mandible consists of the horizontal portion containing the teeth and the vertical portion that includes the angle, ramus, coronoid, and condylar process. The pattern and displacement of the fractures in each anatomical region determine treatment. Orbital fractures are classified by their position on the orbital rim and by their involvement of the internal section of the orbit. Orbital rim fractures are divided into supraorbital, the nasoethmoid medically, and the zygomatic region inferolaterally (Fig. 22.17). The section of the internal orbit consists of the orbital floor, the lateral orbit, and the medial (ethmoidal) orbit. Maxillary fractures are classified according to the patterns of Le Fort, based on the fracture’s location in the maxilla where it is separated from intact upper facial units.
Fractures of the Frontal Bone and Supraorbital Area
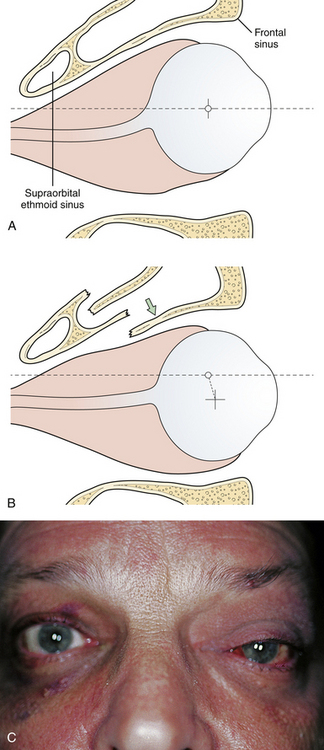
The frontal and ethmoid sinuses render the frontal bone more vulnerable to injury and infection. Each major segment of the frontal sinus (generally two) has a “duct” (usually a broad ostium) that communicates with the middle meatus of the nose. Sinus injury may therefore result in duct obstruction after fracture, mucosal edema, or damage (Fig. 22.18). A cyst-like structure called a mucocele (obstructed mucous cyst) sometimes follows mucosal injury; depending on size, it may create eroding bone pressure and penetrate into the orbit or intracranial cavity. Surgery at a later time is necessary to remedy either of these conditions, as symptoms of pain and sinusitis will persist. Because the posterior wall of the frontal sinus is in contact with the dura, any infection in that area represents an extradural abscess. Posterior wall fractures of the frontal sinus are often accompanied by dural tears. Many of these tears extend along the anterior frontobasilar region of the skull to cause CSF leak or pneumocephalus. A small CSF leak is often masked by epistaxis in the early days after facial injury. When fractures in the posterior wall of the frontal sinus or the anterior base of the skull are noted, a CSF leak should be suspected.
Fractures of the frontal bone commonly extend within the cranial sutures and then involve other regions. When the fracture extends into the supraorbital region, the bone is usually depressed downward and posteriorly, compressing the orbital contents and producing a downward and forward dislocation of the globe (Fig. 22.19). With more limited injuries, linear frontal skull fractures may extend into the orbit and along the cranial base. These fractures can create a CSF leak or obstruct sinus drainage by edema or bone displacement. As fracture patterns become more complex and severe, bone displacement occurs. The anterior base of the skull and the roofs of the orbit are comminuted, and linear fractures extend from the anterior through the middle cranial fossa. The anterior and middle sections of the orbit displace, absorbing energy, and linear fractures extend from the displaced bone through the posterior portion of the orbit and into the middle cranial fossa. These fractures can account for basilar CSF leaks, pituitary disturbances, and dizziness from involvement of the temporal bone and vestibular structures.
Diagnosis
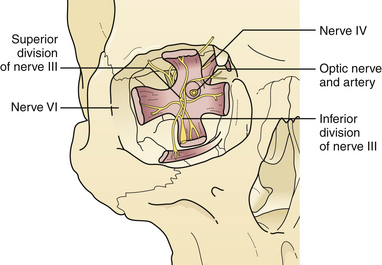
Supraorbital fractures involving the orbital rim are usually displaced inward and downward (see Fig. 22.19), producing a forward and downward displacement of the globe. The globe occasionally bulges forward so that the eyelids cannot close completely. In such cases urgent facial fracture reduction is required to protect the cornea. Fractures of the orbital roof may have a linear extension that enters the superior orbital fissure or optic foramen. Visual acuity is affected if the optic nerve is compressed by a displaced fracture fragment or by edema or nerve shearing, in which case direct pupillary response to light on the injured side is slower than the direct pupil response on the other side. A superior orbital fissure syndrome may also be present, consisting of variable palsy of extraocular muscle motion (cranial nerves III, IV, VI), ptosis, global proptosis, and anesthesia in the first division of the trigeminal nerve (the ipsilateral forehead) (Fig. 22.20 and see section on Orbital Fractures). Patients may experience numbness in the distribution of the supraorbital and supratrochlear nerves, which is usually transient, although lacerations in the area frequently divide the nerve, producing permanent numbness.
Frontal Sinus Fractures
Diagnosis
Frontal sinus fractures are diagnosed on CT scan noting fractures of the front wall, back wall, duct, or both walls. Displacement of bone of the posterior wall more than the thickness of the bone wall usually indicates an underlying dural laceration.
Management
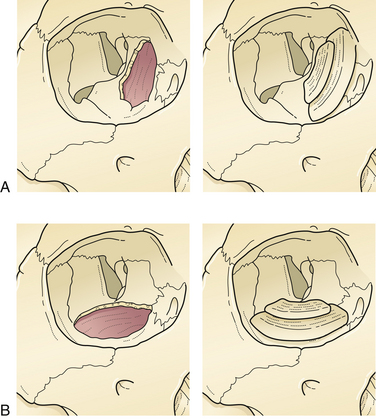
Localized fractures of the anterior wall are managed by returning the bone fragments to the proper position and debriding any devitalized mucosa. If the nasofrontal duct is intact, fluid will flow freely into the nose; replaced bone fragments are stabilized with plate and screw fixation. If the posterior wall is involved, the integrity of the dura is usually assessed by direct inspection at operation. Significant fractures of the anterior and posterior walls are best managed by intracranial exposure and débridement of small bone fragments, and defunction of the sinus. If the posterior wall of the sinus is removed, the sinus is “cranialized” by completely removing the mucosa and plugging the nasofrontal duct with several layers of bone grafts. A sheet bone graft is placed over the bone plugs and over the involved ethmoid sinuses (Fig. 22.21). Involved sinus must be debrided to minimize infection and delayed mucocele formation because obstruction of an ethmoid sinus produces an orbital or epidural abscess. The anterior wall of the frontal sinus is then reconstructed. Complete removal of sinus mucosa requires mucosal stripping and light burring of its bone fragments as the mucosa has minute invaginations (foramina of Breschet) into the bone.
Less complicated frontal sinus fractures may be managed by another more limited procedure which defunctionalizes the sinus “obliteration.” When fractures compromise nasofrontal duct function, the sinus mucosa should be removed and the walls of the sinus should be burred to bleeding bone. The nasofrontal duct is then plugged with several layers of bone plugs taken from the calvaria, and the remainder of the sinus is filled with bone shavings (Fig. 22.22). Unfortunately, regrowth of frontal sinus mucosa may occasionally occur, or the development of a cyst in a lacerated area of mucosa may produce a mucocele. Surgical intervention may be required for infection or erosion of the cyst into adjacent structures.
Orbital Fractures
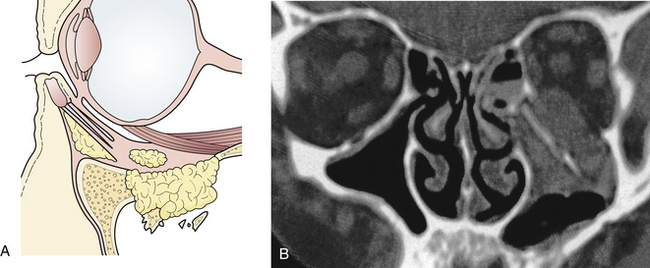
The supraorbital rims are weakened centrally by the presence of the frontal sinus. The supraorbital rim extends to join the temporal bone and the zygoma (see Fig. 22.17). The orbit cavity itself has three sections (see Fig. 22.17): the anterior or rim, the middle, and the posterior orbit. The midsection of the orbit can be divided into four regions (see Fig. 22.17) and the rim into three sections. Fractures occur first in the thin bone of the middle third of the orbit, then the rim. This sequence protects the posterior orbit fractures from much displacement.
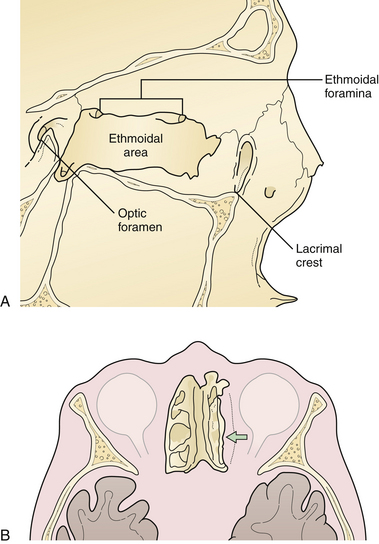
The nasoethmoidal orbital region represents the medial rim and medial wall of the orbit (Fig. 22.23). Posteriorly, the ethmoid air cells weaken the nasoethmoidal region, one of the thinnest portions of the orbital wall. Fractures involving the medial orbital rim displace the bone bearing the attachment of the medial canthal tendon posteriorly and laterally, which may also block the lacrimal system, resulting in tearing. Displacement of the medial orbital rim or the infraorbital rim and floor of the orbit alters the medial attachment of the eyelids and the suspensory ligaments of the globe, permitting globe and canthal ligament dystopia and telecanthus, which can be detected on physical examination.
The medial wall of the orbit is formed by the thin orbital plate of the ethmoid bone. This bone is reinforced by septa within the ethmoid sinus, which gives it some additional strength (see Fig. 22.23). The lateral wall of the orbit consists of the orbital process of the malar bone anteriorly and the greater wing of the sphenoid posteriorly (Fig. 22.24). The zygomaticosphenoid suture is involved in all zygoma fractures with the exception of those confined to the zygomatic arch. Its broad surface forms an excellent area for confirmation of proper zygomatic alignment at the time of reduction. With more comminuted orbital fractures, displacement of multiple walls of the orbit contributes to dramatic orbital deformity. Because soft tissue orbital deformity is not entirely reversible with secondary corrections, the emphasis is on immediate anatomical reconstruction.
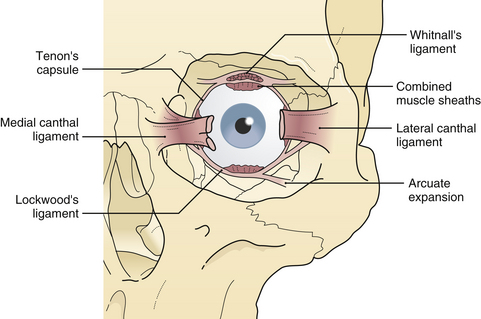
The lateral canthal ligament is attached with the lateral aspect of the eyelids to the zygoma at Whitnall’s tubercle, which is a shallow bulge behind the internal aspect of the lateral orbital rim about 10 mm inferior to the zygomaticofrontal suture. The anterior limb of the lateral canthal tendon is continuous with the galea, and the posterior limb joins the lateral extension of the levator tendon and Lockwood’s suspensory ligament in its attachment to Whitnall’s tubercle (Fig. 22.25). The extraocular muscles travel close to the orbital walls in the posterior half of the orbit, In the anterior half of the orbit, they are protected from orbital wall fractures only by a thin cushion of extramuscular cone fat. Thin “muscular cheek ligaments” extend from the extraocular muscles diffusely to the orbital walls (Fig. 22.26). The fine ligament system, described by Leo Koorneef (see Fig. 22.26), diffusely interconnects the soft tissue of the orbit to provide structural continuity among all the orbital tissues, such as fat, muscle, periosteum, and globe. This interconnection of all orbital soft tissue is why diplopia (extraocular muscle restriction) occurs if a particular section of orbital fat is trapped in a fracture by virtue of these connections. The entrapped fat and ligament system, in the absence of actual extraocular muscle incarceration, may cause diplopia.
The orbital floor is one of the weakest portions of the orbit. There is an initial concave section of the floor immediately behind the inferior orbital rim, and then a convex constriction of the orbit posteriorly. This complex orbital shape must be re-created when reconstructing the orbit. Because the complex curves of bone in relation to the soft tissue determine globe position (Fig. 22.27), it is extremely important to mimic the exact curvature of the middle portion of the orbit and the position of the orbital rim in reconstruction. The concave orbital roof must be reconstructed in its exact arching anatomical position, or the globe will be displaced inferolaterally. The orbital roof is convex from anterior to posterior, and from medial to lateral.
The posterior third of the orbit contains the optic foramen, the superior orbital fissure, and the posterior aspect of the inferior orbital fissure. The superior orbital fissure is bounded by the greater and lesser wings of the sphenoid (see Fig. 22.20). Linear fractures are commonly seen in the posterior portion of the orbit; however, displacement of bone is less common. Usually, the anterior and middle sections of the orbital bones displace, acting as a “shock absorber” protecting posterior orbital bone from severe displacement.
Diagnosis
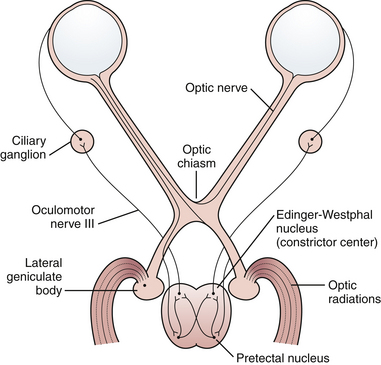
The most common reason for visual acuity deficit after trauma is optic nerve injury. Shearing, contusion, or compression may be involved. These injuries may occur with or without demonstrated fractures of the optic canal. If vision is lost at the moment of impact, decompression of an optic canal fracture usually does not increase the chance of visual recovery. Frequently, steroids are employed but recently their efficacy has been questioned. When bone displacement that compromises the optic canal is demonstrated, or if fluctuating or deteriorating visual deficit is seen, then optic canal decompression should be considered. Immediately after an optic nerve injury, the optic disk usually looks normal. A patient with visual loss may present with a Marcus Gunn pupil, in which the reaction to consensual constriction is present but the reaction to direct stimulus is reduced. Swinging a light from one eye to the other demonstrates paradoxical pupillary dilatation in the affected eye (Fig. 22.28).
Nasoethmoid Orbital Fractures
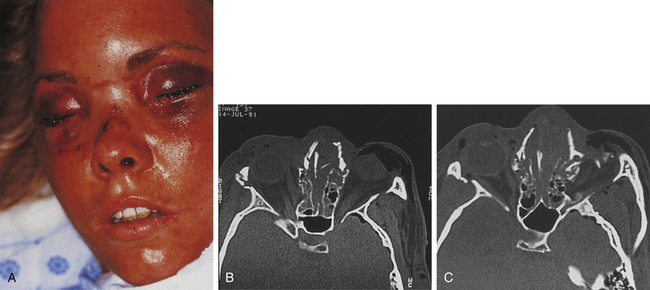
These injuries, which may cause significant long-term deformity of telecanthus and enophthalmos, are often initially obscured by swelling. Patients usually present with bleeding from the nose, a nasal dislocation, and bilateral periorbital and subconjunctival hematomas (Fig. 22.29). The nasal deformity consists of depression of the nasal dorsum and foreshortening of the nose, with an increased angle between the columella and the lip. Severe dislocation of the septum and nasal septal perforation are often present. Forty percent of nasoethmoid fractures are unilateral, and because of their proximity to the frontal sinus and the dura, a CSF leak may be present. Nasoethmoid fractures produce tearing by compromising the drainage of the lacrimal system as it passes through the maxilla. On palpation, pain and tenderness are found over the frontal process of the maxilla, and the palpating finger, inserted deeply over the medial canthal ligament, discloses bony crepitus, movement, and tenderness. Telecanthus may be present if the medial orbital rim fracture fragment has been dislocated laterally, in which case the palpebral fissure shortens.
Diagnosis
The presence of a nasoethmoid fracture can be determined by a bimanual examination (Fig. 22.30). A palpating finger is placed deeply over the canthal ligament opposite a clamp placed intranasally, and the “central” (canthal ligament containing) bone fragment is moved between the finger and clamp. Movement confirms a mobile base fracture.
Lacrimal system injury should be suspected in lacerations of the medial portion of the eyelids. The lacrimal system may also be compromised by fractures involving the bone surrounding the nasolacrimal duct. If the lacrimal system is transected, fluid emerges from a laceration on irrigation of the system with saline by a catheter placed through the lacrimal punctum in the lower lid.
Management
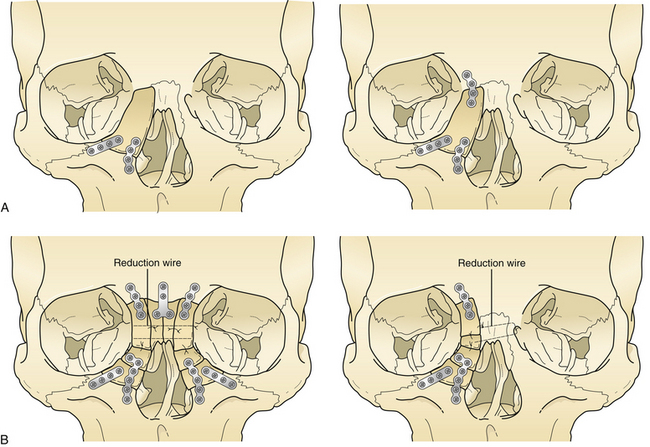
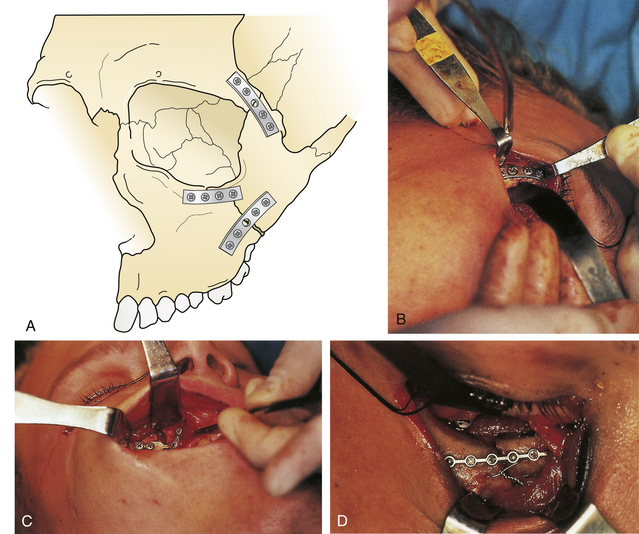
Nasoethmoid orbital fractures require a definitive open reduction consisting of interfragment wiring and plate and screw fixation of the assembled fragments. In some situations, this can be accomplished through a laceration or local incision; otherwise, a broad exposure must be provided by a coronal incision (Fig. 22.31), bilateral lower eyelid incision, and gingival buccal sulcus incision. Usually, the surgeon is careful to avoid detaching the canthal ligament from the bone during fracture reduction. If the canthal ligament is detached by the injury, it must be reattached after assembly of the bone fragments to the proper area of the medial orbital rim. A separate set of transnasal wires, again passed posterior and superior to the lacrimal fossa through the nose, connect the canthal ligament to the bone in its proper position (Fig. 22.32) after the bone reduction. Contoured bone grafts are used to reconstruct the medial and inferior internal orbit (Fig. 22.33). Long straight bone grafts are used to provide contour and to add dorsal height to the nose. These bone grafts are taken from the calvarium, the iliac crest, or a split rib.
Fractures of the Orbital Floor
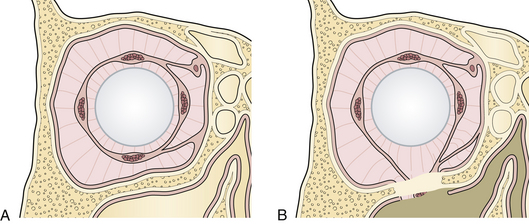
The most frequent fracture of the internal orbit is the blow-out fracture, which is usually confined to the floor and the lower portion of the medial wall (Fig. 22.34). A depressed fracture of this section of the orbit allows the orbital tissue to be displaced downward into the maxillary and ethmoid sinuses. Medial, inferior, and posterior dislocation of the globe occurs. If fat is trapped in the fracture, it may interfere with the motion of the globe because of the fine internal ligament system of the orbit linking all soft tissue (see Figs. 22.34 and 22.26). Less commonly, the inferior rectus muscle may be directly trapped in a small fracture, leading to restriction of globe movement. Patients with orbital fractures usually present with a history of a blunt injury to the orbit. They may have double vision when looking either upward or downward. Extraocular range of motion may be limited. Periorbital and subconjunctival hematomas are present and there may be numbness in the infraorbital nerve distribution. It is imperative that the globe be examined; the possibility of hyphema, retinal detachment, or globe rupture exists with any fracture involving the orbit.
The possible presence of an intraorbital foreign body should also be considered. Orbital fractures are accompanied in 10% to 15% of cases by a globe injury. The visual system and globe are evaluated by visual acuity, visual fields, funduscopic examination, extraocular motion, and intraocular pressure.
Diagnosis
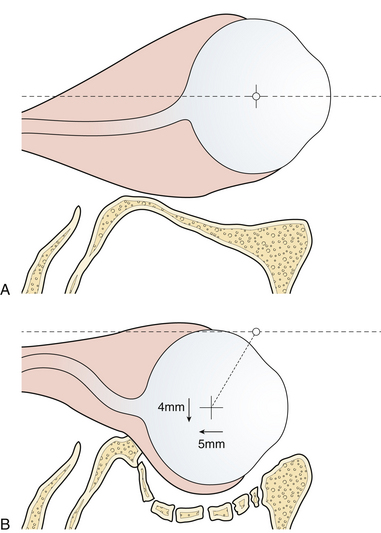
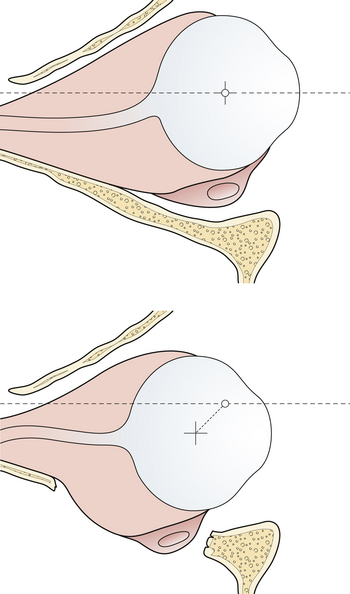
Enophthalmos denotes the backward dislocation of the globe into the orbit (Fig. 22.35). Large fractures of the orbital floor allow the orbital soft tissue to prolapse backward, downward, and medially, resulting in a loss of globe support and a change in globe position. The position of the globe on physical examination is best compared by assessing symmetry in the patient with an inferior view (Fig. 22.36) or with Hertel exophthalmometry. The trauma of the injury may produce periorbital fat atrophy, which may cause globe malposition. Acutely, periorbital injuries produce hemorrhage and edema. Initially, proptosis or exophthalmos appears. Acute enophthalmos is unusual and indicates a dramatic enlargement in the orbit. If the globe prolapses away from the lids, lubrication of the cornea cannot be accomplished; this is an urgent indication for orbital wall repair. Enophthalmos is usually accompanied by inferior displacement (globe dystopia). Posterior displacement of the globe produces a supratarsal hollow and ptosis of the upper eyelid.
Management
In many cases, the symptoms of a small internal orbital fracture resolve substantially within a short period. Frequently, double vision is the result of muscular contusion and resolves with observation. Surgery is usually indicated for double vision only when it occurs in a functional field of gaze and is the result of incarceration of the muscle or the ligament system. There are thus two indications for surgery for blow-out fractures: muscle or ligament entrapment confirmed by CT scan and forced-duction examination, and enlargement of the orbit sufficient to produce enophthalmos. This generally requires more than 2 cm2 of orbital floor involvement, with displacement of that section more than 3 to 4 mm. The size of the fracture can be accurately estimated on CT scans. The orbit should be reconstructed by bone grafts or alloplastic material placed over the edges of the orbital defect so as to support the orbital contents (see Fig. 22.33).
Le Fort Maxillary Fractures
Fractures of the maxilla involve not only the lower maxilla but often the entire midfacial region. These fractures are termed Le Fort maxillary fractures after the classification used by René Le Fort (Fig. 22.37), who described the three “great lines of weakness” of the maxilla through which fractures commonly occur.
Diagnosis
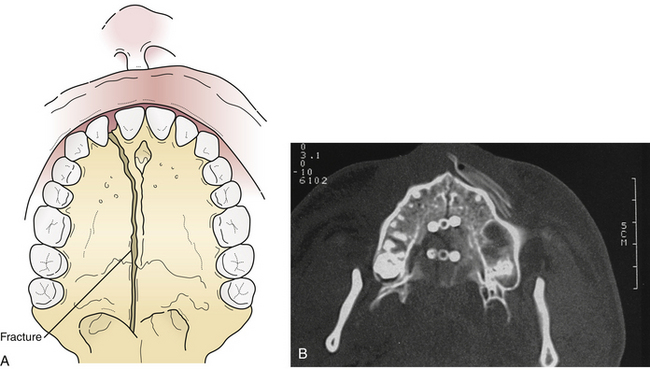
In 10% of the Le Fort fractures the maxillary alveolus itself is split, usually in a sagittal (longitudinal) direction (Fig. 22.38), increasing instability and making preservation of normal occlusion a challenge. Lower maxillary fractures are diagnosed by malocclusion and maxillary mobility. Upper maxillary fractures are diagnosed by maxillary mobility, malocclusion, periorbital hematomas, nasopharyngeal bleeding, pain, and the symptoms of zygomatic, orbital, and nasoethmoidal fractures. Examination for maxillary mobility is essential to confirm the presence of a Le Fort fracture. The level at which the mobility occurs indicates the level of the Le Fort fracture. The maxilla should be grasped with one hand while the head is stabilized with the other. The level at which the mobility occurs indicates the level of the Le Fort fracture. Multiple Le Fort fracture levels may be seen in the same patient. Occasionally, Le Fort fractures are not mobile; they may be either impacted or incomplete.
Management
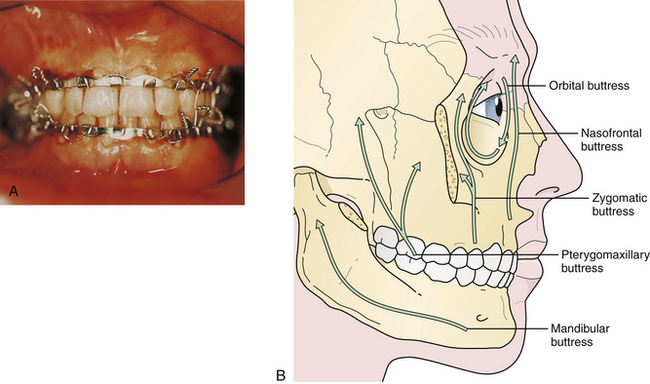
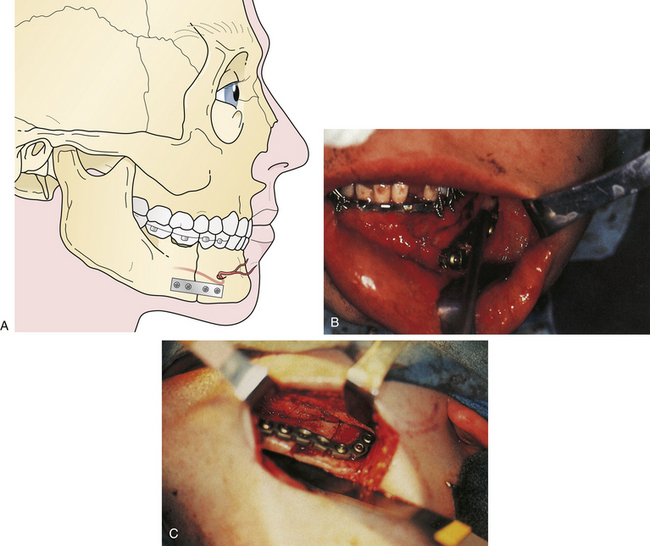
The principal treatment of Le Fort fractures is intermaxillary fixation with the maxilla in occlusion with the mandible. Initial stabilization is generally accomplished by ligating the arch bars to the upper and lower teeth and connecting the maxillary and mandibular arch bars with intermaxillary wires. Fracture sites at the various levels of the midface (as defined by CT scans) are aligned, and then the nasofrontal and zygomaticomaxillary buttresses (Fig. 22.39) are reconstructed with direct plate and screw fixation. This eliminates or decreases the need for intermaxillary fixation postoperatively.
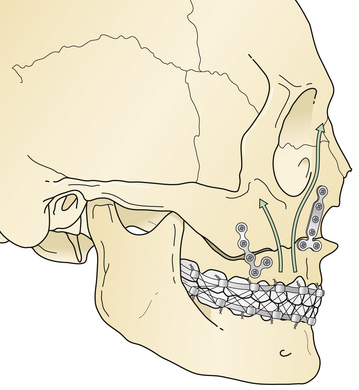
A Le Fort I fracture is treated by placing the patient in intermaxillary fixation, exposing the four Le Fort I level buttresses, reducing the fracture, and using direct plate and screw fixation over the buttress fractures (Fig. 22.40). Bone grafts should span bone gaps of more than 3 to 5 mm.
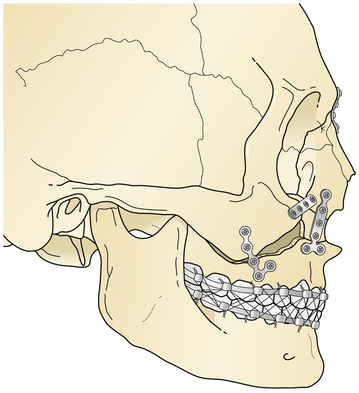
Le Fort II fractures are treated by placing the patient in intermaxillary fixation. The fracture fragments are aligned and stabilized with direct plate and screw fixation (Fig. 22.41). Orbital floor defects are spanned with bone grafts, or perhaps with alloplastic implants for smaller defects. If rigid fixation is used, intermaxillary fixation may frequently be discontinued early postoperatively. Normal occlusion must be confirmed carefully for a 4- to 12-week period postoperatively. Patients with intermaxillary fixation require a liquid diet and should be placed on a soft diet when it is discontinued. The upper nose may need direct fixation through a coronal incision, and the lower orbital rims are reduced and stabilized through bilateral lower eyelid incisions.
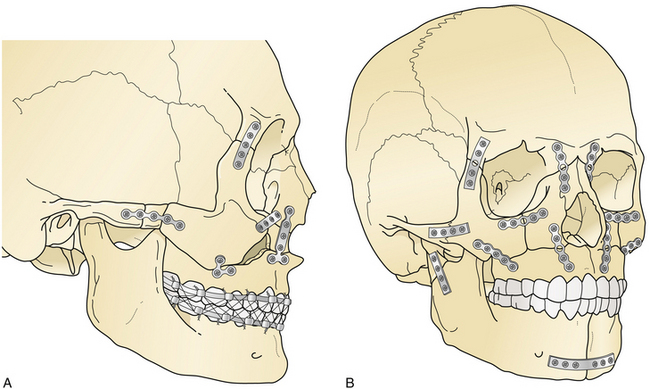
Le Fort III fractures are treated with surgical approaches to zygomatic, nasoethmoidal, and orbital floor fractures and these fractures are connected to the maxilla at the Le Fort I level (Fig. 22.42). Again, fragments are initially aligned with interfragment wires and stabilized with plate and screw fixation. A sagittal fracture of the maxilla is directly reduced through the palatal laceration or incision with plate and screw fixation (see Fig. 22.38). A small plate is also placed at the piriform aperture to unite the two maxillary alveolar segments. In some cases, an acrylic splint is placed in the palatal vault to reduce the occlusal relationships. A “panfacial fracture” (Le Fort fractures, plus fractures of the nasoethmoidal and mandibular areas) is shown repaired with plate and screw fixation (Fig. 22.42B). The mandibular subcondylar and symphysis fractures were stabilized prior to the fixation of the Le Fort fracture.
Fractures of the Nose
Diagnosis
Two types of dislocations occur in the nasal fractures (see Fig. 22.33): (1) posterior dislocation (shortening or flattening of the nose, resulting in a wider nasal bridge); and (2) lateral dislocation (deviated nose). Any patient with a nasal fracture should have the nasal airway inspected. If a significant hematoma exists along the septum, it should be drained to prevent cartilage necrosis. Patients with nasal fractures usually have swelling over the external surface of the nose. A small laceration is often the clue to the presence of a fracture. Pain, crepitation, and periorbital ecchymoses are present but not confirmed sharply to the insertion of the orbital septum as in periorbital fracture. The most reliable sign of a nasal fracture is epistaxis. Radiographic evaluation of the nose is best performed with a CT scan, which can confirm the nasal fracture and also rule out the possibility of adjacent fractures.
Fractures of the Zygoma
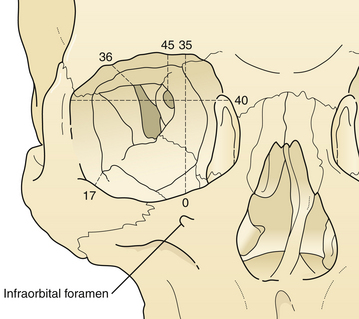
The zygoma forms the lateral and inferior portion of the orbit and supports the lateral areas of the upper midface (Fig. 22.43). The prominent position of the zygoma makes it a frequent recipient of traumatic dislocation. A fracture usually involves the entire zygoma, but may less commonly involve the zygomatic arch alone, which produces a minimal depression in the lateral cheek. Depression of the zygomatic arch may interfere with movement of the coronoid process of the mandible, a symptom requiring reduction. Because complete zygomatic fractures involve the lateral and inferior internal walls of the orbit, they may produce ocular symptoms that require treatment.
Diagnosis
The symptoms of zygomatic fractures are shown in Figure 22.44. The lateral canthus, which attaches to the frontal process of the zygoma, may be dislocated inferiorly, producing an antimongoloid slant to the palpebral fissure. Either swelling or dislocation of the zygoma may interfere with motion of the coronoid process by producing a mild temporary interference with occlusion. Hematomas are observed in the cheek, periorbital area, and upper gingival buccal sulcus. Orbit symptoms produced by the fractures include diplopia, ocular dystopia, and lower eyelid dislocation. Palpation of the orbital rim may demonstrate a “step” deformity or depression. Palpation of the malar eminence, when compared with the normal side, demonstrates retrusion. With a medially dislocated zygomatic fracture, the orbital volume may be constricted, resulting in exophthalmos. With laterally or inferiorly dislocated zygomatic fractures, the orbital volume increases, and enophthalmos occurs.
Management
Initially, dislocated fragments of the zygoma are repositioned and aligned by drilling holes adjacent to the fractures and linking the fragments with small interfragment wires. The fracture fragments are held in position while rigid internal fixation is performed using small plates and screws (Fig. 22.45). If the lateral canthus is detached in the reduction, it should be replaced after bone assembly.
Fractures of the Mandible
Diagnosis
The diagnosis of the mandibular fracture is suggested by malocclusion, pain, swelling, tenderness, crepitus, fractured teeth, gaps or discrepancies in the level of the dentition, asymmetry of the dental arch, presence of intraoral lacerations, broken or loose teeth, or numbness in the distribution of the mental nerve. An odor is frequently present. Fractured, missing, or dislocated teeth are frequently seen. An “open bite” occurs if the fracture sufficiently dislocates a segment of jaw so that the teeth cannot be brought into occlusion (Fig. 22.46). The open bite may occur anteriorly, laterally, or bilaterally. On opening, the jaw may deviate toward one side because of the fractures in the subcondylar area, which prevent the balancing effect of the lateral pterygoid muscle. Fractures in the condylar and subcondylar area may result in a laceration of the ear canal that produces bleeding, confusing the injury with that of a middle cranial fossa fracture. Instability of the alveolar section of the mandible relative to the mandibular body implies the presence of an alveolar fracture. Separation of the alveolus from the basilar bone of the mandible creates dramatic dental instability.
The mandible has strong muscular attachments that contribute to displacement after injury. The direction of the fracture line may oppose fracture displacement produced by the muscles. The Panorex radiograph uses a rotating x-ray tube to obtain a circumferential view for an excellent evaluation of the entire mandible in a single plane. Lateral oblique, posteroanterior, and Towne’s skull views are used to demonstrate the mandible on plain films. A CT scan provides one of the most accurate evaluations of a mandibular fracture but can occasionally miss nondisplaced fractures. CT scans also demonstrate the course of the fracture through the bone, which is essential for treatment planning.
Management
The treatment of mandibular fractures depends on the state of the dentition and the location of the fracture. It begins with closure of intraoral and extraoral lacerations and the application of arch bars and intermaxillary fixation to bring the teeth into occlusion (Fig. 22.47). In some cases, an acrylic splint can be applied to the teeth temporarily to align them. Some fractures are treated with intermaxillary fixation alone for 4 to 6 weeks after “closed reduction.” For displaced fractures in both the horizontal and vertical portions of the mandible, direct open reduction of the fracture with plate and screw fixation is the preferred treatment. A plate is placed along the inferior border of the mandible, avoiding the mental nerve and tooth roots (Fig. 22.48). At least two screws are placed to each side of the fracture in stable bone.
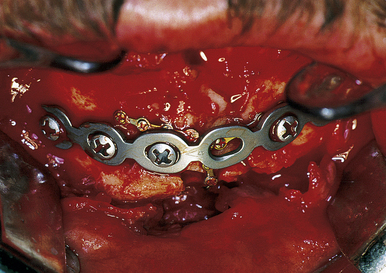
FIGURE 22.47 Rigid fixation in a mandibular fracture utilizes small and large plates for stabilization of all the segments.
Facial Fractures in Children
Less than 5% of all facial fractures occur in children: of that 5%, most occur in those over 5 years of age. The bones of children are less brittle than those of adults, and they displace without fracture in many cases. Fracture healing progresses more rapidly in children than in adults. Sinuses are small and therefore do not weaken the bony structure of the midface. The treatment of fractures in the upper face of a child follows the same principles described for adults. The emphasis is on early or immediate treatment because healing occurs rapidly. It may be difficult to reduce a Le Fort fracture after even 1 week. Intermaxillary fixation is often difficult to apply in children because of mixed dentition and inadequate root structure of the teeth and the shape of the crowns, which produces difficulty in ligating teeth. Arch bars may have to be supported by piriform aperture wires, circummandibular wires, or suspension wires for stabilization. The application of acrylic splints to the dentition can facilitate reduction of the fracture. The use of miniature plate and screw fixation systems in children is preferred. The long-term efficacy of resorbable plates in pediatric facial fractures has yet to be defined. Healing times are shorter in children. Because of the prominence of the frontal skull in children, frontal skull and supraorbital fractures are frequent in the young (0-5 years) age group. Orbital floor fractures are not frequent because of the small size of the maxillary sinus. The frontal sinus is absent in young children. Healing times are generally significantly reduced in children. One would like to minimize the use of fixation materials that will remain because of a possible role in localized growth restriction (perhaps 5%).
Clark N., Birely B., Manson P.N., et al. High-energy ballistic and avulsive facial injuries: classification, patterns, and an algorithm for primary reconstruction. Plast Reconstr Surg. 1996;98:583-601.
Gruss J. Advances in craniofacial fracture repair. Scand J Plast Reconstr Hand Surg. 1995;27(Suppl):67-81.
Manson P.N., Stanwix M., Yaremchuk M., et al. Frontobasilar fractures: anatomy, classification and clinical significance. Plast Reconstr Surg. 124, 2010. 2096–2016
Rodriguez E.D., Stanwix M.G., Nam A.J., et al. Twenty-six-year experience treating frontal sinus fractures: a novel algorithm based on anatomical fracture pattern and failure of conventional techniques. Plast Reconstr Surg. 2008;122(6):1850-1866.
Please go to expertconsult.com to view the complete list of references.
Adekeye E.O., Ord R.A. Giant frontal sinus mucocele. J Maxillofac Surg. 1984;12:184.
Anderson R.I. The medial canthal tendon branches out. Arch Ophthalmol. 1977;95:2051.
Anderson R.I., Panje W.R., Gross C.E. Optic nerve blindness following blunt forehead trauma. Ophthalmology. 1982;89:445.
Angle E.H. Classification of malocclusion. Dent Cosmos. 1989;41:240.
Barton F.E., Berry W.L. Evaluation of the acutely injured orbit. In: Aston S.J., Hornblass A., Meltzer M.A., Rees T.D., editors. Third International Symposium of Plastic and Reconstructive Surgery of the Eye and Adnexa. Baltimore: Williams & Wilkins; 1982:34.
Burstein F., Cohen S., Hudgins R., Boydston W. Mandibular osteosynthesis by miniature screwed plates via a buccal approach. J Maxillofac Surg. 1978;6:14.
Clark N., Birely B., Manson P.N., et al. High-energy ballistic and avulsive facial injuries: classification, patterns, and an algorithm for primary reconstruction. Plast Reconstr Surg. 1996;98:583-601.
Converse J.M., Firmin F., Wood-Smith D., Friedland J.A. The conjunctival approach in orbital fractures. Plast Reconstr Surg. 1972;52:656.
Converse J.M., Smith B. Enophthalmos and diplopia in fracture of the orbital floor. Br J Plast Surg. 1957;9:265.
Crawley W, Manson P. Problems and Complications in Cranioplasty in Craniomaxillofacial Trauma. Perspectives in Plastic Surgery. Philadelphia: Lippincott; 191:458–465.
Donald P.J., Ettin M. The safety of frontal sinus fat obliteration when sinus walls are missing. Laryngoscope. 1986;96:190.
Dufresne C., Manson P., Iliff N. Early and late complications of orbital fractures. Semin Ophthalmol. 1989;4:176-190.
Ferre J., Bordvre P., Huet P., Favre A. Usefulness of the galeal flap in treatment of intensive frontal bone defects: a study of 14 patients. J Craniofac Surg. 1995;6:164-169.
Fila T.G., Novelline R.A., Yaremchuk M.C. Comparison of CT imaging artifact from craniomaxillofacial internal fixation devices. Plast Reconstr Surg. 1993;92:1227-1232.
Fukado Y. Results in 400 cases of surgical decompression of the optic nerve. In: Bleeker G.M., et al, editors. Proceedings of Second International Symposium on Orbital Disorders. Basel: Karger, 1975.
Girotto J., Gamble B., Robertson B., et al. Blindness following reduction of facial fractures. Plast Reconstr Surg. 1998;102:1821-1834.
Girotto J., Makenzie E., Fowler C., et al. Long term physical impairment and functional outcomes following complex facial fractures. Plast Reconstr Surg. 2001;108:312-328.
Gruss J. Advances in craniofacial fracture repair. Scand J Plast Reconstr Hand Surg. 1995;27(Suppl):67-81.
Bakay L., Glasauer F.R. Head Injury. Boston: Little, Brown; 1980.
Becker D.P., Gade G.F., Young H.F., Feuerman T.F. Diagnosis and treatment of head injury in adults. In: Youmans J.R., editor. Neurological Surgery. 3rd ed. Philadelphia: WB Saunders; 1990:2017-2148.
Cooper P.R. Skull fracture and traumatic cerebrospinal fluid fistulas. In: Cooper P.R., editor. Head Injury. 2nd ed. Baltimore: Williams & Wilkins; 1987:89-107.
Eisenberg H.M., Briner R.P. Late complications of head injury. In: McLaurin R.L., Venes J.L., Schut L., Epstein F., editors. Pediatric Neurosurgery. 2nd ed. Philadelphia: WB Saunders; 1989:290-297.
Geisler F.H., Greenberg J. Management of the acute head-injury patient. In: Salcman M., editor. Neurologic Emergencies. 2nd ed. New York: Raven Press; 1990:135-165.
Geisler F.H., Salcman M. The head injury patient. In: Siegel J.H., editor. Trauma—Emergency Surgery and Critical Care. New York: Churchill Livingstone; 1987:919-946.
Gudeman S.K., Young H.F., Miller J.D., et al. Indications for operative treatment and operative technique in closed head injury. In: Becker D.P., Gudeman K.D., editors. Textbook of Head Injury. Philadephia: WB Saunders; 1989:138-181.
Jennett B., Teasdale G. Management of Head Injuries. Philadelphia: Davis; 1981.
Mealey J.Jr. Skull fractures. In: McLaurin R.L., Venes J.L., Schut L., Epstein F., editors. Pediatric Neurosurgery. 2nd ed. Philadelphia: WB Saunders; 1989:263-270.
Thomas L.M. Skull fractures. In: Wilkins R.H., Rengachary S.S., editors. Neurosurgery. New York: McGraw-Hill; 1985:21623-21626.
Wilberger J., Chen D.A. The skull and meninges. Neurosurg Clin North Am. 1991;2:341-350.
Deschamps-Braly J.C., Sawan K., Iliff N., Manson P. Decision making in isolated orbital roof fractures with case report of upper eyelid approach to treatment. Ann Plast Surg. 2010;126(6):308e-309e.
Gurss J.S., Hurwitz J.J., Nik N.A., Kassel E.E. The pattern and incidence of nasolacrimal injury in nasoethmoid orbital fractures: the role of delayed assessment and dacryocystorrhinostomy. Br J Plast Surg. 1985;38:116.
Gruss J.S., Mackinnon S.E., Kassel E., Cooper P.W. The role of primary bone grafting in complex craniomaxillofacial trauma. Plast Reconstr Surg. 1985;75:17.
Gruss J.S., Pollock R.S., Phillips J.H., Antonyshyn O. Combined injuries of the cranium and face. Br J Plast Surg. 1989;42:385-398.
Hendler N., Viernstein M., Schallenberger C., Long D. Group therapy with chronic pain patients. Psychosomatics. 1987;22:333.
Hendler H. The anatomy and psychopharmacology of chronic pain. J Clin Psychiatr. 1982;43:15.
Hendrickson M., Clark N., Manson P. Sagittal fractures of the maxilla: classification and treatment. Plast Reconstr Surg. 1998;101:319-332.
Iliff N., Manson P., Katz J., et al. Mechanisms of extraocular muscle injury in orbital fractures. Plast Reconstr Surg. 1999;103:787-799.
Jackson I.T., Pellett C., Smith J.M. The skull as a bone graft donor site. Ann Plast Surg. 1983;11:527.
Jacobs J.B., Perky M.S. Traumatic pneumocephalus. Laryngoscope. 1980;90:515.
Jones L.T. An anatomical approach to problems of the “eyelids and lacrimal apparatus. Arch Ophthalmol. 1961;66:111.
Kim L.H., Lam L.K., Moore M.H., et al. Associated injuries in facial fractures: review of 839 patients. Br J Plast Surg. 1993;46:65.
Kline R.M., Wolfe S.A. Complications associated with the harvesting of cranial bone grafts. Plast Reconstr Surg. 1995;95:5.
Koorneef L. Spatial Aspects of the Orbital Musculofibrous Tissue in Man: A New Anatomical and Histological Approach. Amsterdam: Swets & Zeitlinger; 1977.
Lessel S. Indirect optic nerve trauma. Arch Ophthalmol. 1989;107:382-386.
Manson P.N. Some thoughts on the classification and treatment of Le Fort fractures. Ann Plast Surg. 1986;17:356.
Manson P.N. Facial bone healing and grafts; a review of clinical physiology. Clin Plast Surg. 1994;21:331-348.
Manson P.N., Cifford C.M., Su C.T., et al. Mechanisms of global support and post traumatic enophthalmos, I: the anatomy of the ligament sling and its relation to intramuscular cone orbital fat. Plast Reconstr Surg. 1986;77:193.
Manson P.N., Crawley W.A., Hoopes J.E. Frontal cranioplasty; risk factors and choice of cranial vault reconstructive material. Plast Reconstr Surg. 1986;76:888.
Manson P.N., Grivas A., Rosenbaum A., et al. Studies on enophthalmos, II: the measurement of orbital injuries and their treatment by quantitative computed tomography. Plast Reconstr Surg. 1986;77:203.
Manson P., Iliff N., Vander Kolk C., et al. Rigid fixation of the orbital fractures. Plast Reconstr Surg. 1990;85:202-212.
Manson P., Clark N., Robertson B., et al. Subunit principles in midface fractures; the importance of sagittal buttresses, soft tissue reductions and sequencing treatment of segmental fractures. Plast Reconstr Surg. 1999;103:1287-1306.
Manson P.N., Iliff N., Robertson B. The hope offered by early surgical treatment to those patients whose blowout fractures demonstrate tight muscle restriction or true muscle incarceration. Plast Reconstr Surg. 2002;109:490-495.
Manson P.N., Stanwix M., Yaremchuk M., et al. Frontobasilar fractures: anatomy, classification and clinical significance. Plast Reconstr Surg. 2010;124:2096-2116.
Markowitz B., Manson P., Sargent L., et al. Management of the medial canthal tendon in nasoethmoid orbital fractures; the importance of the central fragment in treatment and classification. Plast Reconstr Surg. 1991;87:843-853.
Markowitz B., Manson P.N., Yaremchuk M., et al. High-energy orbital dislocations: the possibility of traumatic hypertelorism. Plast Reconstr Surg. 1991;88:20-29.
McKinnon C.A., David D.J., Cooter R.D. Blindness and severe visual impairment in facial fractures: an 11 year review. Br J Plast Surg. 2002;55:1-7.
Pensler J., McCarthy J.G. The calvarial donor site: an anatomic study in cadavers. Plast Reconstr Surg. 1985;75:648.
Philips J., Gruss J., Wells M. Periorbital suspension of lower eyelid and cheek following subciliary exposures of facial fractures. Plast Reconstr Surg. 1991;88:145.
Putterman A.M., Stevens Y., Urist M.J. Nonsurgical management of blow-out fractures of the orbital floor. Am J Ophthalm Surg. 1974;77:232.
Rodriguez E., Bluebond-Langner R., Amable R., Manson P. Treatment of infected frontal sinus fractures: novel utility of the fibula flap. J Craniofac Surg. 2007;18(3):680-683.
Rodriguez E., Bluebond-Langner R., Brazio P., et al. Near total mandible reconstruction with a single fibula flap containing fibrous dysplasia in McCune-Albright syndrome. J Craniofac Surg. 2007;18(6):1479-1482.
Rodriguez E., Bluebond-Langner R., Silverman R., et al. Abdominal wall reconstruction following severe loss of domain: the R Adams Cowley shock trauma center algorithm. Plast Reconstr Surg. 2007;123(3):669-680.
Rodriguez E., Mithani S., Bluebond-Langner R., Manson P. Hand evaluation following forearm perforator ulnar flap harvest. Plast Reconstr Surg. 2007;120(6):1598-1601.
Rodriguez E., Martin M., Bluebond-Langner R., et al. Microsurgical reconstruction of post-traumatic high-energy maxillary defects: establishing the effectiveness of early reconstruction. Plast Reconstr Surg. 2007;120(7):103S-117S.
Rodriguez E., Bluebond-Langner R., Park J., Manson P. Preservation of contour in periorbital and midfacial craniofacial microsurgery: reconstruction of the soft tissue elements and skeletal buttresses. Plast Reconstr Surg. 2008;121(5):1738-1747.
Rodriguez E., Bluebond-Langner R., Devgan L., et al. Correction of the recalcitrant post-traumatic periorbital soft tissue deformity: a novel microsurgical approach. Plast Reconstr Surg. 2008;121(6):1978-1981.
Rodriguez E.D., Stanwix M.G., Nam A.J., et al. Twenty-six-year experience treating frontal sinus fractures: a novel algorithm based on anatomical fracture pattern and failure of conventional techniques. Plast Reconstr Surg. 2008;122(6):1850-1866.
Deschamps-Braly J.C., Sawan K., Iliff N., Manson P. Decision making in isolated orbital roof fractures with case report of upper eyelid approach to treatment. Ann Plast Surg. 2010;126(6):308e-309e.
Rodriguez E.D., Stanwix M.G., Nam A.J., et al. Definitive treatment of persistent frontal sinus infections: elimination of dead space and sinonasal communication. Plast Reconstr Surg. 2009;123(3):957-967.
Raflo G.T. Blow-in and blow-out fractures of the orbit: clinical correlations and proposed mechanisms. Ophthalmic Surg. 1984;15:114.
Rish B.L., Dillon J.D., Meirowsky A.M., et al. Cranioplasty: a review of 1030 cases of penetrating head injury. Neurosurgery. 1979;4:381.
Robertson B., Manson P. The importance of serial debridement and second look procedures in high-energy ballistic and avulsive facial injuries. Operat Techn Plast Reconstr Surg. 1998;5:236-246.
Rumelt M.B., Ernest J.F. Isolated blowout fractures of the medial orbital wall with medial rectus muscle entrapment. Am J Ophthalmol. 1971;73:451.
Vondra J. Fractures of the Base of the Skull. London: Iliffe Books; 1965.
Wolfe S.A. Application of craniofacial surgical precepts following trauma and tumour removal. J Maxillofac Surg. 1982;10:212.
Zide B.M., McCarthy J.G. The medial canthus revisited—an anatomical basis for canthopexy. Ann Plast Surg. 1983;11:1.