Chapter 91 Traumatic Chorioretinopathies
![]() For additional online content visit http://www.expertconsult.com
For additional online content visit http://www.expertconsult.com
Epidemiology
Eye injuries are an important world-wide cause of visual loss, particularly in developing countries. Surveys indicate that there are almost 2.5 million new eye injuries in the United States each year. Of these, between 40 000 and 60 000 patients are diagnosed with trauma-related blindness.1,2 Ocular trauma is the leading cause of monocular blindness2 and the affected population tends to be young, with 58% under 30 years of age.2 Given the potential societal burden, the importance of preventing eye injuries is a point of emphasis in both developed and developing nations.
As noted above, most victims of ocular trauma are in the 18–44 age range.1,2 Most eye injuries to children occur during athletic or recreational activities.3,4 In adults, car accidents, work-related injuries and physical assaults are the most commonly reported causes.5,6 The vast majority of individuals suffering traumatic chorioretinopathies are male (72–90%).2,6 Blunt injuries account for 51–66% of ocular injuries in both prospective and retrospective studies,5 with the majority of retinal lesions being unilateral.1 Previous studies have implicated metal, explosives, and stone as the most common materials contributing to ocular morbidity and blindness in the setting of trauma.6,7
The chorioretinopathies described in this chapter are those due to direct or indirect injury to the eye by blunt forces, without scleral rupture. Penetrating injuries are discussed in other chapters (Chapter 110, Surgery for ocular trauma).
Chorioretinopathies from direct ocular injuries
The blunt force acting on the eye can cause a coup injury at the site of impact, and a contrecoup injury in the opposite part of the eye as a result of shock waves acting at the interface of tissues of different densities. Direct blunt trauma also compresses the eye in the axis of the force and significantly stretches the tissues in the perpendicular plane. This stretching effect may also contribute to indirect injury.8
Commotio retinae
The term commotio retinae was first introduced by Berlin9 in 1873. It is sometimes referred to as Berlin’s edema, as Berlin believed that the whitish appearance of the retina was due to extracellular edema. Commotio retinae is a self-limited opacification of the retina secondary to direct blunt ocular trauma, characterized by a transient whitening at the level of the deep sensory retina (Fig. 91.1). Retinal whitening may take hours to develop before it becomes visible ophthalmoscopically.10 The lesion may involve both the central retina and the periphery.10
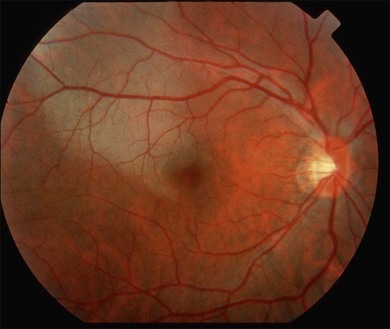
Fig. 91.1 Commotio retina in the macular area. Notice whitish change in the outer retina.
(Courtesy of John Payne, MD, Emory University.)
Commotio retinae has been reproduced in many different animal models in the setting of blunt eye trauma.11–13 Histopathologic studies of animal models have shown that it is characterized by disruption of the photoreceptor outer segments with associated retinal pigment epithelium (RPE) damage.10,14–18 and coworkers19 studied a single human eye with commotio retinae that was enucleated within 24 hours and also demonstrated photoreceptor outer segment disruption and RPE cell injury. Recently, reports11,20 using optical coherence tomography (OCT) have confirmed that the major site of retinal trauma in commotio retinae appears to be at the level of the photoreceptor outer segment/RPE interface, thus correlating well with the histopathology studies.
Commotio retinae is a common finding after blunt eye trauma and accounts for 9.4% of all post-traumatic fundus changes.10 Not surprisingly, the vision is more affected when the fovea is involved. No treatment is available or recommended for commotio retinae, other than observation.10,12
Retinal concussion
Retinal concussion is a milder condition of traumatic retinal opacification. The initial vision is better (usually better than 20/200) and the gray–white change is less dramatic. In addition, the clinical changes are reversible, with vision generally recovering fully with minimal sequelae.11 On fluorescein angiography (FA) the areas of opaque retina block background choroidal fluorescence. Leakage from the retinal vessels is not observed although mild staining is often noted at the level of the RPE, which generally clears within 24 hours.10,13 The retinal whitening on ophthalmoscopy also clears spontaneously in a few days.10,17
Retinal contusion
In retinal contusion, the retinal whitening is more intense and may be associated with hemorrhages and more persistent visual loss. Visual acuity may be variably affected, from mild to severe, in a fashion that does not correlate with the degree of retinal whitening seen clinically. If the macula is affected, acuity is usually permanently damaged.21
In contrast to retinal concussion, more intense staining or leakage is noted at the level of the RPE on FA.21 Acute pigment epithelial damage leads to permanent pigmented scars in the ensuing weeks.
Choroidal rupture
Choroidal rupture is a tear of the inner choroid and overlying Bruch’s membrane and RPE caused by mechanical disruption when the globe is acutely deformed by blunt trauma causing anterior posterior compression, and subsequent horizontal expansion of the eye.10,22 Choroidal ruptures are noted to be present in approximately 5–10% of blunt ocular trauma cases.23 During the blunt trauma, the fortified collagenous sclera and the naturally flexible retina are less likely to rupture. However, the relatively inelastic RPE, Bruch’s membrane, and choriocapillaris, do rupture.10,22 Direct choroidal ruptures are relatively uncommon; they tend to be parallel to the ora serrata and are found at the direct site of impact, which is usually anterior to the equator.10 Indirect choroidal ruptures resulting from compressive injury to the posterior pole of the eye22 are more common (about 80%),22,24 with the crescent-shaped tears occurring concentric to the disc because of the tethering or stabilizing effect of the optic nerve.25 The majority of indirect ruptures occur temporal to the disc and involve the fovea. Many are isolated, but there can be multiple ruptures (Fig. 91.2).24 Patients with underlying compromise or brittleness of Bruch’s membrane, as can be seen in the setting of angioid streaks, are more susceptible to indirect rupture than the normal population.24
Choroidal rupture is often associated with intrachoroidal, subretinal, and intraretinal hemorrhage. The associated hemorrhage and accompanying commotio retinae may conceal the presence of the rupture, which may become visible only after the blood clears in 2–3 months.25 Occasionally they may not be readily evident ophthalmoscopically, and their presence is confirmed after FA or indocyanine green angiography (ICGA). ICGA appears to have an advantage in diagnosis of choroidal rupture as it often highlights broader areas of pathology and disturbance compared to FA.25 Choroidal ruptures appear as hypofluorescent streaks on ICGA.
In histopathologic studies, Aguilar and Green22 showed that choroidal rupture is followed by bleeding, fibrovascular tissue proliferation, and RPE hyperplasia. The overlying retina is variably affected, ranging from loss of the outer layers alone to discontinuity affecting the full retinal thickness. Areas of choroidal rupture are noted to heal with a fibroglial response.
Choroidal rupture is associated with a long-term risk of choroidal neovascularization (CNV) with 5–10% of eyes developing CNV.24,26,27 The development of CNV was most strongly associated with older age, macular location of the choroidal rupture, and a greater length of the choroidal rupture.24 Neovascularization is a consistent and expected part of the process of scar formation, and in some cases this neovascularization regresses without sequelae.22 Management approaches for CNV secondary to traumatic choroidal rupture include observation, laser photocoagulation, submacular surgery, photodynamic therapy, and intravitreal anti-vascular endothelial growth factor treatment.26,28,29
Visual prognosis after choroidal rupture depends on whether the fovea was affected by the choroidal rupture and on whether late complications such as choroidal neovascularization ensue.25,26 Most patients with traumatic choroidal rupture do not achieve final VA of 20/40 or better.24
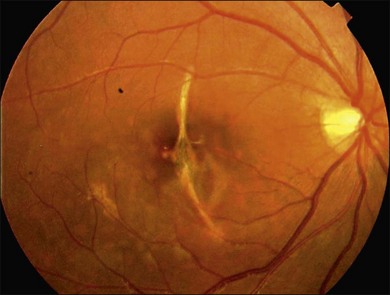
Traumatic macular hole
Blunt eye trauma is one of the main causes of secondary macular hole30 (Fig. 91.3). Traumatic macular holes (TMH) were first described by Knapp in 1869 and by Noyes in 1875.31
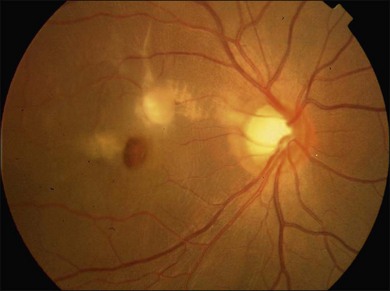
Fig. 91.3 Traumatic macular hole in a 15-year-old boy 4 weeks after being hit by a book during school.
The exact cause of TMH remains controversial. Ho and associates32 outlined four basic historical theories regarding its cause: the traumatic theory, the cystic degeneration theory, the vascular theory, and the vitreous theory. Today, it is recognized that the pathogenesis of macular hole formation is a combination of factors, while vitreous traction may play an important role in some cases of TMH formation.33,34
Macular holes do not always form immediately after trauma, but may appear after several days. In some cases macular edema and cysts develop, and eventually the roof of the cysts erode leading to the formation of a macular hole.30 OCT can help to identify alterations that occur before hole formation and may help to explain the pathogenesis of TMH.35,36
There is no consensus on surgical management for this disease, because some cases demonstrate spontaneous closure of the macular hole and improved visual acuity by 6 months after injury.31,37 Cases that close spontaneously tend to be in younger patients and in patients with smaller holes (less than 1/3 disc diameter) and without a fluid cuff.35 Yamada and associates31 proposed that for young patients with small traumatic macular hole without a fluid cuff, it is probably better to wait at least 6 months from their ocular concussion to allow spontaneous hole closure. Mechanisms of spontaneous TMH closure may include glial cells or RPE cells that proliferate at the margin of the hole and may fill and close the defect.31
Traumatic chorioretinal rupture
Goldzieher introduced the term chorioretinitis plastic sclopetaria, or the shorter form chorioretinitis sclopetaria, in 1901,38 but now the term traumatic chorioretinal rupture (TCR) is deemed to be more acceptable. It refers to a simultaneous break in the retina and choroid resulting from a high-velocity missile passing adjacent to and coming into contact with the globe, entering the orbit without causing a scleral rupture. This injury causes a full-thickness chorioretinal defect and visual loss.39 Simultaneous retraction of the choroid and retina at the site of the break reveals bare sclera. Two mechanisms have been considered: damage adjacent to the pathway of the high-velocity object is responsible for the direct injury, and the indirect injury is caused by the shock waves transmitted to the globe.39
TCR is a rare clinical presentation. Fundus examination may show the chorioretinal defect, bare sclera, pigment proliferation, marked fibrovascular proliferation and scar formation.40 If the foreign body has come to rest deep in the orbit, the TCR is typically oriented radially.41 The visual prognosis depends on the extent and location of intraocular injury. Secondary chorioretinal arterial and venous anastomoses have been reported.42 ICG dye permits visualization of the deeper choroidal vessels and may demonstrate ruptures that are difficult to appreciate clinically.43
Despite severe retinal and choroidal injuries in TCR, the patients have a low chance of retinal detachment, as marked proliferation of fibrous tissue causes firm adherence of retina and choroid to the sclera.40 In addition, the intact posterior hyaloid that is typically present in young patients further mitigates the risk for retinal detachment.41
Management includes observation, and rarely requires surgical intervention to repair a retinal detachment or to remove a non-clearing vitreous hemorrhage.21,41 While confronted with TCR, it is important to make an accurate diagnosis to prevent unwarranted surgical intervention.40
TCR is typically caused by gun injuries resulting in retained intraorbital metallic foreign bodies, which are well tolerated and typically have minimal adverse effect on visual prognosis, and as such can be followed up without surgical intervention.40
Traumatic retinal pigment epithelial tears
RPE tear is a well-known complication of age-related macular degeneration.44 But it is also a rare complication after trauma.45,46 Why? It is thought that the force applied must fit into an extremely narrow window.46 The force must be sufficiently large so as to cause an RPE tear, but not so large as to cause both the RPE and Bruch’s membrane to tear, as in a choroidal rupture. As the elastic torn edge of RPE tends to retract over the adjacent intact RPE, RPE tears are usually prevented from healing, and visual recovery is generally poor.46
Biomicroscopy typically demonstrates a hypopigmented area corresponding to RPE loss and exposed Bruch’s membrane. A scroll of pigmented RPE is usually noted at the margin of the hypopigmented area.45 FA demonstrates a window defect in the area of RPE loss with an adjacent area of blocked fluorescence due to the rolled-up RPE (usually with a sharp edge between these regions).45 OCT is particularly useful in displaying these changes at the level of the RPE.45 Patients with traumatic RPE tears involving the fovea usually have a poor visual prognosis.44
Fok et al.46 reported spontaneous resolution of traumatic RPE tears in a patient who subsequently had good visual recovery. The mechanisms of resolution in this case included a layer of hypopigmented RPE cells, atrophy of the choriocapillaris, and/or deposition of fibrous tissue.
Traumatic retinal tears and detachments
Ocular contusion may result in numerous types of retinal tears, such as horseshoe tears, operculated holes, macular holes, and retinal dialyses.47 Dialysis is the most common type of retinal break in the setting of trauma.48 Contusion leads to direct injury at the site of impact or indirectly as a result of changes in the shape of the eye (anteroposterior shortening followed by equatorial elongation) that may cause peripheral tears immediately, or secondarily following premature separation of the vitreous gel.10
Traumatic retinal tears are predominantly located within the vitreous base region. Cox et al.48 reported that retinal tears were limited to the ora serrata in 59% of cases with traumatic retinal detachments (RD). Only 8% of the traumatic tears were in the equatorial area, whereas approximately 60% of the nontraumatic tears were in the equatorial area. However, Atmaca et al.49 reported that 34.3% of the eyes with traumatic retinal holes and tears were in the equatorial region.
Johnston reported that 84.4% of the patients with traumatic retinal tears developed rhegmatogenous RD.50 The presence of RD immediately following injury is extremely rare, and in general RD progress slowly, occurring weeks to months following the trauma.51 The reason for the slower rate of progression from retinal defect (tear, dialysis) to frank detachment relates to the fact that the vitreous is formed and may be attached in these young patients. For patients developing giant retinal tears following trauma, the progression to detachment is much more rapid, and these types of tears appear to be more common in myopic male patients.52 Goffstein found that 28% of contusion detachments were myopic, nine times higher than expected.53
The treatment of traumatic retinal tears and RD will be discussed in other chapters (Chapter 109, Giant retina tears, and Chapter 110, Surgery for ocular trauma).
Retinal dialyses
Retinal dialysis is a circumferential retinal tear located along its marginal attachment at the ora serrata (Fig. 91.4). Dialyses account for 8–14% of RD.54 Ocular trauma is widely recognized as an important cause of retinal dialysis, resulting from mechanical disruption of the retina by force transmitted via the vitreous base.55,56 Dialyses and giant tears caused 69% of traumatic detachments and 6% of nontraumatic detachments.57 A blow from the fist was the most common mechanism of trauma in these cases.54,57
The most common symptoms are visual field loss or vague blurring of vision.54,55 Other complaints include photopsias, vitreous floaters, or both. Some patients may not have any symptoms at the time of diagnosis.54 The dialysis-associated RD may develop at the time of injury or months or even years later.54,57 Because the vitreous in young patients is formed, RD caused by traumatic dialyses advance very slowly, especially in the inferior fundus. Signs of chronicity, such as demarcation lines and intraretinal cysts, are common.53 In previous reports, the mean size of the traumatic dialyses was 2.4 clock hours as compared to a mean of 1.5 clock hours for the nontraumatic cases.57
A defining feature of spontaneous dialysis is its selective localization/involvement of the inferotemporal quadrant,57 because of developmental asymmetry. However, traumatic dialyses may involve all quadrants. A higher frequency in the superonasal and inferotemporal quadrants has been reported in traumatic dialyses, with an incidence of 16–33.3% and 30.6–72% respectively.54,57 Weidenthal and Schepens55 postulated that the nasal peripheral retina has a greater susceptibility to traumatic dialysis secondary to its relatively narrower vitreous base. The inferotemporal quadrant, being the area least protected based on orbital anatomy, remains susceptible to dialysis with temporally directed trauma.56
Optic nerve avulsion
Optic nerve avulsion is an uncommon yet visually devastating event that usually occurs after nonpenetrating or penetrating ocular trauma, typically when an object intrudes between the globe and the orbital wall and displaces the eye.58,59 Patients typically describe a sudden loss of vision at the time of the trauma. The loss of vision may be relatively minor or severe, even to the point of no light perception. The avulsion is usually associated with other globe and orbital injuries but may occur as an isolated lesion.59 It is usually associated with a decelerating injury of significant momentum.49 Among many different causes, motor vehicle and bicycle accidents are the most common causes, and basketball injuries are the most common sporting injuries.60
Both complete and partial (involving only a segment of the optic disc) avulsions have been described. A complete avulsion implies that the optic nerve fibers are disinserted from the retina, choroid, and vitreous and that the lamina cribrosa is retracted from the scleral rim, resulting in a blind eye with a fixed and dilated pupil. Histopathological examination of the enucleated eye may reveal absence of optic nerve fibers from the lamina cribrosa.59 The diagnosis of optic nerve avulsion is usually apparent if the media is clear. The fundus examination in such cases usually shows an excavation in the optic disc area because of retraction of the optic nerve into its dural sheath.49 However, the fundus is usually obscured by hyphema or vitreous hemorrhage. Optic nerve avulsion can cause secondary substantial intraocular architectural changes, such as fibroglial scarring in the disc, epiretinal membrane and RD, in long-term follow-up.61
Sudden extreme rotation of the globe may be the major mechanism in some cases.58 Other postulated mechanisms include a sudden marked rise of intraocular pressure that forces the nerve out of the scleral canal or a sudden anterior displacement of the globe.
B-scan ultrasonography has been only variably successful in imaging optic nerve avulsion.58 A posterior ocular wall defect in the region of the optic disc characterized by a hypoechoic defect may be apparent.62 A-scan ultrasonography may show a marked widening of the optic nerve suggesting hemorrhage and edema within the nerve sheath.62 FA may demonstrate normal, delayed, or absent filling of the peripapillary retinal vasculature. In general, ancillary testing, such as CT, MRI, or color Doppler ultrasonography, are unlikely to assist in establishing the diagnosis in the presence of intraocular hemorrhage, although avulsions may be detected in certain cases and these studies may be useful in evaluating comorbid conditions.58
There is no known effective medical or surgical treatment for this condition. Final visual outcome is generally poor and dependent on initial post-injury visual acuity. Although administration of high-dose corticosteroids and optic canal decompression have been attempted, these treatments have not proved to be effective.58 Therefore, early diagnosis is important to spare the patient unnecessary interventions.
Chorioretinopathies from indirect ocular injuries
Purtscher’s retinopathy
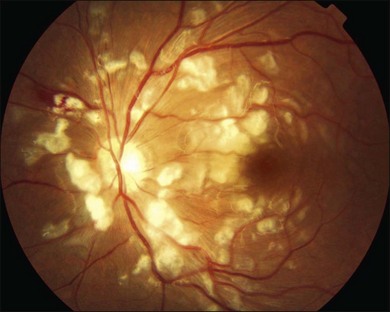
Purtscher’s retinopathy was first described in 1910 by Otmar Purtscher, in a patient with severe head trauma.63 Purtscher noted multiple areas of retinal whitening and hemorrhage in the posterior poles of both eyes.64 The term ‘‘Purtscher-like retinopathy’’ is sometimes used to describe the retinopathy seen in conditions other than trauma, such as acute pancreatitis, fat embolism syndrome, childbirth, connective tissue disorders, and renal failure. Suggested pathophysiological mechanisms have included fat embolization leading to arterial occlusion, angiospasm, or lymphatic extravasation.64,65
Patients with Purtscher’s retinopathy present with loss of acuity in one or both eyes, ranging from minimal impairment to hand motions visual acuity. Acute fundus abnormalities in Purtscher’s retinopathy include Purtscher flecken, cotton-wool spots, retinal hemorrhage, and optic disc swelling (Fig. 91.5). The characteristic Purtscher flecken consists of multiple, discrete areas of retinal whitening in the superficial aspect of the inner retina, between the arterioles and venules. Retinal hemorrhages are often minimal and are typically flame-shaped, but dot and blot hemorrhage may occur. On FA, choroidal fluorescence may be masked by retinal whitening or blood, non-perfusion of the smaller retinal arterioles or capillaries may be seen, and there may be leakage from the retinal vessels in areas of ischemia.64
Without treatment, these findings resolve spontaneously within 1–3 months and may be replaced by mottling of the RPE, temporal disc pallor, or attenuation or sheathing of the retinal vessels.64 Treatment with systemic high-dose steroids may improve visual outcome in some patients but at present there is little evidence to support such treatment.66–68
Terson’s syndrome
Moritz Litten first described the occurrence of vitreous hemorrhage in association with subarachnoid hemorrhage in 1881.69 Intraocular hemorrhage secondary to either subarachnoid hemorrhage or subdural hemorrhage is named Terson syndrome after Albert Terson.70 The reported incidence of Terson syndrome in patients suffering from subarachnoid hemorrhage ranges from 10% to 50%.71 Terson syndrome is associated with a worse outcome than in patients with subarachnoid hemorrhage but without vitreous hemorrhage.72
There are two plausible mechanisms for Terson syndrome. One explanation is that the vitreous hemorrhage may derive from ocular blood. The sudden rise in intracranial pressure may lead to a decrease in venous return to the cavernous sinus or obstruct the retinochoroidal anastomoses and central retinal vein, culminating in venous stasis and hemorrhage.72 Another explanation is that the vitreous hemorrhage may be caused by a large amount of blood entering the subarachnoid space around the optic nerve, subsequently infiltrating the intraocular space through the perivascular space around the central retinal vessels within the optic nerve.71
Histopathologic correlation shows that hemorrhages occur in the vitreous, subhyaloid space, sub-internal limiting membrane (ILM), intraretinal, and subretinal spaces, in association with macular holes, retinal detachments, and optic neuropathy. Subhyaloid hemorrhages have a diffuse morphology, whereas sub-ILM are well demarcated. Continuous and noncontinuous blood flow has been observed along optic nerves, within nerve sheaths, and in the subdural and subarachnoid spaces.73
Current recommendations in the literature suggest 3–6 months of observation after the acute event, followed by vitrectomy if there is no improvement in visual acuity.73 Vitrectomy in Terson syndrome usually results in substantial improvement in visual acuity.74
Shaken-baby syndrome
Shaken-baby syndrome (SBS) is a type of child abuse especially prevalent in infants under 3 years of age. In 1974, Caffey75 first reported cases of battered infants presenting with subdural hemorrhage, intraocular bleeding, and metaphyseal fractures.
The mechanism of retinal hemorrhage has not been clarified; however, it is assumed that the acceleration–deceleration causes relative movements of the vitreous body on the one hand and the retina and vessels on the other. Most studies support a link between the extent of retinal hemorrhage and the severity of the trauma suffered and the prognosis.76
Ocular examination is critical because up to 90% of babies with SBS display unusual ocular findings. Findings in such cases are typically bilateral but can be asymmetrical. Dilated fundus examination may demonstrate posterior pole retinal hemorrhages, involving any of the layers of the retina. Heavy vitreous hemorrhage, retinal folds, choroidal rupture, and/or retinoschisis may be observed. There may be disc edema secondary to elevated intracranial pressure or indirect optic canal damage and optic sheath hemorrhage. Horseshoe tears and retinal detachment may also be found.77
Any suspected child abuse should be reported to law enforcement agencies. Hospital admission of an SBS patient is indicated not only on medical grounds but also to avoid the risk of further abuse. Most intraretinal, subretinal, and preretinal hemorrhages clear spontaneously within 4 weeks after injury. Vitreous hemorrhage may clear or persist longer than a few months. Schisis, choroidal rupture, retinal folds, retinal detachment, and optic nerve trauma may be safely observed until the patient is stabilized and the appropriate surgical strategy is determined. For nonclearing vitreous hemorrhage or subhyaloid hemorrhage overlying the macula, trans-pars plana vitrectomy should be considered, as irreversible visual loss owing to deprivation amblyopia may occur in as little as 4 weeks. Retinal detachment should be repaired with scleral buckling or vitrectomy.77
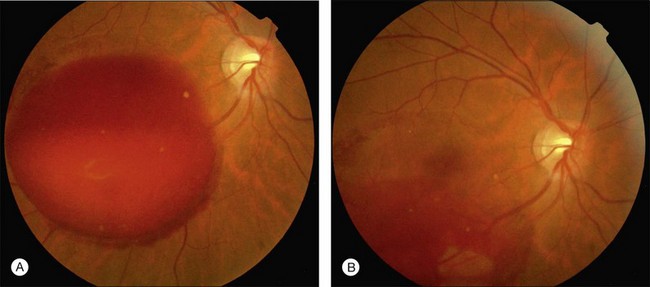
Valsalva retinopathy
Valsalva retinopathy was first described in 1972 by Thomas Duane78 as a particular form of retinopathy, preretinal and hemorrhagic in nature, secondary to a sudden increase in intrathoracic pressure. Increasing intrathoracic pressure against a closed glottis diminishes venous return to the heart, decreasing stroke volume and subsequently increasing venous system pressure. Valves in the venous system of the head and neck that are not functioning properly may allow the direct transmission of intrathoracic or intra-abdominal pressure into the head and neck. This pressure rise causes a decompensation at the level of the retinal capillary bed resulting in either unilateral or bilateral retinal hemorrhages, which are usually found below the internal limiting membrane, but can occasionally break through to become a subhyaloid or intravitreal hemorrhage.79
The anatomic location of the premacular hemorrhage is described as subinternal limiting membrane (ILM), subhyaloid, or a combination of both. Optical coherence tomography (OCT) can be used to distinguish clinically between a subhyaloid and a sub-ILM hemorrhage.80 Therapeutic options in Valsalva retinopathy include conservative management, surgery (vitrectomy), and Nd:YAG laser membranotomy (Fig. 91.6).
Fat embolism syndrome
Fat embolism syndrome (FES) was first described in 1861 by Zeuker.81 FES can be a complication of fractures of long bones, such as the femur, and is associated with various neurologic signs including paralysis, tremor, delirium, stupor, and coma. FES has been diagnosed in 5% of all patients with fractures. Clinical features usually commence between 24 and 48 hours after the injury, and the mortality rate has been reported to be as high as 30%.82 It was suggested that FES is caused by the delayed release of fat droplets from pulmonary fat emboli, by repeated influx of emboli from an inadequately fixed fracture, or by fatty acid hydrolyzed from neutral fat. The pathologic changes in FES are produced by combined mechanical damage induced by fat droplets and biochemical damage induced by fatty acids.82
Retinopathy has been reported in 50% of patients with FES and in 4% of patients with long-bone fractures presenting with a subclinical syndrome. Typical lesions consist of cotton-wool spots and flame-like hemorrhages, and are attributed to microvascular injury and microinfarction of the retina. Retinal lesions disappear after a few weeks, although scotomas may persist.83
Whiplash retinopathy
Kelley described a slight, grayish retinal haze, a crater-like depression of less than 100 µm in diameter, and a slight disturbance in the pigment epithelium in the fovea following a flexion–extension type of head and neck injury in 1978.84 The term whiplash maculopathy now refers to an immediate reduction in visual acuity with corresponding development of a foveolar depression and increased thickness of the peripheral retina. Occasionally, there may be small retinal detachments as well.85 No treatment is required, and in general, the prognosis for normal vision is good.
1 May DR, Kuhn FP, Morris RE, et al. The epidemiology of serious eye injuries from the United States Eye Injury Registry. Graefes Arch Clin Exp Ophthalmol. 2000;238:153–157.
2 Wei Z, Yusheng W. General situation of international eye injury epidemiology. Int J Ophthalmol. 2004;4:877–881.
3 Tomazzoli L, Renzi G, Mansoldo C. Eye injuries in childhood: a retrospective investigation of 88 cases from 1988 to 2000. Eur J Ophthalmol. 2003;13:710–713.
4 Serrano JC, Chalela P, Arias JD. Epidemiology of childhood ocular trauma in a northeastern Colombian region. Arch Ophthalmol. 2003;121:1439–1445.
5 Liggett PE, Pince KJ, Barlow W, et al. Ocular trauma in an urban population: review of 1132 cases. Ophthalmology. 1990;97:581–584.
6 Chunxia J, Shengyong W, Guibo C. An epidemiological analysis on eye injuries. Chin J Dis Control Prev. 2001;5:194–196.
7 Min Wu, Jian Ye. Hospitalized eye injury in a Chinese urban population: a retrospective analysis. Int J Ophthalmol. 2010;10:1861–1863.
8 Delori F, Pomerantzeff O, Cox MS. Deformation of the globe under high speed impact: its relation to contusion injuries. Invest Ophthalmol. 1969;8:290–301.
9 Berlin R. Zur sogenannten. Commotio retinae. Klin Monatsbl Augenheilkd. 1873;11:42–79.
10 Youssri AI, Young LH. Closed-globe contusion injuries of the posterior segment. Int Ophthalmol Clin. 2002;42:79–86.
11 Sony P, Venkatesh P, Gadaginamath S, et al. Optical coherence tomography findings in commotio retina. Clin Experiment Ophthalmol. 2006;34(6):621–623.
12 Viestenz A, Kchle M. Blunt ocular trauma. Part II. Blunt posterior segment trauma. Ophthalmologe. 2005;102:89–99.
13 Pulido JS, Blair NP. The blood–retinal barrier in Berlin’s edema. Retina. 1987;7:233–236.
14 Blight R, Hart JCD. Structural changes in the outer retinal layers following blunt mechanical non-perforating trauma to the globe: an experimental study. Br J Ophthalmol. 1977;61:573–587.
15 Blight R, Hart JCD. Histological changes in the internal retinal layers produced by concussive injuries to the globe: an experimental study. Trans Ophthalmol Soc UK. 1978;98:270.
16 Sipperly JO, Quigley HA, Hass JDM. Traumatic retinopathy in primates: the explanation of commotio retinae. Arch Ophthalmol. 1978;96:2267.
17 Greven CM, Collins AS, Slusher MM, et al. Visual results, prognostic indicators and posterior segment findings following surgery for cataract/lens subluxation–dislocation secondary to ocular contusion injuries. Retina. 2002;22:575–580.
18 Itakura H, Kishi S. Restored photoreceptor outer segment in commotio retinae. Ophthalmic Surg Lasers Imaging. 2011 Mar 3;42 Online:e29–e31.
19 Mansour AM, Green WR, Hogge C. Histopathology of commotio retinae. Retina. 1992;12:24–48.
20 Bradley JL, Shah SP, Manjunath V, et al. Ultra-high-resolution optical coherence tomographic findings in commotio retinae. Arch Ophthalmol. 2011;129(1):107–108.
21 Williams DF, Mieler WF, Williams GA. Posterior segment manifestations of ocular trauma. Retina. 1990;10:35–44.
22 Aguilar JP, Green WR. Choroidal rupture: a histopathologic study of 47 eyes. Retina. 1984;4:269–275.
23 Lavinsky D, Martins EN, Cardillo JA, et al. Fundus autofluorescence in patients with blunt ocular trauma. Acta Ophthalmol. 2011;89(1):e89–e94.
24 Ament CS, Zacks DN, Lane AM, et al. Predictors of visual outcome and choroidal neovascular membrane formation after traumatic choroidal rupture. Arch Ophthalmol. 2006;124:957–966.
25 Kohno T, Miki T, Shiraki K, et al. Indocyanine green angiographic features of choroidal rupture and choroidal vascular injury after contusion ocular injury. Am J Ophthalmol. 2000;129:38–46.
26 Francis JH, Freund KB. Photoreceptor reconstitution correlates with visual improvement after intravitreal bevacizumab treatment of choroidal neovascularization secondary to traumatic choroidal rupture. Retina. 2011;31:422–424.
27 Arrim ZI, Simmons IG. Traumatic choroidal rupture. Emerg Med J. 2009;26:880.
28 Yadav NK, Bharghav M, Vasudha K, et al. Choroidal neovascular membrane complicating traumatic choroidal rupture managed by intravitreal bevacizumab. Eye. 2009;23:1872–1873.
29 Harissi-Dagher M, Sebag M, Gauthier D, et al. Photodynamic therapy in young patients with choroidal neovascularization following traumatic choroidal rupture. Am J Ophthalmol. 2005;139:726–728.
30 Oehrens AM, Stalmans P. Optical coherence tomographic documentation of the formation of a traumatic macular hole. Am J Ophthalmol. 2006;142:866–869.
31 Yamada H, Sakai A, Yamada E, et al. Spontaneous closure of traumatic macular hole. Am J Ophthalmol. 2002;134:340–347.
32 Ho AC, Guyer DR, Fine SL. Macular hole. Surv Ophthalmol. 1998;42:393–416.
33 Smiddy WE, Flynn HW, Jr. Pathogenesis of macular holes and therapeutic implications. Am J Ophthalmol. 2004;137:525–537.
34 Li XW, Lu N, Zhang L, et al. Follow-up study of traumatic macular hole. Zhonghua Yan Ke Za Zhi. 2008;44:786–789.
35 Takahashi H, Kishi S. Optical coherence tomography images of spontaneous macular hole closure. Am J Ophthalmol. 1999;128:519–520.
36 Huang J, Liu X, Wu Z, et al. Comparison of full-thickness traumatic macular holes and idiopathic macular holes by optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2010;248:1071–1075.
37 Chen YJ. Vitrectomy and internal limiting membrane peeling of a traumatic macular hole with retinal folds. Case Report Ophthalmol. 2011;2(1):78–83.
38 Goldzieher W. Beitrag zur Pathologie der orbitalen Schussverletzungen. Z Augenheilkd. 1901;6:277.
39 Pe’rez-Carro G, Junceda-Moreno C. Dual cause of blindness: chorioretinitis sclopetaria and homonymous hemianopsia. Arch Soc Esp Oftalmol. 2006;81:119–122.
40 Ahmadabadi MN, Karkhaneh R, Roohipoor R, et al. Clinical presentation and outcome of chorioretinitis sclopetaria: A case series study. Injury. 2010;41:82–85.
41 Martin DF, Awh CC, McCuen BW, 2nd., et al. Treatment and pathogenesis of traumatic chorioretinal rupture (sclopetaria). Am J Ophthalmol. 1994;117:190–200.
42 Fraser EA, Barr DB. Chorioretinal arterial and venous anastomoses as a result of blunt trauma. Eye. 2004;18:336–338.
43 Maguluri S, Hartnett M. Radial choroidal ruptures in sclopetaria. J Am Coll Surg. 2003;197:689–690.
44 Shima C, Sakaguchi H, Gomi F, et al. Complications in patients after intravitreal injection of Bevacizumab. Acta Ophthalmol. 2008;86:372–376.
45 Chan A, Duker JS, Ko TH, et al. Ultra-high resolution optical coherence tomography of retinal pigment epithelial tear following blunt trauma. Arch Ophthalmol. 2006;124:281–283.
46 Fok AC, Lai TY, Wong VW, et al. Spontaneous resolution of retinal pigment epithelial tears and pigment epithelial detachment following blunt trauma. Eye. 2007;21:891–893.
47 Dolan BJ. Traumatic retinal detachment. Optom Clin. 1993;3:67–80.
48 Cox MS. Retinal breaks caused by blunt nonperforating trauma at the point of impact. Trans Am Ophthalmol Soc. 1980;78:414–466.
49 Atmaca LS, Yilmaz M. Changes in the fundus caused by blunt ocular trauma. Ann Ophthalmol. 1993;25:447–452.
50 Johnston PB. Traumatic retinal detachment. Br J Ophthalmol. 1991;75:18–21.
51 Kennedy CJ, Parker CE, McAllister IL. Retinal detachment caused by retinal dialysis. Aust N Z J Ophthalmol. 1997;251:25–30.
52 Nacef L, Daghfous F, Chaabini M, et al. Ocular contusions and giant retinal tears. J Fr Ophthamol. 1997;203:170–174. (French)
53 Goffstein R, Burton TC. Differentiating traumatic from nontraumatic retinal detachment. Ophthalmology. 1982;89:361–368.
54 Ross WH. Traumatic retinal dialyses. Arch Ophthalmol. 1981;99:1371–1374.
55 Weidenthal DT, Schepens CL. Peripheral fundus changes associated with ocular contusion. Am J Ophthalmol. 1966;62:465–477.
56 Cox MS. Retinal breaks caused by blunt nonperforating trauma at the point of impact. Trans Am Ophthalmol Soc. 1980;78:414–466.
57 Hollander DA, Irvine AR, Poothullil AM, et al. Distinguishing features of nontraumatic and traumatic retinal dialyses. Retina. 2004;24:669–675.
58 Foster BS, March GA, Lucarelli MJ, et al. Optic nerve avulsion. Arch Ophthalmol. 1997;115:623–630.
59 Chaudhry IA, Shamsi FA, Al-Sharif A, et al. Optic nerve avulsion from door-handle trauma in children. Br J Ophthalmol. 2006;90:844–846.
60 Anand S, Harvey R, Sandramouli S. Accidental self-inflicted optic nerve head avulsion. Eye. 2003;17:646–648.
61 Sturm V, Menke MN, Bergamin O, et al. Longterm follow-up of children with traumatic optic nerve avulsion. Acta Ophthalmol. 2010;88:486–489.
62 Sawhney R, Kochhar S, Gupta R, et al. Traumatic optic nerve avulsion: role of ultrasonography. Eye. 2003;17:667–670.
63 Purtscher O. Noch unbekannte befunde nach schadeltrauma. Berl Dtsch Ophthalmol Ges. 1910;36:294–301.
64 Agrawal A, McKibbin MA. Purtscher’s and Purtscher-like retinopathies: a review. Surv Ophthalmol. 2006;51:129–136.
65 Trikha S, Tiroumal S, Hall N. Purtscher’s retinopathy following a road traffic accident. Emerg Med J. 2011;28:453.
66 Wang AG, Yen MY, Liu JH. Pathogenesis and neuroprotective treatment in Purtscher’s retinopathy. Jpn J Ophthalmol. 1998;42:318–322.
67 Atabay C, Kansu T, Nurlu G. Late visual recovery after intravenous methylprednisolone treatment of Purtscher’s retinopathy. Ann Ophthalmol. 1993;25:330–333.
68 Agrawal A, McKibbin M. Purtscher’s retinopathy: epidemiology, clinical features and outcome. Br J Ophthalmol. 2007;91:1456–1459.
69 Litten M. Ueber einige vom allgemein-Klinischen Standpunkt aus interessante Augenveränderungen. Berl Klin Wochenschr. 1881;18:23–27.
70 Terson A. De l’hémorrhagie dans le corps vitre au cours del’hémorrhagie cerebrale. Clin Ophthalmol. 1900;6:309–312.
71 Sakamoto M, Nakamura K, Shibata M, et al. Magnetic resonance imaging findings of Terson’s syndrome suggesting a possible vitreous hemorrhage mechanism. Jpn J Ophthalmol. 2010;54:135–139.
72 McCarron MO, Alberts MJ, McCarron P. A systematic review of Terson’s syndrome: frequency and prognosis after subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2004;75:491–493.
73 Ko F, Knox DL. The ocular pathology of Terson’s syndrome. Ophthalmology. 2010;117:1423–1429.
74 Murjaneh S, Hale JE, Mishra S, et al. Terson’s syndrome: surgical outcome in relation to entry site pathology. Br J Ophthalmol. 2006;90:512–513.
75 Caffey J. The whiplash shaken infant syndrome. Pediatrics. 1974;54:396–403.
76 Matschke J, Herrmann B, Sperhake J, et al. Shaken baby syndrome: a common variant of non-accidental head injury in infants. Dtsch Arztebl Int. 2009;106:211–217.
77 Tang J, Buzney SM, Lashkari K, et al. Shaken baby syndrome: a review and update on ophthalmologic manifestations. Int Ophthalmol Clin. 2008;48:237–246.
78 Duane TD. Valsalva hemorrhagic retinopathy. Trans Am Ophthalmol Soc. 1972;70:298–313.
79 Tildsley J, Srinivasan S. Valsalva retinopathy. Postgrad Med J. 2009;85:110.
80 Shukla D, Naresh KB, Kim R. Optical coherence tomography findings in valsalva retinopathy. Am J Ophthalmol. 2005;140:134–136.
81 Zeuker FA. Beitrage zur Anatomie und Physiologie der Lunge. Dresden: J Braunsdorf; 1861.
82 Lee JE, Jea SY, Oum BS, et al. Effect of fat embolism with triolein emulsion on blood–retinal barrier. Ophthalmic Res. 2009;41:14–20.
83 Akhtar S. Fat embolism. Anesthesiol Clin. 2009;27:533–550.
84 Kelley JS, Hoover RE, George T. Whiplash maculopathy. Arch Ophthalmol. 1978;96:834–835.
85 Uebbing C, Miller J, Arnold C, et al. Soccer player whiplash maculopathy. Am J Emerg Med. 28, 2010. 120.e7–8