Chapter 231 Traumatic Central Cord Syndrome
Early Surgery
Central cord syndrome (CCS) is defined as an acute cervical spinal cord injury (SCI) presenting with a disproportionately greater motor impairment in the upper extremities than in the lower extremities, bladder dysfunction, and a variable degree of sensory loss below the level of injury.1–6 This was first described by Schneider in 1954.5,6 CCS has been reported to occur more frequently among older individuals with cervical spondylosis but has been reported in younger people as well.1,6 CCS is the most common SCI syndrome, accounting for 9% of traumatic SCIs.1,6 It is usually associated with a favorable prognosis including some degree of functional and neurologic recovery. Like other types of SCI, CCS tends to affect men more than women.1 The main cause of CCS is a hyperextension injury in an individual with preexisting chronic spondylosis.1–6 CCS associated with bleeding into the central aspect of the spinal cord portends a less favorable prognosis.1 At the cellular level, CCS is associated with axonal disruption in the lateral columns at the level of injury with relative preservation of the gray matter.
Surgical Management
The role and timing of surgical treatment for traumatic central cord syndrome have been the subjects of multiple retrospective class II and III studies. The timing of surgical decompression remains controversial but is generally accepted to be effective with controlled hemodynamic parameters.3,5 The emerging data from the Surgical Treatment for Acute Spinal Cord Injury Study (STASCIS), the only class I study in this field, shows significantly improved outcome with decompressive surgery within the first 24 hours in patients with isolated SCI.3,7 The continuing data from STASCIS long-term follow up will give us better answers about the effectiveness and timing of surgery for CCS.3,7 Some of the landmark class II and class III studies along with their conclusions and limitations are discussed next.
In their studies, Chen et al. analyzed the effect of time (before and after 4 days) and type (ventral vs. dorsal) of surgery as well as the type of spinal cord pathology on recovery in 49 patients with traumatic central cord syndrome (TCCS).2 The recovery was assessed by American Spinal Injury Association (ASIA) motor scores, the 36-item Short Form Health Survey (SF-36), the Walking Index for Spinal Cord Injury (WISCI), self-reported patient satisfaction (1–5), bladder management scores, presence of spasticity, and presence of neuropathic pain.2 The result of their study showed no correlation between any of the assessed variables and the recovery outcome. Some major limitations of this study include the lack of a comparative control group, the retrospective design of the study, and the arbitrary selection of 4 days to define the early group versus the late group.
However, in another retrospective study, Guest et al.4 reached a conclusion opposite to that of Chen et al.,2 finding early surgery highly effective in improving the outcome in TCCS. The authors analyzed the clinical characteristics, radiographic findings, ASIA score, and length of hospital stay in 50 patients who underwent either early (≤24 hours) or late (>24 hours) surgery.3,4 They found significant improvement in the ASIA score and length of hospital stay (P = .04) in patients with TCCS caused by acute disc herniation or fracture-dislocation who underwent early surgery.3,4 There was not a significant change in ASIA score for CSS secondary to spinal stenosis or spondylosis. The worst outcome was observed in patients older than 60 years in whom initial bladder dysfunction was present.3,4 One reason for the drastic difference in the results between these two studies is that the definition of early surgery was 4 days in the first study and 24 hours in the second study.
In another landmark study, Yamazaki et al. looked retrospectively at 47 patients with CCS.6 They reviewed clinical and radiologic data, including age, Japanese Orthopaedic Association Scale, anteroposterior diameter of spinal canal on CT, signal intensity change on T2-weighted MRI, associated spine diseases, and type of treatment received.6 They concluded that Japanese Orthopaedic Association Scale score on admission, signal changes in the MRI, and associated spine disease were not correlated with patient recovery.6 The only two variables that were found to improve the outcome significantly were the anteroposterior diameter (P = .0402) and the timing of surgery (P < .0001).6 The cut-off time for early versus late surgery was set at 2 weeks, which was considerably later than that in the first two studies. One would expect, on the basis of the Chen et al. study, that a late time setting (i.e., >24 hours) to define early versus late surgery would cause inaccuracy in the outcome and would not support a role for early surgery.2,6 However, possible interaction between multiple limitations such as an inaccurate time setting, a retrospective design, or lack of controlled subjects has resulted in a conclusion that actually favors early surgery. From all the preceding class II and III studies, it becomes obvious that a reliable conclusion could be achieved only if we were to run a prospective systemic study with well-defined and randomized control subjects. The closest to this idealistic goal is the STASCIS, which has released some promising results supporting the role of early surgery in improvement of the outcome in incomplete SCI.
Surgical Treatment for Acute Spinal Cord Injury Study
Run by multiple centers in North America, STASCIS is a long-term prospective study looking at 170 patients with various degrees of SCI.3,7. Of these subjects, 38% had incomplete SCI, 22% were rated ASIA B, and 16% were rated ASIA C.7 The subjects were randomly divided into early (<24 hours) and late (>24 hours) surgery groups.7 The preliminary report at 6 and 12 months follow-up was released in April 2010. The initial results have shown a two-grade improvement in the ASIA score in patients who took the early surgery (24% of subjects) versus late surgery (only 4%) (P = .009).3,7
Case Vignette
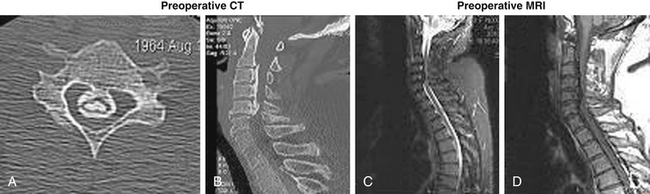
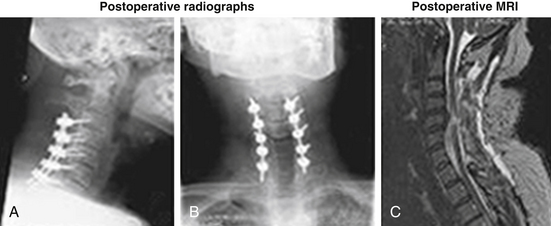
A 45-year-old woman with no significant past medical history had a syncopal episode, struck her head, and became immediately tetraplegic. She arrived in our hospital 6 to 8 hours later. Her neurologic status upon arrival at the emergency room was ASIA A, C6 motor level, T3 sensory level, HR 43/min, BP systolic 108 mm Hg. The preoperative workup included cervical MRI and CT (Fig. 231-1). Methylprednisolone IV infusion was initiated before arrival and was continued over the admission according to the National Acute Spinal Cord Injury Study (NASCIS) II protocol. The patient received dopamine IV continuous drip, keeping her mean arterial pressure over 85 mm Hg, to secure a reasonable blood flow at the injury site. The decompressive surgery (C3-7 laminectomy and dorsal instrumented fusion; Fig. 231-2) was performed in emergency (in the first 10 hours after trauma and the first 2 hours after arrival in our hospital). Some motor activity was noticed on the left side (both upper and lower limbs) very early postoperatively (in the recovery room). The patient’s neurologic status continued to improve, and she was transferred to a rehabilitation facility as an ASIA C patient. At 6 weeks postoperatively, her neurologic status was consistent with that of an ASIA D impaired patient, with a normal motor function on the left side (both upper and lower limbs, except a mild deficit in the hand) but significant motor deficits on the right side. The patient was still using intermittent self-catheterization to void the bladder, but rectal and micturitional sensation was present. Concluding, we assume that all elements from this scenario—early transportation to a medical facility, early diagnosis, early decompressive surgery, a dopamine drip to maintain mean BP over 85 mm Hg, and early administration of methylprednisolone—contributed to this excellent outcome.
Conclusion
The effectiveness and timing for surgery in TCCS have been studied in multiple class II and class III retrospective studies with contradictory conclusions secondary to the limitations of the study designs. The initial data from the ongoing STASCIS projects support a recommendation for early surgery (<24 hours) in hemodynamically stable patients with severe TCCS. On the other hand, the senior author defers surgical treatment in patients with milder TCCS (ASIA D deficits with rapidly improving neurology) for 6 to 8 weeks after injury to allow the posttraumatic edema to resolve. Of note, Bizhan Aarabi at the University of Maryland is spearheading efforts to undertake a prospective randomized controlled trial to examine the timing of surgical intervention for TCCS.1 We await the results of this trial with great interest.
Aarabi B., Koltz M., Ibrahimi D. Hyperextension cervical spine injuries and traumatic central cord syndrome. Neurosurg Focus. 2008;25(5):E9.
Chen A., Yang H., Yang T., et al. Effectiveness of surgical treatment for traumatic central cord syndrome. J Neurosurg Spine. 2009;10:3-8.
Fehlings MG. STASCIS: Early surgery in spinal cord injury improves outcomes, lowers complications. Paper presented at American Association of Neurological Surgeons 76th Annual Meeting: Abstract 600. Presented April 28, 2008.
Fehlings M.G., Arvin B. The timing of surgery in patients with central spinal cord injury. J Neurosurg Spine. 2009;10:1-2.
Guest J., Eleraky M.A., Apostolides P.J., et al. Traumatic central cord syndrome: results of surgical management. J Neurosurg Spine. 2002;97:25-32.
Harrop J.S., Sharan A., Ratliff J. Central cord injury: Pathophysiology, management and outcomes. Spine J. 2006;6:S198-S206.
Yamazaki T., Yanaka K., Fujita K., et al. Traumatic central cord syndrome: analysis of factors affecting the outcome. Surg Neurol. 2005;63:95-100.
1. Aarabi B., Koltz M., Ibrahimi D. Hyperextension cervical spine injuries and traumatic central cord syndrome. Neurosurg Focus. 2008;25(5):E9.
2. Chen A., Yang H., Yang T., et al. Effectiveness of surgical treatment for traumatic central cord syndrome. J Neurosurg Spine. 2009;10:3-8.
3. Fehlings M.G., Arvin B. The timing of surgery in patients with central spinal cord injury. J Neurosurg Spine. 2009;10:1-2.
4. Guest J., Eleraky M.A., Apostolides P.J., et al. Traumatic central cord syndrome: results of surgical management. J Neurosurg Spine. 2002;97:25-32.
5. Harrop J.S., Sharan A., Ratliff J. Central cord injury: Pathophysiology, management and outcomes. Spine J. 2006;6:198S-206S.
6. Yamazaki T., Yanaka K., Fujita K., et al. Traumatic central cord syndrome: analysis of factors affecting the outcome. Surg Neurol. 2005;63:95-100.
7. Fehlings MG. STASCIS: Early surgery in spinal cord injury improves outcomes, lowers complications. Paper presented at American Association of Neurological Surgeons 76th Annual Meeting: Abstract 600. Presented April 28, 2008.
Delayed Surgery
Acute traumatic central cord syndrome (TCCS), first defined by Schneider et al. in 1954,1 is characterized as an incomplete spinal cord injury (SCI) occurring primarily in the subaxial cervical spine, following a compressive injury to the cord, with a variable rate of functional recovery. TCCS accounts for roughly half of all the diverse clinical syndromes that have been described following acute spinal cord trauma. The purported mechanism of injury is thought to arise most commonly following traumatic hyperextension of the neck, causing contusion of the cord due to the compressive, pincer-like effect of buckled ligamentum flavum and dorsally projecting osteophytes in stenotic and spondylotic spines. In all likelihood, this accounts for the preponderance of older patients in whom this condition is diagnosed. However, the damage may also occur with acute disc sequestrations, fracture dislocations of the cervical spine, and radiographically silent neck injuries in a younger patient cohort.2–5 TCCS manifests itself primarily with upper extremity weakness, autonomic perineal dysfunction, and a variable pattern of sensory loss below the level of injury, while the lower extremities tend to be spared or less involved. For years, the pathogenesis of this clinical pattern was thought to be secondary to the largely unvalidated theory of somatotopically organized neural pathway laminations in the spinal cord, whereby greater injury was sustained by the medially oriented corticospinal fibers subserving arm and hand function. An alternative theory suggests that upper-extremity motor utility, especially fine motor movement, is mediated by the lateral corticospinal tracts extending from the medulla to the cervical enlargement, with lower-extremity function and locomotion predicated upon other descending spinal pathways, such as the rubrospinal and/or vestibulospinal tracts.6,7 TCCS tends to occur more frequently in older patients or those with congenitally narrowed spinal canals. The incomplete nature of the injury is characterized by a greater propensity for neurologic recovery, albeit with residual hand dysfunction and spasticity in most patients.
Acute SCI is further defined by the presence of primary and secondary injury mechanisms to the neural structures. Primary injury occurs at the time of trauma and is related to compressive displacement of bone, soft tissue, or blood against the cord. The hallmark of secondary injury, first described by Allen in 1911,8 is its potential reversibility as a progressive cascade of delayed noxious events unfolds in the ensuing hours after the principal insult. It is this secondary injury, with possible exacerbation by continued compression of the neural elements, that provides a potential window for therapeutic intervention. SCI is a devastating neurologic condition with far-reaching clinical, economic, and social consequences, and expanding this opportunity for improved outcomes in TCCS patients has been a tantalizing goal for surgeons over the last decades. However, despite the advent of new and improved therapeutic and diagnostic tools, only modest progress has been made in this direction.
The current standards of care include systemic resuscitation, immobilization, and restoration of spine alignment and stability. The use of high-dose steroids (intravenous methylprednisolone sodium succinate) has been shown to diminish posttraumatic inflammation, cellular oxidative damage, and apoptosis. Following publication of two prospective, randomized control studies (National Acute Spinal Cord Injury Study [NASCIS] II and III), which demonstrated favorable neurologic outcomes with regard to incomplete SCI when large-dose drug administration occurred within specified time constraints, the protocol has been almost universally adopted as an indispensible treatment modality in managing acute SCI.9–11 However, questions have been raised about the validity of the study data, and doubts remain about the efficacy and safety of this treatment option.12–15 A position statement issued by the AANS/CNS Joint Section on Disorders of the Spine and Peripheral Nerves in 2002 recommends use of high-dose steroids for acute SCI as a treatment option only.16 Despite the controversy surrounding the NASCIS studies, chemical intervention is still regarded as a highly promising avenue for the management of acute SCI on the cellular and molecular levels. In a thorough review of the current literature, Baptiste and Fehlings have catalogued several pharmacologic agents and groundbreaking cell-based therapies that are being studied on the preclinical and early clinical levels to ascertain efficacy and safety regarding neuroprotection as well as cellular repair and regeneration.17
Nonsurgical decompression and stabilization (i.e., traction and bedrest) have been mainstays of successful treatment in SCI for many years, mostly because of earlier concerns about higher documented rates of neurologic injury associated with laminectomy.18,19 However, this particular concern has been moderated, as contemporary principles of spine surgery recognize the need for adjunctive, stabilizing instrumentation in most cases of acute SCI requiring posterior decompression alone. A recent retrospective review of 82 patients with TCCS in Sweden found no difference in outcomes between surgery and conservative management.2 Nonetheless, there is a general lack of class I or II evidence advocating conservative management, most of the current scientific support for nonoperative treatment depending on retrospective analyses of clinical databases.20 Furthermore, current trends in health-care economics, with pressures to limit the duration of hospital stay, as well as the possibility of complications related to prolonged bedrest and immobilization have restricted the role of conservative management in TCCS.21,22
Several eloquent animal studies have demonstrated compelling evidence of improved outcomes with early decompression in incomplete SCI models, using spacer placement or circumferential constriction techniques.23,24 Furthermore, Delamarter et al. demonstrated a close association between the extent of neurologic improvement, the severity of histopathologic injury and electrophysiologic recovery, and the duration of compression; thereby suggesting a continuum of secondary spinal cord damage related to the timing of decompression.25 Nevertheless, animal data have been difficult to analyze, owing to differences in injury mechanisms when compared to human trauma; therefore, conclusions reached with these studies cannot readily be applied to the clinical paradigm.
While major advances in instrumentation and osteobiologics since the 1980s have paved the way for decompression and stabilization of increasingly complex spine injuries, thereby improving outcomes, the timing of spinal cord decompression remains controversial. Laboratory data and the intuitive need to relieve local compressive forces have created a groundswell of support for early intervention; however, the possible risks of neurologic, pulmonary, and hemodynamic deterioration associated with early operative treatment are well documented.26,27 The national trend toward evidence-based medicine and outcomes studies has continued to raise the expectation of a comprehensive set of guidelines for appropriate timing of operative intervention; however, the lack of class I data has frustrated these efforts. Class II and class III data in human clinical trials have raised the possibility that there is a tendency toward superior outcome with earlier surgical intervention, yet these studies have not met the exacting criteria for standard of care.
The distinction between early and late surgery remains a further point of controversy. Determination of faster neurologic recovery and sustained long-term benefit is difficult to ascertain without clear and unified definitions of early and late. Prior studies adopted a broader view of the timeline for urgent surgical decompression. Tator et al. compared nonoperative to operative treatment, their surgical cohort being further subdivided into early surgery (within a week of injury) and delayed surgery (up to 4 weeks after injury), and found no difference in hospital length of stay and neurologic outcome between any of the groups studied.19 In reviewing the NASCIS II data, Duh et al, studied the effectiveness of surgery as it relates to the administration of pharmacologic agents. They determined 100 hours after injury to be the cut-off time between early and late surgery. At 1-year follow-up, they did show an association between surgery performed at either less than 25 hours or greater than 200 hours with regard to improved motor outcome. However, none of this was thought to provide clinically relevant information pertaining to the outcomes and timing of surgery in treating acute SCI.28 A recently published retrospective study of 49 patients demonstrated a significant improvement in motor outcome after 6 months with a 4-day cut-off point between early and late surgery.29 A prospective, randomized, controlled study performed on 64 patients with acute cervical SCI by Vaccaro et al. revealed no statistically significant neurologic benefit in surgery performed within 72 hours of injury (early) compared to more than 5 days after injury (late). However, they acknowledge several problems regarding the methodology of this study, including 20 patients lost to long-term follow-up and discrepancies in the mode of muscle testing, which may have weakened the validity of the results. They make the point that earlier surgical interventions are being considered in future study designs.30
More recent studies have drawn the distinction between early and late surgery at increasingly earlier benchmarks, which also sets a higher standard for patient access from the point of injury to the operating room. In a meta-analysis of the clinical literature up to 2000, La Rosa et al. identified 226 patients who underwent surgical decompression within 24 hours of injury and compared them to late (>24 hours) and nonsurgical patient cohorts. Although statistically better outcomes occurred in the early decompression group, the authors note that most of the data were derived from retrospective (class III) evidence, and they were unable to confer any particular advantage upon early surgery compared to late or nonsurgical treatment.31 A retrospective review of 412 patients by Pollard and Apple found no relationship between timing of surgery (either earlier or later than 24 hours after injury) with regard to neurologic recovery. They did, however, document improved outcomes in younger patients and those with Brown-Séquard or central cord syndromes.14 This raises the interesting prospect that SCIs do not all have the same essential characteristics and therefore should not be compared in a broad collective fashion. Outcomes in patients with cauda equina syndrome may differ considerably from those for patients with TCCS.
The Surgical Treatment of Spinal Cord Injury Study (STASCIS), currently underway across North America, is a prospective study to support the supposition that early decompression within 24 hours may be associated with improved neurologic outcome when compared to late surgery (after 24 hours). Further long-term outcome data from this study are still pending, and it is hoped that the results from this undertaking will provide additional insights into the effects of surgical timing on outcome in acute SCI.20 Practically speaking, from legal, ethical, and methodologic perspectives, it would be extremely unlikely that consensus could be reached in the design of an appropriate prospective, randomized, multicenter study to confirm the hypothesis for early surgical intervention with class I data.
Aarabi B., Klotz M., Ibrahimi D. Hyperextension cervical spine and traumatic central cord syndrome. Neurosurg Focus. 2008;25(5):E9.
Aito S., D’Andrea M., Werhagen L., et al. Neurological and functional outcome in traumatic central cord syndrome. Spinal Cord. 2007;45(4):292-297.
Delamarter R.B., Sherman J., Carr J.B. Pathophysiology of spinal cord injury. Recovery after immediate and delayed decompression. J Bone Joint Surg Am. 1995;77:1042-1049.
Fehlings M.G., Perrin R.G. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine (Phila Pa 1976). 2006;31(Suppl):S28-S35.
La Rosa G., Conti A., Cardali S., et al. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using a meta-analytical approach. Spinal Cord. 2004;42:503-512.
Pollard M.E., Apple D.F. Factors associated with improved neurological outcome in patients with incomplete tetraplegia. Spine (Phila Pa 1976). 2002;28:33-38.
Vaccaro A.R., Daugherty R.J., Sheehan T.P., et al. Neurological outcome of early versus late surgery for cervical spinal cord injury. Spine (Phila Pa 1976). 1997;22:2609-2613.
1. Schneider R.C., Cherry G., Pantek H. The syndrome of acute central cervical spinal cord injury: with special reference to the mechanisms involved in hyperextension injuries to the cervical spine. J Neurosurg. 1954;13:546-577.
2. Aito S., D’Andrea M., Werhagen L., et al. Neurological and functional outcome in traumatic central cord syndrome. Spinal Cord. 2007;45(4):292-297.
3. Dvorak M.F., Fisher C.G., Hoekema J., et al. Factors predicting motor recovery and functional outcome after traumatic central cord syndrome: a long-term follow-up. Spine (Phila Pa 1976). 2006;30:2303-2311.
4. Aarabi B., Klotz M., Ibrahimi D. Hyperextension cervical spine and traumatic central cord syndrome. Neurosurg Focus. 2008;25(5):E9.
5. Quencer R.M., Bunge R.P., Egnor M., et al. Acute traumatic central cord syndrome: MRI-pathological correlations. Neuroradiology. 1992;34:85-94.
6. Winn H.R. Youmans neurological surgery. Philadelphia: Saunders; 2004.
7. Jimenez O., Marcillo A., Levi A.D. A histopathological analysis of the human cervical spinal cord in patients with acute traumatic central cord syndrome. Spinal Cord. 2000;38:532-537.
8. Allen A.R. Surgery of experimental lesion of spinal cord equivalent to crush injury of fractured dislocation of spinal column: a preliminary report. JAMA. 1911;57:878-880.
9. Bracken M.B., Shepard M.J., Collins W.F., et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal cord injury: results of the second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;332:1405-1411.
10. Bracken M.B., Shepard M.J., Holford T.R., et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;28:1597-1604.
11. Bracken M.B., Shepard M.J., Holford T.R., et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. J Neurosurg. 1998;89:699-706.
12. Hurlbert R.J. The role of steroids in acute spinal cord injury: an evidence-based analysis. Spine (Phila Pa 1976). 2001;26(24 S):S39-S46.
13. Sayer F.T., Kronvall E., Nilsson O.G. Methylprednisolone treatment in acute spinal cord injury: the myth challenged through a structured analysis of published literature. Spine J. 2006;6:335-343.
14. Pollard M.E., Apple D.F. Factors associated with improved neurological outcome in patients with incomplete tetraplegia. Spine (Phila Pa 1976). 2002;28:33-38.
15. Ito Y., Sugimoto Y., Tomioka M., et al. Does high dose methylprednisolone sodium succinate really improve neurological status in patient with acute cervical cord injury? A prospective study about neurological recovery and early complications. Spine (Phila Pa 1976). 2009;34:2121-2124.
16. Hadley M.N., Walters B.C., Grabb P.A., et al. Guidelines for the management of acute cervical spine and spinal cord injuries. Clin Neurosurg. 2002;49:407-498.
17. Baptiste D.C., Fehlings M.G. Update on the treatment of spinal cord injury. Prog Brain Res. 2007;161:217-233.
18. Frankel H.L., Hancock D.O., Hyslop G. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia. 1969;7:179-192.
19. Tator C.H., Duncan E.G., Edmonds V.E. Comparison of surgical and conservative management in 208 patients with acute spinal cord injury. Can J Neurol Sci. 1987;14:60-69.
20. Fehlings M.G., Perrin R.G. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine (Phila Pa 1976). 2006;31(Suppl):S28-S35.
21. Katoh S., el Masry W.S., Jaffray D. Neurologic outcome in conservatively treated patients with incomplete closed traumatic cervical spinal cord injuries. Spine (Phila Pa 1976). 1996;21:2345-2351.
22. Chen T., Dickman C.A., Eleracky M., et al. The role of decompression for acute incomplete cervical spinal cord injury in cervical spondylosis. Spine (Phila Pa 1976). 1998;23:2398-2403.
23. Dimar J.R.II, Glassman S.D., Raque G.H., et al. The influence of spinal canal narrowing and timing of decompression on neurological recovery after spinal cord contusion in a rat model. Spine (Phila Pa 1976). 1999;24:1623-1633.
24. Rabinowitz R.S., Eck J.C., Harper C.M.Jr. Urgent surgical decompression compared to methylprednisolone for the treatment of acute spinal cord injury: a randomized prospective study in beagle dogs. Spine (Phila Pa 1976). 2008;33:2260-2268.
25. Delamarter R.B., Sherman J., Carr J.B. Pathophysiology of spinal cord injury. Recovery after immediate and delayed decompression. J Bone Joint Surg [Am]. 1995;77:1042-1049.
26. Marshall L.F., Knowlton S., Garfin S.R., et al. Deterioration following spinal cord injury: a multicenter study. J Neurosurg. 1987;66:400-404.
27. Levi L., Wolf A., Rigamonti D., et al. Anterior decompression in cervical spine trauma: does timing of surgery affect the outcome? Neurosurgery. 1991;29:216-222.
28. Duh M.S., Shepard M.J., Wilberger J.E., et al. The effectiveness of surgery on the treatment of acute spinal cord injury and its relation to pharmacological treatment. Neurosurgery. 1994;35:240-248.
29. Chen L., Yang H., Yang T., et al. Effectiveness of surgical treatment for traumatic central cord syndrome. J Neurosurg Spine. 2009;10:3-8.
30. Vaccaro A.R., Daugherty R.J., Sheehan T.P., et al. Neurological outcome of early versus late surgery for cervical spinal cord injury. Spine (Phila Pa 1976). 1997;22:2609-2613.
31. La Rosa G., Conti A., Cardali S., et al. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using a meta-analytical approach. Spinal Cord. 2004;42:503-512.