73 Traumatic Brain Injury (Adult)
• Emergency treatment of traumatic brain injury is aimed at rapidly identifying surgically correctable lesions and preventing secondary insults such as hypoxemia and hypotension.
• Triage to a neurosurgical trauma center is recommended for patients with intracerebral hemorrhage or persistent altered mental status.
• Early computed tomography is used to identify intracranial hemorrhage in patients with a significant mechanism of injury, history of altered mental status, or risk factors such as anticoagulant therapy.
• Osmotic agents such as mannitol or hypertonic saline are important first-line therapies for patients with elevated intracranial pressure.
• Hyperventilation should be avoided except as a temporizing measure in patients with impending herniation.
• A significant proportion of patients with mild head injury will have persistent postconcussive symptoms. All head-injured patients should be counseled about this possibility and be given appropriate outpatient referral.
• Athletes suffering from concussion should return to play only after completing a supervised stepwise rehabilitation program.
Pathophysiology
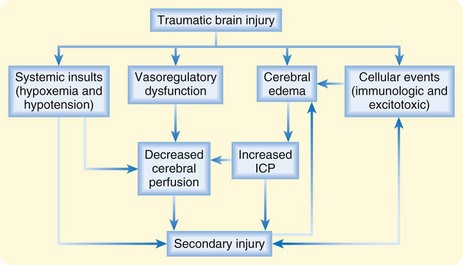
Hemorrhage or edema following TBI leads to rapid increases in intracranial pressure (ICP). Initially, cerebrospinal fluid (CSF) can be shunted out of the skull via the ventricles and cisterns. However, after severe TBI, this capacity is quickly overcome, and a rapid rise in ICP with compromised cerebral blood flow and cerebral ischemia ensues. Ultimately, the brain tissue itself may be forced downward across the rigid tentorium or out of the base of the skull itself, thereby resulting in a herniation syndrome and rapid death. Systemic hypoxia and hypotension occur with high frequency in patients with TBI and are associated with increased mortality (Fig. 73.1).
Differential Diagnosis and Medical Decision Making
Imaging
Non–contrast-enhanced computed tomography (CT) is the initial imaging study of choice in the evaluation of patients with TBI. Plain radiographs are neither sensitive nor specific in identifying intracranial lesions or skull fracture and are therefore not recommended as a diagnostic study. CT has excellent sensitivity in detecting the presence of intracranial hemorrhage, a mass effect such as ventricular compression or midline shift, and the presence of significant cerebral edema. CT also has the advantage of being widely available and rapid. See Box 73.1 for examples of CT findings in patients with TBI.
Box 73.1 Findings on Computed Tomography in Individuals with Traumatic Brain Injury
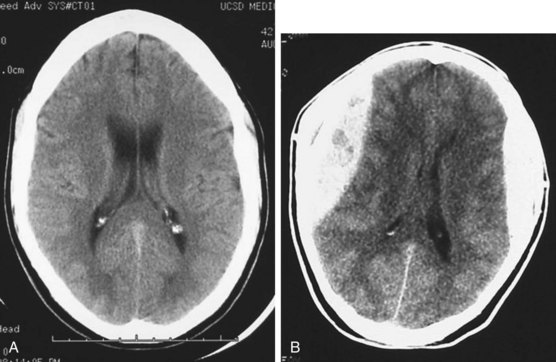
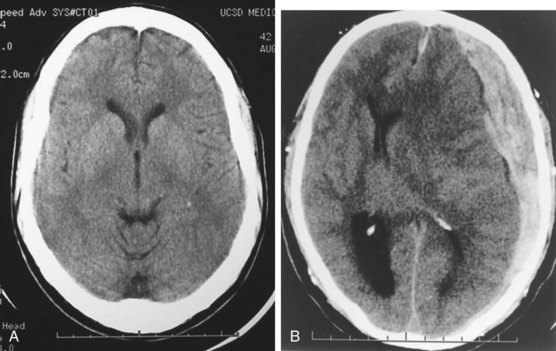
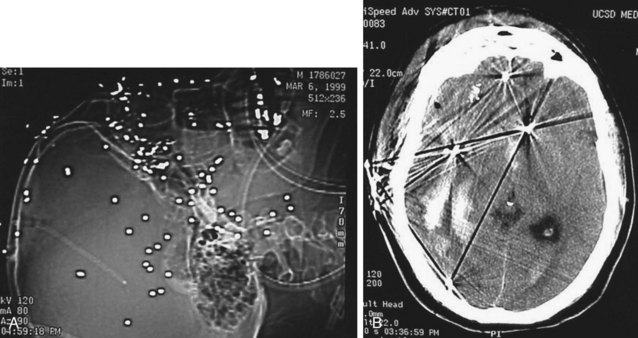
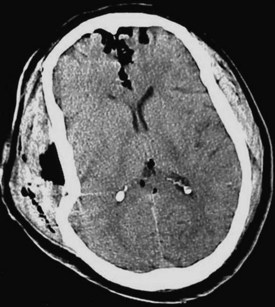
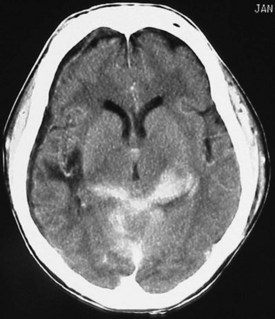
Acute hemorrhage appears hyperdense on computed tomography (CT) scans, with the shape and location of hemorrhage suggesting the underlying pathology. Epidural hematomas are classically lentiform (lens shaped) because of their relationship to arterial injury, with the higher pressure compressing the brain parenchyma (Fig. 73.2). Subdural hematomas are more commonly crescent shaped, with blood from torn veins tracking along the surface of the brain beneath the dura mater (Fig. 73.3). Intraparenchymal hemorrhage can exist as a discrete hematoma or as multiple smaller foci throughout a contused area of brain (Fig. 73.4). In addition to focal areas of hemorrhage, cerebral contusions typically involve cerebral edema, which may progress markedly over a period of several days. Skull fractures may be seen on plain radiographs, but more important is the potential for injury to the underlying brain parenchyma or the existence of intracranial hemorrhage (Fig. 73.5). Subarachnoid hemorrhage appears as hyperdensities within the ventricles, along the falx and tentorium, and around the circle of Willis (Fig. 73.6). One of the most elusive diagnoses is diffuse axonal injury, in which the findings on CT are often much less impressive than the degree of obtundation. Small, punctate hemorrhages along the gray-white interface at the cortical periphery suggest this diagnosis, although the initial scan may be completely normal.
Treatment
Prehospital Management
Half of all patients who die of TBI do so within the first few hours after their injury.
Prehospital assessment of patients with TBI should include rapid airway evaluation, continuous pulse oxygen saturation monitoring, frequent measurement of blood pressure, determination of GCS scores, and pupillary evaluation.1 Prehospital intubation should be avoided in patients who are spontaneously breathing and maintaining greater than 90% oxygen saturation.2 Prehospital airway management may be necessary in patients with a GCS score lower than 9 or those unable to maintain oxygen saturation greater than 90% with supplemental oxygen. If prehospital endotracheal intubation is performed, confirmation of placement should be done with auscultation and end-tidal capnography. Even mild hyperventilation should be avoided in all cases with the exception of patients who have evidence of herniation or acute neurologic deterioration. Hypotension should be treated with isotonic fluids, although protocols involving prehospital hypertonic saline administration are reasonable for patients with GCS scores lower than 9. Rapid transport is a priority, ideally to a facility with immediately available imaging and neurosurgical care.
Airway and Breathing
Trauma data registries have shown increased mortality in TBI patients with hypoxemia.3 This association has led to an aggressive approach to airway management in patients with severe TBI, including oxygen supplementation and early intubation. All patients with TBI should undergo pulse oximetry monitoring and administration of supplemental oxygen to correct the hypoxemia (PaO2 < 60 mm Hg or oxygen saturation < 90%).
In patients with severe TBI, endotracheal intubation is critical. Indications for endotracheal intubation include a GCS score lower than 9, airway protection when airway protective reflexes are in question, hypoxia refractory to supplementary oxygen, and agitated or combative patients who cannot comply with a rapid and thorough assessment, including CT.4
Patients with TBI may be sensitive to the increases in ICP associated with RSI, and some experts recommend neuroprotective adjuncts to traditional RSI medications. Preadministration of lidocaine at a dose of 1.5 to 2 mg/kg may blunt the rise in ICP associated with laryngoscopy and intubation. Coadministration of 2.5 to 3 mcg/kg of fentanyl with the induction agent may prevent the tachycardia and hypertension associated with tracheal intubation.5 Finally, a small dose of a nondepolarizing neuromuscular agent, such as pancuronium, before administration of succinylcholine can protect against fasciculations, which may lead to a rise in ICP. The “defasciculation” dose is typically one tenth of the full paralytic dose of the agent. Box 73.2 presents a sample neuroprotective RSI strategy. It should be noted that although all the aforementioned adjuncts to RSI in patients with TBI are reasonable considerations, they should be implemented as part of a streamlined approach to TBI patients. The higher priority is rapid and safe intubation that avoids hypoxia and aspiration. If implementation of these adjuncts to traditional RSI results in delay or complications, all theoretic benefit is lost. Therefore, it is also reasonable to use traditional RSI in TBI patients without adjunctive medications.6
Box 73.2 Example of a Neuroprotective Induction Strategy for Individuals with Traumatic Brain Injury
The postintubation ventilation strategy significantly influences outcomes in patients with TBI. This reflects the adverse effects of positive pressure ventilation on cardiac output, hypocapnic cerebral vasoconstriction, and retrograde cerebral transmission of intrathoracic pressure via the jugular venous system, all of which can lead to cerebral hypoperfusion and ischemia. PaCO2 should be maintained as close to normal as possible, within the range of 30 to 39 mm Hg.7 Hyperventilation does decrease ICP, but it does so by causing cerebral vasoconstriction and results in decreased cerebral blood.8 Transient hyperventilation, to a PaCO2 in the range of 30 to 35 mm Hg, is therefore reserved as a temporizing measure in the setting of acute deterioration as a means of avoiding herniation. Serial arterial blood gas analysis or PETCO2 monitoring should be performed after intubation to ensure proper ventilation because traditional pulse oximetry monitoring reflects only oxygenation status.
Circulation
The use of pressors is generally discouraged in the initial resuscitation of multiple-trauma victims. Thus, the main focus of therapy is volume replacement with intravenous fluids and blood products. Albumin and nonprotein colloids have no role in the initial resuscitation of patients with TBI.9 Hypertonic saline solutions appear to have osmotic properties that may lower ICP and decrease cerebral edema. However, outcome data have been disappointing,10 and at this time the initial resuscitation fluid of choice remains standard crystalloid (lactated Ringer or 0.9% saline solution).
Avoiding Cerebral Herniation
Patients with cerebral herniation should receive mannitol, 0.25 to 1 g/kg in bolus form, repeated every 4 to 6 hours.3,11 Mannitol is an osmotic diuretic that can decrease brain edema, reduce blood viscosity, and cause a transient increase in circulating volume that improves cerebral blood flow and oxygenation. Hypertonic saline at concentrations of 7.2% to 23.4% has been studied as an alternative or adjunct to mannitol with favorable results. This is a promising therapy that has been beneficial in multiple small studies,12–15 but at the time of this writing, evidence has not yet reached the level to change practice guidelines, and we therefore still recommend mannitol as the first-line hyperosmolar therapy.
Seizure Prophylaxis
Seizures in patients with TBI have a constellation of effects, including elevated ICP, hypoxia, hypercapnia, and massive release of excitatory neurotransmitters, all of which result in secondary insults to an already injured brain. Seizures should be treated immediately with benzodiazepines. There is no evidence that early seizure prophylaxis decreases the likelihood that a delayed seizure disorder will develop, but prophylaxis does decrease the likelihood of early seizures (within the first 7 days after injury). The decision to empirically administer seizure prophylaxis should weigh the likelihood of seizures against the possible side effects of medication. There is currently no consensus regarding which patients should receive prophylaxis, but it is reasonable to consider administration in high-risk patients. Patients at high risk for seizures include those with a GCS score lower than 10, cerebral contusion or hematoma, depressed skull fracture, and penetrating injury.3 The first-line agent for seizure prophylaxis is phenytoin.
Mild Traumatic Brain Injury
Imaging
As discussed earlier, the modality of choice for the initial imaging of patients after TBI is non–contrast-enhanced CT. The prevalence of CT abnormalities is about 5% in patients arriving at the hospital with a GCS score of 15 and increases significantly with a lower initial GCS score.11 Approximately 1% of all patients with mild TBI ultimately require neurosurgical intervention. Several clinical decision rules designed to help clinicians decide which patients with mild TBI require imaging have been validated. Their recommendations differ because of variations in definitions and study populations. That said, certain features are consistently associated with intracranial pathology, such as older age, vomiting, focal neurologic deficit, and persistent alteration in level of consciousness. Recent consensus guidelines have been developed to guide imaging decisions and are summarized in Box 73.3.
Box 73.3
Consensus Guidelines to Direct Imaging Decisions
• Glasgow Coma Scale score lower than 15
• Drug or alcohol intoxication
• Deficits in short-term memory
Adapted from Jagoda AS, Bazarian JJ, Bruns JJ, et al. Clinical policy: neuroimaging and decision making in adult mild traumatic brain injury in the acute setting. Ann Emerg Med 2008;52:714–48.
Follow-Up, Next Steps in Care, and Patient Education
Disposition
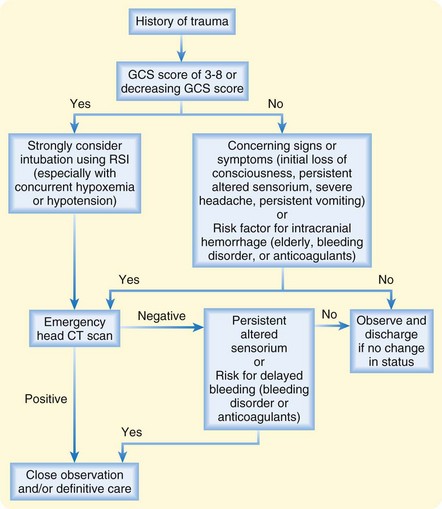
Patients suffering TBI who have abnormalities on head CT or persistent alteration in mental status should be admitted to a hospital with neurosurgical capability and be closely monitored for deterioration (Fig. 73.7). There is good evidence that patients with head injury who have normal findings on head CT and neurologic examination can be safely discharged from the ED without an extended observation period.16,17 It must be noted that the studies that came to this conclusion excluded certain populations, in particular, patients with bleeding disorders and those taking anticoagulant medications. Therefore, the data are insufficient to be certain that this recommendation is safe in these patients.18 We recommend a more careful approach to patients with acquired or inherited bleeding diatheses: either close observation in the hospital or discharge in the care of responsible caregivers who can watch the patient closely for signs of deterioration and have rapid access to return to the hospital for reevaluation. It is reasonable to include patients and their families in this decision and have an open discussion of the uncertainty in our understanding of the risk for deterioration in patients with TBI.
Patient Teaching
It is also important to communicate to patients the possibility of postconcussive syndrome (PCS). This syndrome of persistent neurologic, behavioral, and cognitive symptoms develops in a substantial proportion of patients with mild TBI. Symptoms include headache, memory impairment, difficulty concentrating, anxiety, and depression. Of all patients with mild TBI, about 50% at 3 months and 15% at 1 year19,20 will have persistent PCS. Even though no specific treatments are available for PCS, early psychosocial intervention appears to reduce postconcussion symptoms and limit the emergence of persistent problems. Awareness can help validate symptoms that might otherwise not be attributed to the traumatic incident and lead to referral for neurorehabilitation or psychologic services or support groups.
Concussion and Return to Play
The term concussion refers to short-lived impairment of neurologic function after trauma. Such impairment may or may not involve loss of consciousness and typically resolves over a sequential course, although postconcussive symptoms may be prolonged.21 A primary concern of emergency physicians and trainers is second-impact syndrome, which has been reported in athletes who return to play while still symptomatic from a concussion and sustain another head injury. These athletes, despite having mild symptoms and sustaining apparently mild second injuries, are at risk for the rapid development of brain swelling, herniation, and death. Additionally, athletes who sustain repeated concussions are at higher risk for long-term cognitive deficits. A stepwise progression of rehabilitation after concussion is recommended before return to play (Table 73.1), ideally supervised by an experienced athletic trainer or health care provider.
Table 73.1 Stepwise Progression of Rehabilitation After Concussion
| REHABILITATIVE STAGE | FUNCTIONAL EXERCISE AT EACH STAGE OF REHABILITATION | OBJECT OF EACH STAGE |
|---|---|---|
| 1. No activity | Complete physical and cognitive rest | Recovery |
| 2. Light aerobic exercise | Walking, swimming, or stationary cycling while keeping the intensity at <70% of the maximum predicted heart rate; no resistance training | Increase the heart rate |
| 3. Sport-specific exercise | Skating drills in ice hockey, running drills in soccer; no head impact activities | Add movement |
| 4. Noncontact training drills | Progression to more complex training drills, such as passing drills in football and ice hockey; may start progressive resistance training | Exercise, coordination, and cognitive load |
| 5. Full-contact practice | Following medical clearance, participation in normal training activities | Restore athlete’s confidence; coaching staff assesses functional skills |
| 6. Return to play | Normal game play |
If symptoms recur during any step, the patient should return to step 1. If asymptomatic, the patient may advance to the next step every 24 hours.
Adapted from McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in sport: the 3rd international conference on concussion in sport held in Zurich, November 2008. J Athl Train 2009;44:434–48.
Brain Trauma Foundation. Guidelines for the management of severe head injury 3rd edition. J Neurotrauma. 2007;24(Suppl 1):S1–106.
Jagoda AS, Bazarian JJ, Bruns JJ, et al. Clinical policy: neuroimaging and decision making in adult mild traumatic brain injury in the acute setting. Ann Emerg Med. 2008;52:714–748.
McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in sport: the 3rd international conference on concussion in sport held in Zurich, November 2008. J Athl Train. 2009;44:434–448.
1 Dewall J. The ABCs of TBI. Evidence-based guidelines for adult traumatic brain injury care. JEMS. 2010;35:54–61.
2 National State EMS Officials, National Association of EMS Educators & National Association of Association of EMTs. Guidelines for the prehospital management of traumatic brain injury, 2nd edition. Prehosp Emerg Care. 2008;12(Suppl 1):S1–52.
3 Brain Trauma Foundation. Guidelines for the management of severe head injury 3rd edition. J Neurotrauma. 2007;24(Suppl 1):S1–106.
4 Stevens RD, Lazaridis C, Chalea JA. The role of mechanical ventilation in acute lung injury. Neurol Clin. 2008;26:543–563.
5 Helfman SM, Gold MI, Delisser EA, et al. Which drug prevents tachycardia and hypertension associated with tracheal intubation: lidocaine, fentanyl, or esmolol? Anesth Analg. 1991;72:482–486.
6 Schofer JM. Premedication during rapid sequence intubation: a necessity or waste of valuable time? Calif J Emerg Med. 2006;2(4):75–79.
7 Warner KJ, Cuschieri J, Copass MK, et al. Emergency department ventilation effects outcome in severe traumatic brain injury. J Trauma. 2008;64:341–347.
8 Davis DP, Kene M, Vilke GM, et al. Head-injured patients who “talk and die”: the San Diego perspective. J Trauma. 2007;62:277–281.
9 Roberts I, Alderson P, Bunn F, et al. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. (4):2004. CD001208
10 Bulger EM, May S, Brasel KJ. Out-of-hospital hypertonic resuscitation following severe traumatic brain injury. JAMA. 2010;304:1455–1464.
11 Wakai A, Roberts I, Schierhout G. Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev. (4):2005. CD001049
12 Ware ML, Nemani VM, Meeker M, et al. Effects of 23.4% sodium chloride solution in reducing intracranial pressure in patients with traumatic brain injury: a preliminary study. Neurosurgery. 2005;57:727–736.
13 Vialet R, Albanese J, Thomachot L, et al. Isovolume hypertonic solutes (sodium chloride or mannitol) in the treatment of refractory posttraumatic intracranial hypertension: 2 mL/kg 7.5% saline is more effective than 2 mL/kg 20% mannitol. Crit Care Med. 2003;31:1683–1687.
14 Rockswold GL, Solid CA, Paredes-Andrade E, et al. Hypertonic saline and its effects on intracranial pressure, cerebral perfusion pressure, and brain tissue oxygenation. Neurosurgery. 2009;65:1035–1042.
15 Harutjunyan L, Holz C, Rieger A, et al. Efficiency of 7.2% hypertonic saline hydroxyethyl starch 200/0.5 versus mannitol 15% in the treatment of increased intracranial pressure in neurosurgical patients—a randomized clinical trial. Crit Care. 2005;9:530–540.
16 Livingston DH, Lavery RF, Passannante MR, et al. Emergency department discharge of patients with a negative cranial computed tomography scan after minimal head injury. Ann Surg. 2000;232:126–132.
17 af Geijerstam JL, Oredsson S, Britton M, et al. Medical outcome after immediate computed tomography or admission for observation in patients with mild head injury: randomised controlled trial. BMJ. 2006;333:465.
18 Jagoda AS, Bazarian JJ, Bruns JJ, et al. Clinical policy: neuroimaging and decision making in adult mild traumatic brain injury in the acute setting. Ann Emerg Med. 2008;52:714–748.
19 Middleboe T, Andersen HS, Birket-Smith M, et al. Minor head injury: impact on general health after 1 year. A prospective follow-up study. Acta Neurol Scand. 1992;85:5–9.
20 Kushner D. Mild traumatic brain injury: toward understanding manifestations and treatment. Arch Intern Med. 1998;158:1617–1624.
21 McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in sport: the 3rd international conference on concussion in sport held in Zurich, November 2008. J Athl Train. 2009;44:434–448.