Chapter 233 Traumatic Asymptomatic Vertebral Artery Injury Secondary to Facet Fracture Dislocation
Do Nothing
Vertebral artery injury (VAI) is a complication of cervical spine trauma that can have potentially disastrous consequences without proper identification and management in some patients. Although the natural history of asymptomatic VAI is just beginning to be understood, these lesions are most likely asymptomatic, with a benign course. In 1961, using angiography, Carpenter first described vertebral artery thrombosis in a patient who suffered blunt cervical spine trauma.1 For many years, angiography remained the only means of diagnosing this condition. As a result, few VAIs were diagnosed following cervical spine trauma, given the risks of performing angiography in potentially unstable trauma patients. The study of VAI, therefore, was limited to a few case reports, and a detailed knowledge of the condition, in addition to its natural course, was limited.2–4
While angiography remained the gold standard for the diagnosis of VAI, more liberalized screening protocols began to be used, beginning in the late 1990s, to identify trauma patients at risk for VAI; once VAI was diagnosed, it was believed that systemic anticoagulation was necessary to treat it unless absolute contraindications existed. More recently, as a result of advanced, less invasive, imaging techniques such as computed tomographic angiography (CTA), VAI has been noted to be more common than was previously thought, with an incidence ranging between 17% and 46% of patients suffering from cervical spine fractures.5–8 In addition, it has been noted that approximately 0.5% to 0.77% of all trauma patients will have a VAI and that a vast majority (70–77%) of patients with VAI will have an associated cervical spine fracture.9–12
Mechanism of Injury
VAI results from two main mechanisms. The first is a stretch-type injury of the artery caused by a facet dislocation or from hyperflexion or hyperextension injuries. The second is the result of fractured bone fragments directly injuring the vessel wall. The injury can range from minor trauma resulting in the raising of a subintimal flap to progressively more severe forms of dissection—subintimal or subadventitial—to overt injury of all three vessel wall layers resulting in pseudoaneurysm formation. The most severe form—arterial transection—is usually fatal. Cothren et al. have modified their trauma-associated carotid injury rating scale (Box 233-1) to neatly classify VAI severity.10 The same group identified three findings in patients who have suffered a cervical spine fracture—cervical subluxation, fracture extending into the foramen transversarium, and upper cervical spine (C1-2) fractures—that identify more than 97% of patients with VAI (1c). Oetgen et al. more recently evaluated foramen transversarium fractures in a detailed manner and found that multiple foramen transversarium fractures, in addition to comminution of fragments, increased the risk of VAI as diagnosed by CTA.13
BOX 233-1 Grading Scale for Vertebral Artery Injuries
Grade I: Irregularity of the vessel wall or a dissection/intramural hematoma with <25% luminal stenosis
Grade II: Intraluminal thrombus or raised intimal flap is visualized, or dissection/intramural hematoma with ≥25% luminal narrowing
From Cothren CC, Moore EE, Biffl WL, et al: Cervical spine fracture patterns predictive of blunt vertebral artery injury. J Trauma 55:811–813, 2003. Reproduced with permission.
Diagnosis
Numerous groups have developed screening protocols to identify patients who are at risk of developing either VAI or carotid artery injury.14–16 In general, the presence of any of the following features were used to select patients for most of the screening protocols: a cervical spine fracture or subluxation, Horner syndrome, skull base fracture, Lefort 2 or 3 facial fractures, extensive neck soft tissue injury, or the presence of neurologic deficits that are unexplained by intracranial imaging findings.
The gold standard for the diagnosis of VAI is angiography. However, angiography is invasive and carries a small but significant risk of complications. Since the late 1990s, CTA17 and magnetic resonance angiography (MRA)18 were enthusiastically promoted as possible replacements for conventional angiography. Beginning in the early part of this decade, however, reports comparing the sensitivity and specificity of CTA and MRA with those of traditional angiography concluded that these newer tests were not appropriate alternatives because they missed a substantial proportion of VAI in addition to having a high false positive rate.19–20 Two important papers are worth mentioning here. Over a five-year period, Biffl et al. evaluated 46 patients with carotid and/or VAI and found that the sensitivity of CTA was only 68% and the specificity was 67%.19 With regard to MRA, the same group reported a sensitivity of 75% and a specificity of 67% when compared with conventional angiography. Similarly, Miller et al. described 43 VAIs in a group of 216 patients meeting screening protocol requirements.20 All 43 patients underwent four-vessel cerebral angiography, and 30 of these 43 underwent CTA as well.20 In these 30 patients, CTA revealed only 16 VAIs, leading to a sensitivity of only 53%.20
In the same study, Miller et al. also compared MRA to four-vessel cerebral angiography and found a 47% sensitivity rate for the former study.20 However, these early studies were performed with four-channel detector technology, with limited three-dimensional reconstruction capability.
Newer Technology Computed Tomographic Angiography
With newer technology that incorporated 8- and 16-slice multidetector systems, the ability to accurately detect arterial injury was vastly improved. Schneidereit et al. and Berne et al. in 2006 published the first reports on the use of 8-slice and 16-slice CTA, respectively, as the initial imaging modality to screen for blunt cerebrovascular injury.21,22 The reports concluded that newer CT technology was effective in diagnosing more instances of both carotid artery injury and VAI; in addition, these authors stated that no patient with a negative CTA developed ischemic symptoms that prompted study with catheter angiography.21,22 Langner et al. in 2008 performed CTA as part of their whole-body imaging protocol in 368 patients and discovered three VAIs; they concluded that since no patient with a negative CTA developed clinical symptomatology indicative of VAI, CTA was a sufficient screening study.23 Similarly, Biffl et al. detected nine VAIs in a group of 331 patients via CTA that were then confirmed by catheter angiography; however, patients with normal-appearing CTAs did not undergo confirmatory catheter angiography.24 Instead, the group concluded that none of the patients who had normal CTA went on to develop clinical symptomatology related to cerebrovascular injury.24 Utter et al. studied a group of 82 patients who underwent 16-slice CTA and had normal findings, which was confirmed in 75 of these patients using catheter angiography, giving CTA a negative predictive value of 92%.25 In the four aforementioned studies, CTA and four-vessel cerebral angiography were not compared head to head; as a result, it was difficult to draw conclusions related to the ability of CTA to replace four-vessel cerebral angiography as the screening modality of choice. In addition, these groups all relied on the presentation of clinical symptomatology to define the false negativity of CTA.
However, Eastman et al. in 2006 laid many of these concerns to rest when they prospectively evaluated 162 patients with 16-slice CTA, of whom 146 subsequently underwent follow-up catheter angiography using their screening protocol for trauma victims.6 CTA successfully diagnosed 25 of the 26 VAIs subsequently seen on catheter angiography for a sensitivity of 96%.6 Malhotra et al. studied 7000 trauma patients over a 40-month period and used a more conservative screening protocol than other groups, resulting in 119 patients selected for screening with CTA, of whom 92 later underwent catheter angiography.26 Thirteen patients were diagnosed with VAI, and the authors reported 75% sensitivity and 92% specificity for CTA.26 However, CTA sensitivity increased over the latter half of the study period, leading this group to advise caution in the use of CTA as a sole means of diagnosing VAI, because there may be a learning curve associated with CTA evaluation, especially with regard to reconstructed images.26
Natural History
Until recently, the failure to diagnose a significant number of VAIs limited the ability to predict, with any degree of certainty, the natural history of VAI, especially in asymptomatic cases. Louw et al. studied a group of 12 patients in South Africa who suffered from cervical spine dislocation and found that 9 of these patients (75%) suffered from VAI.27 Only 2 of 9 patients suffered from neurologic deficits, but these resolved spontaneously during the follow-up period.27 In the early 1990s, Willis et al. reported on 26 patients who had suffered from either cervical spine fracture or subluxation over a 21-month period and who were enrolled in a prospective study to evaluate the incidence of VAI using catheter angiography.8 Twelve of 26 patients (46%) were found to have VAI; in 9, it consisted of occlusion, and the remaining three suffered from intimal flap, arterial dissection, and pseudoaneurysm, respectively.8 None of the 9 patients with occluded VAI were treated, and none suffered from a delayed neurologic deficit attributable to the VAI during the course of their hospitalization. However, the study did not evaluate patients for the development of neurologic symptoms attributable to the VAI following discharge.8 Vaccaro et al.28 and Parbhoo et al.29 have reported on patients suffering from VAI secondary to cervical spine trauma; on the basis of follow-up MRA, they have concluded that the vast majority of injured vessels do not recanalize.
In a prescreened group of trauma patients who were at high risk of developing VAI, Miller et al. in 2001 reported a 14% rate of stroke attributable to VAI in a group of 50 patients.15 Similarly, Biffl et al. in 2000 reported a 24% rate of stroke among a group of 38 patients,14 and then in 2002, the same group reported a 20% rate of stroke when they evaluated patients over an 11-year period.11 However, these rates are illustrative only of patients who develop neurologic deficits around the time of the traumatic injury, and it is still uncertain what the rate of stroke may be in asymptomatic patients who suffer from VAI and who are followed for long periods of time subsequent to their injury. It is logical to theorize that patients with lower-grade injuries may be at higher risk of subsequent stroke, but numerous reports have shown that there is no correlation between the grade of VAI and stroke.11,14,15
A recent report by Stein et al, may cast more light on the long-term outcome of VAI. This group evaluated 68 VAIs in 61 patients out of more than 12,000 admissions to a major urban trauma center.12 Six of these 68 injuries (8.8%) resulted in stroke referable to either one or both injured vertebral arteries around the time of the initial trauma. At first follow-up (<1 month) to evaluate the VAI over the long term in 20 injuries, no injury worsened radiographically in either the medically treated group or the untreated group; all injuries in both groups either improved or were found to be stable radiographically. These results were echoed at later follow-up (>1 month).
Management
The most controversial issue related to VAI is management. At this time, there is no class I evidence to support or refute any form of therapy. Some researchers11,14,15,20 have advocated varying degrees of anticoagulation, antiplatelet agents, and/or endovascular intervention for most VAI lesions unless contraindications exist, while others9,12,27,29 have advocated observation in asymptomatic cases. There are two major centers advocating medical and/or endovascular treatment for VAIs, and these recommendations are borne out of the following studies from those groups.
In one of the first studies comparing medical treatment with heparin or antiplatelet agents and observation, Biffl et al. in 200014 evaluated a group of 38 trauma patients with 47 total VAIs in Denver, Colorado. Of the 21 patients who were treated with heparin, 3 (14%) suffered stroke attributable to the VAI; and in the group of 17 patients with VAI who did not receive medical treatment, 6 patients suffered stroke (35%). This difference did not reach statistical significance (P = .13). The authors concluded that heparin and, if heparin was contraindicated, antiplatelet agents prevent stroke in patients who suffer from VAI and who may be asymptomatic. Heparin was given as a bolus dose of 15 U/kg/hour and then was administered to maintain a partial thromboplastin time of between 40 and 50 seconds. The study had many limitations. First, the study did not adequately define what was meant by “mild” or “severe” deficit in patients with neurologic signs and symptoms related to VAI. Second, the number of patients studied was too small to draw conclusions. Furthermore, radiographic improvement of VA characteristics, as a result of what the authors believed was the result of heparin treatment, had no bearing on clinical improvement.
The same group evaluated patients over an 11-year period11 to report on the effect of treatment on follow-up arteriography. In this report, there was no difference in the rate of healing of vessels in the group of patients given heparin (or antiplatelet drugs) or those who were given no treatment.11 The authors continued to call for follow-up arteriography in this report, since they noted that 8% of grade I and 43% of grade II VAI progressed to pseudoaneurysm formation leading to endovascular treatment of these lesions; in addition, they noted that grade III and IV injuries scarcely changed over time. It is unclear, however, what the nature of changes were with regard to the anatomy of pseudoaneurysm formation in grade I and II injuries. We are also not aware as to the decision-making process of balancing the benefit of preventing further neurologic events and the risks inherent in stenting or angioplasty, which may cause strokes, worsen the vessel injury, or condemn patients to be maintained on antiplatelet therapy for years. There was no statistically significant difference (P = .15) in neurologic outcome between the three treatment groups: heparin, antiplatelet drugs, or no treatment.11 In addition, there was a 1% rate of stroke as a result of follow-up catheter angiography in addition to a 22% incidence of bleeding complications—intracranial, gastrointestinal, and retroperitoneal—in the group of patients receiving heparin, leading the authors to omit the starting bolus dose.
A center in Memphis, Tennessee, has reported on the management of VAI.15,20 The group evaluated 64 VAIs in 50 patients over a 4-year period and noted that 7 of these patients (14%) developed stroke.15 Eighty-eight percent of the patients received some form of antithrombotic treatment; 62% received heparin, and 26% received antiplatelet drugs.15 Only four patients out of 50 received no treatment, one as a result of VAI resulting in vessel occlusion, one as a result of spinal cord contusion, and the other two as a result of poor prognosis.15 There was an 8% rate of complications associated with heparin use. Of the seven patients who developed stroke, two improved to resume normal life, and two died. The authors go on to conclude that since there were no strokes in the group treated with heparin, one stroke in the group treated with aspirin, and six strokes in patients who were not treated, patients with VAIs should be treated with heparin, and if that is contraindicated, some form of antiplatelet regimen should be instituted. However, the conclusions drawn by this study are weakened as a result of several study limitations. First, a comparison between asymptomatic VAIs that were then assigned to either treatment or no treatment groups was not performed. Second, the strokes included in the no treatment group included those in patients who received no treatment in addition to those in patients who did not receive treatment before the onset of ischemia. In addition, it is not clear whether the patients in the no treatment group were more critically ill or had severe brain injury that ultimately resulted in worse neurologic outcomes than those who were treated, further confounding the results. Obviously, it is very difficult to analyze, much less conclude, which mode of management is best if patients presenting with neurologic deficits are included in the analysis, thereby limiting the scope of treatment in these patients as compared with others.
The same group later used their previous group of patients15 as a historical control and compared them with 43 patients who suffered a total of 49 VAIs,20 the majority (74%) receiving treatment with antiplatelet drugs, 19% receiving systemic anticoagulation, and 7% receiving no treatment. The authors noted a 0% rate of stroke in these 43 patients and compared it to their historical control,15 in which they noted a 14% rate of stroke; with this observation, they concluded that treatment with antiplatelet medication reduces the rate of stroke in VAI, even though there was no difference in the stroke rate (0%) in any of the three groups. In addition, comparison with the historical control is flawed as a result of the inherent shortcomings in the first study, as mentioned previously.
Biffl W.L., Moore E.E., Elliott J.P., et al. The devastating potential of blunt vertebral arterial injuries. Ann Surg. 2000;231:672-681.
Cothren C.C., Moore E.E., Ray C.E.Jr., et al. Cervical spine fracture patterns mandating screening to rule out blunt cerebrovascular injury. Surgery. 2007;141:76-82.
Eastman A.L., Chason D.P., Perez C.L., et al. Computed tomographic angiography for the diagnosis of blunt cervical vascular injury: is it ready for primetime? J Trauma. 2006;60:925-929.
Fassett D.R., Dailey A.T., Vaccaro A.R. Vertebral artery injuries associated with cervical spine injuries: a review of the literature. J Spinal Disord Tech. 2008;21(4):252-258.
Louw J.A., Mafoyane N.A., Small B., et al. Occlusion of the vertebral artery in cervical spine dislocations. J Bone Joint Surg [Br]. 1990;72(4):679-681.
Miller P.R., Fabian T.C., Bee T.K., et al. Blunt cerebrovascular injuries: diagnosis and treatment. J Trauma. 2001;51:279-286.
Schneidereit N.P., Simons R., Nicolaou S., et al. Utility of screening for blunt vascular neck injuries with computed tomographic angiography. J Trauma. 2006;60(1):209-215.
1. Carpenter S. Injury of neck as cause of vertebral artery thrombosis. J Neurosurg. 1961;18:849-853.
2. Deen H.G.Jr., McGirr S.J. Vertebral artery injury associated with cervical spine fracture. Report of two cases. Spine (Phila Pa 1976). 1992;17:230-234.
3. Schwarz N., Buchinger W., Gaudernak T., et al. Injuries to the cervical spine causing vertebral artery trauma: case reports. J Trauma. 1991;31:127-133.
4. Sim E., Schwarz N., Biowski-Fasching I., et al. Color-coded Duplex sonography of vertebral arteries. 11 cases of blunt cervical spine injury. Acta Orthop Scand. 1993;64:133-137.
5. Cothren C.C., Moore E.E., Ray C.E.Jr., et al. Cervical spine fracture patterns mandating screening to rule out blunt cerebrovascular injury. Surgery. 2007;141:76-82.
6. Eastman A.L., Chason D.P., Perez C.L., et al. Computed tomographic angiography for the diagnosis of blunt cervical vascular injury: is it ready for primetime? J Trauma. 2006;60:925-929.
7. Taneichi H., Suda K., Kajino T., et al. Traumatically induced vertebral artery occlusion associated with cervical spine injuries: prospective study using magnetic resonance angiography. Spine (Phila Pa 1976). 2005;30:1955-1962.
8. Willis B.K., Greiner F., Orrison W.W., Benzel E.C. The incidence of vertebral artery injury after midcervical spine fracture or subluxation. Neurosurgery. 1994;34:435-441.
9. Fassett D.R., Dailey A.T., Vaccaro A.R. Vertebral artery injuries associated with cervical spine injuries: a review of the literature. J Spinal Disord Tech. 2008;21(4):252-258.
10. Cothren C.C., Moore E.E., Biffl W.L., et al. Cervical spine fracture patterns predictive of blunt vertebral artery injury. J Trauma. 2003;55(5):811-813.
11. Biffl W.L., Ray C.E.Jr., Moore E.E., et al. Treatment-related outcomes from blunt cerebrovascular injuries: the importance of routine follow-up arteriography. Ann Surg. 2002;235:699-707.
12. Stein D.M., Boswell S., Sliker C.W., et al. Blunt cerebrovascular injuries: does treatment always matter? J Trauma. 2009;66(1):132-143.
13. Oetgen M.E., Lawrence B.D., Yue J.J. Does the morphology of foramen transversarium fractures predict vertebral artery injuries? Spine (Phila Pa 1976). 2008;1;33(25):E957-E961.
14. Biffl W.L., Moore E.E., Elliott J.P., et al. The devastating potential of blunt vertebral arterial injuries. Ann Surg. 2000;231:672-681.
15. Miller P.R., Fabian T.C., Bee T.K., et al. Blunt cerebrovascular injuries: diagnosis and treatment. J Trauma. 2001;51:279-286.
16. Biffl W.L., Moore E.E., Ryu R.K., et al. The unrecognized epidemic of blunt carotid arterial injuries: early diagnosis improves neurologic outcome. Ann Surg. 1998;228:462-470.
17. Rogers F.B., Baker E.F., Osler T.M., et al. Computed tomographic angiography as a screening modality for blunt cervical arterial injuries: preliminary results. J Trauma. 1999;46:380-385.
18. Weller S.J., Rossitch E.Jr., Malek A.M. Detection of vertebral artery injury after cervical spine trauma using magnetic resonance angiography. J Trauma. 1999;46:660-666.
19. Biffl W.L., Ray C.E.Jr., Moore E.E., et al. Noninvasive diagnosis of blunt cerebrovascular injuries: a preliminary report. J Trauma. 2002;53(5):850-856.
20. Miller P.R., Fabian T.C., Croce M.A., et al. Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann Surg. 2002;236(3):386-393. discussion 393–395
21. Schneidereit N.P., Simons R., Nicolaou S., et al. Utility of screening for blunt vascular neck injuries with computed tomographic angiography. J Trauma. 2006;60(1):209-215.
22. Berne J.D., Reuland K.S., Villarreal D.H., et al. Sixteen-slice multi-detector computed tomographic angiography improves the accuracy of screening for blunt cerebrovascular injury. J Trauma. 2006;60(6):1204-1209.
23. Langner S., Fleck S., Kirsch M., et al. Whole-body CT trauma imaging with adapted and optimized CT angiography of the craniocervical vessels: do we need an extra screening examination? AJNR Am J Neuroradiol. 2008;29(10):1902-1907.
24. Biffl W.L., Egglin T., Benedetto B., et al. Sixteen-slice computed tomographic angiography is a reliable noninvasive screening test for clinically significant blunt cerebrovascular injuries. J Trauma. 2006;60(4):745-751. discussion 751–752
25. Utter G.H., Hollingworth W., Hallam D.K., et al. Sixteen-slice CT angiography in patients with suspected blunt carotid and vertebral artery injuries. J Am Coll Surg. 2006;203(6):838-848.
26. Malhotra A.K., Camacho M., Ivatury R.R., et al. Computed tomographic angiography for the diagnosis of blunt carotid/vertebral artery injury: a note of caution. Ann Surg. 2007;246(4):632-642.
27. Louw J.A., Mafoyane N.A., Small B., et al. Occlusion of the vertebral artery in cervical spine dislocations. J Bone Joint Surg [Br]. 1990;72(4):679-681.
28. Vaccaro A.R., Klein G.R., Flanders A.E., et al. Long-term evaluation of vertebral artery injuries following cervical spine trauma using magnetic resonance angiography. Spine (Phila Pa 1976). 1998;23:789-795.
29. Parbhoo A.H., Govender S., Corr P. Vertebral artery injury in cervical spine trauma. Injury. 2001;32(7):565-568.
Endovascular Management
During the routine evaluation of patients with multiple traumatic injuries coupled with the proliferation of fast, safe, and sensitive diagnostic techniques, clinicians are increasingly presented with asymptomatic vertebral artery traumatic injuries. In addition, they are faced with multiple treatment options. These injuries include dissections, intimal tears, thrombosis, and arteriovenous fistula. The latter is most common after penetrating injuries. The mechanism responsible for vertebral artery dissections is neck extension, especially with a rotational component.1 Interestingly, these two mechanisms are also responsible for unilateral facet dislocations, explaining the high incidence of vertebral artery injuries associated with facet dislocations. Bilateral facet dislocation caused by neck hyperflexion followed by extension and may compromise both vertebral arteries simultaneously.
The variety and intensity of the symptoms may be related to the diversity of anatomic variations, which include single vertebral arteries, codominant vertebral arteries, a unilateral vertebral artery terminating in a posterior inferior cerebellar artery, absence of the distal V4 segment, and absence of or insufficient contribution from the anterior circulation through the posterior communicating artery. Symptoms of vertebrobasilar insufficiency include ataxia, dizziness, vertigo, and decreased level of consciousness.2
The need to screen asymptomatic patients is debated, as is the best method for screening. The gold standard is digital subtraction angiography, which constitutes an invasive procedure associated with a potential morbidity and mortality rate as high as 5%. The widespread use of multislice CT with simultaneous intravenous contrast injection (CT angiography) allows the rapid acquisition of images that can be obtained simultaneously during an evaluation for other traumatic injuries. CT angiography constitutes a noninvasive technology that provides a “snapshot” of the anatomy with the possibility of performing orthogonal reconstructions, which facilitate image interpretation. Its sensitivity has been reported to be as high as 100% with a specificity of 98% compared to catheter angiography.3
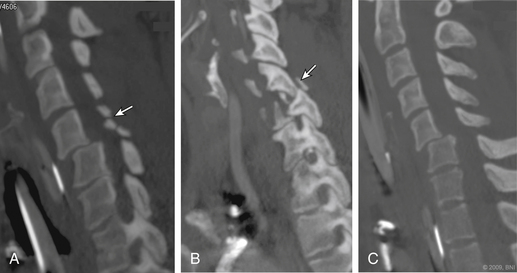
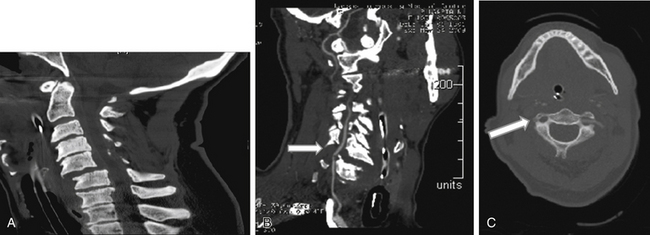
In a prospective study, Hoit et al.4 used catheter-based angiography to identify vascular injuries (carotid and vertebral) after blunt trauma. The incidence of these injuries increased from 0.6% to 28.9% when high-risk criteria were used to identify patients with vascular injury. These high-risk features included skull base fractures through the carotid canal, anterior clinoid process fractures, and spine fractures caused by hyperextension or hyperflexion/rotation (Fig. 233-1). Other features included lateralizing neurologic symptoms or Horner syndrome.
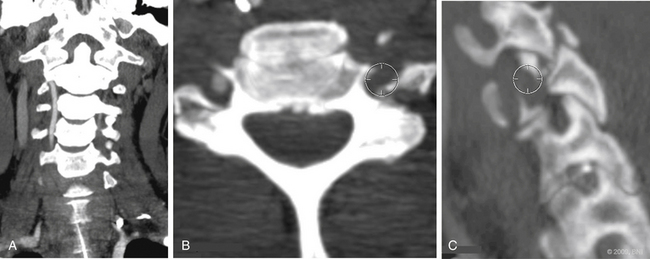
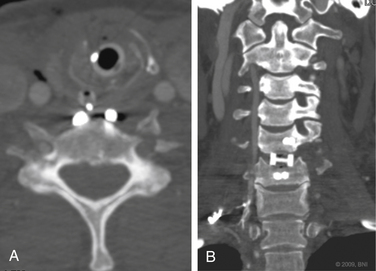
Vertebral artery injury is more common in patients with a spinal cord injury rated as A or B on the American Spinal Injury Association scale (Fig. 233-2) compared with neurologically intact patients.5 Nine of 12 patients with a facet joint dislocation had vertebral artery occlusion6 (Fig. 233-3). Interestingly, after the fracture-dislocation is reduced, there is no evidence of recanalization of the artery (Fig. 233-4). In the presence of fractures through the foramen transversarium, the incidence of vertebral artery injury increases to 88%.7 Screening patients in the high-risk group is recommended, owing to their potential for developing delayed and devastating neurologic deficits.8
As reported by Fasset et al.,2 no class I evidence supports any specific treatment for arterial dissections. The treatment armamentarium is large and includes formal anticoagulation therapy with intravenous heparin followed by administration of oral anticoagulation therapy, a single antiplatelet agent, or dual antiplatelet agents (usually aspirin and Plavix [Bristol-Myers Squibb/Sanofi Pharmaceuticals, New York, NY]).
On the basis of historical controls from the same group, aggressive screening of high-risk patients and early administration of anticoagulation therapy (primarily with heparin) or, if it is contraindicated by severe head injury or other major systemic trauma, an antiplatelet regime decreases the incidence of stroke in patients with a blunt vertebral artery injury from 14% to 0%.9 We usually recommend aspirin for 3 months and follow-up with imaging to evaluate the progression of the dissection.10 The use of antiplatelets and anticoagulation requires consensus within the trauma team to balance the risk of hemorrhage from associated injuries.
Conclusion
Vascular imaging of the vertebral artery is recommended for high-risk patients. CT angiography with orthogonal reconstructions is a sensitive, minimally invasive technique for identifying vertebral artery injuries. Imaging characteristics of the injury as well as specific patient characteristics such as dominance of the injured artery, presence of collateral circulation, and flow restriction caused by the dissection, may prompt different treatments. Sequential follow-up is recommended to evaluate worsening of the injury. No reliable evidence is available to choose between antiplatelet and anticoagulation therapies. Because of their safety profile, antiplatelet agents seem to be the drug of choice in the treatment of vertebral artery injuries. In the presence of embolic events, formal anticoagulation therapy is recommended until the injury is stabilized. Stenting should be limited to lesions that occur in dominant arteries or to when the flow is significantly reduced and collateral circulation is minimal or there are continuing neurologic symptoms on medical therapy. Decisions should be made on a case-by-case basis, considering the aspects of care described herein.
Chen C.J., Tseng Y.C., Lee T.H., et al. Multisection CT angiography compared with catheter angiography in diagnosing vertebral artery dissection. AJNR Am J Neuroradiol. 2004;25(5):769-774.
Fassett D.R., Dailey A.T., Vaccaro A.R. Vertebral artery injuries associated with cervical spine injuries: a review of the literature. J Spinal Disord Tech. 2008;21(4):252-258.
Hoit D.A., Schirmer C.M., Weller S.J., et al. Angiographic detection of carotid and vertebral arterial injury in the high-energy blunt trauma patient. J Spinal Disord Tech. 2008;21(4):259-266.
Miller P.R., Fabian T.C., Bee T.K., et al. Blunt cerebrovascular injuries: diagnosis and treatment. J Trauma. 2001;51(2):279-285.
Parbhoo A.H., Govender S., Corr P. Vertebral artery injury in cervical spine trauma. Injury. 2001;32(7):565-568.
1. Parbhoo A.H., Govender S., Corr P. Vertebral artery injury in cervical spine trauma. Injury. 2001;32(7):565-568.
2. Fassett D.R., Dailey A.T., Vaccaro A.R. Vertebral artery injuries associated with cervical spine injuries: a review of the literature. J Spinal Disord Tech. 2008;21(4):252-258.
3. Chen C.J., Tseng Y.C., Lee T.H., et al. Multisection CT angiography compared with catheter angiography in diagnosing vertebral artery dissection. AJNR Am J Neuroradiol. 2004;25(5):769-774.
4. Hoit D.A., Schirmer C.M., Weller S.J., et al. Angiographic detection of carotid and vertebral arterial injury in the high-energy blunt trauma patient. J Spinal Disord Tech. 2008;21(4):259-266.
5. Torina P.J., Flanders A.E., Carrino J.A., et al. Incidence of vertebral artery thrombosis in cervical spine trauma: correlation with severity of spinal cord injury. AJNR Am J Neuroradiol. 2005;26(10):2645-2651.
6. Louw J.A., Mafoyane N.A., Small B., Neser C.P. Occlusion of the vertebral artery in cervical spine dislocations. J Bone Joint Surg Br. 1990;72(4):679-681.
7. Woodring J.H., Lee C., Duncan V. Transverse process fractures of the cervical vertebrae: are they insignificant? J Trauma. 1993;34(6):797-802.
8. McCormick M.T., Robinson H.K., Bone I., et al. Blunt cervical spine trauma as a cause of spinal cord injury and delayed cortical blindness. Spinal Cord. 2007;45(10):687-689.
9. Miller P.R., Fabian T.C., Bee T.K., et al. Blunt cerebrovascular injuries: diagnosis and treatment. J Trauma. 2001;51(2):279-285.
10. Ashley W.W.Jr., Rivet D., Cross D.T.III, Santiago P. Development of a giant cervical vertebral artery pseudoaneurysm after a traumatic C1 fracture: case illustration. Surg Neurol. 2006;66(1):80-81.
Medical (Anticoagulation) Management
Anatomy
The vertebral artery has a rich collateral blood supply, the contralateral vertebral artery being the major contributor. The posterior inferior cerebellar arteries serve as the other significant collaterals, whereas intramuscular collaterals may have a secondary role as an alternative to the occluded vertebral artery.1
Vascular Injury Type
The vascular wall comprises three layers: the intima, the media, and the adventitia. When the vessel’s wall is subject to blunt trauma and specifically to the shear forces involved in a cervical fracture-dislocation, the most vulnerable layer is the intima. The intima may tear to create an intimal tear or flap, which in turn may protrude into the lumen, causing partial or complete occlusion. The pathophysiology of a dissection involves blood flow through the intimal defect into the arterial wall. The vascular wall defect and exposed subintimal tissue in these injury patterns are predisposing factors for thrombus formation, resulting in arterial thrombotic occlusion or thromboembolic events. Pseudoaneurysm is the result of blood flow through a defect involving all the layers of the vascular walls (partial transaction) and formation of an extravascular hematoma. Complete transection of the vertebral artery is uncommon in the setting of blunt cervical trauma yet frequently fatal when it occurs.2,3
In 1999, Biffl et al.4 suggested a classification for blunt arterial injuries based on their angiographic appearance. This classification has commonly been used to describe the spectrum of arterial nonpenetrating injury. Grade I includes injuries in which there is an arteriographic appearance of irregularity of the vessel wall or a dissection/intramural hematoma with less than 25% luminal stenosis. In grade II injury, intraluminal thrombus or raised intimal flap is visualized, or there is dissection/intramural hematoma with 25% or more luminal narrowing. Grade III are the pseudoaneurysms, and grade IV includes the vessel occlusions. Grade V is the most severe form of arterial injury: transaction.
Facet Fracture-Dislocation as a Cause of Vertebral Artery Injury
Facet fracture dislocations of the cervical spine are classified as either distraction-flexion cervical injury for bilateral facet dislocation or rotation-flexion for unilateral facet dislocation. While the reported incidence of vertebral artery injury among all blunt trauma admissions ranges from 0.20% to 0.77%,5 there is wide consensus that facet fracture dislocations are one of the injury patterns most frequently associated with vertebral artery injury. Reports estimate the frequency of vertebral artery injury in patients presenting with subaxial fracture dislocations from 21% to 75%.6–10 The high rate of vascular injury found in patients suffering cervical fracture dislocation injuries warrants a very high index of suspicion as well as a low threshold for imaging studies and justifies the use of more invasive imaging modalities when indicated.
The vertebral artery segment most commonly injured in a facet fracture dislocation is V2. The injury mechanism is direct stretching of the vascular wall secondary to the bony displacement. The thinner intima layer is then torn, while the more resistant media and adventitia remain intact. A less common injury pattern is a fracture line through the foraminal wall with direct injury from a bone fragment (Fig. 233-5). In that case, all the layers of the vessel wall are at risk, and pseudoaneurysm or partial transaction may result.7 As a result of the shearing mechanism, the vascular injuries that are typically found in conjunction with facet fracture dislocation are the intimal tears and the grade I, II, and IV dissections.8,11–13
The neurologic status was found to have an influence on the prevalence of traumatic vertebral artery thrombosis. While rate of vertebral artery thrombosis in patients with incomplete motor neurologic damage at the level of injury (American Spinal Injury Association [ASIA] C and D) was found to be similar to that of the neurologically intact (ASIA E), those with complete neurologic injury (ASIA A) were found to have higher incidence of vertebral artery thrombosis.14
Asymptomatic Vertebral Artery Injury
The majority of patients who suffer from unilateral traumatic vertebral artery injury present with no symptoms that can be directly attributed to the vascular trauma.15,16 Only 12% to 20% of the patients are reported to have such symptoms.8,17 Factors contributing to the presence of symptoms include bilateral vertebral injury, insufficient vertebral artery on the contralateral side, and thromboembolic events such as basilar thromboembolism or direct progression of the thrombus into the basilar artery.18,19 The left vertebral artery is more often the dominant one (75% of the patients), but no higher incidence of symptomatic injury was found with left-sided injuries.20 Patients who suffer from fracture-dislocation of the cervical spine have a high rate of spinal cord injury, making the diagnosis of motor or sensory deficit related to a vertebral artery injury challenging. Coexisting head injury or depressed level of consciousness may render a clinical diagnosis even more difficult. When symptoms occur, they are referred to as vertebrobasilar ischemia and include motor deficits that are not compatible with the spinal injury level: ataxia and visual deficits; dizziness, tinnitus, vertigo and nausea; dysarthria; dysphagia; and hoarseness. Bilateral injury may present with altered mental status, pinpoint pupils, and even respiratory arrest.21
Delayed onset of symptoms has been reported by a few authors, probably due to a long-lasting thrombotic event with or without embolization. The time period from the initial trauma to the appearance of symptoms ranged from a few days to a few months.5 These reports, as well as the evolving understanding that vertebral artery injury is not a rare event in the presence of cervical spine injury, have led to the recommendations for significantly more aggressive screening and treatment protocols.
Diagnosis
Despite much debate regarding the need for screening of the vertebral arteries in every blunt trauma patient, most authors agree that a facet fracture-dislocation, with or without vertebral foramen fracture, is a clear indication for imaging of the vertebral arteries. It is not only the high incidence of injury in this patient population that warrants screening, but also the treatment regimens for the fracture-dislocation type of injury, which include manipulation of the injured segment and placement of hardware in very close proximity to the vessel, especially with the posterior surgical approach.22–24
The gold standard for the diagnosis of cervical vascular injuries is conventional catheter angiography, specifically digital subtraction angiography. However, angiography is an invasive procedure that carries the risk for iatrogenic vascular injury, entry site hematoma and arterial-venous fistula, contrast nephropathy, stroke, and even death. The complication rate with invasive angiography is reported to be as high as 4%.25 Critically ill and polytrauma patients tend to develop those 5complications at a higher rate.26 Angiography is also resource- and labor-consuming and not available in all facilities. Many studies, including comparative and animal studies, have shown promising results with magnetic resonance angiography (MRA) for screening patients with cervical trauma for vascular injury.27–31 MRA is time consuming and not inexpensive, but most of the patients who suffer cervical fracture dislocation will undergo MRI of the cervical spine as part of their imaging studies prior to surgical treatment of the injury; therefore, adding an MRA is relatively simple.
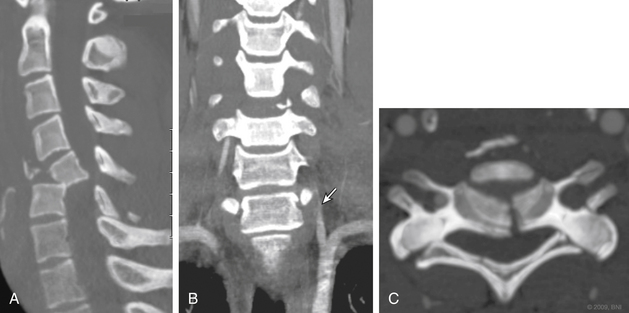
Even less expensive and more readily available in most centers is the computerized tomographic angiography (CTA). All patients who suffer significant cervical trauma will undergo a CT, and the contrast media dosage required for CTA is significantly lower than that of formal angiography. CTA has an advantage of being less time-consuming than both angiography and MRA and less costly. CTA has not been accepted yet as equivalent to angiography as a screening tool for vascular injury in the presence of cervical spine trauma, but with the advances in CT technology, several recent studies have shown CTA to have a sensitivity that approaches that of angiography32–34 (Fig. 233-6).
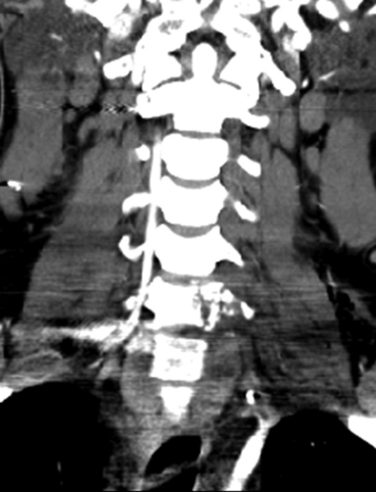
FIGURE 233-6 Coronal computerized tomographic angiography shows C6-7 fracture-dislocation and injury to left vertebral artery.
A few reports discuss the possibility of using Doppler ultrasound (DUS) as a screening imaging technique for vertebral artery imaging. These authors emphasize the noninvasive nature of the examination as well as the availability and compatibility with ventilators and fixation devices. High correlation was found between DUS results and angiography but only when cases of pseudoaneurysm were excluded. DUS was effective for V2 lesions and can probably be used as a primary screening tool for patients who cannot, or should not, undergo CTA, MRA, or angiography.35
In 2002, the AANS/CNS Joint Section published recommendations for the diagnosis and management of vertebral artery injuries after nonpenetrating cervical trauma. Either angiography or MRA was recommended for diagnosis.15 Some of the more pertinent studies advocating the use of CTA were not yet available for review at that time; with the encouraging data on CTA, inclusion of this modality in the recommendations is to be expected.
Treatment
The treatment of asymptomatic vertebral artery injury remains controversial. Delayed occurrence of symptoms related to the vascular injury, including thrombus propagation into the basilar system and other thromboembolic events, were reported. The time interval between the injury and symptomatic presentation varied from days to weeks or even months.36,37 Those reports led some of the authors to recommend the use of anticoagulative agents, either intravenous heparin or antiplatelet preparations, intended to prevent late thrombotic and embolic events.8,38 The largest series of vertebral artery injury cases was published by Berne and Norwood in 200933; they concluded that anticoagulation or antiplatelet therapy was probably justified for asymptomatic patients with grade I to II injury, and less so with grade IV injury. Surgical and fibrinolytic therapies, advocated for the treatment of symptomatic vertebral artery injury, are not recommended for asymptomatic patients with grade I, II, and IV lesions. Grade III lesions (pseudoaneurysms) present a higher risk for future rupture and embolization; thus, more aggressive treatment protocols have been proposed, including endovascular procedures, regardless of presence of symptoms.39–42 Systemic anticoagulation carries the risk of intracranial or other major bleeding in the polytraumatized patient and could impede surgery for reduction and stabilization of the cervical spine. In the face of the inconclusive evidence found in the literature, the AANS/CNS Joint Section Guidelines did not recommend using anticoagulation in asymptomatic vertebral artery injury.15
The most common type of vertebral artery injury associated with cervical fractures is type IV, in which evidence for the benefit of anticoagulation is the weakest.16,33 Type III injuries might benefit from endovascular procedures, but considering the need for postprocedural anticoagulation, this decision should be coordinated with the spine surgeon.43 Not enough data exist to clearly outweigh the risks of perioperative anticoagulation or antiplatelet therapy against their potential benefits in type I and II injuries. The strength and quality of evidence for the use of anticoagulation in asymptomatic vertebral artery injury do not seem to justify delay of spine stabilization and decompression procedures.
Prognosis and Follow-Up
Little data exist regarding the prognosis of asymptomatic vertebral artery injury. At 7 to 10 days follow-up angiography after diagnosis of the injury, Biffl et al. found that a majority of grade I injuries will be healed or unchanged, in contrast to 29% to 47% of grade II injuries that will develop pseudoaneurysms.44 Grade II injuries are unlikely to resolve, and grade IV injuries have low rate of recanalization.45 At 4 months follow-up, Edwards et al. found 72% of grade I injuries completely healed. Grade II injuries were evenly distributed: 33% improved, 33% were stable, and 33% progressed to pseudoaneurysms. Grade III injuries either remained unchanged (50%) or enlarged (40%). Grade IV injuries did not improve.46 These data support follow-up imaging for grade III injuries in the absence of new symptoms and for grades I and II at 7 to 10 days to determine future therapy.38
American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Section on Disorders of the Spine and Peripheral Nerves. Management of vertebral artery injuries after nonpenetrating cervical trauma. Neurosurgery. 2002;50(Suppl 3):S173-S178.
Biffl W.L., Moore E.E. Identifying the asymptomatic patient with blunt carotid arterial injury. J Trauma. 1999;47(6):1163-1164.
Biffl W.L., Moore E.E., Offner P.J., et al. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma. 1999;47(5):845-853.
Biffl W.L., Moore E.E., Offner P.J., et al. Optimizing screening for blunt cerebrovascular injuries. Am J Surg. 1999;178(6):517-522.
Cothren C.C., Moore E.E., Biffl W.L., et al. Cervical spine fracture patterns predictive of blunt vertebral artery injury. J Trauma. 2003;55(5):811-813.
Inamasu J., Guiot B.H. Vertebral artery injury after blunt cervical trauma: an update. Surg Neurol. 2006;65(3):238-245.
1. Six E.G., Stringer W.L., Cowley A.R., Davis C.H.Jr. Posttraumatic bilateral vertebral artery occlusion: case report. J Neurosurg. 1981;54(6):814-817.
2. Biffl W.L., Moore E.E. Identifying the asymptomatic patient with blunt carotid arterial injury. J Trauma. 1999;47(6):1163-1164.
3. Biffl W.L., Moore E.E., Mestek M. Patients with blunt carotid and vertebral artery injuries. J Trauma. 1999;47(2):438-439.
4. Biffl W.L., Moore E.E., Offner P.J., et al. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma. 1999;47(5):845-853.
5. Inamasu J., Guiot B.H. Vertebral artery injury after blunt cervical trauma: an update. Surg Neurol. 2006;65(3):238-245.
6. Parbhoo A.H., Govender S., Corr P. Vertebral artery injury in cervical spine trauma. Injury. 2001;32(7):565-568.
7. Willis B.K., Greiner F., Orrison W.W., Benzel E.C. The incidence of vertebral artery injury after midcervical spine fracture or subluxation. Neurosurgery. 1994;34(3):435-441.
8. Miller P.R., Fabian T.C., Bee T.K., et al. Blunt cerebrovascular injuries: diagnosis and treatment. J Trauma. 2001;51(2):279-286.
9. Louw J.A., Mafoyane N.A., Small B., et al. Occlusion of the vertebral artery in cervical spine dislocations. J Bone Joint Surg Br. 1990;72(4):679-681.
10. Giacobetti F.B., Vaccaro A.R., Bos-Giacobetti M.A., et al. Vertebral artery occlusion associated with cervical spine trauma. A prospective analysis. Spine (Phila Pa 1976). 1997;22(2):188-192.
11. Cothren C.C., Moore E.E., Ray C.E.Jr., et al. Screening for blunt cerebrovascular injuries is cost-effective. Am J Surg. 2005;190(6):845-849.
12. Miller P.R., Fabian T.C., Croce M.A., et al. Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann Surg. 2002;236(3):386-395.
13. Utter G.H., Hollingworth W., Hallam D.K., et al. Sixteen-slice CT angiography in patients with suspected blunt carotid and vertebral artery injuries. J Am Coll Surg. 2006;203(6):838-848.
14. Torina P.J., Flanders A.E., Carrino J.A., et al. Incidence of vertebral artery thrombosis in cervical spine trauma: correlation with severity of spinal cord injury. AJNR Am J Neuroradiol. 2005;26(10):2645-2651.
15. American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Section on Disorders of the Spine and Peripheral Nerves. Management of vertebral artery injuries after nonpenetrating cervical trauma. Neurosurgery. 2002;50(Suppl 3):S173-S178.
16. Sack J.A., Etame A.B., Shah G.V., et al. Management and outcomes of patients undergoing surgery for traumatic cervical fracture-subluxation associated with an asymptomatic vertebral artery injury. J Spinal Disord Tech. 2009;22(2):86-90.
17. Biffl W.L., Moore E.E., Elliott J.P., et al. The devastating potential of blunt vertebral arterial injuries. Ann Surg. 2000;231(5):672-681.
18. Prabhu V., Kizer J., Patil A., et al. Vertebrobasilar thrombosis associated with nonpenetrating cervical spine trauma. J Trauma. 1996;40(1):130-137.
19. Rodriguez M., Tyberghien A., Matgé G. Asymptomatic vertebral artery injury after acute cervical spine trauma. Acta Neurochir (Wien). 2001;143(9):939-945.
20. Fassett D.R., Dailey A.T., Vaccaro A.R. Vertebral artery injuries associated with cervical spine injuries: a review of the literature. J Spinal Disord Tech. 2008;21(4):252-258.
21. Taylor M.W., Senkowski C.K. Bilateral vertebral artery dissection after blunt cervical trauma: case report and review of the literature. J Trauma. 2002;52(6):1186-1188.
22. Cothren C.C., Moore E.E., Biffl W.L., et al. Cervical spine fracture patterns predictive of blunt vertebral artery injury. J Trauma. 2003;55(5):811-813.
23. Inamasu J., Guiot B.H. Vascular injury and complication in neurosurgical spine surgery. Acta Neurochir (Wien). 2006;148(4):375-387.
24. Biffl W.L., Moore E.E., Offner P.J., et al. Optimizing screening for blunt cerebrovascular injuries. Am J Surg. 1999;178(6):517-522.
25. Citron S.J., Wallace R.C., Lewis C.A., et al. Quality improvement guidelines for adult diagnostic neuroangiography. Cooperative study between ASITN, ASNR, and SIR. J Vasc Interv Radiol. 2003;14(9 Pt 2):S257-S262.
26. Kerwin A.J., Bynoe R.P., Murray J., et al. Liberalized screening for blunt carotid and vertebral artery injuries is justified. J Trauma. 2001;51(2):308-314.
27. Ren X., Wang W., Zhang X., et al. The comparative study of magnetic resonance angiography diagnosis and pathology of blunt vertebral artery injury. Spine (Phila Pa 1976). 2006;31(18):2124-2129.
28. Ren X., Wang W., Zhang X., et al. Clinical study and comparison of magnetic resonance angiography (MRA) and angiography diagnosis of blunt vertebral artery injury. J Trauma. 2007;63(6):1249-1253.
29. Veras L.M., Pedraza-Gutiérrez S., Castellanos J., et al. Vertebral artery occlusion after acute cervical spine trauma. Spine (Phila Pa 1976). 2000;25(9):1171-1177.
30. Biffl W.L., Ray C.E.Jr., Moore E.E., et al. Noninvasive diagnosis of blunt cerebrovascular injuries: a preliminary report. J Trauma. 2002;53(5):850-856.
31. Biffl W.L. Diagnosis of blunt cerebrovascular injuries. Curr Opin Crit Care. 2003;9(6):530-534.
32. Berne J.D., Norwood S.H., McAuley C.E., Villareal D.H. Helical computed tomographic angiography: an excellent screening test for blunt cerebrovascular injury. J Trauma. 2004;57(1):11-17.
33. Berne J.D., Norwood S.H. Blunt vertebral artery injuries in the era of computed tomographic angiographic screening: incidence and outcomes from 8292 patients. J Trauma. 2009;67(6):1333-1338.
34. Núñez D.B.Jr., Torres-León M., Múnera F. Vascular injuries of the neck and thoracic inlet: helical CT-angiographic correlation. Radiographics. 2004;24(4):1087-1098.
35. Wessels T., Mosso M., Krings T., et al. Extracranial and intracranial vertebral artery dissection: long-term clinical and duplex sonographic follow-up. J Clin Ultrasound. 2008;36(8):472-479.
36. Tulyapronchote R., Selhorst J.B., Malkoff M.D., Gomez C.R. Delayed sequelae of vertebral artery dissection and occult cervical fractures. Neurology. 1994;44(8):1397-1399.
37. Vaccaro A.R., Klein G.R., Flanders A.E., et al. Long-term evaluation of vertebral artery injuries following cervical spine trauma using magnetic resonance angiography. Spine (Phila Pa 1976). 1998;23(7):789-794.
38. Arthurs Z.M., Starnes B.W. Blunt carotid and vertebral artery injuries. Injury. 2008;39(11):1232-1241.
39. Islamoğlu F., Posacioğlu H., Memiş A., Durmaz I. Endovascular transcatheter occlusion for traumatic vertebral artery pseudoaneurysm. Eur J Vasc Endovasc Surg. 2000;20(6):576-578.
40. Saket R.R., Razavi M.K., Sze D.Y., et al. Stent-graft treatment of extracranial carotid and vertebral arterial lesions. J Vasc Interv Radiol. 2004;15(10):1151-1156.
41. Biffl W.L., Moore E.E., Ray C., Elliott J.P. Emergent stenting of acute blunt carotid artery injuries: a cautionary note. J Trauma. 2001;50(5):969-971.
42. Biffl W.L., Moore E.E., Offner P.J., Burch J.M. Blunt carotid and vertebral arterial injuries. World J Surg. 2001;25(8):1036-1043.
43. Dill-Macky M.J., Khangure M., Song S. Traumatic cervical distraction complicated by delayed reduction due to traumatic vertebral artery pseudo-aneurysm. Australas Radiol. 1999;43(3):372-377.
44. Biffl W.L., Ray C.E.Jr., Moore E.E., et al. Treatment-related outcomes from blunt cerebrovascular injuries: importance of routine follow-up arteriography. Ann Surg. 2002;235(5):699-706.
45. Vaccaro A.R., Urban W.C., Aiken R.D. Delayed cortical blindness and recurrent quadriplegia after cervical trauma. J Spinal Disord. 1998;11(6):535-539.
46. Edwards N.M., Fabian T.C., Claridge J.A., et al. Antithrombotic therapy and endovascular stents are effective treatment for blunt carotid injuries: results from long term follow up. J Am Coll Surg. 2007;204(5):1007-1013.