Chapter 66 Trauma Surgery
Thoracic and Thoracolumbar Junction
Approximately 160,000 patients a year in the United States suffer traumatic spinal column injuries, with 10% to 30% of them having a concurrent spinal cord injury.1–4 The majority of these injuries consist of cervical and lumbar (L3-5) spine fractures. However, between 15% and 20% of traumatic fractures occur at the thoracolumbar junction (T11-L2), whereas 9% to 16% occur in the thoracic spine (T1-10).5,6 Forces along the long stiff, kyphotic thoracic spine catalyze an abrupt switch into the mobile lordotic lumbar spine at the thoracolumbar junction. Biomechanically, this transition zone is susceptible to injury and is the most commonly injured portion of the spine. High-energy trauma (i.e., motor vehicle accidents) is the leading cause of injury over this region, followed by falls and sports-related injuries.7 Men are at four times higher risk than women. Because of the higher-energy mechanisms of injury, involvement of other organ systems is encountered in up to 50% of thoracolumbar trauma patients.7 These high-energy injuries, such as those causing thoracic-level paraplegia, have a first-year mortality rate of 7%.3,8
Anatomy
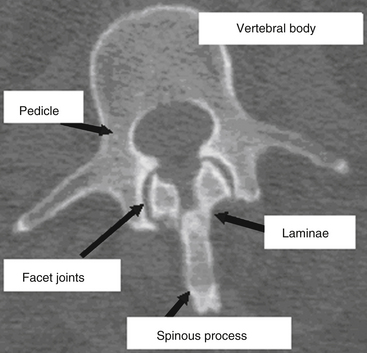
The vertebral column provides humans with the ability to maintain an upright posture, protects the neural and visceral organs (i.e., heart, lungs, abdominal contents), and helps with motility. It consists of 29 vertebrae arranged in 4 major curves, 2 primary curves (thoracic and sacral), and 2 compensatory or secondary curves (cervical and lumbar).9 The vertebral column also provides a protective environment for the spinal cord and neural elements. The vertebral body, pedicles, and dorsal elements surround the spinal cord, permitting the spinal nerves to exit through the paired neural foramina. The laminae are formed as dorsomedial extensions of the pedicles and fuse in the midline to create the spinous processes (Fig. 66-1). Nomenclature for the thoracic spine varies; however, in this chapter the thoracic spine is considered to span T1 through T10, and the thoracolumbar junction T11 through L2.
Primary spinal curves are present at birth, are maintained through life, and are relatively rigid or stiff. Secondary curves are more flexible and result from development or adaptation. The first secondary curve is the development of cervical lordosis at approximately 3 to 9 months of age as the infant begins supporting his or her head and sitting upright. The lumbar lordosis develops later (between 12 and 18 months), as the child begins to ambulate and assumes an upright posture.9 A thorough knowledge of the thoracic spine and thoracolumbar junction anatomy facilitates a greater understanding of the biomechanical, radiographic, and surgical techniques that are used to treat these fractures.
Thoracic Spine
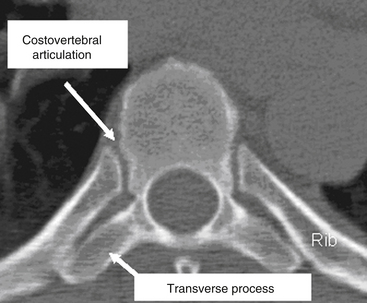
The thoracic spine differs from the cervical and lumbosacral spines as a result of its articulation with the rib cage (T1-12), extensive ligamentous support network, coronal facet joint orientation, and small spinal canal-to-neural element ratio (Fig. 66-2). The thoracic laminae increase in width and thickness from T1 to T12, and this prevents hyperextension.10 The anterior longitudinal ligament (ALL) provides further stability by increasing the tensile strength from T1 to T12. Moreover, the dorsal, ventral, and lateral diameters of the vertebral bodies increase from T2 to T12.11–15 The thoracic kyphotic curve results from the greater height of the dorsal vertebral wall as opposed to the ventral vertebral wall. The transverse pedicle diameters decrease from 9 mm at T1 to 5 mm at T5 and then increase in size distally to T12.14 In the sagittal plane, pedicle width increases from T1 to T11. However, in the transverse or axial plane, the thoracic vertebrae have a triangular configuration and appear heart shaped. There is significant variability in what is considered the “normal” sagittal curvature of the thoracic spine. This value has been reported to be between 20 and 45 degrees,13,16,17 with each individual vertebral body contributing approximately 3.8 to 3.9 degrees of kyphosis through its wedged-shaped angulation. This variability is further influenced by age (increases with age) and sex; women have a greater degree of kyphosis than men.16,17 There is also a significant degree of variability on a segmental basis, particularly at the transitional regions with the lordotic cervical and lumbar spines.12,18,19 The apex of thoracic kyphosis is typically located at the seventh thoracic vertebra, but varies with each individual. The thoracic spine typically has a mild, right-sided lateral curvature.9,17 The etiology of the right-sided lateral curve is debated but is believed to be either the result of hand dominance (right-hand majority) or created by pulsations of the thoracic aorta.9
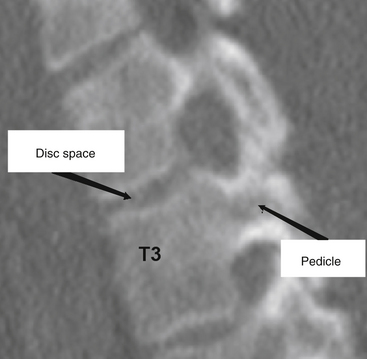
The thoracic pedicles are situated toward the rostral portion of the vertebral body, close to the superior disc space (Fig. 66-3). The pedicle angle decreases from T1 to T12; it is 251 degrees at T1 and 0 degrees at T12.11,17,20 The pedicle location on the vertebral body progressively migrates as the spine is descended in a caudal direction. The medial pedicle cortical wall is approximately two to three times thicker than the lateral wall.21
The thoracic transverse processes project laterally from the dorsal articular pillars and decrease in length caudally.5 However, unlike the lumbar spine, the relationship between the transverse process and the midpoint of the pedicle is not as clearly defined. McCormick22 showed that there is a significant degree of variability in the relationship of the transverse processes to the pedicles. The midpoint of the T1 transverse process is approximately 5 mm rostral to the center of the pedicle, whereas at T12 the transverse process-to-pedicle relationship changes to approximately 6 mm caudal.20 Comparatively, this transverse process to pedicle relationship is greater than 1 cm at both T1 and T2, and is approximately 0 cm when one analyzes T6 and T7.20
The thoracic spine facet articulations are considered apophyseal joints and are composed of a ligamentous capsule with a synovial lining. These ligaments in the thoracic spine are thicker than their cervical counterparts. In the thoracic spine, the costovertebral (rib-vertebra) facets are located anterior to the transverse processes. The isthmus of bone between each pair of superior and inferior facets is called the pars interarticularis, a site of fracture and bony nonunion for those with a condition known as spondylolysis. The joints are located at the rostral and caudal borders of the laminae and situated medial to the transverse processes (Fig. 66-4). The caudal facet’s ventral surface articulates with the rostral facet’s dorsal surface. Thoracic facet joints are oriented in a coronal plane and therefore limit the degree of flexion and extension of the thoracic spine.23
There are several key anatomic features that are essential for understanding the relationship of the ribs and the thoracic vertebrae. First, the ribs articulate with the thoracic vertebrae at two separate locations. The head of the rib articulates with the transverse process of the body at the costotransverse articulation, except at T1, T11, and T12. This articulation is supported with a large superior costotransverse ligament, which connects the rostral rib segment to the caudal transverse process (see Fig. 66-2). It also articulates with the disc space adjacent to the body above by virtue of the rib head of the same-numbered vertebral body articulating with it through the two costal hemifacets (T2-10).
The strong ligamentous structures that compose the costovertebral joint make the thoracic disc the strongest of all the vertebral discs.24 The superior hemifacet (rostral on the vertebral body and caudal to the rib) is located over the pedicle, such that the sixth rib articulates with the fifth and sixth thoracic vertebral bodies and overlies the sixth vertebral pedicle. Because of the rostral location of the thoracic pedicle on the vertebral body, the sixth rib overlies the T5 to T6 disc space. Understanding the anatomic relationship of the rib head with the pedicle allows the surgeon to remove the rib head and identify the neurovascular bundle, along with the neural foramina and thecal sac, at that level.
The spinal canal diameter varies throughout the vertebral column and is the narrowest in the midthoracic region (T3-9).11,13,25 The transverse spinal canal diameter decreases from T1 to T3 and then increases caudally into the lumbar region. The anteroposterior (AP) diameter, however, is more varied.13,25 Therefore, in the thoracic region a minor degree of canal encroachment can compromise the narrow canal and may result in neurologic compromise.26,27 Furthermore, the thoracic spinal cord has the most tenuous blood supply.13 Thus, small canal size, limited blood supply, and the high degree of energy required to create a thoracic fracture combine to result in a 90% incidence of neurologic deficit in patients who sustain a thoracic fracture.28
Thoracolumbar Junction
The transition from a relatively rigid thoracic kyphosis to a mobile lumbar spine occurs at the thoracolumbar junction. This transition generally occurs at T11 to T12, although in elderly female patients the thoracolumbar inflexion point migrates caudally as a result of their increased degree of thoracic kyphosis.12,17,19
The caudal thoracic ribs (T11 and T12) afford less stability at the thoracolumbar junction region compared with the rostral thoracic region because there is no connection to the sternum and thus they are “free floating.” Only a single rib articulation is present on the T11 and T12 vertebral bodies, and there are no accessory ligamentous attachments, such as the rib’s tubercle to the vertebral body by the costotransverse ligament, or the ligamentous attachment to the transverse process.9 The surrounding thoracolumbar ligaments, such as the interspinous and thoracolumbar fascia, are strongest caudally and provide a significant amount of stability.14
The thoracolumbar junction facet joints are again of the apophyseal type and are composed of a ligamentous capsule with a synovial lining. As mentioned previously, the joints of the midthoracic region are oriented in the coronal plane, limiting flexion and extension while providing substantial resistance to AP translation.5 In the lumbosacral region, the facet joints are oriented in a more sagittal alignment, which increases the degree of potential flexion and extension at the expense of limiting lateral bending and rotation. Depending on the spatial orientation of the spinal column (i.e., flexion or extension), the facet joints may support a third of the axial load. These joints, however, provide substantial support and resistance to approximately 35% to 45% of the torsional and shear forces experienced in this region.6,29
At birth, the spinal cord terminates at the end of the vertebral column or lumbosacral junction. However, the end of the spinal cord, or conus medullaris, migrates rostrally as the infant develops.30 In neonates the spinal cord terminates between the first and third lumbar vertebrae, whereas in adults it is positioned between the twelfth thoracic vertebra and the second lumbar vertebra.30
Imaging
It is not uncommon in clinically unstable trauma patients for fractures not to be identified early in the resuscitative period. It has been reported that between 5% and 15% of multisystem trauma patients have occult fractures not diagnosed on their initial evaluation.31–33 Although thoracic vertebral fractures make up only a minor proportion of traumatic fractures, they are extremely difficult to visualize compared with other vertebral or appendicular fractures. Approximately 20% to 50% of superior thoracic spine fractures are not diagnosed on admission plain radiographs.5,34,35 Therefore, all suspected spine trauma patients should be immobilized on admission until a thorough and detailed spinal evaluation can be performed. If appropriate stabilization precautions are not taken in this patient population, unforeseen neurologic compromise may result.6
Initial radiographic assessment includes AP and lateral spine films. The AP film should be examined for loss of vertical body height, fracture of the oval-shaped pedicles, increased interpedicular distance, transverse process or rib fractures, and malalignment of vertebral bodies or spinous processes without a history of scoliosis. The lateral radiograph should be examined for loss of body height, disruption of the rostral or caudal end plate, dorsal cortical wall fracture with retropulsed bone, fracture of spinous processes, widening of interspinous distance, and subluxation or angulation of vertebral bodies.36 Malalignment in any plane, but especially in the AP plane, suggests the possibility of a fracture-dislocation.12 Plain radiographs may not be accurate in determining the involvement of the posterior vertebral wall with a thoracic fracture.37,38
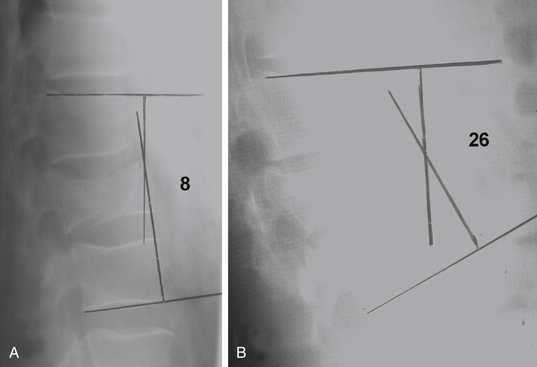
Plain radiographs are particularly useful in assessing the patient’s overall sagittal and coronal balance. If a deformity exists, a useful radiographic technique to determine the degree of deformity is measurement of the Cobb angle, which is the subtended angle measured between a perpendicular line drawn from the superior end plate of the vertebral body above the injured vertebral body and the inferior end plate one level below the injured body (see Fig. 66-20). This method of measuring spinal sagittal angulation has been shown to have the highest degree of intraobserver and interobserver reliability.39
In the presence of a vertebral body injury, the entire spine should be imaged in an orthogonal manner because of the high incidence (5–20%) of noncontiguous spine fractures.40–42 The rostral thoracic spine can be difficult to visualize on lateral plain radiographs because of the patient’s shoulders and body habitus, and a swimmer’s view may provide better visualization of the cervicothoracic junction down to the T3 vertebral body.43 Radiographically, a typical superior end-plate thoracic fracture shows loss of vertebral height, with or without malalignment, a widened paraspinal line, and possibly a widened mediastinum.35 Because of difficulties in imaging the upper thoracic region (T1-4), a high index of suspicion is required on the physician’s part to avoid missing injuries at this level. The physician should have a low threshold for ordering supplemental imaging modalities to assist in the diagnosis, such as CT and MRI.
Computed Tomography
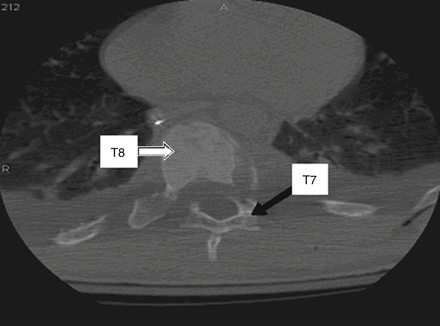
CT is more sensitive in detecting fractures than plain radiographs44 (see Fig. 66-4). It can also define the three-dimensional anatomy of complex fractures through reformatting in the sagittal and coronal planes. CT better delineates the bony structures once an injury is identified.45–47 CT reveals the integrity of the middle column and the degree of canal compromise, as well as subluxations or fractures of facets and laminae. The presence of two vertebral bodies on the same axial cut of a CT scan may indicate a fracture-dislocation, but first the radiographer must ensure that the gantry has been angled parallel to the vertebral end plates. Sagittal reconstructions are helpful in visualizing flexion-distraction injuries and fracture-dislocations. Serial CT scans of lumbar fractures have confirmed spontaneous remodeling and the reabsorption of retropulsed bone fragments in the spinal canal at long-term follow-up.48–50
CT image reconstruction is also invaluable at the cervicothoracic junction because of the overlap of the scapula, shoulders, and surrounding tissues. In the obtunded patient, this technique has been reported to identify more than 10% of fractures not visualized on plain radiographs.51 CT, however, has a limited capacity to visualize disc herniations, epidural or subdural hematomas, ligamentous disruption, and spinal cord parenchymal changes.52
Magnetic Resonance Imaging
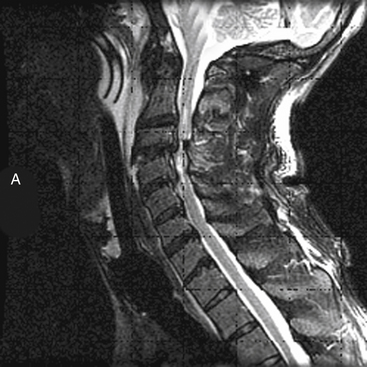
MRI has further improved the ability to visualize and comprehend the pathoanatomy of soft tissues, ligaments, and intervertebral discs, and the neural element disruption that occurs after spine injury. Unfortunately, MRI is not always available because of its expense, because it takes longer to implement, and because it cannot be performed on patients with ferromagnetic implants. Today it has supplanted CT myelography as the imaging tool of choice for the neuraxis because it is faster and noninvasive and allows improved visualization of the spinal cord parenchyma.53 MRI provides the physician with the ability to identify edema and/or hemorrhage of the spinal cord53 (Fig. 66-5). These images have been correlated with neurologic outcomes, where the presence of hemorrhage in the spinal cord parenchyma is associated with minimal neurologic recovery.54
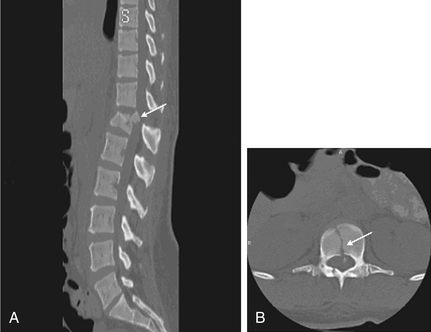
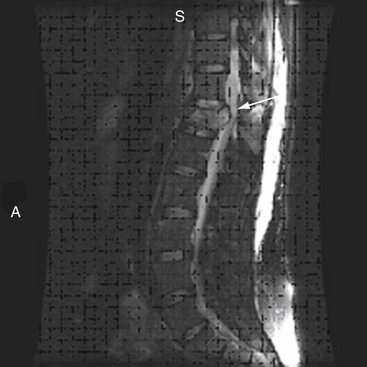
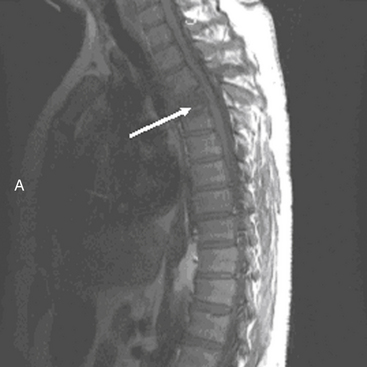
MRI evaluation is especially useful at the thoracolumbar junction because of the variable location of the cauda equina and conus medullaris in the adult population at this level.55 A neurologic examination can be difficult to interpret at the conus-cauda equina transition level as a result of the presence of lumbar spinal nerve sparing, the presence of concurrent injuries, sedation, or indwelling catheters, and delayed reflex recovery (Fig. 66-6). Accurate neural visualization may help in clarifying the pathoanatomy in these clinical situations.
Biomechanics
The vertebral body is the primary load-bearing structure of the spine, with the intervertebral disc transferring all forces applied to the adjacent vertebral bodies.56,57 The anulus fibrosus of the intervertebral disc supports a significant portion of all applied axial and lateral loads and resists tension and shearing.58
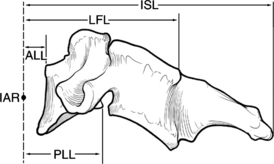
The spinal ligamentous structures are essential in maintaining overall sagittal balance. The posterior longitudinal ligament (PLL) is a relatively weak ligament that provides some restriction to hyperflexion, along with the ligamentum flavum. The thicker ALL functions in resisting spinal hyperextension and distraction.59 This thick ligament has fatigue loading values that are approximately double those of any other spinal ligaments,57–59 and its strength increases caudally from C3 to the sacrum.59 The intrinsic strength of the spinal ligaments is only an isolated factor in the overall stability of the spinal column. The lever arm by which these ligaments act on the spine also significantly affects the overall stability of the vertebral column (Fig. 66-7).
Abnormal motion patterns and coupling can be an indication of clinical instability, which ultimately must be treated in some manner. Clinical instability can be quantitatively measured in respect to the moving segment’s instantaneous axis of rotation (IAR). The IAR is an axis about which a vertebra rotates at some instant of time.46 This axis is a geometric concept and does not apply to a specific anatomic location.60 However, in the normal thoracic and thoracolumbar spine, the IAR is located in the ventral vertebral body. For normal spinal units, the IAR for each of the rotary modes (flexion, extension, lateral bending, and axial torsion) is confined to a relatively small area somewhere within the spinal unit.14 The facet capsules are very strong ligaments and act with a short lever arm by their relationship to the IAR, whereas the intraspinous ligaments are relatively weak but act with a great lever arm because of their increased distance from the IAR. Based on the ligaments’ relative strengths, it would seem that the intraspinous ligaments are not important, but both ligaments significantly affect the strength and structure of the spine.
The thoracic spine differs from the remainder of the spinal column because it is supported by and maintains articulations with the ribs (see Fig. 66-2). The intact rib cage increases the axial load-resisting capacity of the thoracic spine by a magnitude of four. The rib cage and facet articulations limit rotation, and therefore most thoracic spine fractures occur from a flexion or axial compression force vector.61 The majority of stability in flexion is provided by the costovertebral articulations.62 A significant factor in the degree and extent of fracture character is the rate of force impact loading.63
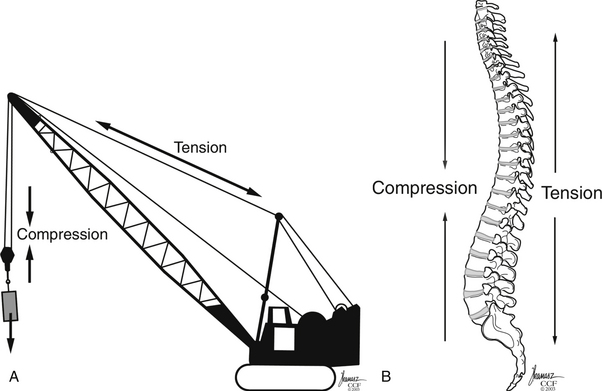
Gravitational forces exert a significant axial load on the vertebral column in the standing adult human. The center of gravity of the body, located where all forces are counterbalanced such that there is no net movement, is approximately 4 cm anterior to the first sacral vertebra.14 This results in a ventral bending (angular) vector acting on the spinal column. This bending force draws or attracts the ventral spinal column closer to the center of gravity such that a lower energy state may be achieved by the paraspinous musculature. The dorsal ligamentous complex and dorsal paraspinal musculature, acting as a tension band, counteracts these forces, such that the net sum of the vectors acting on the spine equals zero. Therefore the dorsal ligamentous, osseous, and muscular components are essential for overall support of the spine to prevent a change in the spine’s sagittal alignment. Trauma resulting in disruption of the spinal ligaments or osseous structures may change the net vector sum from zero, resulting in the potential for spinal imbalance. These new vectors acting on the spine, if not corrected, may result in a gradual spinal deformity with or without associated pain or neurologic deterioration. Whiteside used the analogy of a construction crane to illustrate this mechanical principle64 (Fig. 66-8). In this analogy, the weight to be lifted is ventral to the crane, where the boom (anterior vertebral column) is under compression and the guidewires (posterior columns) are under tension. Failure of either supporting structure independent of the other will result in mechanical failure or collapse.
The thoracic and thoracolumbar vertebrae are at increased risk for development of compression fractures after trauma as a consequence of axial loads resulting from the natural kyphotic curvature of the thoracic spine.65 The kyphotic posture results in the placement of axial forces on the ventral portion of the vertebral body. An axial load causes all points that are ventral to the IAR of the spine to come closer together while simultaneously all points that are dorsal are spread apart. Therefore, if the strength of the ventral vertebral body is exceeded, a fracture of the vertebral body occurs, resulting in a vertebral compression fracture (VCF). The traumatic forces may also exceed the strength of the dorsal vertebral body and ligamentous elements, resulting in disruption of the dorsal tension band. The destruction of the ventral vertebral stabilizing elements (i.e., vertebral body, disc, ligaments, anulus) causes the IAR to migrate dorsally to the region with intact supporting structures.65,66 Dorsal migration of the IAR causes the previous mechanical advantage of a longer level arm from which the dorsal ligaments and muscles acted to be shortened. This migration of the IAR also simultaneously increases the distance of the center of gravity to the IAR, thereby placing further distraction on the dorsal spinal column and compression on the ventral spinal column65,66 (see Fig. 66-7).
Concurrent Injuries
Thoracic Spine Fractures
The thoracic spine’s structural integrity—the interaction of its osseous structures and ligaments and the rib cage—provides more protection against potential fracture than the remaining vertebral column. Hence, when a fracture occurs over this region, the physician must be aware that a high degree of energy was required to produce this lesion. These forces on impact are transmitted to the soft tissue and viscous elements contained within and around the thoracic cavity, resulting in a high incidence of concurrent injuries. The incidence of concurrent injuries is reported to be greater than 80%, involving the thorax, appendicular skeleton, and abdominal region.35,42,67 These high-energy impacts also affect areas remote from the trauma, such as the cranial vault. Petitjean et al.67 reported a 65% incidence of head injuries after high-velocity impacts that resulted in incomplete thoracic spinal cord injury, with 12% of these injuries classified as severe (Glasgow Coma Scale score <8).
Tearing or rupture of the aorta has been associated with thoracic vertebral fractures.68 Hemodynamic instability may result from injury to any vascular structure, or even from blood loss secondary to a thoracic vertebral fracture.67,69,70 Hemothorax has also been reported to occur in 24% to 32% of patients with thoracic fractures.71,72 Pulmonary injuries have been reported in 85% of patients and typically consist of pulmonary contusions.28 Infrequently, perforation of the esophagus and tracheal injuries have also been associated with thoracic fractures.73–75 The mechanism of perforation is believed to be ischemia of the esophageal tissue after becoming devascularized as a result of a deceleration-traction injury.73,75
Thoracolumbar Junction Fractures
The thoracolumbar region is more vulnerable to concurrent injuries than the thoracic region because it is not provided the protection of the thoracic rib cage. Petitjean et al.67 reported a 71% incidence of associated blunt abdominal injuries after thoracolumbar fractures. Typically, these consist of hollow viscus injuries (e.g., intestinal perforation), mesenteric avulsions, or solid organ injuries.28,67,76
The most common mechanism of abdominal injury is the distraction or seat belt injury.28,67,77 Blunt abdominal aortic dissections are associated with distraction-rotation injuries of the thoracolumbar region.2,77 These aortic injuries can range from an intimal tear to a full-thickness laceration. CT provides accurate imaging of this injury in the stable and asymptomatic patient. A large degree of energy is involved in this distraction-type mechanism, accounting for the large number of associated injuries. Multiple-level thoracic and lumbar fractures are also associated with a high incidence of abdominal injuries.2
Axial load injuries, particularly in patients who have jumped or fallen and landed on their feet, may manifest as both thoracolumbar fractures and calcaneal fractures. Isolated transverse process fractures of the lumbar spine should not be overlooked as a minor injury. Miller et al.78 reported a 48% incidence of concurrent abdominal injuries associated with transverse process fractures. Therefore, a physician treating vertebral column injuries must be aware not only of the presence of spine fractures but also of the possibility of concurrent, nonspinal, soft tissue and bony injuries.
Classification
Injuries to the thoracic and lumbar spine account for more than 50% of all spine fractures and a large portion of acute spinal cord injuries.17 Given this frequency and the significant impact of these injuries, significant advances have been made in the surgical treatment of thoracolumbar trauma. Nevertheless, although there has been progress in the invention and evolution of spinal instrumentation and surgical techniques, medical decision making in spine trauma remains controversial. To this day, fracture treatment can vary widely, from bracing to invasive 360-degree fusions, based on geographic, institutional, or individual preferences with little scientific basis.
A number of classification systems have been developed in an attempt to better define thoracolumbar trauma and aid treatment decision making. These systems are typically based on either anatomic structures (Denis three-column system) or on proposed mechanisms of injury (Ferguson and Allen).79,80
In 1931 Watson-Jones pioneered one of the first spinal fracture grouping systems by type.81 This schematic categorization was based on diagnostic and treatment of flexion injuries.82 This was followed by Nicholl,83 who developed the first detailed thoracic and thoracolumbar spine fracture classification scheme and attempted to define unstable versus stable fractures after trauma in a series of flexion and flexion-rotation injuries. This classification system was originally intended to guide the treatment and work status of injured miners. Nicholl emphasized the importance of the dorsal interspinous ligament in spinal stability.83 Later, Holdsworth,84 after clinical failures in immobilizing pure flexion fractures following the recommendations of Watson-Jones, further studied the importance of the spinal ligamentous complexes after thoracolumbar junction injuries. He classified fractures, according to their mechanism of injury, into four main types: flexion, flexion and rotation, extension, and compression. Holdsworth84 further classified these fractures as unstable if the posterior ligamentous complex, consisting of the intervertebral disc, spinous ligaments, facet capsules, and ligamentum flavum, was disrupted. He noted that in compression, flexion, and extension injuries, the dorsal ligamentous complex is typically not ruptured and these fractures were therefore considered stable. However, he reported that flexion and rotational injuries were at a much greater risk for disruption of the dorsal ligamentous complex and subsequent instability. Subsequently, Rennie and Mitchell85 added a fifth category of thoracolumbar junction fractures consisting of flexion-distraction fractures or seat belt injuries, based on the description and reporting of Chance.86
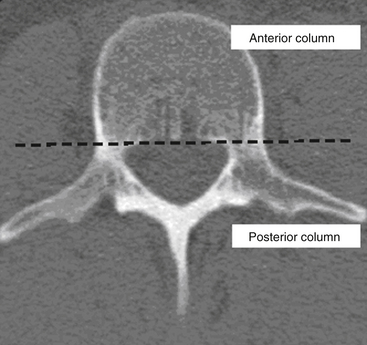
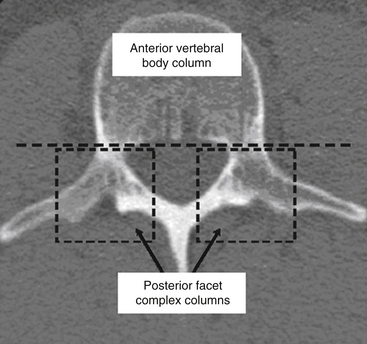
Kelly and Whiteside87 reported that without dislocation of the dorsal elements of the spinal column, neurologic injuries rarely occur. They classified fractures based on structural criteria and considered the spine to consist of not one, but two separate supportive columns (Fig. 66-9). The primary weight-bearing ventral column is composed of the vertebral bodies, and a second structural column consists of the posterior neural arches and ligaments. The structural classification scheme provided surgeons the ability to predict the degree of instability of the spine based on the degree of resulting structural damage after trauma. Based on this assessment, treatments were devised to enhance neurologic and spinal stability. Later, Louis88 modified this structural classification scheme by proposing a third column. Louis’s three-column concept consisted of one ventral column, the vertebral bodies, and two dorsal columns involving each facet articulation (Fig. 66-10).
Technologic advances in the form of superior imaging studies have allowed a greater understanding of the pathoanatomy of spine trauma. Several biomechanical studies have documented that an isolated rupture of the posterior spinal ligamentous complex is insufficient to create instability.89–91 Nonetheless, if the PLL, along with the posterior anulus fibrosus, is also disrupted, then the vertebral column will become unstable, particularly in flexion.
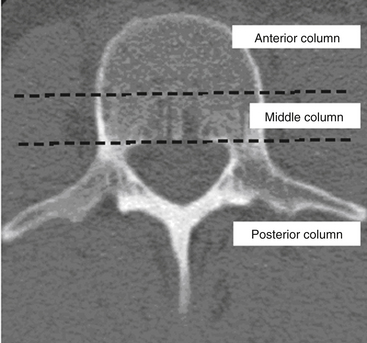
Denis79 used the enhanced CT imaging techniques, along with in vitro biomechanical data, to modify spinal column theories further into a different three-column classification scheme (Fig. 66-11). In this classification, the ventral column consists of the ALL, the anterior anulus fibrosus, and the anterior half of the vertebral bodies. The middle column consists of the PLL, the dorsal anulus fibrosus, and the dorsal half of the vertebral bodies. Last, the posterior column, analogous to what Holdsworth defined as the dorsal ligamentous complex, consists of the bony neural arch, posterior spinous ligaments, and ligamentum flavum, as well as the facet joints. According to the Denis classification scheme, rupture of the dorsal ligamentous complex creates instability only if there is concurrent disruption of at least the PLL and dorsal anulus.
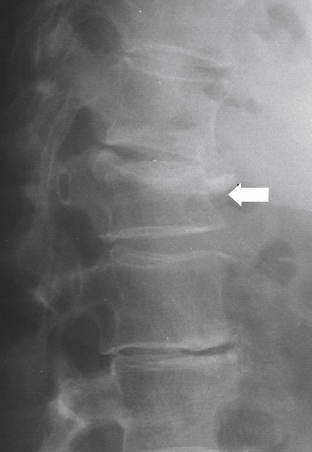
Denis79 defined failure of the anterior column alone under compression (compression fracture) with an intact posterior column as a stable fracture (Fig. 66-12). Burst fractures were defined as being generated through an axial compressive load, and involved failure of the anterior and middle columns (Fig. 66-13). Severe tensile injuries resulted in seat belt fracture or flexion-distraction injuries, which involve a disruption of the posterior and middle columns with an intact anterior column that serves as a fulcrum or hinge (Fig. 66-14). The last category in Denis’s scheme is fracture-dislocations, which are defined as a mechanical failure of all three columns, making them extremely unstable injuries (Fig. 66-15).
External immobilization and, when appropriate, operative reduction and stabilization, may prevent this progressive deformity. Neurologic instability may occur after a severe burst fracture in which the middle column has ruptured under axial loads. This disruption and retropulsion of bone fragments into the spinal canal predisposes patients to an increased risk for neurologic injury, especially with increased spinal motion. Denis79 reported that in 20% of patients with severe burst fractures and dorsal ligamentous injuries that were treated nonoperatively with external immobilization, a subsequent neurologic deficit developed. Neurologic and mechanical instability may develop after a burst fracture or fracture-dislocation, with or without an initial neurologic deficit. These are very unstable injuries and according to Denis’s analysis require decompression and internal stabilization.
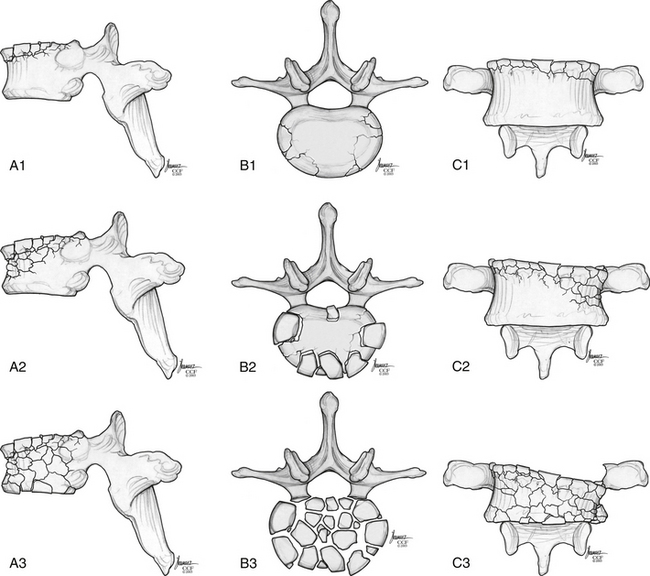
Magerl et al.92 developed a comprehensive classification scheme based on pathomorphologic criteria. This system is modeled on the AO (Arbeitsgemeinschaft für Osteosynthesefragen) long bone fracture classification (developed by Müeller, Schneider, and Willenegger) and consists of a grid with three major fracture types, subdivided into three more groups, which are further subgrouped into three more categories.92 The main categories are (A) compression injuries of the vertebral bodies, (B) distraction injuries that affect the anterior and posterior elements, and (C) axial torque or multidirectional injuries with translation that also affects the anterior and posterior elements. The severity of the fractures in this scheme increases as they progress from type A through type C. Although this classification system is extremely organized and comprehensive, it is underused clinically because of its complexity.
McCormack et al.93 created a classification system based on a load-sharing principle that uses a graded point system reflecting the integrity of the vertebral bodies or anterior and middle columns. Points are based on the amount of vertebral body comminution, spread of fragments at the fracture site, and amount of corrected traumatic kyphosis (Fig. 66-16). Trauma associated with the highest degree of energy should result in the greatest dispersion and displacement of bone fragments. This classification can be applied before surgery to quantitatively estimate the extent of disruption of the anterior and middle columns. Patients with high point values (>6) have a large void or gap, resulting in the least supportive anterior and middle columns. Fractures with the greatest dispersion of fragments and least bone-to-bone contact result in the highest degree of cantilever bending loads on the pedicle screw implants, predisposing posterior instrumentation to failure.15,94 This classification scheme assists the surgeon in deciding if ventral spine support is necessary after dorsal instrumentation, based on the premise that inadequate anterior column support will result in excessive loads being transferred to the dorsal elements (and instrumentation), thus increasing the risk for failure.
In 2009, the Spine Trauma Group created the Thoracolumbar Injury Classification and Severity Score (TLICS) system to address many of its predecessors’ limitations.95–97 The TLICS system defines injuries according to injury morphology, integrity of the posterior ligamentous complex, and neurologic status of the patient. It is the first thoracolumbar injury classification system to use injury morphology combined with the patient’s neurologic status and the critically important status of the posterior ligamentous structures in medical decision making.
Consequently, the TLICS system was designed to aid in medical decision making by providing both diagnostic and prognostic information with a weighted injury severity score. Stable injury patterns (TLICS <4) may be treated nonoperatively with brace immobilization and active patient mobilization. Unstable injury patterns (TLICS >4) may be treated operatively with the guiding principles of deformity correction, neurologic decompression if necessary, and spine stabilization followed by active patient mobilization.95
So far, the TLICS system has shown good to excellent intraobserver and interobserver reliability in a number of countries, with both orthopedic surgeons and neurosurgeons, and throughout a spectrum of spine treatment providers with varying levels of experience.95 The TLICS system has been tested in the setting of an academic trauma center, verifying that physicians in training (residents and fellows) can readily be taught the TLICS system and incorporate it into patient care.95 Furthermore, use of the TLICS system has yielded greater than 90% agreement in decision making for the management of thoracolumbar trauma across a number of providers.98
Although the TLICS system has demonstrated success, it has inherent limitations. To date, many of the investigations into the TLICS system have been performed by individuals involved with its development.99 In addition, a prospective application of the TLICS system to the treatment of spine injuries is needed to define any improvements in care and patient outcomes compared with the use of conventional systems.
Treatment Options and Strategies
No definitive treatment algorithm has been universally accepted for thoracic spine injuries, despite the numerous classification systems that exist. Stability of the vertebral column over the thoracic and thoracolumbar region, like the remainder of the spine, depends on the integrity of the osseous and ligamentous components. Once these structures are disrupted, the stability of the vertebral column can become compromised, resulting in an unstable spine. One difficulty in treating these fractures is that spinal instability is difficult to assess, based on clinical and radiographic findings. White and Panjabi give the most detailed description of instability: “the loss of the ability of the spine under physiological loads to maintain relationships between vertebrae in such a way that there is either damage or subsequent irritation to the spinal cord or nerve roots, and in addition, there is development of incapacitating deformity or pain due to structural changes.”14 However, even this definition leaves a large degree of ambiguity because of the large spectrum of spinal disorders.
Nonoperative Strategies
Treatment of two-column injuries, such as burst fractures, depends to a significant extent on the patient’s neurologic status. In neurologically intact patients, nonoperative treatment is generally recommended.14 A period of bed rest followed by mobilization in a thoracolumbosacral orthosis (TLSO) brace and continued close monitoring for increased kyphosis and neurologic changes are recommended.
Gertzbein demonstrated in a large study that kyphotic deformity greater than 30 degrees correlated with increased back pain.6,56,82,100 This result has not been duplicated in other studies. There is an array of highly morbid complications that can arise from nonoperative treatment for a kyphosis of greater than 30 degrees. More specifically, acute to subacute neurologic deterioration from avoidance of surgery becomes the most serious untoward event. This has been proven by Denis et al.45 who witnessed how, in 21% (6/29) of their patients in this series, a concerning and debilitating neurologic deficit developed after nonoperative treatment was undertaken. In a prospective study by Mumford et al. of 41 patients with a nonoperatively treated burst fracture, a neurologic deficit developed in 1 patient.101
Notwithstanding the aforementioned surgical experiences for kyphotic deformities, other authors such as Reid et al.102 and Cantor et al.40 have not necessarily noted an abrupt, or even progressive, decline in neurologic status by recommending a nonsurgical approach for patients with thoracolumbar burst fractures. It appears that the incidence of neurologic worsening lies between 0% and 20%. The inherent stability of the preserved ligaments and osseous structures usually prevents acute instability; however, a low potential for chronic or glacial instability still remains. Glacial instability usually presents as mechanical pain but could also present as a neurologic deficit.
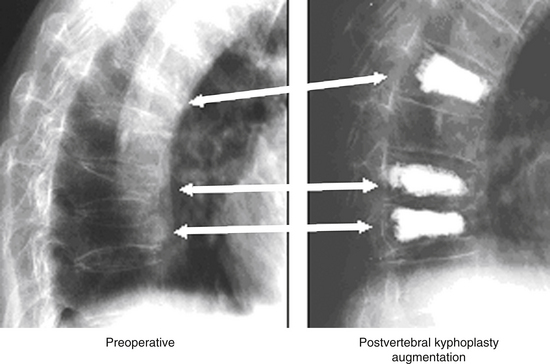
In the elderly, symptomatic compression fractures associated with severe pain and functional morbidity, which have not responded to a minimum of 6 weeks of conservative management, have been treated with polymethylmethacrylate (PMMA) augmentation, either through vertebroplasty or through a cavitation and end-plate elevation procedure (Fig. 66-17). These techniques have been associated with significant improvements in patient function and pain relief.93,103,104
Burst fractures, as defined by Denis,45,79 are vertebral body fractures involving the anterior and middle columns, such that the ALL and vertebral body, including the dorsal vertebral body cortex, are disrupted (see Figs. 66-4, 66-6, 66-11, and 66-13). As stated earlier, a burst fracture in a neurologically intact patient without posterior ligamentous or dorsal element fractures is usually considered a stable injury. James et al.105 confirmed this clinically in their reported series of patients with burst-type fractures with an intact dorsal bony architecture, all of whom became stable with a bracing regimen. Thoracic burst fractures (T1-10) make up a minor subset of burst injuries, representing approximately 5% to 10% of the total number of burst fractures. Thoracic burst fractures are inherently more stable because of the presence of the costovertebral ligamentous complex, along with the support of the rib cage.106 Therefore, as with VCFs, conservative therapy using bracing and postural reduction has been the mainstay of treatment.89,90,107
The incidence and degree of kyphosis and neurologic deterioration after a thoracic or thoracolumbar burst fracture are not known. In the neurologically intact patient with a mild kyphotic deformity (<15 degrees) and minimal dorsal ligamentous disruption, a bracing strategy may be appropriate108 (Fig. 66-18). This is corroborated by Rajasekaran,109 who in 2009 reviewed the major series of burst fracture management in the previous 20 years and found that the results of conservative treatment were equal to those of surgery and also carried fewer complications. He mentions, however, that surgery for burst fractures may have definite advantages in patients with polytrauma or in the rare event of neurologic deterioration. Thus, nonoperative treatment can be safely adopted in the management of thoracolumbar burst fractures with a normal neurologic status. However, significantly divergent splaying of the spinous processes on lateral plain radiographs, which implies dorsal ligamentous complex disruption, suggests the potential for progressive instability, and in such cases surgical intervention should be considered.
Brown et al.110 advocated nonoperative treatment of burst fractures with less than 50% of vertebral body collapse, less than 30 degrees of kyphotic deformity, and no more than 3 cm of offset from the standard sagittal vertical angle on lateral scoliosis films. These recommendations are supported by the findings of Cantor et al.,40 where evidence of dorsal ligamentous complex disruption was present if there was greater than 30 degrees of kyphosis, greater than 50% loss of ventral vertebral body height, splaying of the spinous processes, facet fracture or subluxation, and pars interarticularis fracture. Brown et al.’s management protocol recommended immediate casting of the hemodynamically stable patient in a hyperextension body cast, followed by serial radiographs. Casts were maintained for 6 to 12 weeks, followed by a Jewett orthosis and then serial radiographs every 4 weeks. This management strategy facilitated early mobilization, reduced hospital stays and costs, and, when successful, avoided the risks of surgical therapy.
Nonoperative management therefore may be used in the neurologically intact patient, even with a large degree of spinal canal stenosis from retropulsed bone fragments, as long as there is no significant kyphosis representing significant disruption of the dorsal osteoligamentous complex.111 CT has confirmed spontaneous remodeling through reabsorption of retropulsed bone fragments in the spinal canal in patients immobilized nonoperatively.48 However, if there is a decline in the patient’s neurologic status during nonoperative treatment, operative intervention is indicated in the presence of documented instability or neural compression.101
Operative Strategies
Wood et al.112 published the first prospective, randomized study comparing operative and nonoperative treatment of a thoracolumbar burst fracture in patients without a neurologic deficit. They found that operative treatment of patients with a stable thoracolumbar burst fracture and normal findings on the neurologic examination provided no major long-term advantage compared with nonoperative treatment.
There has, however, been a trend toward performing short-segment instrumentation at the thoracolumbar junction in an effort to preserve motion at adjacent levels. In this case, the McCormack classification seems useful in determining which patients would require an anterior stabilization in addition to the posterior short-segment instrumentation.113
If surgical management is chosen, the next step is determining the most appropriate approach: anterior, posterior, or both.114,115 Many factors, including fracture morphology and neurologic status, can play a role in this decision. Patients with complete neurologic deficit who are no longer in spinal shock have very little chance of significant neurologic recovery. The primary goal of surgery in this group is realignment and stabilization, typically through a posterior approach.38
Another option is anterior decompression and fusion with instrumentation. Surgeon preference often plays a role, as does fracture morphology. Concomitant lamina fractures with posterior canal compromise generally necessitate beginning with a posterior approach because of possible neural entrapment and dural tears.116
Flexion-distraction injuries result in disruption of the posterior and middle columns in tension.117 Very often, the anterior column remains intact, acting as a hinge. Surgical intervention for these fractures typically involves a posterior approach. Anterior approaches are not routinely used in these injuries, to preserve the intact anterior column.
Ventral versus Dorsal Approaches
When a partial neurologic deficit is present, improving residual canal compromise is also a goal of surgery. This situation most typically occurs with burst fractures. If performed early enough (generally within 72 hours), posterior instrumentation allows for distraction and correction of sagittal alignment and successful indirect decompression of the spinal canal. Laminectomy with transpedicular decompression also can improve the canal clearance achieved through a posterior approach. A ventral or dorsolateral approach may effectively decompress the neural elements in the setting of spinal cord compression. The ventral approach is particularly useful for decompressing midline ventral lesions and correcting severe kyphotic deformities.11,48 Bohlman et al.26,27 reported superior neurologic outcomes after a ventral, compared with a dorsal, procedure in the setting of an incomplete thoracic spinal cord injury. The major limitation of a ventral exposure is the potential surgical violation of the pleural and/or peritoneal cavities. McCormick22 reported the effective use of an extrapleural technique for the exposure of the ventral thoracic and thoracolumbar spine, thus avoiding violation of the pleural cavity.
Dorsal decompression by laminectomy after thoracic and thoracolumbar injuries has been shown to be ineffective and should not be performed as an isolated treatment strategy.11,118 The surgical removal or destruction of the dorsal ligamentous complex, along with the dorsal osseous elements, may permit temporary neurologic recovery. However, the vertebral column may not be able to maintain its alignment with the loss of the dorsal tension band, and instability, along with the potential progression of a kyphotic deformity, may ensue (Fig. 66-19). The immediate result of removing the dorsal osseous components is dorsal migration of the spinal cord if the spine has a lordotic alignment. However, the normal, untraumatized spinal column at the thoracolumbar junction maintains a slightly kyphotic posture. Spine trauma typically results in an increase in the kyphotic deformity or angulation. Therefore, removing the dorsal tension band after trauma in the setting of ventral compression from bone fragments may result in further neurologic compromise, caused by tethering of the neural elements over ventral bony elements (bowstring effect).
Biomechanically, ventral as opposed to dorsal reconstruction and instrumentation strategies provide superior mechanical stability over an equal number of spinal segments to compressive loads.57,119 A patient with midline ventral spinal cord compression, an incomplete neurologic injury, and an unstable thoracic or thoracolumbar junction burst fracture is most effectively treated with a ventral decompression and reconstruction procedure (Fig. 66-20). A variety of ventral instrumentation systems are available that may be applied to the ventral spinal elements after an adequate decompression and fusion procedure. Biomechanically, ventral bicortical screws provide a 25% to 50% increase in screw purchase strength compared with unilateral screws.120
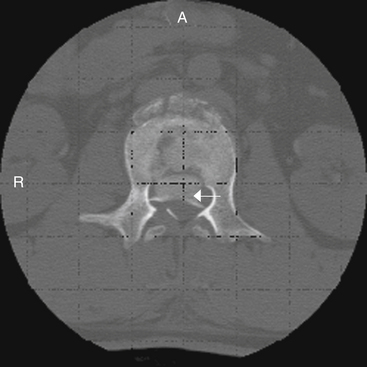
An advantage of a dorsal approach is that it provides excellent visualization and access to the dorsal thecal sac. This is useful in certain fracture types because the reported incidence of thecal sac lacerations (with the potential for nerve root incarceration) after traumatic thoracic and thoracolumbar burst fractures is 7% to 16%.121,122 The lumbar nerve roots are prone to entrapment through these lacerations, and a ventral approach will not provide access to repair this deficiency.123 The presence of a central split or greenstick fracture of the spinous process on preoperative transaxial CT may be an indicator of a dural laceration, depending on its size and displacement during impact.124
Surgical Stabilization Methods
Harrington rods were the first spinal implants widely used for the treatment of vertebral fractures.125 The dorsal spinal elements are distracted to reduce and realign the vertebral column by ligamentotaxis.9 Unfortunately, this artificially applied distraction force can result in the loss of the normal spinal curvature (loss of lordosis or exaggeration of kyphosis), resulting in muscles and ligaments working at a biomechanical disadvantage.126 Harrington rods perform relatively poorly in three-column injuries because of a predisposition to overdistraction and the relatively high incidence of rod breakage and hook cutout (7–10%).
Pedicle screw fixation allows for instrumentation of vertebrae with fractured or absent laminae. In addition, pedicle screw fixation allows for rigid bony purchase through all three columns. Because of this increased rigidity, it is often possible to use fewer segments for stable fixation, allowing the preservation of more motion segments. Preserving motion segments is of less importance in the thoracic spine because little motion is lost compared with the cervical and lumbar segments. However, limiting instrumentation of distal segments is important in thoracolumbar injuries.106
The osseous structures are fused concomitantly with posterior instrumentation. Some surgeons fuse only the injured vertebral segments with subsequent staged removal of hardware. Others fuse the entire length of the instrumentation, which results in loss of motion at additional segments. As mentioned, this is of less importance in the thoracic spine. With modern segmental fixation, fewer segments need to be instrumented to provide stability, and generally, the entire instrumented region is fused.127
The condition of the anterior column also can affect instrumentation choices. If severe comminution or kyphosis is present, extending the length of the dorsal instrumentation should be considered. This will allow for added anterior column support toward the construct as a whole. This is often an issue with burst fractures, with which ventral strut graft fusion may be required.
Neurologic Decompression for Incomplete Injuries
Neurologic injuries are present in 42% of patients with thoracolumbar junction fractures.56 Approximately 90% of patients with a thoracic fracture, excluding a VCF, have a concurrent neurologic injury, with 63% having a complete neurologic deficit.128 Compression of the spinal cord after thoracic or thoracolumbar trauma is typically caused by a ventral retropulsed bone fragment from the vertebral body. Complete loss of neurologic function below the level of the injury usually occurs at the moment of trauma, and therefore removing the retropulsed bone in the setting of a complete neurologic injury (American Spinal Injury Association [ASIA] type A) rarely, if ever, enhances neurologic recovery.48 However, in the presence of an incomplete neurologic deficit with persistent compression of the neural elements, a decompressive procedure may maximize the potential for neurologic recovery.8
Hence, timing of surgery becomes an important issue in the treatment of thoracic spine fractures. Progressive neurologic deficit in the presence of continued canal compromise is an accepted indication for immediate decompression and stabilization. Quite often, patients with thoracic spine fractures have concomitant injuries, making the timing of spinal stabilization difficult to plan. Some studies suggest that patients with thoracic spine fractures treated within 72 hours, regardless of concomitant injuries, do much better physiologically after surgery than those in whom stabilization is delayed. Early fixation results in less time in the intensive care unit, less ventilator support, a decreased rate of pulmonary complications, and less overall time in the hospital. Boerger et al.48 performed a meta-analysis of the world literature to analyze surgical outcome after decompression of the spinal cord after thoracolumbar burst fractures. Patients with an incomplete neurologic deficit who underwent an operative decompression and stabilization procedure in less than 72 hours were shown to have an enhanced neurologic recovery compared with nonoperatively treated patients.48,129 Operative therapy has also been shown to decrease pain, reduce periods of postural reduction or bed rest, and improve sagittal alignment.56 At present, surgical decompression in patients with a complete neurologic injury has not demonstrated true benefit in terms of overall neurologic improvement.27
Additional Operative Considerations
Fracture-dislocations are the most unstable of traumatic vertebral column injuries. The fractures are created by significant high-energy loads that result in a complete dissociation of the osseous structures and ligamentous complexes. Typically these carry a complete loss of neurologic function45,130 (see Fig. 66-15). Flexion-distraction-dislocation injuries have a significant distraction component and differ from seat belt–type injuries in that there is a large rotatory or torque component that causes a disruption of all three spinal columns.79 An injury of this type in the thoracic region almost always results in a complete spinal cord injury. Such injuries are associated with the highest incidence of concurrent neurologic injuries.79 Denis reported that over 50% of patients with this fracture subtype had a complete paraplegia.79
Patients with a complete neurologic injury involving the thoracic spine may frequently benefit from surgical intervention to realign a deformity, provide immediate stability, and prevent further or future neurologic injury. This expedites their transfer to a facility so they can begin rehabilitation and can subsequently be placed erect without further injury to the spinal cord proper.131 Postural reduction and bed rest mandate at least 6 to 12 weeks of prolonged immobilization before the spine heals enough to attain any degree of intrinsic stability.130
Indirect augmentation of the anterior column through a dorsal transpedicular approach, without removal or replacement of the contiguous intervertebral discs, does not appear to provide sufficient anterior column support. Alanay et al.132 demonstrated that short-segment transpedicular screw fixation through a dorsal approach failed equally (approximately 50%) with and without transpedicular bone graft augmentation. The lateral extracavitary approach provides access to both the ventral and dorsal portion of the vertebral column through a single dorsal incision. However, this approach has limited usefulness in thoracic and lumbar trauma because of its high incidence of associated morbidities. For example, Resnick and Benzel133 reviewed their series of 33 trauma patients treated with this approach and reported a 55% incidence of morbidity.
Summary
Successful management of thoracolumbar spine injuries protects the patient from further spinal deformity and neurologic deficit. As we have seen here, there is no clear consensus as to the absolute indications for surgical intervention in patients with many types of thoracolumbar fractures.
Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976). 1983;8:817-831.
Haber T.R., Filmy W.T., O’Brien M. Thoracic and lumbar fractures: diagnosis and management. In: Bridwell K.H., DeWald P.R., editors. The textbook of spinal surgery. Philadelphia: JB Lippincott; 1991:857-910.
Magerl F., Aebi M., Gertzbein S.D., et al. A comprehensive classification of thoracic and lumbar fractures. Eur Spine J. 1994;3:184-201.
McCormack B.M., Benzel E.C., Adams M.S., et al. Anatomy of the thoracic pedicle. Neurosurgery. 1995;37:303-308.
Patel A.A., Dailey A., Brodke D.S., et al. Spine Trauma Study Group: thoracolumbar spine trauma classification: the Thoracolumbar Injury Classification and Severity Score system and case examples. J Neurosurg Spine. 2009;10:201-206.
Rajasekaran S. Thoracolumbar burst fractures without neurological deficit: the role for conservative treatment. Eur Spine J. 2010;19(Suppl 1):40-47.
White A.A., Panjabi M.M. Clinical biomechanics of the spine. Philadelphia: JB Lippincott; 1978.
Wood K., Buttermann G., Mehbod A., et al. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit: a prospective, randomized study. J Bone Joint Surg [Am]. 2003;85:773-781.
1. Evans L. Risk of fatality from physical trauma versus sex and age. J Trauma. 1988;28:368-378.
2. Inaba K., Kirkpatrick A.W., Finkelstein J., et al. Blunt abdominal aortic trauma in association with thoracolumbar spine fractures. Injury. 2001;32:201-207.
3. National Spinal Cord Injury Statistical Center. Spinal cord injury facts and figures at a glance 2008. The National SCI Statistical Center website. 2008. nscisc.uab.edu/public_content/facts_figures_2008.aspx. Accessed August 16, 2011
4. Price C., Makintubee S., Herndon W., et al. Epidemiology of traumatic spinal cord injury and acute hospitalization and rehabilitation charges for spinal cord injuries in Oklahoma. Am J Epidemiol. 1994;139:37-47.
5. El-Khoury G.Y., Whitten C.G. Trauma to the upper thoracic spine: anatomy, biomechanics and unique imaging features. AJR Am J Roentgenol. 1993;160:95-102.
6. Gertzbein S.D. Fractures of the thoracic and lumbar spine. Baltimore: Williams & Wilkins; 1992.
7. Hu R., Mustard C.A., Burns C. Epidemiology of incident spinal fracture in a complete population. Spine (Phila Pa 1976). 1996;21:492-499.
8. Chapman J.R., Anderson P.A. Thoracolumbar spine fractures and neurologic deficits (review). Orthop Clin North Am. 1994;25:595-612.
9. Gray H. Anatomy, descriptive and surgical. In: Pick T.P., Howden R., editors. Revised American edition from the fifteenth English edition. New York: Bounty Books, 1977.
10. Fessler R.G., Greenwald D., Peace D. Surgical exposures of the cervicothoracic and upper thoracic spine. In: Benzel E.C., Stillerman C.B., editors. The thoracic spine. St. Louis: Quality Medical Publishing; 1999:197-207.
11. Berry J.L., Moran J.M., Berg W.S., et al. A morphological study of human lumbar and selected thoracic vertebrae. Spine (Phila Pa 1976). 1987;12:362-366.
12. Boyle J.J., Singer K.P., Milne N. Morphological survey of the cervicothoracic junctional region. Spine (Phila Pa 1976). 1996;21:544-548.
13. Panjabi M.M., Takata K., Goal V., et al. Thoracic human vertebrae: quantitative three-dimensional anatomy. Spine (Phila Pa 1976). 1991;26:888-901.
14. White A.A., Panjabi M.M. Clinical biomechanics of the spine. Philadelphia: JB Lippincott; 1978.
15. Zindrick M.R., Wiltse L.L., Doornic A., et al. Analysis of the morphometric characteristics of the thoracic and lumbar pedicles. Spine (Phila Pa 1976). 1987;12:160-166.
16. Fon G., Pitt M.J., Thies A.C. Thoracic kyphosis: range in normal subjects. AJR Am J Roentgenol. 1980;134:979-983.
17. Singer K.P., Jones T.J., Breidahl P.D. A comparison of radiographic and computer-assisted measurements of thoracic and thoracolumbar sagittal curvature. Skeletal Radiol. 1990;19:21-26.
18. Breathnach A.S. Fraser’s anatomy of the human skeleton, ed 5. London: J&A Churchill; 1958.
19. Schmorl G., Junghans H. The human spine in health and disease. New York: Grune & Stratton; 1971. pp 290–291
20. McCormack B.M., Benzel E.C., Adams M.S., et al. Anatomy of the thoracic pedicle. Neurosurgery. 1995;37:303-308.
21. Kothe R., O’Holleran J.C., Liu W., et al. Internal architecture of the thoracic pedicle, an anatomic study. Spine (Phila Pa 1976). 1996;21:264-270.
22. McCormick P.C. Retropleural approach to the thoracic and thoracolumbar spine. Neurosurgery. 1995;37:908-914.
23. Ebraheim N.A., Xu R., Ahmad M., et al. Projection of the thoracic pedicle and its morphometric analysis. Spine (Phila Pa 1976). 1997;22:233-238.
24. Andriacchi T.P., Schultz A.B. A model for the studies of mechanical interactions between the human spine and rib cage. J Biomech. 1974;7:497-507.
25. Scoles P.V., Linton A.E., Latimer B., et al. Vertebral body and posterior element morphology: the normal spine in middle life. Spine (Phila Pa 1976). 1988;13:1082-1086.
26. Bohlman H.H. Traumatic fractures of the upper thoracic spine with paralysis. J Bone Joint Surg [Am]. 1974;56:1299.
27. Bohlman H.H., Freehafer A., Dejak J. The results of treatment of acute injuries of the upper thoracic spine with paralysis. J Bone Joint Surg [Am]. 1985;67:360-369.
28. Beaunoyer M., St-Vil D., Lallier M., et al. Abdominal injuries associated with thoraco-lumbar fractures after motor vehicle collision. J Pediatr Surg. 2001;36:760-762.
29. Dickman C.A., Fessler R.G., MacMillan M., et al. Transpedicular screw-rod fixation of the lumbar spine: operative technique and outcome in 104 cases. J Neurosurg. 1992;77:860-870.
30. Malas M.A., Salbacak A., Büyükmumcu M., et al. An investigation of the conus medullaris termination level during the period of fetal development to adulthood. Kaibogaku Zasshi. 2001;76:453-459.
31. Chan R.N.W., Ainscow D., Sikorski J.M. Diagnostic failures in the multiple injured. J Trauma. 1980;20:684-687.
32. Enderson B.L., Reath D.B., Meadows J. The tertiary trauma survey: a prospective study of missed injury. J Trauma. 1990;30:666-669.
33. Laasonen E.M., Kivioja A. Delayed diagnosis of extremity injuries in patients with multiple injuries. J Trauma. 1991;31:257-260.
34. Stanislas M.J.C., Latham J.M., Porter K.M., et al. A high-risk group for thoracolumbar fractures. Injury. 1998;29:15-18.
35. van Beek E.J., Been H.D., Ponsen K.K., et al. Upper thoracic spinal fractures in trauma patients: a diagnostic pitfall. Injury. 2000;31:219-223.
36. Arigtuaco E.J., Binet E.F. Radiology of thoracic and lumbar fractures. Clin Orthop Relat Res. 1984;189:43-57.
37. Ballock R.T., Mackersie R., Abitbol J.J., et al. Can burst fractures be predicted from plain radiographs? J Bone Joint Surg [Br]. 1992;74:147-150.
38. Bradford D.S., Akbamia B.A., Winter R.B. Surgical stabilization of fractures and fracture dislocations of the thoracic spine. Spine (Phila Pa 1976). 1977;2:185-196.
39. Kuklo T.R., Polly D.W., Owens B.D., et al. Measurement of thoracic and lumbar fracture kyphosis: evaluation of intraobserver, interobserver, and technique variability. Spine (Phila Pa 1976). 2001;26:61-65.
40. Cantor J.B., Lebwohl N.H., Garvey T., et al. Non-operative management of stable thoracolumbar burst fractures with early ambulation and bracing. Spine (Phila Pa 1976). 1993;19:1731-1740.
41. Posner I., White A.A.III, Edwards W.T. A biomechanical analysis of the clinical stability of the lumbar and lumbosacral spine. Spine (Phila Pa 1976). 1982;7:374-389.
42. Saboe L.A., Reid D.C., Davis L.A., et al. Spine trauma and associated injuries. J Trauma. 1991;31:43-48.
43. Daffner R.H., Deeb Z.L., Rothfus W.E. Thoracic fractures and dislocations in motorcyclists. Skeletal Radiol. 1987;16:280-284.
44. Denis F., Burkus J.K. Shear fracture dislocations of the thoracic and lumbar spine associated with forceful hyperextension (lumberjack paraplegia). Spine (Phila Pa 1976). 1992;17:152.
45. Denis F., Armstrong G.W., Searls K., et al. Acute thoracolumbar burst fractures in the absence of neurologic deficit. Clin Orthop Relat Res. 1984;189:142-149.
46. Haher T.R., O’Brien M., Felmly W.T., et al. Instantaneous axis of rotation as a function of the three columns of the spine. Spine (Phila Pa 1976). 1992;17(Suppl 6):S149-S154.
47. McAfee P.C., Yuan H.A., Fredrickson B.E., et al. The value of computed tomography in thoracolumbar fractures: an analysis of one hundred cases and a new classification. J Bone Joint Surg [Am]. 1983;65:461-473.
48. Boerger T.O., Limb D., Dickson R.A. Does ‘canal clearance’ affect neurological outcome after thoracolumbar burst fractures? J Bone Joint Surg [Br]. 2000;82:629-635.
49. Dai L.Y. Remodeling of the spinal canal after thoracolumbar burst fractures. Clin Orthop Relat Res. 2001;382:119-123.
50. Klerk L.W.L., Fontijne P.J., Stijnen T., et al. Spontaneous remodeling of the spinal canal after conservative management of thoracolumbar burst fractures. Spine (Phila Pa 1976). 1998;23:1057-1060.
51. Jelly L.M., Evans D.R., Easty M.J., et al. Radiography versus spiral CT in the evaluation of cervicothoracic junction injuries in polytrauma patients who have undergone intubation. Radiographics. 2000;20(Spec No):S251-S259.
52. Flanders A.E. Thoracolumbar trauma imaging overview. Instr Course Lect. 1999;48:429-431.
53. Karnaze M.G., Gado M.H., Sartor K.J., et al. Comparison of MR and CT myelography in imaging the cervical and thoracic spine. AJR Am J Roentgenol. 1988;150:397-403.
54. Schaefer D.M., Flanders A., Northrup B.E., et al. Magnetic resonance imaging of acute cervical spine trauma: correlation with severity of neurological injury. Spine (Phila Pa 1976). 1989;14:1090-1095.
55. Brightman R.P., Miller C.A., Rea G.L., et al. Magnetic resonance imaging of trauma to the thoracic and lumbar spine. Spine (Phila Pa 1976). 1992;17:541-550.
56. Gertzbein S.D. Neurologic deterioration in patients with thoracic and lumbar fractures after admission to the hospital. Spine (Phila Pa 1976). 1994;19:1723-1725.
57. Panjabi M.M., White A.A., Johnson R.M. Cervical spine mechanics as a function of transection of components. J Biomech. 1975;8:327-336.
58. Maiman D.J., Pintar F.A. Anatomy and clinical biomechanics of the thoracic spine. Clin Neurosurg. 1992;38:296-324.
59. Myklebust J.B., Pintar F., Yoganandan N. Tensile strength of spinal ligaments. Spine (Phila Pa 1976). 1971;13:526-531.
60. Haher T.R., Bergman M., O’Brien M., et al. The effect of the three columns of the spine on the instantaneous axis of rotation in flexion and extension. Spine (Phila Pa 1976). 1991;16(Suppl 8):S312-S318.
61. Hanley E.N., Eskay M.L. Thoracic spine fractures. Orthopedics. 1989;12:689-696.
62. Haber T.R., Filmy W.T., O’Brien M. Thoracic and lumbar fractures: diagnosis and management. In: Bridwell K.H., DeWald P.R., editors. The textbook of spinal surgery. Philadelphia: JB Lippincott; 1991:857-910.
63. Tran N.T., Watson N.A., Tencer A.F., et al. Mechanism of the burst fracture in the thoracolumbar spine. Spine (Phila Pa 1976). 1995;20:1984-1988.
64. Whiteside T.E. Traumatic kyphosis of the thoracolumbar spine. Clin Orthop Relat Res. 1977;128:78-92.
65. Haher T.R., Tozzi J.M., Lospinuso M.F., et al. The contribution of the three columns of the spine to spinal stability: a biomechanical model. Paraplegia. 1989;27:432-439.
66. Haher T.R., Felmy W., Banich H., et al. The contribution of the three columns of the spine to rotational stability: a biomechanical model. Spine (Phila Pa 1976). 1989;14:663-669.
67. Petitjean M.E., Mousselard H., Pointillart V., et al. Thoracic spinal trauma and associated injuries: should early spinal decompression be considered? J Trauma. 1995;39:368-372.
68. Sturm J.T., Hines J.T., Perry J.F. Thoracic spinal fractures and aortic rupture: a significant and fatal association. Ann Thorac Surg. 1990;50:931-933.
69. Dalvie S.S., Burwell M., Noordeen M.H.H. Haemothorax and thoracic spinal fracture: a case for early stabilization. Injury. 2000;31:269-270.
70. van Raaij T.M., Slis H.W., Hooglanb P.H., et al. Massive haemothorax following vertebral fracture. Injury. 2000;31:202-203.
71. Argenson C., Bouleau P., de Peretti F., et al. Les fractures du rachis thoracique (T1-T10): a propos de 105 cas. Rev Chir Orthop. 1989;75:370-386.
72. Freysz M., Adamon O., Wilkening M., Sautreaux J.L., et al. Hémothorax et fractures de la colonne dorsale. Sem Hop. 1983;59:2229-2231.
73. Chiliimindris C.P. Rupture of the thoracic esophagus from blunt trauma. J Trauma. 1977;17:968-971.
74. Monzon J.R., Ryan B. Thoracic esophageal perforation secondary to blunt trauma. J Trauma. 2000;49:1129-1131.
75. Stothert J.C.Jr., Buttorff J., Kaminski D.L. Thoracic esophageal and tracheal injury following blunt trauma. J Trauma. 1980;20:992-995.
76. Rabinovici R., Ovadia P., Mathiak G., et al. Abdominal injuries associated with lumbar spine fractures in blunt trauma. Injury. 1999;30:471-474.
77. Gumley G., Taylor T.K., Ryan M.D. Distraction fractures of the lumbar spine. J Bone Joint Surg [Br]. 1982;64:520-525.
78. Miller C.D., Blyth P., Civil I.D. Lumbar transverse process fractures: a sentinel marker of abdominal organ injuries. Injury. 2000;31:773-776.
79. Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976). 1983;8:817-831.
80. Ferguson R.L., Allen B.L.Jr. A mechanistic classification of thoracolumbar spine fractures. Clin Orthop Relat Res. 1983;189:817-831.
81. Watson-Jones R. Manipulative reduction of crush fractures of the spine. BMJ. 1931;1(3659):300-302.
82. Prabhakar M.M., Rao B.S., Patel L. Thoracolumbar burst fracture with complete paraplegia: rationale for second-stage anterior decompression and fusion regarding functional outcome. J Orthop Traumatol. 2009;10(2):83-90.
83. Nicholl E.A. Fractures of the dorso-lumbar spine. J Bone Joint Surg [Br]. 1949;31:376-394.
84. Holdsworth F.W. Fractures, dislocations, and fracture-dislocations of the spine. J Bone Joint Surg [Br]. 1963;45:6-20.
85. Rennie W., Mitchell N. Flexion distraction fractures of the thoracolumbar spine. J Bone Joint Surg [Am]. 1973;55:386-390.
86. Chance C.Q. Note on a type of flexion fracture of the spine. Br J Radiol. 1948;21:452.
87. Kelly R.P., Whiteside T.E.Jr. Treatment of lumbodorsal fracture-dislocations. Ann Surg. 1968;167:705-717.
88. Louis R. Les theories de l’instabilise. Rev Chir Orthop. 1977;63:423-435.
89. Bedhrook G.M. Stability of spinal fractures and fracture dislocations. Paraplegia. 1971;9:23-32.
90. Bedbrook G.M. Treatment of thoracolumbar dislocation and fracture with paraplegia. Clin Orthop Relat Res. 1979;112:267-284.
91. Reuber M., Schultz A., Denis F., et al. Bulging of lumbar intervertebral discs. J Biomech Eng. 1982;104:187-192.
92. Magerl F., Aebi M., Gertzbein S.D., et al. A comprehensive classification of thoracic and lumbar fractures. Eur Spine J. 1994;3:184-201.
93. McCormack T., Karaikovic E., Gaines R.W. The load sharing classification of spine fractures. Spine (Phila Pa 1976). 1994;19:1741-1744.
94. McLain R.F., Spasrling E., Benson D.R. Early failure of short-segment pedicle instrumentation for thoracolumbar fractures. J Bone Joint Surg [Am]. 1993;75:162-167.
95. Patel A.A., Dailey A., Brodke D.S., et al. Spine Trauma Study Group: thoracolumbar spine trauma classification: the Thoracolumbar Injury Classification and Severity Score system and case examples. J Neurosurg Spine. 2009;10:201-206.
96. Vaccaro A.R., Lehman R.A.Jr., Hulbert R.J., et al. A new classification of thoracolumbar injuries: the importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine (Phila Pa 1976). 2005;30:2325-2333.
97. Whang P.G., Vaccaro A.R., Poelstra K.A., et al. The influence of fracture mechanism and morphology on the reliability and validity of two novel thoracolumbar injury classification systems. Spine (Phila Pa 1976). 2007;32:791-795.
98. Patel A.A., Vaccaro A.R., Albert T.J., et al. The adoption of a new classification system: time-dependent variation in interobserver reliability of the thoracolumbar injury severity score classification system. Spine (Phila Pa 1976). 2007;32:E105-E110.
99. Rampersaud Y., Fisher C., Wilsey J., et al. Agreement between orthopedic surgeons and neurosurgeons regarding a new algorithm for the treatment of thoracolumbar injuries: a multicenter reliability study. J Spinal Disord Tech. 2006;19:477-482.
100. Gertzbein S.D. Scoliosis Research Society: multicenter spine fracture study. Spine (Phila Pa 1976). 1992;17:528-540.
101. Mumford J., Weinstein J.N., Spratt K.F., et al. Thoracolumbar burst fractures: the clinical efficiency and outcome of nonoperative management. Spine (Phila Pa 1976). 1993;18:955-970.
102. Reid D.C., Ho R., Dans L.A., et al. The nonsurgical treatment burst fractures of the thoracolumbar junction. J Trauma. 1988;28:1188-1194.
103. Barr J.D., Barr M.S., Lemley T.J., et al. Percutaneous vertebroplasty for pain relief and spinal stabilization. Spine (Phila Pa 1976). 2000;25:923-928.
104. Lieberman I.H., Dudeney S., Reinhardt M.K., et al. Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine (Phila Pa 1976). 2001;26:1631-1638.
105. James K.S., Wenger K.H., Schlegel J.D., et al. Biomechanical evaluation of the stability of thoracolumbar burst fractures. Spine (Phila Pa 1976). 1994;19:1731-1740.
106. Bransford R., Bellabarba C., Thompson J.H., et al. The safety of fluoroscopically-assisted thoracic pedicle screw instrumentation for spine trauma. J Trauma. 2006;60:1047-1052.
107. Frankel H.L., Hancock D.O., Hyslop G. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia. 1969;7:179-192.
108. Weinstein J.N., Collalto P., Lehmann T.R. Thoracolumbar “burst” fractures treated conservatively: a long-term follow-up. Spine (Phila Pa 1976). 1988;13:33-38.
109. Rajasekaran S. Thoracolumbar burst fractures without neurological deficit: the role for conservative treatment. Eur Spine J. 2010;19(Suppl 1):40-47.
110. Brown C.W., Gorup J.M., Chow G.H. Nonsurgical treatment of thoracic burst fractures in controversies. In: Zdeblick T.A., Benzel E.C., Anderson P.A., et al, editors. Spine surgery. St. Louis: Quality Medical Publishing; 1999:86-96.
111. Hashimoto T., Kaneda K., Abumi K. Relationship between traumatic spinal canal stenosis and neurologic deficits in thoracolumbar burst fractures. Spine (Phila Pa 1976). 1988;13:1268-1272.
112. Wood K., Buttermann G., Mehbod A., et al. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit: a prospective, randomized study. J Bone Joint Surg [Am]. 2003;85:773-781.
113. Sharan A.D., Choe H., Snyder L. Postoperative spinal imaging. In: Schwartz E.D., Flanders A.E., editors. Spinal trauma: imaging, diagnosis, and management. Philadelphia: Lippincott Williams & Wilkins; 2006:304-325.
114. Knop C., Fabian H.F., Bastian L., et al. Late results of thoracolumbar fractures after posterior instrumentation and transpedicular bone grafting. Spine (Phila Pa 1976). 2001;26:88-99.
115. Sasso R.C., Best N.M., Reilly T.M., et al. Anterior-only stabilization of three-column thoracolumbar injuries. J Spinal Disord Tech. 2005;18(Suppl):S7-S14.
116. Pickett J., Blumenkopf B. Dural lacerations and thoracolumbar fractures. J Spinal Disord. 1989;2:99-103.
117. Gertzbein S.D., Court-Brown C.M. Flexion-distraction injuries of the lumbar spine: mechanism of injury and classification. Clin Orthop Relat Res. 1988;227:52-60.
118. Morgan T.H., Wharton G.W., Austin G.N. The results of laminectomy in incomplete spinal cord injury. J Bone Joint Surg [Am]. 1970;52:1115-1130.
119. Shono Y., McAfee P.C., Cunningham B.W. Experimental study of thoracolumbar burst fractures. Spine (Phila Pa 1976). 1994;19:1711-1722.
120. Breeze S.W., Doherty B.J., Noble P.S., et al. A biomechanical study of anterior thoracolumbar screw fixation. Spine (Phila Pa 1976). 1998;23:1829-1831.
121. Keenen T.L., Anthony J., Benson D.R. Dural tears associated with lumbar burst fractures. J Orthop Trauma. 1990;4:243-245.
122. Silvestro C., Francavigilia N., Bragazzi R. On the predictive value of radiologic signs for the presence of dural lacerations related to fractures of the lower thoracic and lumbar spine. J Spinal Disord. 1991;4:49-53.
123. Miller C.A., Dewey R.C., Hunt W.E. Impaction fracture of the lumbar spine. J Neurosurg. 1980;53:765-771.
124. Miller C.A., Hunt W.E. Variation of impaction fracture of the lumbar spine. J Neurosurg. 1982;56:603.
125. Cotler J.M., Vernace J.V., Michalski J.A. The use of Harrington rods in thoracolumbar fractures. Orthop Clin North Am. 1986;17:87-103.
126. Jacobs R.R., Casey M.P. Surgical management of thoracolumbar spine injuries. Clin Orthop Relat Res. 1984;189:22-35.
127. McCullen G., Vaccaro A.R., Garfin S.R. Thoracic and lumbar trauma: rationale for selecting the appropriate fusion technique. Orthop Clin North Am. 1998;29:813-828.
128. Meyer P.R.J. Fractures of the thoracic spine: T1 to T10. In: Meyer P.R.J., editor. Surgery of spine trauma. New York: Churchill Livingstone; 1989:525-571.
129. Kaneda K., Abumi K., Jujiya M. Burst fractures with neurologic deficits of the thoracolumbar-lumbar spine: results of anterior decompression and stabilization with anterior instrumentation. Spine (Phila Pa 1976). 1984;9:788-795.
130. Convery F.R., Minteer M.A., Smith R.W., et al. Fracture-dislocation of the dorsal-lumbar spine: acute operative stabilization by Harrington instrumentation. Spine (Phila Pa 1976). 1978;3:160-166.
131. Shikata J., Yamamuro T., Iida H., et al. Surgical treatment for paraplegia resulting from vertebral fractures in senile osteoporosis. Spine (Phila Pa 1976). 1990;15:485-489.
132. Alanay A., Emre A., Muharrem Y., et al. Short-segment pedicle instrumentation of thoracolumbar burst fractures: does transpedicular intracorporeal grafting prevent early failure? Spine (Phila Pa 1976). 2001;26:213-217.
133. Resnick D.K., Benzel E.C. Lateral extracavitary approach for thoracic and thoracolumbar spine trauma: operative complications. Neurosurgery. 1998;43:796-803.