Chapter 63 Trauma Surgery
Occipitocervical Junction
Occipitocervical junction injuries can be classified in several ways (Box 63-1). One useful system describes occipitocervical junction injuries as isolated ligamentous injuries, isolated fractures, or mixed ligamentous and bony injuries. Occipitocervical junction trauma can also be described by the site and/or level(s) of injury. At most sites, classification systems have been developed for specific injury patterns (e.g., C2 odontoid fractures). Finally, occipitocervical junction injuries can be described on the basis of their stability. Stability is generally determined with clinical and radiographic assessment, sometimes using dynamic flexion/extension radiographs. A stable injury does not demonstrate significant radiographic deformity, pain, or neurologic dysfunction with normal physiologic loads and movement. An example of a stable injury would be an isolated C2 spinous process fracture that meets the preceding criteria. Some injuries are clearly unstable, such as occipitocervical dislocations. Other injuries may initially appear stable but have a reasonable chance of developing delayed instability with time, gravity, movement, and/or relaxation of paraspinal muscle spasm. This category reflects the reality that clinical and radiographic assessment of long-term stability may be indeterminate.
BOX 63-1 Occipitocervical Junction Injury Classification Systems
The preceding classification systems are helpful in injury assessment and planning management. However, the management of a patient with occipitocervical junction trauma is best determined by considering the nature of the injury (including associated injuries), patient characteristics (e.g., age, medical risk factors, bone quality, desire and ability to tolerate use of a halo orthosis), and the physician’s experience. Although much less common, penetrating trauma to the occipitocervical junction presents unique issues that relate to the specific location and trauma modality (e.g., bullet, knife). This class of injuries is not specifically discussed in this chapter but is addressed in Chapter 73. Although most of the principles from blunt trauma are applicable to penetrating trauma, it is important to point out some important differences. Compared with blunt trauma, penetrating trauma typically results in less ligamentous injury and, therefore, for a similar fracture, may be more stable. However, penetrating trauma more commonly results in trauma to vascular or other important regional structures.
General Principles
The initial management of occipitocervical junction injuries is focused on basic trauma management principles, including establishment and maintenance of airway, breathing, and circulation; careful immobilization and transportation; and recognition and management of any associated injuries. These principles have evolved over time and have been published in numerous settings.1,2
Occipitocervical junction injuries are frequently recognized on routine cervical spine imaging. However, these injuries may be difficult to detect on initial diagnostic studies. Clinical suspicion based on history and physical examination can aid recognition. Routine radiographs and clinical assessment are often inadequate to fully characterize the injury, and more specialized imaging is usually indicated. Coronal, curved coronal, sagittal, and/or three-dimensional CT reconstruction views can be extremely helpful in characterizing the presence and nature of injury. MRI may be difficult or impossible to obtain acutely but can often provide essential information on spinal canal compromise and may suggest the presence and degree of ligamentous injury. Dynamic imaging with plain radiographs, CT, and/or MRI can be valuable in assessing stability but should be performed carefully. Occasionally, stability is checked with real-time fluoroscopy during careful flexion and extension controlled by a qualified examiner. For example, fluoroscopic flexion/extension imaging may be helpful when there is urgent need to assess the stability of the cervical spine in an unresponsive patient but there is still controversy about its interpretation.3
Once occipitocervical junction injuries are diagnosed, management decisions are based on several factors, including the extent and stability of injury, the presence or progression of neurologic deficits, and patient-specific factors that influence the risks with different treatments. Nonoperative management typically includes some type of rigid (halo) or semirigid (collar) orthosis. Operative management is generally indicated for injuries that are unstable, have significant potential for delayed instability, have progressive neurologic deficits, and/or cause significant deficits or symptoms that are not controlled with nonoperative measures. Operative planning may include obtaining additional imaging (e.g., dedicated studies for image guidance), ensuring the availability of appropriate instrumentation, and arranging neurophysiologic monitoring where appropriate.
Diagnosis and Management
Occipitocervical Dislocations
Occipitocervical dislocations are relatively uncommon ligamentous injuries that usually result from hyperflexion and distraction during high-impact blunt trauma.4,5 These injuries are highly unstable, frequently fatal, and usually result in significant neurologic injury from stretching, compression, and/or distortion of the spinal cord, brainstem, and cranial nerves.6 In addition, significant morbidity and mortality can result from associated cerebrovascular injury, which varies among trauma series, diagnosis test used (CT, conventional angiogram), and severity of injuries.7,8 Recognition and rapid management of these injuries may limit further injury, but even with appropriate care, neurologic deficits can progress. Although these were initially felt to be rare, several series of trauma fatalities have revealed an incidence between 8% and 19%.4
Lateral cervical spine radiographs may recognize occipitocervical dislocations (sensitivity, 0.57), especially in severe injuries. However, these injuries can be difficult to diagnose with plain radiographs alone, especially with less-severe dislocations. In addition, the frequent presence of coexisting significant head trauma can delay recognition of spinal injury. Diagnostic clues include prevertebral soft tissue swelling, an increase in the dens-basion distance, and separation of the occipital condyles and C1 lateral masses (Fig. 63-1). CT imaging with reconstruction views (sensitivity 0.84) usually provides a better assessment of fractures and alignment than plain radiographs do. The presence of subarachnoid hemorrhage supports but does not confirm the diagnosis. MRI imaging can be helpful for diagnosis (sensitivity 0.86), to assess the extent of spinal cord compression and injury, and to demonstrate compressive hematoma lesions.2
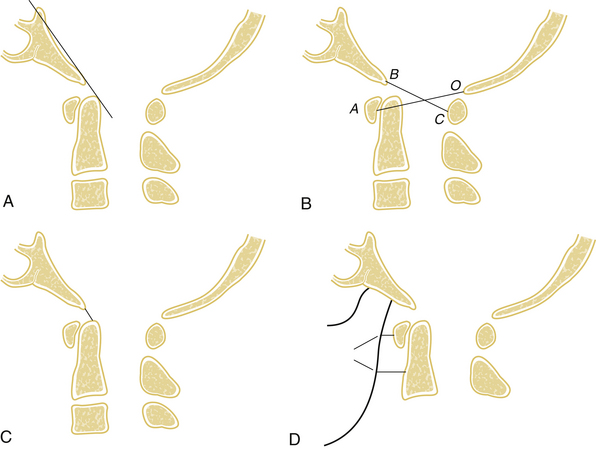
On the basis of the injury pattern, occipitoatlantal dislocations have been classified by Traynelis et al.9 into four types: type I (anterior), type II (longitudinal), type III (posterior), and “other” (complex). A number of diagnostic radiographic criteria have been described that assess the relationship between the skull base and cervical spine (Fig. 63-2). Although developed for lateral plain radiographs, these criteria can also be used on sagittal reconstruction CT views, provided that there are no significant artifactual distortions. The Wackenheim clival line extends along the dorsal surface of the clivus and should be tangential to the tip of the dens.10 Ventral or dorsal translation of the skull in relation to the dens will shift the clival line to either intersect or run dorsal to the dens, respectively. The Powers ratio is based on the relationship of the B–C line (from the basion to the C1 dorsal arch) and the O–A line (between the opisthion and the C1 ventral arch).11 Normal B–C/O–A ratios average 0.77, while pathologic ratios (>1) typically represent occipitocervical dislocations. However, false negatives can occur with longitudinal or dorsal dislocations.12 The Wholey dens-basion technique assesses the distance from the basion to the dens tip.13 Although variability is common, the average distance in adults is about 9 mm, and pathologic distances are greater than 15 mm.14 The Dublin method, the least reliable method, measures the distance from the mandible (posterior ramus) to the ventral part of C1 (normally 2–5 mm) and C2 (normally 9–12 mm).15
Initial management of these injuries focuses on immobilization, almost always with a halo orthosis. Cervical collars are potentially dangerous because they may produce distraction and thereby promote further injury. Similarly, traction can cause neurologic worsening (2 of 21 patients) and should be avoided or used with extreme caution.1,2 Nonoperative management does not provide definitive treatment of these injuries because of the significant ligamentous disruption that cannot be expected to heal even with prolonged rigid (halo) external immobilization (11 of 40 patients had a nonunion and/or neurologic deterioration).2 Operative stabilization consists of an occipitocervical arthrodesis with rigid internal fixation (discussed later and in Chapter 143). Decompression and restoration of alignment may also be necessary to maximize neurologic recovery.
Transverse Ligament Injuries
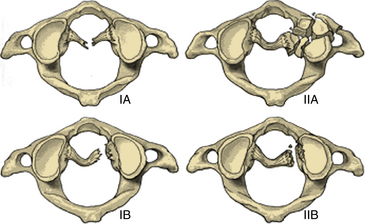
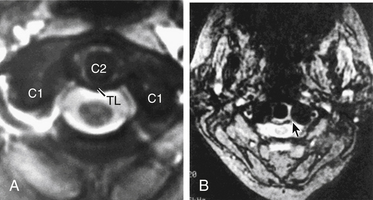
Transverse ligament injuries are suggested or diagnosed with radiographic imaging. A widened atlantodental interval on flexion lateral cervical radiographs (>3 mm in adults, >5 mm in children) suggests transverse ligament insufficiency. Thin-cut CT imaging with reconstruction views may suggest the diagnosis by demonstrating a C1 lateral mass avulsion fracture at the ligamentous insertion. Thin-cut MRI with attention to the transverse ligament when using gradient echo sequences can directly demonstrate a transverse ligament injury.16 If the diagnosis is uncertain, dynamic (flexion/extension) imaging is appropriate for cooperative patients. On the basis of CT and MRI, traumatic transverse ligament injuries can be classified into two categories (Fig. 63-3). Type I injuries involve disruptions of the midportion (IA) or periosteal insertion laterally (IB). Type II injuries involve fractures that disconnect the C1 lateral mass tubercle for insertion of the transverse ligament via a comminuted fracture (IIA) or an avulsion fracture (IIB).17
The management of transverse ligament injuries is based on the type of injury. Type I injuries are pure ligamentous injuries that cannot be expected to heal with nonoperative external fixation. Therefore, operative stabilization with a dorsal C1-2 arthrodesis and fixation is indicated. The surgical options include C1-2 dorsal wiring, C1-2 Halifax clamps, C1-2 transarticular screws, and/or C1-2 segmental screw fixation (see later section and Chapter 143). Type II injuries have a much higher chance of healing with halo immobilization (up to 74%).17 If a nonunion is still present after a prolonged period of immobilization (>3 months), then operative stabilization is generally appropriate.
Rotatory C1-2 Subluxations
Rotatory C1-2 subluxations are ligamentous injuries that are more common in children and adolescents. These injuries typically present with neck pain and a fixed, rotated “cock-robin” head position. Open-mouth radiographs may demonstrate an asymmetry of the C1 and C2 lateral masses. CT imaging can confirm the rotatory subluxation diagnosis and demonstrate coexisting fractures. C1-2 axial rotation greater than 47 degrees confirms the diagnosis. Three-view CT imaging (15 degrees to the left, neutral, and 15 degrees to the right) can also be helpful in establishing the diagnosis.18,19 MRI may detect a coexistent transverse ligament injury.
Occipital Condyle Fractures
Occipital condyle fractures generally occur with axial trauma and are almost always unilateral (>90%). These injuries are classified into three types according to Anderson and Montesano.20 Type I injuries are comminuted fractures that result from axial trauma. Type II fractures are extensions of linear basilar skull fractures. Type III injuries, the most common, are avulsion fractures of the condyle that can result from a variety of mechanisms. The incidence of occipital condyle fractures has been estimated to be between 1% and 3% of blunt craniocervical trauma cases.21 Although plain radiographs (usually open-mouth radiographs) may occasionally identify the injury, they have an unacceptably low sensitivity (estimated at 3.2%) and should not be relied on when the diagnosis is suspected. CT imaging with reconstruction views provides the best assessment of fracture pattern and alignment.21,22
Occipital condyle fractures are generally stable and therefore are typically managed with an external nonrigid orthosis (collar) until the fracture heals (often 12 weeks). Type III fractures are felt to be more prone to instability, and when significant displacement or clinical concern exists, halo immobilization may be appropriate.23 Operative stabilization with an occipitocervical fusion is generally reserved for situations in which there are associated cervical fractures or significant ligamentous injuries.
C1 Fractures
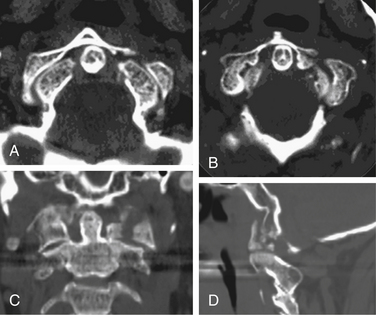
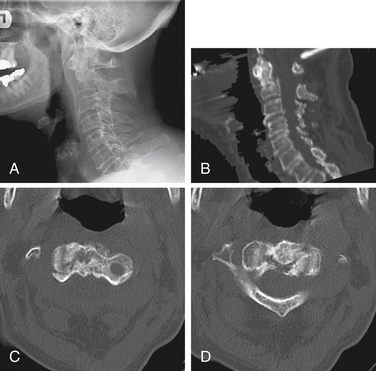
Isolated C1 fractures account for approximately 5% of cervical spine fractures. These injuries occur with axial trauma with or without lateral bending.24 Open-mouth radiographs may suggest the injury, but CT imaging with reconstruction views provides the best assessment of fracture pattern and alignment. Fractures can include almost any part of the ring or lateral masses of C1. Aside from unilateral lateral mass fractures, the fractures usually occur at multiple sites (Fig. 63-4). Jefferson fractures are four-part fractures with bilateral ventral and dorsal ring fractures. The assessment of these injuries is focused on evaluating the integrity of the transverse ligament and on recognizing any additional fractures.
The management of C1 fractures is based on the integrity of the transverse ligament that can be assessed indirectly with several radiographic criteria such as a widened atlantodental interval (>3 mm) and increased spread of the lateral masses of C1 over C2 (>6.9 mm, rule of Spence)25 or directly through high-resolution MRI (Fig. 63-5). If the transverse ligament is intact, isolated C1 fractures are generally stable and can be treated with an external orthosis (e.g., SOMI) primarily for symptom control until the fracture heals. With transverse ligament insufficiency, operative stabilization is indicated by using a C1-2 fusion technique such as dorsal C1-2 wiring techniques, C1-2 transarticular screws, C1 lateral mass-to-C2 pars/pedicle screws, or ventral C1-2 screw fixation (see Chapter 143). The surgical choice is based primarily on patient anatomy and fracture pattern as well as the surgeon’s experience and preference. Postoperatively, most operations employing rigid internal fixation can be managed with a nonrigid external orthosis (e.g., a collar, SOMI), but C1-2 dorsal wiring without additional instrumentation generally warrants the use of a halo.26
C2 Fractures
Odontoid Fractures
C2 odontoid fractures can occur from a number of mechanisms but most often are caused by hyperextension injuries. Although lateral cervical spine radiographs may demonstrate some fractures, especially those with displacement, this technique can easily miss fractures, especially those with degenerative changes or minimal displacement. Open-mouth radiographs are very helpful for diagnosing most odontoid fractures, but these also may be inconclusive. Thin-cut CT images with sagittal and coronal view reconstruction views are the best way to diagnose and characterize odontoid fractures as well as to find associated fractures and plan treatment.27,28
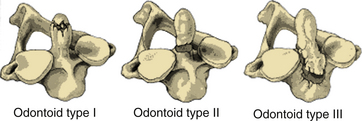
Anderson and D’Alonzo classified odontoid fractures into three types based on the location of the fracture line through the odontoid tip (type I), odontoid base (type II), or C2 body (type III)29 (Fig. 63-6). Type I fractures are essentially avulsion fractures of the odontoid tip and are rare, generally stable, and usually managed with an external semirigid (collar) or rigid (halo) orthosis. Type II fractures are the most common type of odontoid fracture. These fractures are unstable and prone to nonunion because they occur in an area of relatively reduced osseous vascularity. Therefore, rigid halo immobilization or surgical stabilization is often necessary. Hadley et al. described type IIA fractures that are comminuted fractures at the base of the dens with associated free fragments.30 These fractures are considered particularly unstable, and surgical stabilization is advisable, usually with a dorsal C1-2 fusion. Type III fractures involve the vertebral body and are discussed later.
C2 Body Fractures
The C2 body can be defined as the C2 bone mass caudal to the dens and ventral to the pars interarticularis bilaterally. Benzel et al.31 have classified C2 body fractures on the basis of the orientation of the fracture line: coronal, sagittal, or transverse (also known as horizontal rostral). The transverse type of C2 body fracture is a more appropriate description of type III odontoid fractures. The coronal and sagittal types represent “vertical” fractures. Of the vertical fractures, the coronal type was much more common (4:1 ratio) and resulted from multiple (four) different mechanisms. Sagittal type C2 body fractures were caused by axial loading trauma. Figure 63-7 shows an example of a C2 body fracture.
A majority of C2 body fractures can be managed nonoperatively. Depending on the alignment, degree of displacement, and fracture location, either a collar or a halo may be advisable. Occasionally, surgical intervention with a dorsal C1-2 fusion is indicated, particularly for highly unstable fractures and in patients who are prone to nonunion.
Other C2 Fractures
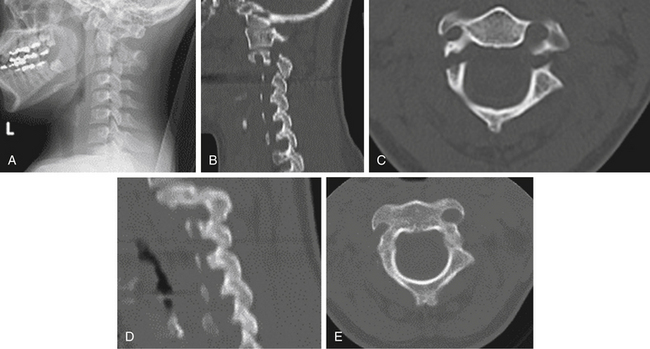
Traumatic spondylolisthesis fractures of the axis (also known as hangman fractures) are characterized by bilateral fractures through the C2 pars/pedicle (Fig. 63-8). Although these fractures may be unstable, they do not generally cause significant compromise of the spinal canal or neurologic injury. Effendi et al.32 have classified these injuries into three groups based on mechanism. Type I fractures are single hairline fractures of the pedicle of axis and occur with axial loading and hyperextension. Type II fractures have displacement of the ventral fragment with an abnormal disc below the axis and are hyperextension injuries with rebound hyperflexion. Type III fractures have displacement of the ventral element with the body of the axis in the flexed position, and the facet joints at C2-3 are dislocated and locked and are primarily flexion injuries with rebound extension. Levine and Edwards33 modified the system by adding a type IIA, which represents flexion-distraction injuries with mild or no displacement but very severe angulation. Type I and II injuries are generally stable and can usually be managed in a collar. With significant displacement (>4–6 mm), halo immobilization may be advisable. Type IIA injuries are more likely to be unstable, especially with displacement greater than 4 to 6 mm or angulation more than 11 degrees. If one or both of these findings are present, surgical stabilization may be necessary. Type III injuries are unstable and typically require surgical stabilization. Isolated C2 laminar or spinous process fractures are stable and therefore are usually managed with an orthosis (e.g., a collar).
Combination Occipitocervical Junction Injuries
Combined C1-2 fractures occur with axial trauma with or without lateral bending. Although plain radiographs may indicate a combined fracture, a CT with multiplanar reconstruction views is usually necessary to fully characterize the fractures and alignment and to plan treatment. Compared with isolated C1 and C2 fractures, combined C1-2 fractures are typically associated with a higher rate of instability, nonunion, and neurologic injury. Treatment of these injuries is based on the degree and location of bony and ligamentous injuries. Because of the instability, rigid external (halo) and/or internal fixation is usually required. Standard surgical procedures (e.g., dorsal C1-2 interspinous fusion) might not be possible because of the extensive fractures. Advances in instrumentation and surgical technique have allowed the development and increased use of newer types of surgical stabilization such as C1-2 transarticular screws or C1-2 segmental fixation.34,35
Surgical Procedures
General Principles
Postoperative Care
Postoperatively, a rigid external orthosis (halo) is used if instrumentation is not used or if concern exists regarding the instrumentation or bone quality. Otherwise, some type of nonrigid orthosis is advisable in most cases. Because standard cervical collars do not immobilize the occipitocervical junction well, special orthoses are often used (e.g., SOMI braces). Currently, there is no consistent medical evidence to support or refute the use of bone stimulator devices.36 Although not officially studied, bone stimulators have been used as a primary adjunct in patients who are prone to nonunion (e.g., smokers) or in an attempt to salvage a nonunion. Patients with spinal cord injury require special attention to nutrition, skin care, pulmonary toilet, deep vein thrombosis prophylaxis, and often psychiatric support. Patients with poor nutrition are prone to wound-healing problems, and sutures may need to be left for a prolonged period (2 to 3 weeks or more).
Occipitocervical Junction Fusions
Structural unicortical strips are harvested from the iliac crest along with cancellous bone. Local autograft from the cranium or dorsal spinal elements is significantly less effective in achieving fusion. Allograft is least likely to achieve fusion and generally should not be relied on. Instrumentation options include inverted U-rods with wiring, inverted-Y-plate/screw constructions, and specialized cranial plate attachments for polyaxial cervical screws.37,38 The midline bone is thickest and allows placement of longer screws with better purchase. The construct should be extended to at least C2 and sometimes lower to achieve optimal fixation. However, advances in instrumentation have made the longer constructs to the lower cervical spine or cervicothoracic junction uncommon unless additional subaxial cervical spine injuries exist. Dorsal C0-1 transarticular screw fixation has recently been described by Grob39 and by Gonzales et al.40 The utility of this procedure is still evolving. The instrumentation options and techniques are discussed further in Chapter 111. Postoperatively, a collar or SOMI brace is used until bony fusion occurs (usually 12 weeks). Halo immobilization is used when the bone quality or fixation is suboptimal.
Dorsal C1-2 Fixation
Instrumentation options include C1-2 wiring alone or with additional screw instrumentation, C1-2 Halifax clamp fixation, C1-2 transarticular screw fixation, and C1-2 segmental screw fixation.34,35 The wiring options include the Brooks, Gallie, and Sonntag interspinous fusion operations.41–47 The relative advantages and disadvantages of the various options are listed in Box 63-2. Postoperatively, a collar or SOMI brace is used until bony fusion occurs (usually 12 weeks). Halo immobilization is used when the bone quality or fixation is suboptimal.
Odontoid Screw Fixation
Ventral odontoid screw fixation is appropriate for many unstable C2 odontoid fractures that require operative fixation. The main advantages of odontoid screw fixation are the preservation of C1-2 mobility and the relatively short and well-tolerated nature of the procedure. However, the procedure is not possible for many patients and fractures because of anatomic limitations. For example, patients with short necks, barrel chests, inability to tolerate cervical extension, insufficient transverse ligaments, oblique fracture lines, and/or significantly comminuted fractures are poor candidates for this procedure. For these patients, a dorsal C1-2 fusion is typically chosen.
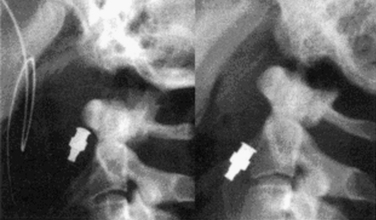
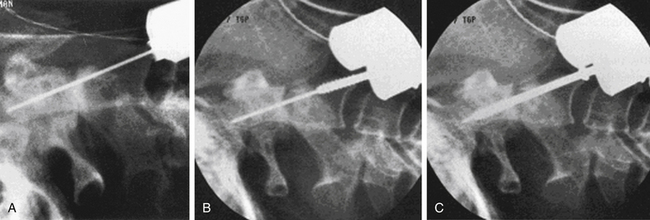
At surgery, the patient is positioned supine with the head extended, usually in a fixed position with a Mayfield head holder. Biplanar fluoroscopy is generally used. Using a lower cervical incision about at C5-6, a standard high ventral cervical approach is followed to the C2-3 region. A variety of standard or specialized retractors can be used to maintain exposure. By using a Kerrison rongeur or high-speed drill, a midline trough is made in the ventral-rostral C3 vertebral body. Next, by using the trough, a power drill with a 2-mm bit is used to drill a pilot hole from the ventral caudal border of C2, across the fracture line, and to the tip of the odontoid process. The appropriate-length screw is determined by preoperative radiograph or CT measurements, intraoperative fluoroscopy, and/or measuring the length of the drill bit. If reduction of the fracture is needed, then a lag screw of an appropriately shorter length should be selected. Even without significant fracture displacement, lag screws can promote fusion by providing a compressive force across the fracture line. The screw is threaded into the pilot hole in the same trajectory. One or two screws may be placed, but one is typically used because the outcomes appear similar.48,49 Several odontoid screw systems exist with specialized instrumentation and screws.50 One system includes cannulated screws that can be placed over a threaded drill bit (Fig. 63-9).51 See Chapter 143 for additional details on the instrumentation.
Patients are managed with a postoperative external orthosis (collar) until the fracture heals (usually 10–12 weeks). Success rates are high, with fusion rates between 81% and 96%.48,49 In the event of a nonunion, a dorsal C1-2 technique can be used.
Ventral C1-2 Fixation
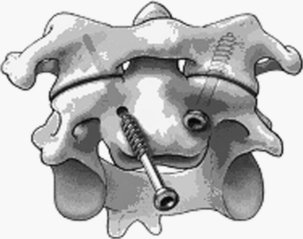
At surgery, the positioning, approach, and exposure are similar to odontoid screw placement. An entry point is marked with a pilot hole at the groove between the C2 body and superior articular facet. This point is just medial to the vertebral artery. The screw trajectory is about 20 degrees lateral and rostral as needed to engage the C1 lateral mass securely. The screws are placed with fluoroscopic guidance (ideally biplanar) (Fig. 63-10). Although placing an onlay bone graft may be possible, the technique does not allow direct placement of bone between C1 and C2 and aims to have fusion occur at the articulation between the C1 and C2 lateral masses. Therefore, the C1-2 facet should be scraped with a small curette as possible to promote arthrodesis. Postoperatively, a collar or SOMI brace is used until bony fusion occurs (usually 12 weeks). Halo immobilization is used when the bone quality or fixation is suboptimal.
Ventral C2-3 Fixation
Ventral C2-3 discectomy and fusion are used for some traumatic C2 hangman fractures that demonstrate sufficient instability to warrant operative fixation.52 Using a high cervical incision, a standard high ventral cervical approach and limited C2-3 discectomy are performed. If there is canal compromise from osteophytes or a herniated disc, then a more extensive dorsal osteophytectomy and/or discectomy is performed. The alignment, if abnormal, is optimized as much as possible. After preparation of the end plates, a C2-3 arthrodesis is performed with structural iliac crest autograft or allograft. Then C2-3 ventral cervical plating is performed (see Chapters 143 and 144). Extra attention is required for the C2 screws because of the unique anatomy. A narrow low-profile plate is preferred to facilitate placement. The patient is managed with a postoperative external orthosis (collar) for 6 or more weeks depending on the degree of preoperative instability.
Summary
Operative procedures generally require rigid intraoperative fixation via a halo ring/adaptor or Mayfield head clamp. Surgical intervention is focused on decompressing significant compressive lesions (e.g., bone, hematoma), restoring alignment, and achieving stabilization with arthrodesis and usually internal fixation. Advances in instrumentation and surgical technique in the past three decades (e.g., image guidance, surgical innovation) have led to the development of better, stronger internal fixation constructs that can spare motion (e.g., odontoid screw fixation), reduce the number of levels to be fused, and avoid or minimize use of uncomfortable orthoses such as halos, which have inherent risks themselves (e.g., pulmonary compromise, skull pin site complications). These instrumentation techniques are discussed further in Chapter 143. Achieving bony fusion is an important goal of stabilization, and careful attention to technique is required. Although many trauma patients are good fusion candidates (young, healthy patients), liberal use of autograft is advised in most cases, because many patients may be or become critically ill and malnourished because of spinal or systemic injuries. Furthermore, nonunions can be difficult to manage and may require more substantial operative intervention. Overall, the treatment of occipitocervical injuries must be individualized on the basis of patient and injury characteristics, the surgeon’s knowledge of the different operative risk factors, complication avoidance and management, instrumentation options, and experience.
Benzel E.C., Hart B.L., Ball P.A., et al. Fractures of the C-2 vertebral body. J Neurosurg. 1994;81:206-212.
Effendi B., Roy D., Cornish B., et al. Fractures of the ring of the axis. A classification based on the analysis of 131 cases. J Bone Joint Surg [Br]. 1981;63:319-327.
Garrett M., Consiglieri G., Kakarla U.K., et al. Occipitoatlantal dislocation. Neurosurgery. 2010;66:A48-A55.
Greene K.A., Dickman C.A., Marciano F.F., et al. Acute axis fractures. Analysis of management and outcome in 340 consecutive cases. Spine (Phila Pa 1976). 1997;22:1843-1852.
Hadley M.N., Browner C.M., Liu S.S., Sonntag V.K. New subtype of acute odontoid fractures (type IIA). Neurosurgery. 1988;22:67-71.
Hadley M.N., Walters B.C., Grabb P.A., et al. Management of acute central cervical spinal cord injuries. Neurosurgery. 2002;50:S166-S172.
Melcher R.P., Puttlitz C.M., Kleinstueck F.S., et al. Biomechanical testing of posterior atlantoaxial fixation techniques. Spine (Phila Pa 1976). 2002;27:2435-2440.
1. Hadley M.N., Walters B.C., Grabb P.A., et al. Guidelines for the management of acute cervical spine and spinal cord injuries. Clin Neurosurg. 2002;49:407-498.
2. Hadley M.N., Walters B.C., Grabb P.A., et al. Management of acute central cervical spinal cord injuries. Neurosurgery. 2002;50:S166-S172.
3. Duane T.M., Scarcella N., Cross J., et al. Do flexion extension plain films facilitate treatment after trauma? Am Surg. 2010;76(12):1351-1354.
4. Alker A.J., Oh Y.S., Leslie E.V. High cervical spine and craniocervical junction injuries in fatal traffic accidents: a radiological study. Orthop Clin North Am. 1978;9:1003-1010.
5. Muhonen M.G., Menezes A.H. Weaver syndrome and instability of the upper cervical spine. J Pediatr. 1990;116:596-599.
6. Bucholz R.W., Burkhead W.F. The pathological anatomy of fatal atlanto-occipital dislocations. J Bone Joint Surg [Am]. 1979;61:248-250.
7. Ringer A.J., Matern E., Parikh S., Levine N.B. Screening for blunt cerebrovascular injury: selection criteria for use of angiography. J Neurosurg. 2010;112:1146-1149.
8. Fleck S.K., Langner S., Baldauf J., et al. Incidence of blunt craniocervical artery injuries: use of whole body CT trauma imaging with adapted CT angiography. Neurosurgery. 2011;69:615-624.
9. Traynelis V.C., Marano G.D., Dunker R.O., Kaufman H.H. Traumatic atlanto-occipital dislocation: case report. J Neurosurg. 1986;65:863-870.
10. Wackenheim A. Roentgen diagnosis of the craniovertebral region. New York: Springer-Verlag; 1974.
11. Powers B., Miller M.D., Kramer R.S., et al. Traumatic anterior atlanto-occipital dislocation. Neurosurgery. 1979;4:12-17.
12. Garrett M., Consiglieri G., Kakarla U.K., et al. Occipitoatlantal dislocation. Neurosurgery. 2010;66:A48-A55.
13. Wholey M.H., Bruwer A.J., Baker H.L. The lateral roentgenogram of the neck (with comments on the atlanto-odontoid-basion relationship). Radiology. 1958;71:350-356.
14. Dickman C.A., Greene K.A., Sonntag V.K.H. Traumatic injuries of the craniovertebral junction. In: Dickman C.A., Spetzler R.A., Sonntag V.K.H., editors. Surgery of the craniovertebral junction. New York: Thieme; 1998:175-196.
15. Dublin A.B., Marks W.M., Weinstock D., Newton T.H. Traumatic dislocation of the atlanto-occipital articulation (AOA) with short-term survival: with a radiographic method of measuring the AOA. J Neurosurg. 1980;52:541-546.
16. Dickman C.A., Mamourian A., Sonntag V.K., Drayer B.P. Magnetic resonance imaging of the transverse atlantal ligament for the evaluation of atlantoaxial instability. J Neurosurg. 1991;75:221-227.
17. Dickman C.A., Greene K.A., Sonntag V.K.H. Injuries involving the transverse atlantal ligament: classification and treatment guidelines based upon experience with 39 injuries. Neurosurgery. 1996;38:44-50.
18. Dvorak J., Hayek J., Zehnder R. CT-functional diagnostics of the rotatory instability of the upper cervical spine. Part 2. An evaluation on healthy adults and patients with suspected instability. Spine (Phila Pa 1976). 1987;12:726-731.
19. Dvorak J., Panjabi M.M., Hayek J. Diagnosis of hyper- and hypomotility of the upper cervical spine using functional computerized tomography. Orthopade. 1987;16:13-19.
20. Anderson P.A., Montesano P.X. Morphology and treatment of occipital condyle fractures. Spine (Phila Pa 1976). 1988;13:731-736.
21. Leone A., Cerase A., Colosimo C., et al. Occipital condylar fractures: a review. Radiology. 2000;216:635-644.
22. Mody B.S., Morris E.W. Fracture of the occipital condyle: case report and review of the world literature. Injury. 1992;23:350-352.
23. Maserati M.B., Stephens B., Zohny Z., et al. Occipital condyle fractures: clinical decision rule and surgical management. J Neurosurg Spine. 2009;11(4):388-395.
24. Hadley M.N., Dickman C.A., Browner C.M., Sonntag V.K. Acute traumatic atlas fractures: management and long-term outcome. Neurosurgery. 1988;23:31-35.
25. Spence K.F.Jr., Decker S., Sell K.W. Bursting atlantal fracture associated with rupture of the transverse ligament. J Bone Joint Surg [Am]. 1970;52:543-549.
26. Sonntag V.K., Hadley M.N., Dickman C.A., Browner C.M. Atlas fractures: treatment and long-term results. Acta Neurochir Suppl (Wien). 1988;43:63-68.
27. Greene K.A., Dickman C.A., Marciano F.F., et al. Acute axis fractures. Analysis of management and outcome in 340 consecutive cases. Spine (Phila Pa 1976). 1997;22:1843-1852.
28. Hadley M.N., Dickman C.A., Browner C.M., Sonntag V.K. Acute axis fractures: a review of 229 cases. J Neurosurg. 1989;71:642-647.
29. Anderson L.D., D’Alonzo R.T. Fractures of the odontoid process of the axis. J Bone Joint Surg [Am]. 1974;56:1663-1674.
30. Hadley M.N., Browner C.M., Liu S.S., Sonntag V.K. New subtype of acute odontoid fractures (type IIA). Neurosurgery. 1988;22:67-71.
31. Benzel E.C., Hart B.L., Ball P.A., et al. Fractures of the C-2 vertebral body. J Neurosurg. 1994;81:206-212.
32. Effendi B., Roy D., Cornish B., et al. Fractures of the ring of the axis. A classification based on the analysis of 131 cases. J Bone Joint Surg [Br]. 1981;63:319-327.
33. Levine A.M., Edwards C.C. The management of traumatic spondylolisthesis of the axis. J Bone Joint Surg [Am]. 1985;67:217-226.
34. Harms J., Melcher R.P. Posterior C1-C2 fusion with polyaxial screw and rod fixation. Spine (Phila Pa 1976). 2001;26:2467-2471.
35. Resnick D.K., Benzel E.C. C1–C2 pedicle screw fixation with rigid cantilever beam construct: case report and technical note. Neurosurgery. 2002;50:426-428.
36. Resnick D.K., Choudhri T.F., Dailey A.T., et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 17: bone growth stimulators and lumbar fusion. J Neurosurg Spine. 2005;2:737-740.
37. Apostolides P.J., Dickman C.A., Golfinos J.G., et al. Threaded Steinmann pin fusion of the craniovertebral junction. Spine (Phila Pa 1976). 1996;21:1630-1637.
38. Hurlbert R.J., Crawford N.R., Choi W.G., Dickman C.A. A biomechanical evaluation of occipitocervical instrumentation: screw compared with wire fixation. J Neurosurg. 1999;90:S84-S90.
39. Grob D. Transarticular screw fixation for atlanto-occipital dislocation. Spine (Phila Pa 1976). 2001;26:703-707.
40. Gonzalez L.F., Crawford N.R., Chamberlain R.H., et al. Craniovertebral junction fixation with transarticular screws: biomechanical analysis of a novel technique. J Neurosurg. 2003;98:202-209.
41. Brooks A.L., Jenkins E.B. Atlanto-axial arthrodesis by the wedge compression method. J Bone Joint Surg [Am]. 1978;60:279-284.
42. Dickman C.A., Crawford N.R., Paramore C.G. Biomechanical characteristics of C1-2 cable fixations. J Neurosurg. 1996;85:316-322.
43. Dickman C.A., Sonntag V.K., Papadopoulos S.M., Hadley M.N. The interspinous method of posterior atlantoaxial arthrodesis. J Neurosurg. 1991;74:190-198.
44. Gallie W.E. Skeletal traction in treatment of fractures and dislocations of the cervical spine. Ann Surg. 1937;106:770-776.
45. Gallie W.E. Fractures and dislocations of the cervical spine. Am J Surg. 1939;46:495-499.
46. Melcher R.P., Puttlitz C.M., Kleinstueck F.S., et al. Biomechanical testing of posterior atlantoaxial fixation techniques. Spine (Phila Pa 1976). 2002;27:2435-2440.
47. Naderi S., Crawford N.R., Song G.S., et al. Biomechanical comparison of C1-C2 posterior fixations. Cable, graft, and screw combinations. Spine (Phila Pa 1976). 1998;23:1946-1955.
48. Jenkins J.D., Coric D., Branch C.L.Jr. A clinical comparison of one- and two-screw odontoid fixation. J Neurosurg. 1998;89:366-370.
49. Subach B.R., Morone M.A., Haid R.W.Jr., et al. Management of acute odontoid fractures with single-screw anterior fixation. Neurosurgery. 1999;45:812-819.
50. Apfelbaum R.I., Lonser R.R., Veres R., Casey A. Direct anterior screw fixation for recent and remote odontoid fractures. J Neurosurg. 2000;93(Suppl 2):227-236.
51. Dickman C.A., Foley K.T., Sonntag V.K., Smith M.M. Cannulated screws for odontoid screw fixation and atlantoaxial transarticular screw fixation. Technical note. J Neurosurg. 1995;83:1095-1100.
52. Francis W.R., Fielding J.W., Hawkins R.J., et al. Traumatic spondylolisthesis of the axis. J Bone Joint Surg [Br]. 1981;63:313-318.