Chapter 67 Trauma Surgery
Lumbar and Sacral Fractures
The upper lumbar spine (L1-2) should be considered as part of the thoracolumbar junction (T11-L2). The thoracolumbar junction is unique in that it transitions from a relatively immobile thoracic spine to the more mobile lumbar spine. Biomechanically, this difference results in a region of high stress at the interface between these two segments. The majority of the thoracic spine is resistant to rotational forces because of the stabilizing effect of the rib cage.1 However, the thoracolumbar spine lacks attachments to the rib cage and has a transitional facet structure that is unable to resist rotational forces. As a result, 60% of all spinal fractures occur between T12 and L2 and 90% occur between T11 and L4.2,3
Fractures of the mid to caudal lumbar spine (L3-5) account for approximately 4% of all spinal fractures. The transition into the lumbar spine results in larger vertebral bodies, designed to sustain greater axial loads, and significantly more muscular attachments, adding to its stability.4,5 However, the facets in the upper lumbar spine are more oblique in orientation, transitioning to a sagittal orientation at the lumbosacral junction. This results in more translational mobility.6 The change in facet orientation, the increased mobility, and the lack of a thoracic cage actually make the lumbar spine more susceptible to injury than the thoracic spine.
The sacrum with its intimate attachments to the pelvis is a very stable structure.7 The relatively immobile sacroiliac joint and strong ventral and dorsal ligamentous attachments between the sacrum and pelvis account for this stability. The sacrum forms a portion of the dorsal pelvic arch and, as a result, most sacral fractures occur in conjunction with pelvic fractures. The sacrum is critical to pelvic ring stability. Removal of the sacrum distal to the S1-2 interspace weakens the pelvic ring by 30%, whereas resection up to S1, which requires removal of half of the sacroiliac joint, weakens the pelvic ring by 50%.8
Spine Stability
A discussion of spine fractures necessitates a definition of spine stability since the goal of any clinically applicable characterization of fracture pattern and subsequent treatment paradigm relies on the concept of restoring the spine to its preinjury functional capacity. White and Panjabi define spinal stability as the ability of the spine to maintain physiologic loads without pain, deformity, or neurologic deficit.9 Conversely, Benzel describes instability as the inability to limit excessive or abnormal spinal displacement.10 These terms are somewhat nebulous, and we agree with the concept that spinal stability should be considered as part of a dynamic process that takes into account multiple parameters, such as actual segment loading, activity level, and chronicity. A detailed discussion of spinal stability is outside the scope of this chapter, but the spine surgeon must have a general concept of acute and chronic instability. With trauma, the initial decision of whether to operate depends in part on whether the fracture is acutely unstable. As described later in this chapter, multiple classification systems based on radiographic and clinical examination help to determine if the injury is acutely unstable. However, the potential of chronic instability should not be ignored. Recognizing injury patterns that lead to chronic instability and that pose a risk of posttraumatic deformity is essential to the long-term care of trauma patients.
Mechanism of Injuries
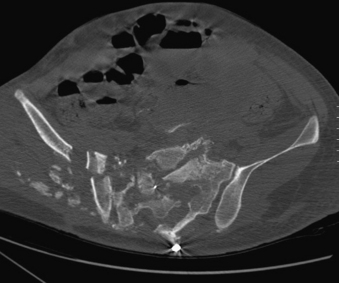
In general, the mechanism of injury to the lumbosacral spine is blunt, although penetrating trauma does occur, especially in a military or combat situation. For penetrating injuries, the key component to the extent and pattern of injury is the amount of kinetic energy (KE) released at the time of impact. The formula KE = 1/2 mv2, where m is mass and v is velocity, explains that the higher the velocity of the projectile, the more kinetic energy it disperses on impact, and, hence, the greater the resulting damage. Most spine injury classifications and subsequent treatment paradigms are based on blunt trauma, with penetrating trauma patterns imposed within that system. Like any injury, the mechanism should be considered when determining a treatment plan, with the understanding that penetrating injuries of the spine are different from their blunt counterparts; this difference is primarily determined by the kinetic energy imparted by the projectile (Fig. 67-1).
Prehospital Management
All patients involved in major trauma should be treated as having a potential spinal injury until proven otherwise. This treatment includes multiperson patient transfers, log rolling, and the use of a cervical orthosis and a backboard. Every attempt should be made to maintain the spinal column in as near neutral alignment as possible. Initial management in the field should follow an advanced life support protocol. A stable airway, adequate ventilation, and hemodynamic stability should be maintained. Preventing hypoxia and hypotension is critical to preserving neural element function after trauma. Once a patient is stabilized, a neurologic examination should be performed, keeping in mind that concomitant head injury, intoxication, or injury to the extremities may make a thorough examination difficult or impossible.11 A “normal” neurologic examination in the field does not rule out a spinal injury.
Initial Hospital Management
Because the thoracolumbar spine is relatively resistant to injury, the amount of force required to result in a fracture makes these traumas high risk for associated neurologic and retroperitoneal injury. It has been reported that 4.4% of all patients arriving at a level I trauma center have a fracture in the thoracolumbar spine and approximately 19% to 50% have a neurologic deficit.12–15 Even with modern spinal immobilization techniques, the concern still exists for an unstable fracture that could result in a new neurologic injury or neurologic deterioration. In a study by Reid et al., fractures of the thoracolumbar spine that were diagnosed in a delayed fashion had a higher incidence of a new neurologic deficit than those diagnosed at the time of admission (10.5% vs. 1.4%).16 Unfortunately, delay in the diagnosis of thoracolumbar injuries is not uncommon. In a retrospective review, Dai et al. found that 28 of 147 patients with acute thoracolumbar injuries had a thoracolumbar fracture that was not diagnosed at the time of admission. Although some diagnoses were delayed secondary to resuscitation efforts or acute surgical intervention, 37% of these patients (7/19) with fractures did not undergo initial radiographic evaluation because of a lack of clinical suspicion and another 26% (5/19) had radiographs but their fractures were either misclassified or not diagnosed at all.17 Because of the potential for neurologic injury from a missed injury, some authors have suggested that the thoracolumbar spine should be imaged in all patients with polytrauma.16,18
Certain injury mechanisms should raise the suspicion of associated spinal fractures. Patients jumping or falling from heights with significant lower-extremity injury, calcaneal injuries in particular, are at high risk for accompanying lumbar and thoracolumbar spine injuries. Patients involved in motor vehicle accidents and wearing lap belts only are at risk for flexion distraction injuries. Lumbar fractures have been associated with abdominal and urologic trauma, particularly in cases of lap belt injuries.19 Sacral fractures are often associated with injury to the pelvic ring.
A neurologic examination should be carried out in a systematic and standardized fashion. Results should be communicated using the American Spinal Injury Association (ASIA) grading system (described elsewhere in this book). This facilitates communication between treating specialties. Progressive neurologic deterioration is a widely accepted indication for acute intervention. It is, therefore, imperative that baseline neurologic function be accurately established. In the confused or obtunded patient, facial grimace or withdrawal to painful stimuli can serve as a gross motor and sensory examination. A rectal examination should be performed to assess perianal sensation, rectal tone, and a bulbocavernosus reflex. Radiographic studies of other areas of injury should be evaluated closely for patterns that may indicate a direction of force through the thoracolumbar spine.
The pattern of neurologic injury observed depends on the location of spinal injury. In the majority of patients, the conus medullaris lies directly opposite the L1 vertebral body. The conus medullaris contains the anterior horn cells of the L5 through S5 nerves. Both upper motor neuron (conus injury) and lower motor neuron (cauda equina) injury can occur. The injury pattern runs the spectrum from a complete injury below L1 to incomplete syndromes resulting in partial motor and sensory preservation with sacral dysfunction. The sacral nerve roots are the most sensitive to injury and the least likely to improve after injury. Injury to the sacral spinal cord and nerve roots can result in loss of bowel and bladder function; sexual function is often less severely affected. Injuries from L2-5 can result in isolated root injuries or cauda equina syndrome. Root injuries can appear as monoradiculopathy or polyradiculopathy. Cauda equina syndrome appears as variable sensory, motor, bowel, and/or bladder dysfunction. Sensory and motor loss tend to be asymmetrical. This tendency is in contradistinction to the conus medullaris syndrome, in which deficits are usually more symmetrical.20 Sphincteric dysfunction is common and is often permanent.
Radiographic Evaluation
In compliant patients with unaltered metal status, no distracting injuries, and lack of midline spinal tenderness, the spine can be cleared without obtaining screening plain radiographs.21 However, if the patient does not meet any of the preceding criteria, radiographic evaluation is mandatory. One should bear in mind that between 5% and 20% of all spine fractures are multiple, and 5% occur at noncontiguous levels.22 Commonly, radiographic evaluation includes anteroposterior (AP) and lateral views of the cervical, thoracic, lumbar, and sacral spine and an open-mouth odontoid view. Flexion-extension studies are generally avoided in the workup of acute fractures. Spinal alignment should be assessed in both planes. The margins of the vertebral bodies and the spinolaminar line, facets, interspinous and interpedicular distances, and position of the transverse process should be studied. Acute kyphotic angulation or loss of lordosis may be indicative of an acute bony or ligamentous injury. Loss of disc height at the level above a vertebral body fracture is often observed in acute flexion injuries, but it may also be seen in cases of degenerative disc disease. Bare, or “naked,” facets may be indicative of posterior ligamentous injury as a result of a distraction-type injury. Abnormalities of the soft tissues, such as a paraspinal mass or loss of the psoas stripe, can help identify areas of adjacent bony injury.
Per advanced trauma life support (ATLS) guidelines, all patients sustaining high-energy trauma should have an AP radiograph of the pelvis. However, these images are often inadequate for evaluating the sacrum due to sacral inclination, bowel gas, and, sometimes, the overlying anterior pelvis.23 Nork et al. identified L5 transverse process fractures and a paradoxic inlet view on AP pelvic radiographs as factors suggestive of sacral fractures.24 Others have identified the foraminal stepladder sign, caused by a displaced and overriding transverse fracture, and disruption of the anterior sacral foraminal lines or sacral arcuate line as diagnostic clues.25–27 Sacral fracture should be suspected in any patient with a pelvic ring injury associated with a neurologic deficit.28–30
Computed Tomography
In many institutions, AP and lateral radiographs are the standard screening tools for evaluating thoracolumbar fractures. However, plain radiographs have been criticized for their lack of sensitivity, diagnostic inaccuracies, and for the amount of time required for adequate views. Many have advocated the use of CT as the primary means of evaluating the thoracolumbar spine. Multiple studies have shown that CT scans are more sensitive for detecting fractures in the spine than plain radiographs.31–34 However, CT carries with it concerns about cost and radiation exposure. In 2004, Brandt et al. found that for 50 patients undergoing radiographic evaluation, the average time for a CT of the chest/abdomen/pelvis was 55 minutes ± 32 minutes with a cost of $654. For thoracic, lumbar, and sacral plain radiographs, the average time was 113 minutes ± 43 minutes. The cost of the radiographic evaluation of the spine in addition to dedicated CT scanning of the spine and viscera was $1487.35 Wintermark et al. found that an average of 4.3 views were needed to adequately evaluate the thoracolumbar spine; 9% of the thoracolumbar films had to be retaken because of insufficient quality. Time needed to perform conventional radiographs was 33 minutes, with 70% (23/33 minutes) devoted to imaging the thoracolumbar spine. This compares with the median time to perform a cervical, thoracic, abdominal, and cranial CT of 40 minutes, including 7 minutes for reformatting and reconstructions of the films.36 There is no level I evidence regarding the use of CT as a standard for diagnosing spine fractures, but a single series of CT scans that can be reformatted specifically to evaluate the spine has proven to be at least equal to if not superior to, plain radiographs in several studies.37
Obtaining appropriate radiographs of sacral injuries is problematic. In one series, 49% of sacral fractures were missed on initial hospital presentation, including 24% with an “unexplained” neurologic deficit that was later explained by the presence of a fracture.38 The sacrum is poorly visualized on standard AP views of the pelvis, so the treating physician must rely on other cues to prompt a more detailed survey of this region. CT with coronal and axial reformations is the most sensitive modality for defining complex pelvic and sacral fractures. For all radiographic measurement parameters, the Spine Trauma Study Group advocates the use of thin-section (1.0–1.5 mm) axial CT scans with coronal and sagittal reconstruction rather than plain radiographs.39
Magnetic Resonance Imaging
MRI can be useful in the study of neurologic deficit to determine the extent of compression on the neural elements. Although current MRI techniques are in many ways more sensitive than CT in detecting areas of acute injury, they do not provide the bony detail provided by conventional CT. Increased signal on T2-weighted imaging may be used to detect fractures that are not well visualized on plain radiographs or CT. Short tau inversion recovery (STIR) sequences are quite sensitive for the detection of ligamentous and intervertebral disc injury not otherwise apparent by plain radiographs or CT.
Classification of Injuries
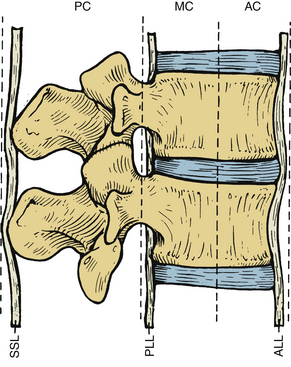
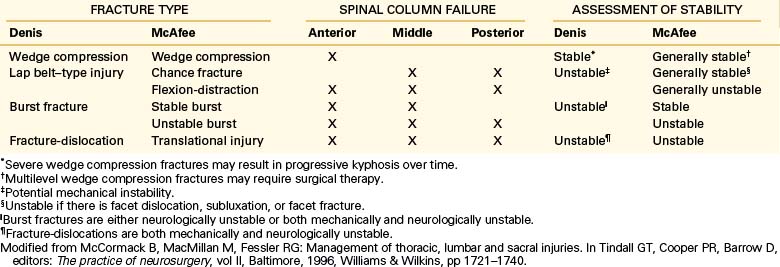
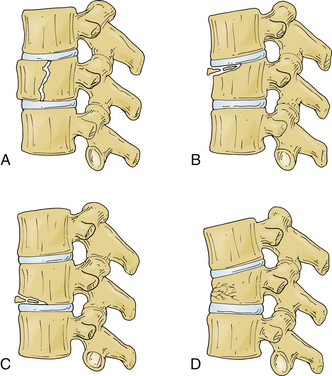
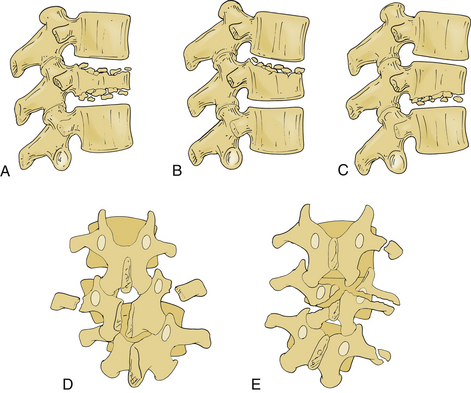
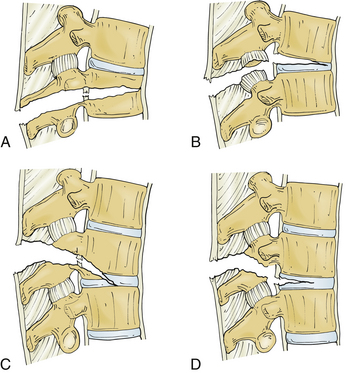
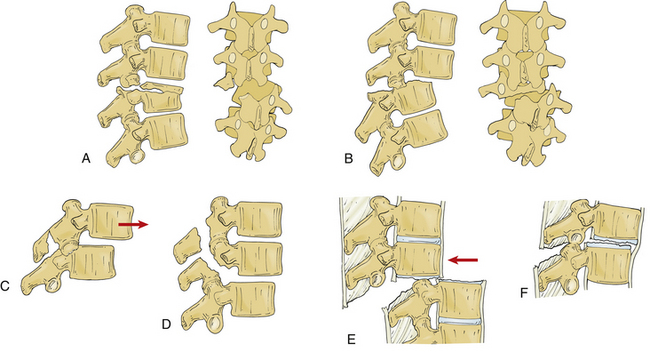
Several classification systems have been developed to describe fractures of the thoracic and lumbar spine. In 1983, both Denis and McAfee et al. independently published three-column models that have become widely accepted (Fig. 67-2).2,40 These models are very similar but do have some fundamental differences (Table 67-1).41 Each model divides the vertebra into three columns: anterior column (anterior longitudinal ligament, ventral half of the vertebral body, and ventral half of the anulus fibrosus), middle column (posterior longitudinal ligament, dorsal half of the vertebral body, and dorsal half of the anulus fibrosus), and posterior column (supraspinous and infraspinous ligaments, ligamentum flavum, articular processes, joint capsules, spinous processes, and laminae). In Denis’s model, instability requires injury to at least two columns with an emphasis on preservation of the middle column for the maintenance of stability. Fractures are divided into four groups: wedge compression (Fig. 67-3), burst fracture (Fig. 67-4), lap belt–type injury (Fig. 67-5), and fracture-dislocation (Fig. 67-6). Instability varies in magnitude: first degree (mechanical), second degree (neurologic), and third degree (mechanical and neurologic). McAfee’s system places more emphasis on preservation of the posterior column for the maintenance of stability and defines six fracture patterns: wedge compression, Chance fracture, flexion-distraction injury, stable burst, unstable burst, and translational injury.
In 1994, Magerl et al. proposed the Arbeitsgemeinschaft für Osteosynthesefragen (AO) system, based on the review of 1445 consecutively treated thoracolumbar injuries. There are three main types of fractures (types A, B, C), with a progression in the severity of injury from type A to type C. Each category contains subclassifications. Type A injuries are compressive forces acting on the vertebral body. Type B injuries are characterized by a distraction mechanism with disruption of the anterior and posterior elements. Type C lesions are rotational injuries involving the anterior and posterior elements.42 Although more accurately descriptive than the three-column classification, the AO system is often viewed as cumbersome to apply in a clinical setting.
In 2005, the Spine Trauma Study Group devised a thoracolumbar injury classification and severity score scale.43 This system relies on three characteristics to describe the injury: injury morphology, the integrity of the posterior ligamentous complex, and neurologic status (Table 67-2) Points are assigned to the various subcategories. The total score among the three categories determines the injury severity score. Scores of 5 or higher suggest the need for operative treatment due to the unstable nature of the injury, and scores of 3 or lower suggest nonoperative management. A score of 4 may be treated either way. If multiple fractures are present, the injury with the greatest score dictates the treatment.
TABLE 67-2 Thoracolumbar Injury Classification and Severity Score Scale
| Category | Points |
|---|---|
| Injury Morphology | |
| Compression | 1 |
| Burst | 1+ |
| Translation/rotational | 3 |
| Distraction | 4 |
| Neurologic Status | |
| Intact | 0 |
| Nerve root | 2 |
| Cord/conus medullaris | |
| Incomplete | 3 |
| Complete | 2 |
| Cauda equina | 3 |
| Posterior Ligamentous Complex | |
| Intact | 0 |
| Injury suspected/indeterminate | 2 |
| Injured | 3 |
A score <3 is generally considered a stable fracture, and a score ≥5 is considered an unstable fracture and typically requires surgical stabilization.
Treatment of Lumbar Spine Fractures
Nonsurgical treatment, usually consisting of a period of bedrest followed by bracing, in patients who are neurologically intact has a long track record, even in the face of a fracture that by the classification schemes is dubbed “unstable.”44 Depending on the type of fracture, the period of bedrest may be as little as 2 weeks or as long as 4 to 6 weeks, depending on the surgeon’s comfort with the fracture pattern. In the acute setting, the use of a specialty bed, specifically a rotating bed, has been found to maintain stability of the spine and decrease pressure on the skin to avoid decubitus ulcers. Although this modality inevitably increases the length of hospitalization, it avoids the risk and costs of surgery. This course of treatment must be accompanied by aggressive pulmonary toilet and mechanical prophylaxis and chemoprophylaxis to prevent deep vein thrombosis.
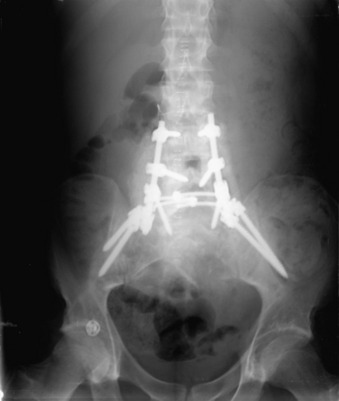
Although the literature has established very few standards for treating these patients, a few thoughts are worth discussing. In general, stopping a construct at a transition from a mobile to an immobile section of the spine may result in a junctional kyphosis. In the lumbosacral spine, this means that when a multilevel lumbar construct is undertaken, crossing the thoracic junction should be considered if the construct would end at L1. Long thoracolumbar constructs or sacral fractures that result in lumbar-sacral disassociation often require fixation to the pelvis in the form of iliac screws (Fig. 67-7). Whenever the treatment is this extensive, care should be given to restore normal anatomic alignment so that appropriate sagittal balance is maintained.
The next question with surgical intervention becomes a matter of timing: early or delayed. In a retrospective review, Schlegel et al. found that patients treated after 72 hours were at 4.3 times higher risk of being admitted to the ICU, 2.8 times higher risk of being on a ventilator, 12.2 times higher risk for development of pulmonary complications, 4.8 times more likely to develop bed sores, and 3.2 times more likely to develop urinary tract infections than those who had surgery within 48 hours of injury.45 A concern about performing early surgery is an increased risk of complications. To examine this problem, McLain and Benson reported on 75 patients treated with spinal instrumentation for fractures and found that surgery within 24 hours was as safe as treatment for those provided care between 24 and 72 hours after injury.46 When specifically looking at the thoracolumbar spine, Chipman et al. found that early surgery (<72 hours) in patients with higher injury severity scores (≥15) was associated with shorter periods in the ICU, shorter hospital stays, fewer ventilator days, and fewer complications.47 A study by McHenry et al. reviewing 1032 spine fractures echoes these results. They found that surgery performed within 2 days of injury, one of the only variables of the injured patient that the surgeon can control, may decrease the risk of developing respiratory failure.48 However, not all the studies on early surgery show an improved outcome. Kerwin et al. compared 361 patients that underwent surgical fixation of their spinal fracture within 48 hours with patients who underwent surgery after 48 hours. The only differences found between the two groups was a statistically significant higher mortality rate in the early-surgery group coupled with a shorter length of hospitalization, which the authors suggest may be related to the higher early mortality.49 In general, each case should be evaluated individually. Although early surgery may be beneficial in a neurologically incomplete injury, the overall status of the patient must be taken into account in order to maximize postoperative function.
Outcomes
The ultimate goal of treatment is the restoration of function to a preinjury baseline. To determine the current status of treatment strategies, McLain reported on 70 patients treated with spinal instrumentation for thoracic, lumbar, and thoracolumbar fractures.50 At 5-year follow-up, he found that 70% of patients were employed at 56% of their previous level of employment. The most significant correlation with return to work status was the degree of neurologic injury. Seventy percent of intact patients returned to full-time employment, whereas only 23% of those with neurologic deficits did. Twelve and one half percent of the intact patients were considered disabled or unemployed, whereas 63% of those with neurologic deficits were considered disabled or unemployed. Although arguments can be made for various treatment modalities and their timing, the preservation and/or recovery of neurologic function should be of the highest priority.
Sacral Fractures
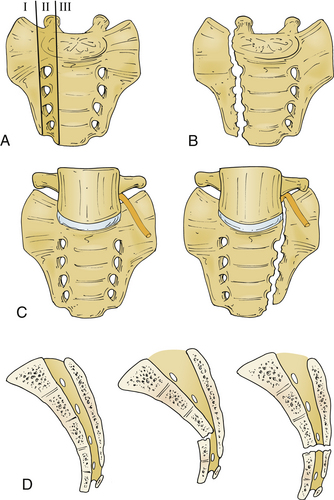
Multiple different classification systems for sacral fractures have been proposed. However, the Spine Trauma Study Group advocates for the use of the Denis system and its modifications.39,51,52 In 1988, Denis et al. published his classification of sacral fractures based on a retrospective review of 236 patients with sacral fractures.28 This system divides the sacrum into three anatomic regions: alar region (zone 1), foraminal region (zone II), and region of the central sacral canal (zone III) (Fig. 67-8).
Management of Sacral Fractures
The management of sacral fractures remains controversial. Complicating this issue is the fact that 80% of patients with neurologic injury at the time of presentation may recover regardless of whether operative reduction and internal fixation/neural decompression is performed.29,39,52–54 Cadaveric studies of sacral and pelvic fractures have demonstrated high rates of nerve root avulsion, suggesting that neurologic injuries (other than a cauda equina syndrome) that do not resolve may frequently be root avulsion, for which decompression is not useful.55
Stability of sacral fractures is generally defined as greater than 1 cm of displacement.39,56 However, translation, kyphosis, and comminution can affect treatment decisions, operative approaches, and clinical outcomes. Treatment strategies that involve open reduction and internal fixation appear to produce better results than closed reduction and percutaneous iliosacral screw fixation.57 Prior to treating a sacral fracture, stability of the pelvic ring should be assessed since these fractures have a high rate of association with pelvic fractures. Typically, these injuries will require treatment separate from or in conjunction with the sacral fracture, usually with an orthopaedic traumatologist.
Most sacral fractures can be treated with bedrest and pelvic immobilization. In the acute setting, application of an external fixator may help to tamponade life-threatening bleeding. However, this may not be enough to stabilize the pelvis in the long term. Treatment for stable, minimally displaced fractures revolves around nonsurgical management with early ambulation and weight bearing as tolerated. Reduction of an alar or zone I fracture may be necessary to decompress an L5 nerve root injury.58 In some cases, stabilization of the pelvis along with bedrest will suffice, particularly in cases where the dorsal ligamentous complex of the pelvis is intact. Treatment for unstable zone I fractures involves open reduction and stabilization of the pelvis. Zone II injuries causing radiculopathy are best treated by bedrest. Residual symptoms despite conservative therapy can be treated by sacral laminectomy and foraminotomy as needed. Weakness from S1 nerve root entrapment may require early decompression. Transverse zone III fractures are usually not associated with pelvic instability. High transverse fractures are the exception and may require instrumentation and surgical decompression of the neural elements. Transverse fractures may also lead to compression of the cauda equina. Early decompression is advocated in an attempt to restore bowel, bladder, and sexual function.7 However, early management of sacral fractures is often complicated by significant comorbidity. Damage to the internal iliac vessels and presacral venous plexus can be associated with significant hemorrhage and is a contraindication to anterior approaches to the sacrum.
Denis F. The three-column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976). 1983;8:817-831.
Denis F., Davis S., Comfort T. Sacral fractures: an important problem retrospective analysis of 236 cases. Clin Orthop Relat Res. 1988;227:67-81.
Sheridan R., Peralta R., Rhea J., et al. Reformatted visceral protocol helical computed tomographic scanning allows conventional radiographs of the thoracic and lumbar spine to be eliminated in the evaluation of blunt trauma patients. J Trauma. 2003;55:665-669.
Vaccaro A.R., Kim D.H., Brodke D.S., et al. Diagnosis and management of sacral spine fractures. Instr Course Lect. 2004;53:375-385.
Vaccaro A.R., Lehman R.A.Jr., Hurlbert R.J., et al. A new classification of thoracolumbar injuries: the importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine (Phila Pa 1976). 2005;30:2325-2333.
White A.A., Panjabi M.M. Clinical biomechanics of the spine, ed 3, Philadelphia: Lippincott, 1990.
1. Andriacchi T., Schultz A., Belytschko T., Galante J. A model for studies of mechanical interactions between the human spine and rib cage. J Biomech. 1974;7:497-507.
2. McAfee P.C., Yuan H.A., Fredrickson B.E., Lubicky J.P. The value of computed tomography in thoracolumbar fractures: an analysis of one hundred consecutive cases and a new classification. J Bone Joint Surg [Am]. 1983;65:461-473.
3. Post M.J.D. Radiographic evaluation of the spine: current advances with emphasis on computed tomography. New York: Masson; 1980.
4. Levine A.M. The surgical treatment of low lumbar fractures. Semin Spine Surg. 1990;2:41-53.
5. Levine A.M., Edwards C.C. Low lumbar burst fractures: reduction and stabilization using the modular spine fixation system. Orthopedics. 1988;11:1427-1432.
6. Kaye J.J., Nance E.P. Thoracic and lumbar spine trauma. Radiol Clin North Am. 1990;28:361-377.
7. Perin N.I., Stanley M.I. Sacral fractures. In Youmans J.R., editor: Neurological surgery, ed 4, Philadelphia: WB Saunders, 1996.
8. Gunterberg B. Effects of major resection of the sacrum: clinical studies on urogenital and anorectal function and a biomechanical study on pelvic strength. Acta Orthop Scand. 1976;162(Suppl):1-38.
9. White A.A., Panjabi M.M. Clinical biomechanics of the spine, ed 2, Philadelphia: Lippincott, 1990.
10. Benzel E.C. Stability and instability of the spine: biomechanics of spine stabilization. New York: Thieme; 2001.
11. Bohlman H. The neck. In: D’Ambrosia R.D., editor. Musculoskeletal disorders, regional examination differential diagnosis. Philadelphia: JB Lippincott, 1985.
12. Cooper C., Dunham D.C., Rodrigues A. Falls and major injuries are risk factors for thoracolumbar spine injuries: cognitive impairment and multiple injuries impede the detection of back pain and tenderness. J Trauma. 1995;38:692-695.
13. Sheridan R., Peralta R., Rhea J., et al. Reformatted visceral protocol helical computed tomographic scanning allows conventional radiographs of the thoracic and lumbar spine to be eliminated in the evaluation of blunt trauma patients. J Trauma. 2003;55:665-669.
14. Brandser E.A., El-Khoury G.Y. Thoracic and lumbar spine trauma. Radiol Clin North Am. 1997;35:533-537.
15. Saboe L.A., Reid D.C., Davis L.A., et al. Spine trauma and associated injuries. J Trauma. 1991;31:43-48.
16. Reid D.C., Henderson R., Saboe L., Miller J.D. Etiology and clinical course of missed spine fractures. J Trauma Inj Infect Crit Care. 1987;27:980-986.
17. Dai L.Y., Yao W.F., Cui Y.M., Zhou Q. Thoracolumbar fractures in patients with multiple injuries: diagnosis and treatment: a review of 147 cases. J Trauma Inj Infect Crit Care. 2004;56:348-355.
18. Born C.T., Ross S.E., Iannacone W.M., et al. Delayed identification of skeletal injury in multisystem trauma: the “missed” fracture. J Trauma. 1989;29:1643-1646.
19. Kauffler S., Hayes J.T. Lumbar fracture dislocations: a study of twenty-one cases. J Bone Joint Surg [Am]. 1996;48:788-795.
20. Portenoy R.K., Lipton R.B., Foley K.M. Back pain in the cancer patient: an algorithm for evaluation and management. Neurology. 1987;37:134-138.
21. Radiographic assessment of the cervical spine in asymptomatic trauma patients. Neurosurgery. 2002;50:S30-S35.
22. Calenoff L., Chessare J.W., Rogers L.F., et al. Multiple level spinal injuries: importance of early recognition. AJR Am J Roentgenol. 1978;130:665-669.
23. Laasonen E.M. Missed sacral fractures. Ann Clin Res. 1977;9:84-87.
24. Nork S.E., Jones C.B., Harding S.P., et al. Percutaneous stabilization of U-shaped sacral fractures using iliosacral screws: technique and early results. J Orthop Trauma. 2001;15:238-246.
25. Ebraheim N.A., Biyani A., Salpietro B. Zone III fractures of the sacrum: a case report. Spine (Phila Pa 1976). 1996;21:2390-2396.
26. Northrop C.H., Eto R.T., Loop J.W. Vertical fracture of the sacral ala: significance of non-continuity of the anterior superior sacral foraminal line. AJR Am J Roentgenol. 1975;124:102-106.
27. Jackson H., Burke J.T. The sacral foramina. Skeletal Radiol. 1984;227:282-288.
28. Denis F., Davis S., Comfort T. Sacral fractures: an important problem retrospective analysis of 236 cases. Clin Orthop Relat Res. 1988;227:67-81.
29. Gibbons K.J., Solonuik D.S., Razack N. Neurological injury and patterns of sacral fractures. J Neurosurg. 1990;72:889-893.
30. Slatis P., Huittinen V.M. Double vertical fractures of the pelvis: a report of 163 cases. Acta Chir Scand. 1972;138:799-807.
31. Gestring M.L., Gracias V.H., Feliciano M.A., et al. Evaluation of the lower spine after blunt trauma using abdominal computed tomographic scanning supplemented with lateral scanograms. J Trauma Inj Infect Crit Care. 2002;53:9-14.
32. Brown C.V., Antevil J.L., Sise M.J., Sack D.I. Spiral computed tomography for the diagnosis of cervical, thoracic, and lumbar spine fractures: its time has come. J Trauma Inj Infect Crit Care. 2005;58:890-895.
33. Buduhan G., McRitchie D.I. Missed injuries in patients with multiple trauma. J Trauma. 2000;49:600-605.
34. Hauser C.J., Visvikis G., Hinrichs C., et al. Prospective validation of computed tomographic screening of the thoracolumbar spine in trauma. J Trauma Inj Infect Crit Care. 2003;55:228-234.
35. Brandt M.M., Wahl W.L., Yeom K., et al. Computed tomographic scanning reduces cost and time of complete spine evaluation. J Trauma Inj Infect Crit Care. 2004;56:1022-1026.
36. Wintermark M., Mouhsine E., Theumann N., et al. Thoracolumbar spine fractures in patients who have sustained severe trauma: depiction with multi-detector row CT. Radiology. 2003;227:681-689.
37. Diaz J.J.Jr., Cullinane D.C., Altman D.T., et al. Practice management guidelines for the screening of thoracolumbar spine fracture. J Trauma Inj Infect Crit Care. 2007;63:709-718.
38. Fishman E.K., Magid D., Brooker A.F., Siegelman S.S. Fractures of the sacrum and sacroiliac joint: evaluation by computerized tomography with multiplanar reconstruction. South Med J. 1988;81:171-177.
39. Kuklo T.R., Potter B.K., Ludwig S.C., et al. Radiographic measurement techniques for sacral fractures consensus statement of the Spine Trauma Study Group. Spine (Phila Pa 1976). 2006;31:1047-1055.
40. Denis F. The three-column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976). 1983;8:817-831.
41. McCormack B., MacMillan M., Fessler R.G. Management of thoracic, lumbar and sacral injuries. In: Tindall G.T., Cooper P.R., Barrow D.L., editors. The practice of neurosurgery. Baltimore: Williams & Wilkins, 1996.
42. Magerl F., Aebi M., Gertzbein S.D., et al. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184-201.
43. Vaccaro A.R., Lehman R.A.Jr., Hurlbert R.J., et al. A new classification of thoracolumbar injuries: the importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine (Phila Pa 1976). 2005;30:2325-2333.
44. Rechtine G.R. Nonsurgical treatment of thoracic and lumbar fractures. Instr Course Lect. 1999;48:413-416.
45. Schlegel J., Bayley J., Yuan H., Fredricksen B. Timing of surgical decompression and fixation of acute spinal fractures. J Orthop Trauma. 1996;10:323-330.
46. McLain R.F., Benson D.R. Urgent surgical stabilization of spinal fractures in polytrauma patients. Spine (Phila Pa 1976). 1999;24:1646-1654.
47. Chipman J.G., Deuser W.E., Beilman G.J. Early surgery for thoracolumbar spine injuries decreases complications. J Trauma Inj Infect Crit Care. 2004;56:52-57.
48. McHenry T.P., Mirza S.K., Wang J., et al. Risk factors for respiratory failure following operative stabilization of thoracic and lumbar spine fractures. J Bone Joint Surg [Am]. 2006;88(5):997-1005.
49. Kerwin A.J., Griffen M.M., Tepas J.J.3rd, et al. Best practice determination of timing of spinal fracture fixation as defined by analysis of the National Trauma Data Bank. J Trauma Inj Infect Crit Care. 2008;65:824-830.
50. McLain R.F. Functional outcomes after surgery for spinal fractures: return to work and activity. Spine (Phila Pa 1976). 2004;29:470-477.
51. Roy-Camille R., Saillant G., Gagna G., Mazel C. Transverse fracture of the upper sacrum: suicidal jumper’s fracture. Spine (Phila Pa 1976). 1985;10:838-845.
52. Strange-Vognsen H.H., Lebech A. An unusual type of fracture in the upper sacrum. J Orthop Trauma. 1991;5:200-203.
53. Fardon D.F. Displaced transverse fracture of the sacrum with nerve root injury: report of a case with successful operative management. J Trauma. 1979;19:119-122.
54. Byrnes D.P., Russo G.L., Ducker T.B., Cowley R.A. Sacrum fractures and neurological damage: report of two cases. J Neurosurg. 1977;19:459-462.
55. Huittinen V.M. Lumbosacral nerve injury in fracture of the pelvis. Acta Chir Scand. 1976;429(Suppl):6-8.
56. Vaccaro A.R., Kim D.H., Brodke D.S., et al. Diagnosis and management of sacral spine fractures. Instr Course Lect. 2004;53:375-385.
57. Templeman D., Goulet J., Duwelius P.J., et al. Internal fixation of displaced fractures of the sacrum. Clin Orthop Relat Res. 1996;329:180-185.
58. Wiltse L.L., Guyer R.D., Spencer C.W., et al. Alar transverse process impingement of the L5 spinal nerve: the far-out syndrome. Spine (Phila Pa 1976). 1984;9:31-41.