Chapter 50D Trauma of the Nervous System
Peripheral Nerve Trauma
Today, up to 5% of all admissions to level I trauma centers have a peripheral nerve, nerve root, or plexus injury (Noble et al., 1998). In general, injuries to the upper extremity are more common than those to the lower extremity, accounting for two-thirds of all peripheral nerve injuries. Of the four major peripheral nervous system plexuses—cervical, brachial, lumbar, and sacral—the brachial plexus is by far the most commonly affected.
Anatomy of the Spinal Nerves of the Peripheral Nervous System
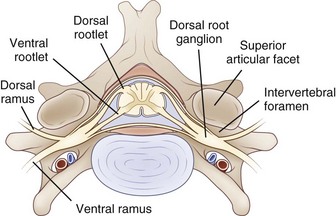
The PNS is composed of those neural elements that extend between the central nervous system (CNS—in this case the spinal cord) and their target organs (Fig. 50D.1). The peripheral motor system axons (somatic efferents) originate from the anterior horn cells and exit the spinal cord to form the ventral (anterior) rootlets. Axons of the peripheral sensory system (somatic afferent) extend from specialized sensory organs within skin, muscle, and viscera to their cell bodies, the dorsal root ganglia (DRG), which lie within the bony intervertebral foramen. These sensory fibers make up the dorsal (posterior, somatic afferent) rootlets that enter the posterior horn of the spinal cord. The mixed spinal nerves are formed when anterior and posterior rootlets combine within the neural foramen just distal to the DRG. The short spinal nerve then divides into two branches: (1) a large anterior branch (ventral ramus) that extends forward to supply the trunk muscles and gives rise to the roots of the plexus and (2) a small posterior branch (dorsal ramus) that extends backward to supply paravertebral muscles and skin of the neck.
Axon
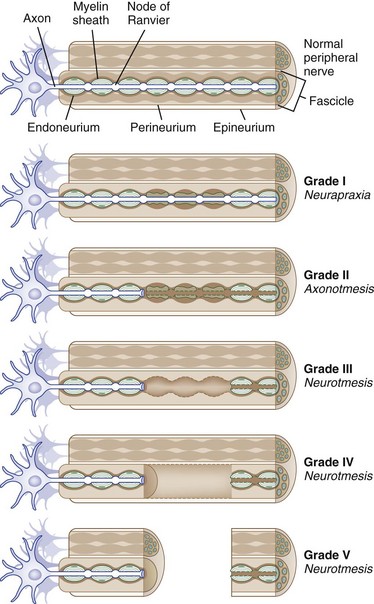
The core element of the nerve is the axon, a thin tube of axoplasm that extends from the nerve cell body to the target organ. Unmyelinated axons are partially ensheathed by invaginations of the Schwann cell membrane, whereas myelinated axons are enveloped in concentric lamellae of myelin composed of compacted spiraled Schwann cell membrane to form a sheath (Fig. 50D.2). The myelin sheath is laid down in segments called internodes, each derived from one Schwann cell. The small gap of uncovered axoplasm between sheaths is called the node of Ranvier, the site where the largest part of ion flow takes place to transmit the action potential. In saltatory conduction, action potentials leap from node to node, rather than traveling in a continuous conduction process along the entire length of the axolemma. In this way, a myelinated large-caliber axon in human adults may conduct electrical impulses at up to 73 meters per second, whereas a small unmyelinated axon may conduct as slowly as 0.5 meter per second (Kimura, 2005).
Peripheral Nerve Trunks
The connective tissue within a peripheral nerve trunk is composed of the endoneurium, perineurium, and epineurium (Fig. 50D.3). These tissues provide structure, tensile strength, and elasticity.
Classification of Nerve Trauma
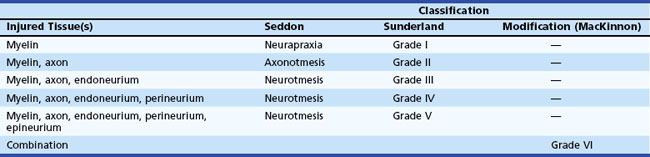
Based on observations made in Great Britain in the Second World War, Seddon devised a three-tiered classification system for nerve trauma (Table 50D.1 and Fig. 50D.3). In this system, the mildest form of injury is due to a transient focal block in conduction along the nerve fiber; injury is confined to the myelin sheath and spares the axon. He called this neurapraxia (Greek for “nonaction of nerve”). This type of injury has an excellent prognosis for complete and spontaneous recovery within a 6-week period. Indeed, many patients return to normal within hours. A clinical example of neurapraxia is wrist drop secondary to prolonged external pressure that compresses the radial nerve at the spiral groove of the humerus.
A second classification system for nerve trauma was devised by Sunderland to include additional information regarding the degree of injury to connective tissue. This system is divided into five grades: grades I and II are identical to Seddon’s neurapraxia and axonotmesis, respectively (see Table 50D.1 and Fig. 50D.3). However, Sunderland subdivided Seddon’s neurotmesis into three further levels of injury: grade III entails injury to the myelin sheath, axon, and endoneurium, with sparing of the perineurium and epineurium; grade IV describes an injury to all nerve trunk elements except the epineurium; and grade V entails complete transection of all neural and connective tissue elements of the nerve trunk. Only Sunderland’s classification system is used in the rest of this chapter.
Peripheral Nerve Degeneration and Regeneration
Large myelinated peripheral axons may respond to injury or disease in three ways: segmental demyelination, wallerian degeneration, and axonal degeneration. Segmental demyelination and wallerian degeneration are relevant to traumatic nerve injury and are discussed in more detail in this chapter, whereas axonal degeneration is more characteristically seen in metabolic and toxic nerve disorders such as diabetes mellitus and renal failure (see Chapter 76).
Wallerian Degeneration
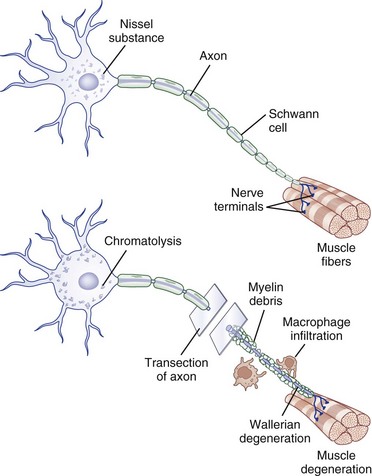
Injury to a peripheral nerve triggers a complex process that involves the axon, its cell body, its cellular connections, and the surrounding connective tissues. Wallerian degeneration follows grade II to grade V injuries and can be divided into changes that involve the segments of nerve distal and proximal to the zone of injury (Fig. 50D.4).
Distal Segment Changes
After nerve injury, the earliest changes occur in the axon distal to the site of injury, where there is disruption of retrograde and anterograde flow of signals within the axon (Makwana and Raivich, 2005). Rapid inflow of extracellular ions such as calcium and sodium occurs through the disruption in the axonal plasma membrane, which activates a cascade of events that shares features with programmed cell death or apoptosis. Axonal injury also leads to recruitment of leukocytes and initiates the cytokine-mediated signaling cascade and changes in the neighboring non-neuronal cells. This in turn triggers the synthesis of neurotrophins, chemokines, extracellular matrix molecules, proteolytic enzymes, and interleukins (Hall, 2005; Radtke and Vogt, 2009). The entire axonal process of wallerian degeneration takes approximately 1 week. By day 3, the Schwann cells retract from the node of Ranvier, and activated Schwann cells and macrophages begin to digest myelin. The process of neuronal degeneration quickly undergoes a transition to neuronal regeneration from the proximal stump. Schwann cell proliferation facilitates sprouting of new nerve branches from the injured axon terminus and initiates the sequence of events that lead to rebuilding of neural contacts to distal muscles.
Proximal Segment
Depending on the severity of injury, a limited degree of axon breakdown extends proximally from the site of injury up to the level of the first node of Ranvier. Although very proximal injury may lead to apoptosis of the cell body itself, the more common consequence of axonal injury is for the cell body to go through the process of chromatolysis. This involves the breakup and dispersion of the rough endoplasmic reticulum, the eccentric displacement of the cell nucleus, and increased nuclear expression of transcription factors that switch the pattern of gene expression from axon maintenance to protein synthesis (Dieu et al., 2005).
Nerve Regeneration
In contrast with partial or mild nerve injury, in which the surviving motor axon begins the process of collateral sprouting almost immediately, in severe or complete injury, neuronal regeneration starts from the proximal stump only after wallerian degeneration is completed (Burnett and Zager, 2004). Schwann cells play a key role in neuronal regeneration. They dedifferentiate and up-regulate the expression of adhesion molecules and neurotrophins (i.e., cadherins, immunoglobulin superfamily factors, laminin) which promote the migration of nerve sprouts that form at the regenerating axon tip. These sprouts then form cords aligned around the original basal lamina tubes of the myelinated axons (bands of Büngner) that provide a pathway along which new axons are destined to grow. The contribution of axon regeneration to clinical recovery overlaps with collateral sprouting at approximately 3 months, but axon regeneration is the main recovery mechanism from 6 to 24 months after the injury, depending in part on the distance the nerve must grow to reach its target muscle.
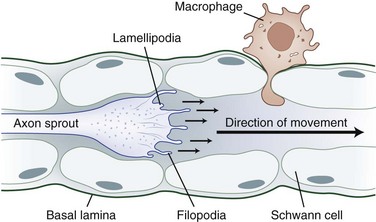
The tip of the axon sprout, called the growth cone, travels by way of filopodia and lamellipodia (Fig. 50D.5). Neurotropism, the term used to describe guidance of a regenerating axon, is accomplished by guidance molecules (i.e., semaphorins, ephrins, netrins, slits) that act to attract or repulse the growth cone to prevent misdirected growth of axon sprouts (Marx, 1995). To pass through endoneurial tubules plugged with cellular debris, the growth cone secretes plasminogen activators that dissolve cell-cell and cell-matrix adhesions. Axonal sprouts grow from the proximal to the distal stump at a rate of approximately 1 to 2 mm/day (or approximately 1 inch/month). This rate of regrowth varies depending on the location of the lesion; with proximal lesions, growth may be as fast as 2 to 3 mm/day, while that with distal lesions is about 1 mm/day.
Peripheral axons and non-neuronal cells contain growth-inducing and trophic molecules that provide the fertile milieu required for nerve regeneration. These include the neurotrophins (nerve growth factor, brain-derived neurotrophic factor, neurotrophins 3 and 4), glial cell line–derived neurotrophic factors (neurturin, artemin, persephin), insulin-like neurotrophic factor, interleukin-6, leukemia inhibitory factor, ciliary neurotrophic factor, fibroblast growth factors, and several transcription factor activators (Dieu et al., 2005; Tucker and Mearow, 2008).
Mechanisms of Traumatic Nerve injury
Compression
Mechanical compression may lead to secondary ischemic injury, as in the case of acute compartment syndromes that compromise nerve microcirculation while sparing distal-extremity pulses. When the intracompartmental pressure exceeds intraarterial pressure, nerve (and muscle) ischemia results in axon-loss lesions of variable severity. An example is the medial brachial fascial compartment syndrome after axillary arteriography or axillary regional block. Severe or irreversible single or multiple axon-loss mononeuropathies can occur within 4 hours if untreated by immediate exploration of the puncture site and decompression (Tsao and Wilbourn, 2003).
Cold Injury
Peripheral nerves are especially prone to damage from excessively cold temperatures (“frostbite” and “trench foot”). In frostbite, tissue freezing occurs through formation of ice crystals in both superficial and deep tissues. The severity of the injury depends on the duration of exposure, the environmental temperature, and the wind chill factor and ranges from mild partial skin freezing to complete tissue mummification (Imray, 2009). Motor weakness develops with prolonged exposure to temperatures around 10°C; sensory fibers withstand temperatures as low as 7°C; and loss of both functions occurs between 0°C and 5°C. Duration of freezing is directly related to severity of injury. With transient or mild exposure, the underlying pathophysiology is primarily that of demyelinating conduction block, but continued exposure to low ambient temperatures causes increasingly severe degrees of axon injury. The endoneurium becomes particularly edematous, which raises intraneural pressure to the point at which blood flow is compromised, thereby further compounding the injury. If the cold-induced injury is not complete, function is restored in the order it was lost—for example, recovery of first sensory and then motor modalities.
Electrical
A severe neurological injury may follow contact with a high-voltage electric current in the home or workplace, or from a lightning strike. Damage is produced mainly by heating, which may cause the tissues to explode. With high-voltage (>1000 V) injuries, frank necrosis of all tissues including nerve may develop, with subsequent loss of the limb or part thereof. Low-voltage (<1000 V) events are associated with a better prognosis, and the injury may or may not include cutaneous burns. Most peripheral nerve injuries occur in association with third- and/or fourth-degree electrical burns and include mononeuropathies, polyneuropathies, and plexopathies, which may be either early or delayed in onset and frequently bilateral. The median and ulnar nerves most frequently are affected. Symptoms and signs may be similar to those of focal compression neuropathies, occurring at sites of minimal limb cross-sectional area where nerves cross bony protuberances. Joule’s law predicts that heat production will be maximal at such sites, and it has been proposed that perineurial fibrosis may occur at these sites, giving rise to neuropathies that may be relieved by surgical decompression (Smith et al., 2002).
Injection
One of the most severe forms of peripheral nerve injury is that due to injection of drug (prescribed or illicit). Unfortunately, these injuries are relatively common and either are a direct effect of needle insertion into the nerve or (more often) are due to the drug itself or its associated buffer and solvent. The presence of drug in and around a nerve may stimulate an intense inflammatory reaction that may lead to extensive epineurial scarring and distortion of the architecture of the nerve trunk. In general, an intact blood-nerve barrier (i.e., an intact perineurial-endoneurial border) will prevent diffusion of drug or toxin into the nerve fibers themselves. Direct injection of drug into nerve, however, may cause severe endoneurial fibrosis with neural ischemia and axon loss (Strasberg et al., 1999). The radial and sciatic nerves are the most frequently affected, but injection injuries also have been described in the median, femoral, and ulnar nerves. The first symptom often is immediate onset of pain, paresthesia, and paralysis, but in some affected persons, the onset apparently is delayed. Patients who fail to show signs of recovery within 3 to 5 months or who have persistent pain may benefit from surgical intervention.
Evaluation of Nerve Trauma
Clinical and Electrodiagnostic Examination
The clinical assessment of traumatic nerve injury should ascertain an account of the injury itself (including the exact circumstance) with details regarding the localization, severity, and particularly the precise time it occurred (Box 50D.1 and Table 50D.2). The electrodiagnostic examination confirms the site of injury and its underlying pathophysiology and severity and may uncover additional information such as subclinical abnormalities or evidence of early recovery. All these elements determine if and when surgical repair is required and provide a timeline for any interventions. For example, if a period of 4 years has elapsed from the time of injury, it is very unlikely that any significant motor recovery will take place despite surgical intervention. The reduction in ability to regenerate following neurorrhaphy or grafting results from several mechanisms. First, the proximal stumps (motor neuron axons) lose the ability to grow. Second, the distal stump loses the ability to support and guide axonal growth. Finally, atrophy in muscle and tendon contractures often limit the impact of the few axons that can reinnervate functioning neuromuscular junctions. Regarding mechanism, intraoperative transection of a nerve by a scalpel is likely to portend a better prognosis than is possible with a laceration by a chain saw. Early (at <3 months) return of motor and sensory function is a good prognostic sign and supports grade I lesions. Elderly patients and those with metabolic disorders such as diabetes and renal failure are less likely to enjoy good functional outcome after nerve injury.
Box 50D.1 Important Elements of the History in Patients with Peripheral Nerve Injury
 Patient’s age and “handedness”
Patient’s age and “handedness”
 Exact time (as near as possible) injury occurred, stated as the date and time, not as a phrase such as “3 days ago Monday”)
Exact time (as near as possible) injury occurred, stated as the date and time, not as a phrase such as “3 days ago Monday”)
 Time interval between injury and initial evaluation
Time interval between injury and initial evaluation
 Description of injury (to differentiate among laceration, compression, traction, etc.); presence of associated injury (e.g., head trauma, vascular repair, compartment syndromes)
Description of injury (to differentiate among laceration, compression, traction, etc.); presence of associated injury (e.g., head trauma, vascular repair, compartment syndromes)
 Description of motor weakness (maximum at onset? progressive? improving? if so, over what time period?)
Description of motor weakness (maximum at onset? progressive? improving? if so, over what time period?)
 Absence or presence of pain or sensory changes
Absence or presence of pain or sensory changes
 Any recovery of sensorimotor function
Any recovery of sensorimotor function
 Presence of systemic disease (e.g., diabetes, preexisting peripheral neuropathy, entrapment neuropathy)
Presence of systemic disease (e.g., diabetes, preexisting peripheral neuropathy, entrapment neuropathy)
 Score on a quantitative pain scale (i.e., Visual Analog Scale)
Score on a quantitative pain scale (i.e., Visual Analog Scale)
Table 50D.2 Neurological Examination in Patients with Peripheral Nerve Injury
| Feature/Component | Focus/Description |
|---|---|
| Wound | Open or closed, other tissues or vascular structures involved, presence of infection |
| Estimated distance from injury site and target organ | |
| Cranial nerves | Routine, plus assessment for Horner syndrome (partial ptosis, miosis, and variable degree of anhidrosis for preganglionic lesions) or cranial nerve deficits often associated with brachial plexus injury (including spinal accessory nerve) |
| Skin | Should be closely examined for trophic and atrophic changes, including vasomotor instability |
| Motor | Should contain a reliable and consistent measure of motor function (e.g., British Medical Research Council, Louisiana State University Medical Center grading scale) |
| Sensory | Should provide quantitative data of both large and small fiber function (e.g., touch-pressure via on Frey filaments, Weber two-point discrimination, protective sensibility or appreciation for pain, cold, warmth, or pressure) |
| Deep tendon reflexes and presence of Tinel sign over nerve distal to injury |
The electrodiagnostic examination consists of nerve conduction studies (NCSs) and needle electromyography (EMG). The two main components of the NCSs are assessment of sensory nerve action potentials (SNAPs) and compound muscle action potentials (CMAPs). When the study is performed to assess for complex lesions of the brachial plexus or lower-extremity nerve lesions, it is essential that the electrodiagnostician examine particularly relevant NCSs and an expanded EMG in addition to those done in routine studies (Ferrante and Wilbourn, 2002).
If the lesion is due to focal demyelinating conduction block (grade I injury), and nerve stimulation is applied both distal and proximal to the lesion site, the examiner should detect a lower proximal compared to distal CMAP amplitude. The difference in proximal and distal CMAP amplitude and the reduction in distal motor unit potential voluntary recruitment on EMG are proportional to the amount of motor fibers blocked. Even with primarily demyelinating lesions, a limited amount of axon loss (i.e., fibrillation potentials) may occur if some axons at the site of the conduction block have incurred injury (Wilbourn, 2002).
Imaging Studies
In trauma cases, plain films of skull base, spine, and long bones may disclose fractures at sites that may compromise local nerve structures. Myelography, sometimes with computed tomography (CT), and magnetic resonance imaging (MRI) with gadolinium of the spine is used to diagnose nerve root avulsions (Fig. 50D.6); contrast material may be seen passing through the torn meningeal sheaths of avulsed nerve roots (pseudomeningoceles). Radiology confirmation of nerve root avulsion may be confounded by localized hematoma formation within or around the neural foramina, which may prevent extravasation of contrast material, thereby obscuring the typical appearance of pseudomeningoceles.
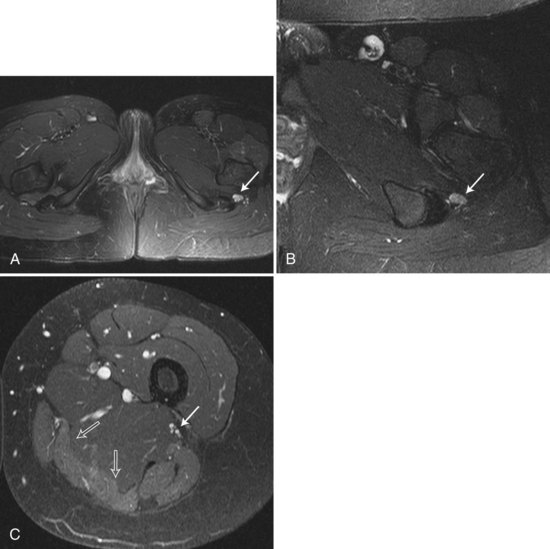
MR neurography of peripheral nerve uses diffusion-weighted, T2-weighted, and short T1/tau inversion recovery (STIR) images based on the longitudinally oriented water diffusion properties of nerve as opposed to surrounding tissues (Grant et al., 2002). Signal hyperintensity can be seen on T2-weighted and STIR images of traumatized nerve segments; abnormal high signal intensity is seen both at and distal to the injury site (Fig. 50D.7).This MRI modality can detect neuroma formation at nerve repair sites, and it may even be possible to identify which fascicles within a nerve trunk are injured and which are spared. These signal changes may be transient in cases of mild nerve injury or may be prolonged (up to many years) in severe preganglionic brachial plexus avulsion injuries. Early (within 1 week) signal hyperintensity also may be seen in denervated muscle on T2-weighted and STIR images, which will persist if nerve discontinuity prevents reinnervation.
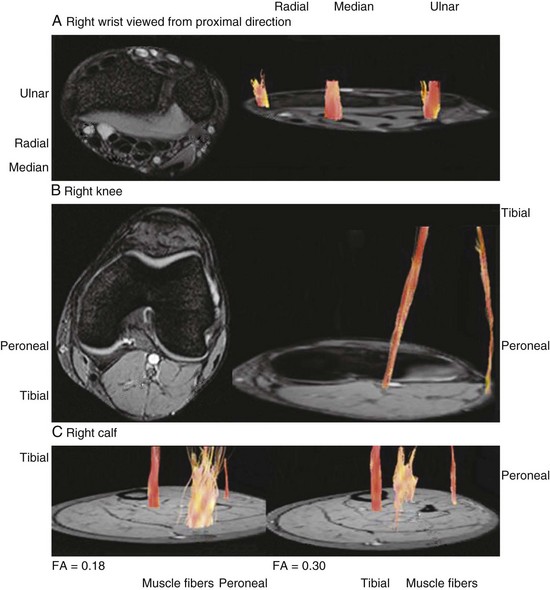
Higher-tesla MRI scanners (3.0 T) offer enhanced resolution that may provide images with imaging quality equal to or greater than that of MR neurography. Another emerging technique, diffusion tensor imaging (DTI) and tractography of peripheral nerves (also performed on 3.0 T MRI), is based on the principle that water molecules have anisotropic diffusion properties (or a preferred orientation) in white-matter fiber tracts, compared with isotropic diffusion (equal in all directions) in surrounding tissues (Hiltunen et al., 2005; Sheikh, 2010) (Fig. 50D.8).
High-frequency ultrasonography of peripheral nerve and muscle is a relatively inexpensive and accessible diagnostic method to provide further information in the assessment of nerve injury (Umans, 2010; Visser, 2010). Although the resolution of ultrasound imaging is below that of MRI, this test can be performed in the office setting, has the advantage of real-time imaging, and is of particular use in patients who cannot tolerate MRI. Ultrasonography enables more dynamic observation of in vivo nerve segments (e.g., retroepicondylar subluxation of the ulnar nerve) and focal structural changes that accompany nerve injury (e.g., swelling, nerve continuity, neuroma formation).
Other Diagnostic Modalities
Somatosensory evoked potentials (SSEPs) are cortical potentials elicited by the stimulation of a sensorimotor nerve trunk or cutaneous nerve. SSEPs obtained by stimulation above the level of nerve injury may give information about the integrity of posterior sensory tracts in the spinal cord and the presence or absence of posterior nerve root avulsion. The presence of an intact SSEP, however, does not reliably indicate that the nerve roots are intact, because SSEPs are so sensitive they may yield positive waveforms in the presence of only a few hundred intact nerve root axons (Spinner and Kline, 2000). Furthermore, they do not provide enough localizing information to be of great assistance in postganglionic peripheral nerve injuries and do not provide information about injury to anterior (motor) rootlets in the spinal canal.
Rehabilitation
Rehabilitation is an essential component of the management of surgical and nonsurgical nerve injuries and should be initiated early to maximize the chances of functional recovery and minimize disability. European studies of the consequences of median and ulnar nerve injuries showed that lost productivity represents the largest portion of the costs associated with these injuries, and that more than half of patients had not returned to work 1 year after the injury (Rosberg et al., 2005). Rehabilitation provides a unique opportunity for a comprehensive evaluation of the consequences of the nerve injury, realistic goal setting, and the identification and aggressive management of factors that may have a negative impact on functional recovery (e.g., associated musculoskeletal or CNS injuries, pain, depression). Unfortunately, a lack of randomized controlled trials has prevented evaluation of the impact of rehabilitation intensity and duration, or of the benefits of specific techniques or programs.
One of the goals of rehabilitative interventions is to prevent contractures and muscle atrophy, which may interfere with recovery even when nerve regeneration occurs. This can be achieved through passive or active mobilization and the use of orthoses. The nerve gliding technique, although somewhat controversial, can be used to maintain the normal longitudinal mobility of nerves as the extremity flexes and extends. Perineural adhesions may form if the extremity is splinted and will result in pain from local nerve traction. Gentle gliding or range-of-motion exercises to the point of mild paresthesia have been advocated. When paresthesias cannot be elicited, gliding has been effective. Another goal is to restore function either with direct intervention on the impaired limb (e.g., use of active orthoses, strengthening of nonimpaired muscles) or by teaching compensatory techniques. Electrical stimulation may limit the consequences of denervation on muscle properties and improve functional recovery (Marqueste et al., 2004).
Surgical Repair of Nerve Trauma
Indications for and Timing of Surgical Repair
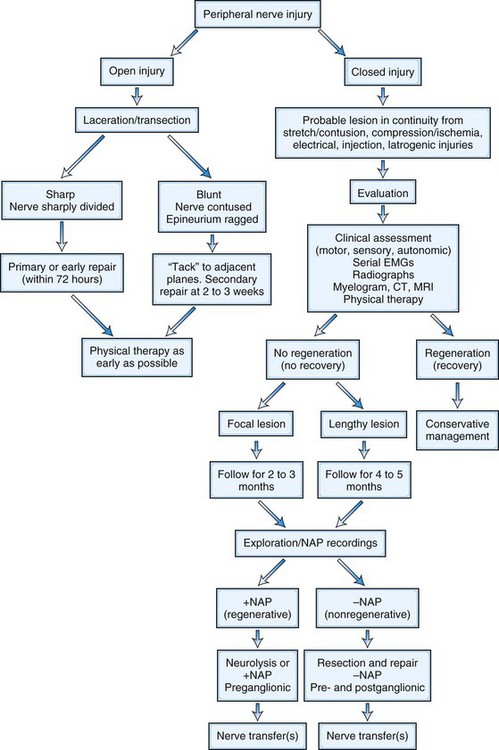
A treatment algorithm for traumatic nerve injuries has been proposed by Spinner and Kline (2000) and is presented in Fig. 50D.9. Surgical intervention is not indicated in patients with neurapraxic (or grade I) nerve lesions, because spontaneous recovery with an excellent outcome is likely. However, for axon-loss lesions (grades II to V), this algorithm divides peripheral nerve injury into two major categories: open and closed. The open category is further divided into early repair (within 72 hours for primary repair) and limited initial exploration to tack the proximal and distal nerve stumps to an adjacent structure such as fascia to allow easier identification at definitive repair carried out within 3 to 4 weeks (delayed primary repair). The closed category, which comprises a majority of nerve injuries seen in the outpatient setting, is subdivided into delayed repair (3 to 6 months) and nonsurgical management. The decision to use surgical repair is made on a patient-to-patient basis and depends on the mechanism and degree of injury, the timing of injury, and the presence or absence of spontaneous clinical or electrodiagnostic improvement.
Intraoperative Nerve Monitoring
Intraoperative nerve action potential (NAP) recording enables the surgeon to locate abnormal sites of mixed sensory and motor nerve conduction caused by axon loss and to define the healthy margins of nerve. After external neurolysis, stimulating and recording electrodes are placed directly onto the nerve surface proximal and distal to an obvious or suspected site of nerve trauma that has remained in continuity. The NAP is proportional to the number of functioning axons. Thus, a 5-µV NAP elicited in the distal nerve segment indicates continuity of at least 3000 to 5000 moderate-diameter myelinated axons at the recording site, the presence of which correlates with good spontaneous recovery (Spinner and Kline, 2000). Such incomplete lesions usually are treated with external or internal neurolysis. Conversely, the absence of a NAP at 6 weeks or more after the injury generally portends poor spontaneous recovery and constitutes an indication for surgical repair.
Intraoperative NAPs can be used to determine whether nerve root avulsion is present. With a plexus lesion in which nerve roots are injured at a preganglionic level but intact postganglionically, a NAP can be recorded because the large myelinated sensory fibers have been spared wallerian degeneration. In contrast, an absent NAP suggests injury to the postganglionic segment. When preganglionic injury is suspected, stimulation of the proximal portion of the spinal nerve to obtain a somatosensory evoked response can be performed. With preganglionic injury, these evoked responses are absent (Kline and Hudson, 1995). Finally, when NAPs and SEPs initially are absent or reduced, reassessment after several months may show regenerating responses. NAP and SEP amplitudes typically recover earlier than those of CMAPs, because the latter type requires maturation of the motor end plate (Harper, 2005).
Direct recording of motor evoked potentials by transcranial magnetic or electrical stimulation of the motor cortex and recording from distal muscles or motor nerves is another method of assessing anterior root or proximal nerve integrity (Burkholder et al., 2003; Sutter et al., 2007; Turkof et al., 1997). This technique has several limitations, including sensitivity to inhaled anesthetics or boluses of intravenous anesthesia, large stimulation artifact, submaximal stimulation, and contraindication with cochlear implants and open skull fractures. With further refinement, however, motor evoked potentials may play a greater role as a routine intraoperative recording technique.
Surgical Techniques
Neurolysis (External and Internal)
The presence of scar tissue either around or within the nerve is thought to impede regeneration by creating compression and entrapment. External neurolysis frees the nerve from surrounding scar tissue and may be sufficient to manage in-continuity lesions with recordable NAPs. Incomplete lesions may involve scar tissue extending within epifascicular or interfascicular epineurium, and internal neurolysis, using the surgical microscope, frees the nerve trunk or fascicles from such scar tissue. If the epifascicular epineurium is the only site of scar formation, a longitudinal incision called an epineurotomy may be sufficient. Interfascicular scars, however, may require more intricate separation of involved from uninvolved nerve fascicles. The latter procedure is usually performed as part of the preparation for nerve graft repairs, during which NAPs are used to identify damaged nerve fascicles. Review of outcomes after neurolysis of in-continuity radial and median nerve injuries at a major nerve trauma center showed good motor functional recovery in 98% and 95% of cases, respectively (Kim et al., 2001a, 2001b). Nonetheless, these results can be misleading because no studies have analyzed the recovery of lesions in continuity with and without external neurolysis. External neurolysis has been compared with neurectomy (see Interventional Strategies, later) for management of persistent pain associated with neuromas of sensory nerves and was found to provide no relief. Accordingly, the main application of external neurolysis to nerves lacking any function on electrodiagnostic testing and physical examination should be for physiological and anatomical exploration before grafting. Similarly, internal neurolysis may be necessary to evaluate the NAPs of individual fascicles to repair fascicular injury. In this case, fascicles devoid of NAPs can be grafted, leaving the NAP-positive fascicles intact. The removal of scarring within nerves through internal neurolysis carries risk to the fascicles, however, and may actually worsen the fibrosis around fascicles. As with external neurolysis, few objective data exist to support this practice except as a means for fascicular transfers, fascicular grafting, and the resection of nerve sheath tumors.
Primary Neurorrhaphy
Primary repair (primary neurorrhaphy) of a grade V lesion involves the direct suturing of the proximal to the distal stump and is the procedure of choice (Fig. 50D.10). Emphasis is placed on the reduction of tension on the site of neurorrhaphy, because this is thought to undermine regeneration through scar formation. The role of early exploration, particularly with nerve laceration, is to tack the nerve (thus preventing nerve retraction) and then to facilitate future approximation of nerve ends. In addition, with early exploration, individual fascicles have not yet atrophied and thus are easier to reanastomose. Delayed exploration, by contrast, allows for better demarcation of the injured nerve endings because the damaged segment of nerve will undergo fibrosis, making identification of healthy nerve easier.
Overall, good functional outcome is achieved in approximately 70% of primary neurorrhaphies but may range as high as 80% to 90% (Kim et al., 2001a, 2001b; Murovic, 2009). The end-to-side repair technique can be employed to repair facial motor nerve injuries and may also be helpful in the repair of brachial plexus injuries that lack a proximal stump. In this technique, the donor nerve is partially transected and grafted to the recipient nerve; it has been shown that sensory nerve sprouts will form spontaneously from the donor nerve, but it requires a deliberate surgical injury to motor axons to generate motor axon sprouts (Siemionow and Brzezicki, 2009).
Nerve Grafting
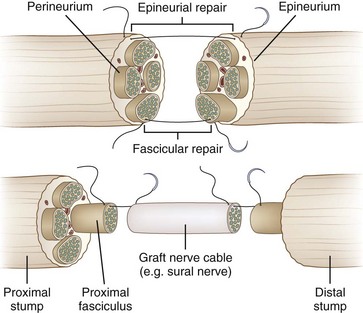
If a significant gap exists between the proximal and the distal stumps, it may not be possible to perform primary neurorrhaphy. Such gaps can occur in severe neurotmesis lesions such as high-velocity gunshot wounds or in axonotmetic stretch injuries in which long regions of the nerve may be damaged. Excessive stretch leads to scar formation, especially with passive stretch across bony protuberances and joints. In such situations, nerve grafting is the preferred choice. This repair strategy entails suturing a piece of nerve harvested from elsewhere between the proximal and distal stumps of the injured nerve. In most centers, an autologous nerve graft is used—a healthy segment of nerve is removed from the patient’s body and sutured between the stumps at the trauma site (Siemionow and Brzezicki, 2009). Although vascularized (pedicled) grafts have been described, conventional grafts are obligatorily devascularized. Accordingly, a longer graft means a longer distance traveled in entirely devascularized tissue. Thus, the surgeon continually balances the need to minimize tension on the anastomosis with the desire to minimize graft length and mobilization. In general, grafts are sutured using a fascicular rather than an epineurial approach. Common donor nerves are those that are “nonessential” and largely sensory in function, such as the sural nerve, the lateral antebrachial cutaneous nerve, and the lateral femoral cutaneous nerve. It is important to counsel the patient that once such a nerve is sacrificed, an area of permanent sensory loss in the sensory distribution of the donor nerve may result. The harvest of an autologous graft often leaves a long scar and carries the risk of painful end-neuroma formation.
In the case of large gaps in proximal nerves, it is often difficult to find sufficient autologous graft material to perform long polyfascicular repairs, and it is equally frustrating to sacrifice a sensory nerve to repair short gaps in small distal nerves. These concerns have motivated a search for alternatives to conventional grafts. Creative approaches have used muscle tissue, with an interposed segment of the distal stump to serve as a source for Schwann cells, or allografts accompanied by immunosuppression (Mackinnon, 1996). Cadaveric grafts have also been introduced and do not require immunosuppression but add expense. The main alternative to autologous grafts is manufactured nerve substitutes. A variety of these have been made available in the last decade; they can broadly be divided into collagen-based graft material and synthetic aliphatic polyesters.
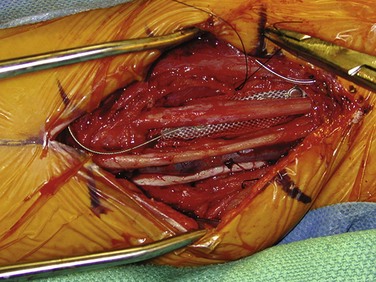
To prepare the stumps for grafting, they are sectioned perpendicular to the long axis of the nerve. Smaller soft nerves may be wrapped with polyethylene film or paper to lend substance to the nerve. Under the microscope, the epineurium is dissected away with microscissors, leaving the fascicles free. The remaining gap is measured, and the harvested graft is cut to fit in order to minimize graft length. We prefer to perform anastomosis with two 8-0 nylon sutures positioned 180 degrees from each other. This maintains the alignment of the graft with individual fascicles and prevents rotation. Use of more sutures may lead to increased scarring at the anastomosis. At the completion of polyfascicular grafting, we apply fibrin glue to stabilize and protect the anastomosis (Fig. 50D.11).
The success rate of nerve grafting depends on the experience and skill of the nerve trauma team, the particular nerve involved, the distance between the proximal and distal stumps, and the type of nerve graft or conduit used. The single most important prognostic factor is the time interval between the nerve injury and reinnervation of the target motor or sensory organ. A primate study showed that the optimal time to successful reinnervation of the target organ using nerve grafts is within 100 days of sustaining the nerve lesion (Krarup et al., 2002).
Overall, a satisfactory functional outcome is achieved in about 50% of nerve graft cases. This is an approximate figure, however, and many factors are involved. For example, at one major nerve trauma center, good functional recovery of motor function was observed in 68% to 75% of median and 80% of radial nerve injuries that were repaired by nerve grafting (Kim et al., 2001a, 2001b). Retrospective review of functional outcomes after repair of sciatic nerve injuries at the same center reported good outcomes after repair of tibial but not peroneal (less than 36%), division injuries (Kline et al., 1998).
Nerve Transfer
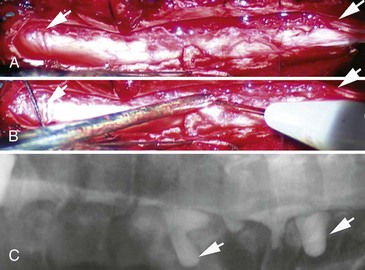
Avulsion injuries of proximal nerves and nerve roots create a special problem in nerve repair because often no proximal stump is present with which an anastomosis may be made to a distal stump or an intervening nerve graft (see Fig. 50D.6, A and B). A specialized nerve transfer technique called neurotization has been developed to improve outcome with these functionally debilitating injuries. Neurotizations in the upper extremity are usually performed to provide shoulder abduction, elbow flexion, and hand sensation. The technique entails transfer of the proximal stump of a healthy nerve to the distal stump of an injured (transected) nerve so that a target muscle of particular functional or mechanical importance becomes reinnervated. An example is the transfer of the spinal accessory nerve to the distal stump of the musculocutaneous nerve in a case of proximal upper-extremity nerve root avulsion; the patient gains useful elbow flexion in the process. The choice of other “axon donor” nerves depends on the exact injury. Candidate nerves include the suprascapular nerve, thoracodorsal nerve, medial pectoral nerve, and intercostal nerve. Some common “axon recipient” nerves include the musculocutaneous nerve, suprascapular nerve, and axillary nerve.
In cases of flail arm following nerve root avulsion, in which more donor nerves are needed, transfer of the phrenic nerve and contralateral C7 root has been attempted. Because denervation of these nerves can result in significant morbidity, special attention must be paid to their role preoperatively. Phrenic nerve transfer should not be performed at the same time as intercostal transfer and should be done only if diaphragmatic function is intact. Moreover, if the patient has sustained multiple rib fractures, respiratory dynamics may be entirely dependent on diaphragmatic function. In the case of contralateral C7 transfers, contralateral plexus deficits pose a significant problem to persons with only one functioning arm. Thus, preoperative C7 root blocks should be applied to ascertain the degree of dysfunction to be anticipated. Successful attempts have been made to perform fascicular transfers. In this case, motor fascicles can be harvested from nerves more distally, thereby bringing the proximal donor stump closer to the denervated muscle. The best example of this approach, the Oberlin procedure, involves transfer of a nerve fascicle to the flexor carpi ulnaris muscle from the ulnar nerve to the musculocutaneous nerve, with reports of elbow flexion strength obtained in nearly 90% (Tung et al., 2003). Positive results have also been reported with the transfer of tibial fascicles to the deep peroneal nerve and in cases of lumbosacral plexus avulsion, lateral fascicles or branches of the femoral nerve transferred to the hamstring fascicles of the sciatic nerve. More recently, the outcome of bypass coaptation of the C3 and C4 anterior rami (distal to their contribution to the phrenic nerve) to the upper and lower trunk of the brachial plexus in 26 patients following C5-C6 avulsion (Erb-Duchenne palsy), C8-T1 avulsion (Klumpke palsy), and C5-T1 nerve root avulsion showed satisfactory proximal and distal muscle recovery in most patients except the adult flail arm (C5-T1) group, where proximal muscle function was regained, but distal muscle improvement was modest. Another benefit in this population was improvement of the postavulsion pain that typically plagues adult patients with nerve root avulsion (Yamada et al., 2009).
Certain researchers have assessed the feasibility of direct reimplantation of avulsed nerve roots back into the spinal cord, with mixed results. Although some subjects regain useful function of proximal limb muscles, more distal hand muscles are not successfully reinnervated, and the procedure carries a risk of injury to spinothalamic tracts within the spinal cord (Carlstedt et al., 2000). A similar problem also arises when the very terminal segments of a nerve have lost contact with the target organ, thus failing to leave a distal stump for reanastomosis. A technique has been developed in which the distal nerve stump is split into fascicles which are then implanted directly into the end-plate region of the target muscle belly (Becker et al., 2002). It is even possible to transfer an entire muscle together with its blood and nerve supply to the site of a functionally more important muscle that is nonfunctioning through prior injury. Examples are the use of a free muscle transfer to restore elbow flexion by way of the spinal accessory nerve or to restore finger flexion via the intercostal nerves, and replacement of a chronically denervated biceps muscle by a neurotized and vascularized gracilis muscle that has been harvested from the lower extremity (Barrie et al., 2004; Doi et al., 2000).
Management of Neuropathic Pain Associated with Nerve Trauma
With nerve root avulsion injuries, patients may suffer a severe pain syndrome that is notoriously difficult to treat. A vivid description of this deafferentation pain was provided by a physician who suffered a brachial plexus avulsion injury himself when he was struck by a window cleaner falling from the fourth floor of a building as he was passing: “The pain is continuous; it does not stop either day or night. It is either burning or compressing (like a vise) or dragging (a sense of weight) in character, or a combination of all these at the same time” (Murray and Wilbourn, 2002).
Pharmacological Options
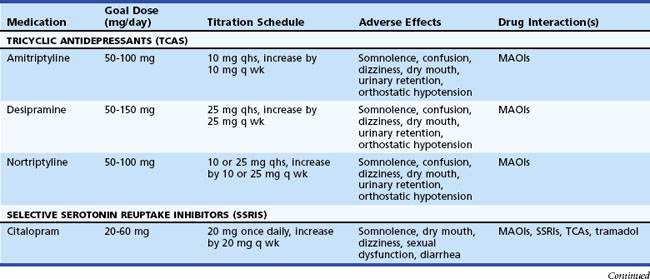
Table 50D.3 summarizes various medications used in the treatment of neuropathic pain. A pharmacological strategy is to establish effective longer-acting antiepileptic or antidepressant monotherapy, while breakthrough pain is judiciously treated with shorter-acting medications. Refractory pain may require the addition of another antiepileptic or antidepressant medication, preferably with a different mechanism of action (e.g., mixing a sodium channel blocker, γ-aminobutyric acid (GABA) inhibitor, and serotonin-norepinephrine reuptake inhibitor). More aggressive pain control, particularly in the acute setting, may require the use of narcotics (e.g., morphine, fentanyl patches).
Interventional Strategies
With peripheral neuromodulation, electrodes most commonly are implanted directly on the peripheral nerve or in the epidural space (Fig. 50D.12). Epidural electrodes referred to as spinal cord stimulators or dorsal column stimulators are positioned over the lower thoracic cord to treat lower-extremity neuropathic pain and over the cervical cord for upper-extremity pain. Alternatively, in cases of partial denervation, they can be implanted subdermally in the region of subjective pain. Once a device is implanted, a controller is given to the patient that allows adjustment of the amplitude of the stimulation. More complex programming is performed in the physician’s office, including adjustments to the frequency, pulse width, voltage window, and specific active leads in the system. In cases of severe refractory causalgia, deep brain stimulation electrodes can be implanted within important brain relay nuclei in the pain pathways. Motor cortex stimulation also has been employed with some success to manage refractory appendicular pain. Nonetheless, the efficacy of motor cortex stimulation currently is a subject of considerable debate. In our experience, direct implantation of stimulators on damaged peripheral nerves is the most effective means to modulate pain from these nerves.
Future Directions in Treatment of Nerve Trauma
Emerging alternative conduits to bridge the gap between separated nerve stumps are being developed with a view to bridging long gaps and also to reduce the need for grafting with autografts or allografts (and its associated requirement for immune suppression). Much work has been carried out in animal models using synthetic, semisynthetic, and biological devices, but some of these devices have been employed in human short-gap nerve repair, achieving results that approach those of standard nerve grafting. Biological conduits include arteries, veins, empty epineural sheath, and muscle, whereas artificial conduits include resorbable and nonresorbable synthetic polymers and/or extracellular matrix components. Although the results have been variable, most such devices have not been quite as effective as autogenous grafts; nevertheless, they hold promise for the future (Battiston et al., 2005; Siemionow and Brzezicki, 2009). A number of modifications have been employed to improve the effectiveness of conduits, including seeding the lumen of the entubulation device with a monolayer of Schwann cells, bone stromal cells, laminin, collagen, or with molecules such as cytokines or neurotrophic factors (nerve growth factor, vascular endothelial growth factor) that are known to promote regrowth of axons (Yan et al., 2009).
Stem cell–derived Schwann cells hold promise in improving nerve regeneration. A number of different sources for such cells have been investigated, including bone marrow, adipose tissue, amniotic fluid, hair follicles, and skin-derived precursor cells (Walsh, 2010). Advancement of this technology, however, requires further molecular characterization and a better understanding of the cellular microenvironment and factors that influence differentiation.
Finally, neurophysiological, neuropsychological, and functional neuroimaging studies have identified reorganization of central motor and sensory pathways in the aftermath of peripheral nerve injury and repair. Chronic denervation leads to extensive reorganization of representational maps within the sensory and motor cortex, including a limited degree of reorganization within subcortical structures (Anastakis, 2008; Chen et al., 2002; Iwase et al., 2001; Lundborg, 2003; Taylor, 2009). Further study into the dynamic relationship between central reorganization and peripheral nerve regeneration may lead to more refined rehabilitative therapy.
Anastakis D.J., Malessy M.J., Chen R., et al. Cortical plasticity following nerve transfer in the upper extremity. Hand Clin. 2008;24(4):425-444.
Barrie K.A., Steinmann S.P., Shin A.Y., et al. Gracilis free muscle transfer for restoration of function after complete brachial plexus avulsion. Neurosurg Focus. 2004;16:E8.
Battiston B., Ceuna S., Ferrero M., et al. Nerve repair by means of tubulization: literature review and personal clinical experience comparing biological and synthetic conduits for sensory nerve repair. Microsurgery. 2005;25:258-267.
Becker M., Lassner F., Fansa H., et al. Refinements in nerve to muscle neurotization. Muscle Nerve. 2002;26:362-366.
Burkholder L.M., Houlden D.A., Midha R., et al. Neurogenic motor evoked potentials role in brachial plexus surgery. J Neurosurg. 2003;98:607-610.
Burnett M.G., Zager E.L. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus. 2004;16:1-7.
Carlstedt T., Anand P., Hallin R., et al. Spinal nerve root repair and reimplantation of avulsed ventral roots into the spinal cord after brachial plexus injury. J Neurosurg. 2000;93:237-247.
Chen R., Cohen L.G., Hallett M. Nervous system reorganization following injury. Neuroscience. 2002;111:761-773.
Dieu T., Johnstone B.R., Newgreen D.F. Genes and nerves. J Reconstr Microsurg. 2005;21:179-186.
Doi K., Muramatsu K., Hattori Y., et al. Restoration of prehension with the double free muscle technique following complete avulsion of the brachial plexus. Indications and long-term results. J Bone Joint Surg. 2000;82:652-666.
Ferrante M., Wilbourn A.J. Electrodiagnostic approach to the patient with suspected brachial plexopathy. Neurol Clin. 2002;20:423-450.
Grant G.A., Britz G.W., Goodkin R., et al. The utility of magnetic resonance imaging in evaluating peripheral nerve disorders. Muscle Nerve. 2002;25:314-331.
Hall S. Mechanisms of repair after traumatic injury. In: Dyck P., Thomas P. Peripheral Neuropathy. fourth ed. Philadelphia: W. B. Saunders; 2005:1405-1410.
Harper C.M. Preoperative and intraoperative electrophysiologic assessment of brachial plexus injuries. Hand Clin. 2005;21:39-46.
Hiltunen J., Suortti T., Arvela S., et al. Diffusion tensor imaging and tractography of distal peripheral nerves at 3 T. Clin Neurophysiol. 2005;116:2315-2323.
Imray C., Grieve A., Dhillon S., The Caudwell Xtreme Everst Research Group. Cold damage to the extremities: frostbite and non-freezing cold injuries. Postgrad Med J. 2009;85:481-488.
Iwase Y., Mashiko T., Ochiai N., et al. Postoperative changes on functional mapping of the motor cortex in patients with brachial plexus injury; comparative study of magnet encephalography and functional magnetic resonance imaging. J Orthop Sci. 2001;6:397-402.
Kim D.H., Kam A.C., Chandika P., et al. Surgical management and outcome in patients with radial nerve lesions. J Neurosurg. 2001;95:573-583.
Kim D.H., Kam A.C., Chandika P., et al. Surgical management and outcomes in patients with median nerve lesions. J Neurosurg. 2001;95:584-594.
Kimura J. Nerve conduction and needle electromyography. In: Dyck P., Thomas P. Peripheral Neuropathy. fourth ed. Philadelphia: W. B. Saunders; 2005:899-908.
Kline D.G., Hudson A.R. Nerve action potential recordings. In: Nerve Injuries. Philadelphia: W.B., Saunders; 1995:102-115.
Kline D.G., Kim D., Midha R., et al. Management and results of sciatic nerve injuries: a 24-year experience. J Neurosurg. 1998;89:13-23.
Krarup C., Archibald S.J., Madison R.D. Factors that influence peripheral nerve regeneration: an electrophysiological study of the monkey median nerve. Ann Neurol. 2002;51:69-81.
Lundborg G. Richard P. Bunge memorial lecture. Nerve injury and repair—a challenge to the plastic brain. J Peripher Nerv Syst. 2003;8:209-226.
Mackinnon S.E. Nerve allotransplantation following severe tibial nerve injury. J Neurosurg. 1996;84:671-676.
Makwana M., Raivich G. Molecular mechanisms in successful peripheral regeneration. FEBS J. 2005;272:2628-2638.
Marqueste T., Alliez J.R., Alluin O., et al. Neuromuscular rehabilitation by treadmill running or electrical stimulation after peripheral nerve injury and repair. J Appl Physiol. 2004;96:1988-1995.
Marx J. Helping neurons find their way. Science. 1995;268:971-973.
Murovic J.A. Upper-extremity peripheral nerve injuries: a Louisiana State University Health Sciences Centre literature review with comparison of the operative outcomes of 1837 Louisiana State University Health Sciences Center median, radial, and ulnar nerve lesions. Neurosurgery. 2009;65(4 Suppl):A11-A17.
Murovic J.A. Lower extremity peripheral nerve injuries: a Louisiana State University Health Sciences Center literature review with comparison of the operative outcomes of 806 Louisiana State University Health Sciences Center sciatic, common peroneal, and tibial nerve lesions. Neurosurgery. 2009;65:A18-A23.
Murray B., Wilbourn A.J. Brachial plexus. Arch Neurol. 2002;59:1186-1188.
Noble J., Munro C.A., Prasad V.S., et al. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45:116-122.
Radtke C., Vogt P.M. Peripheral never regeneration: a current perspective. Eplasty. 2009;9:e47.
Rosberg H.E., Carlsson K.S., Hojgard S., et al. Injury to the human median and ulnar nerves in the forearm—analysis of costs for treatment and rehabilitation of 69 patients in southern Sweden. J Hand Surg [Br]. 2005;30:35-39.
Sheikh K.A. Noninvasive imaging of nerve regeneration. Exp Neurol. 2010;223:72-76.
Siemionow M., Brzezicki G. Current techniques and concepts in peripheral nerve repair. Int Rev Neurobiol. 2009;87:141-172.
Smith M.A., Muehlberger T., Dellon A.L. Peripheral nerve compression associated with low-voltage electrical injury without associated significant cutaneous burn. Plast Reconstr Surg. 2002;109:137-144.
Spinner R.J., Kline D.G. Surgery for peripheral nerve and brachial plexus injuries or other nerve lesions. Muscle Nerve. 2000;23:680-695.
Strasberg J.E., Atchabahian A., Strasberg S.R., et al. Peripheral nerve injection injury with antiemetic agents. J Neurotrauma. 1999;16:99-107.
Sutter M., Eggspuehler A., Grob D., et al. The diagnostic value of multimodal intraoperative monitoring (MIOM) during spine surgery: a prospective study of 1,017 patients. Eur Spine J. 2007;16(Suppl 2):162-170.
Taylor K.S., Anastakis D.J., Davis K.D. Cutting your nerve changes your brain. Brain. 2009;132:3122-3133.
Tsao B.E., Wilbourn A.J. The medial brachial fascial compartment syndrome following axillary angiography. Neurology. 2003;61:1037-1041.
Tucker B.A., Mearow K.M. Peripheral sensory axon growth: from receptor binding to cellular signalling. Can J Neurosci. 2008;35:551-566.
Turkof E., Millesi H., Trukof R. Intraoperative electroneurodiagnostics (transcranial electrical motor evoked potentials) to evaluate the functional status of anterior spinal roots and spinal nerves during brachial plexus injury. Plast Reconstr Surg. 1997;99:1632-1641.
Tung T.H., Novak C.B., Mackinnon S.E. Nerve transfer to the biceps and brachialis branches to improve elbow flexion after brachial injuries. J Neurosurg. 2003;98:313-318.
Umans H., Kessler J., de la Lama M., et al. Sonographic assessment of volar digital nerve injury in the context of penetrating trauma. Am J Radiol. 2010;194:1310-1313.
Visser L.H. High resolution sonography of the superficial radial nerve with two case reports. Muscle Nerve. 2010;39:392-395.
Walsh S.K., Gordon T., Addas B.M., et al. Skin derived precursor cells enhance peripheral nerve regeneration following chronic denervation. Exp Neurol. 2010;223(1):221-228.
Wilbourn A.J. Brachial plexopathies. In: Katirji B., Kiaminski H., Preston D., et al. Neuromuscular Disorders in Clinical Practice. Boston: Butterworth-Heinemann; 2002:884-906gen.
Yamada S., Lonser R.R., Colohan A.R., et al. Bypass coaptation for cervical root avulsion: indications for optimal outcome. Neurosurgery. 2009;65:A203-A211.
Yan H., Zhang F., Chen M.B., et al. Chapter 10: Conduit luminal additives for peripheral nerve repair. Int Rev Neurobiol. 2009;87:199-225.