Objectives
• Compare and contrast injuries associated with blunt and penetrating trauma.
• Use assessment findings to identify potential complications and sequelae of traumatic injuries.
![]()
Be sure to check out the bonus material, including free self-assessment exercises, on the Evolve web site at
http://evolve.elsevier.com/Urden/priorities/.
Trauma is a leading cause of death for all age groups younger than 44 years. Injury costs the United States hundreds of billions of dollars annually. It is one of the most pressing health problems in the United States today.
Injury as a result of trauma is no longer considered to be an accident. The term motor vehicle accident (MVA) has been replaced with motor vehicle crash (MVC), and the term accident has been replaced with unintentional injury. A program for prevention, recognition, and treatment of intimate partner violence is described in Box 25-1.
Clinicians working with trauma patients, however, are uniquely poised to impact the person who presents to the trauma center following a traumatic event that may be related to drugs or alcohol. The American College of Surgeons Committee on Trauma recommends that all patients presenting to a trauma center be screened for alcohol use and history that could have contributed to the traumatic event that brought them to the trauma center.1 The program of alcohol screening, brief interventions, and recommendations for rehabilitation (SBIRT) reduces recidivism and cost for trauma care.2 There are several alcohol use screening tools available, including the Alcohol Use Disorders Identification Test (AUDIT), and the CAGE tool, which is an acronym for Cut down, Annoyed, Guilty, Eye opener morning alcoholic drink. The AUDIT is outlined in Table 25-1.
TABLE 25-1
AUDIT ALCOHOL SCREENING QUESTIONNAIRE
| QUESTION | SCORE* |
| 1. How often do you have a drink containing alcohol? | Never |
| 2. How many standard drinks containing alcohol do you have on a typical day when drinking? | |
| 3. How often do you have six or more drinks on one occasion? | |
| 4. During the past year, how often have you found that you were not able to stop drinking once you had started? | |
| 5. During the past year, how often have you failed to do what was normally expected of you because of drinking? | |
| 6. During the past year, how often have you needed a drink in the morning to get yourself going after a heavy drinking session? | |
| 7. During the past year, how often have you had a feeling of guilt or remorse after drinking? | |
| 8. During the past year, have you been unable to remember what happened the night before because you had been drinking? | |
| 9. Have you or someone else been injured as a result of your drinking? | |
| 10. Has a relative or friend, doctor or other health worker been concerned about your drinking or suggested you cut down? | |
| Total Points |
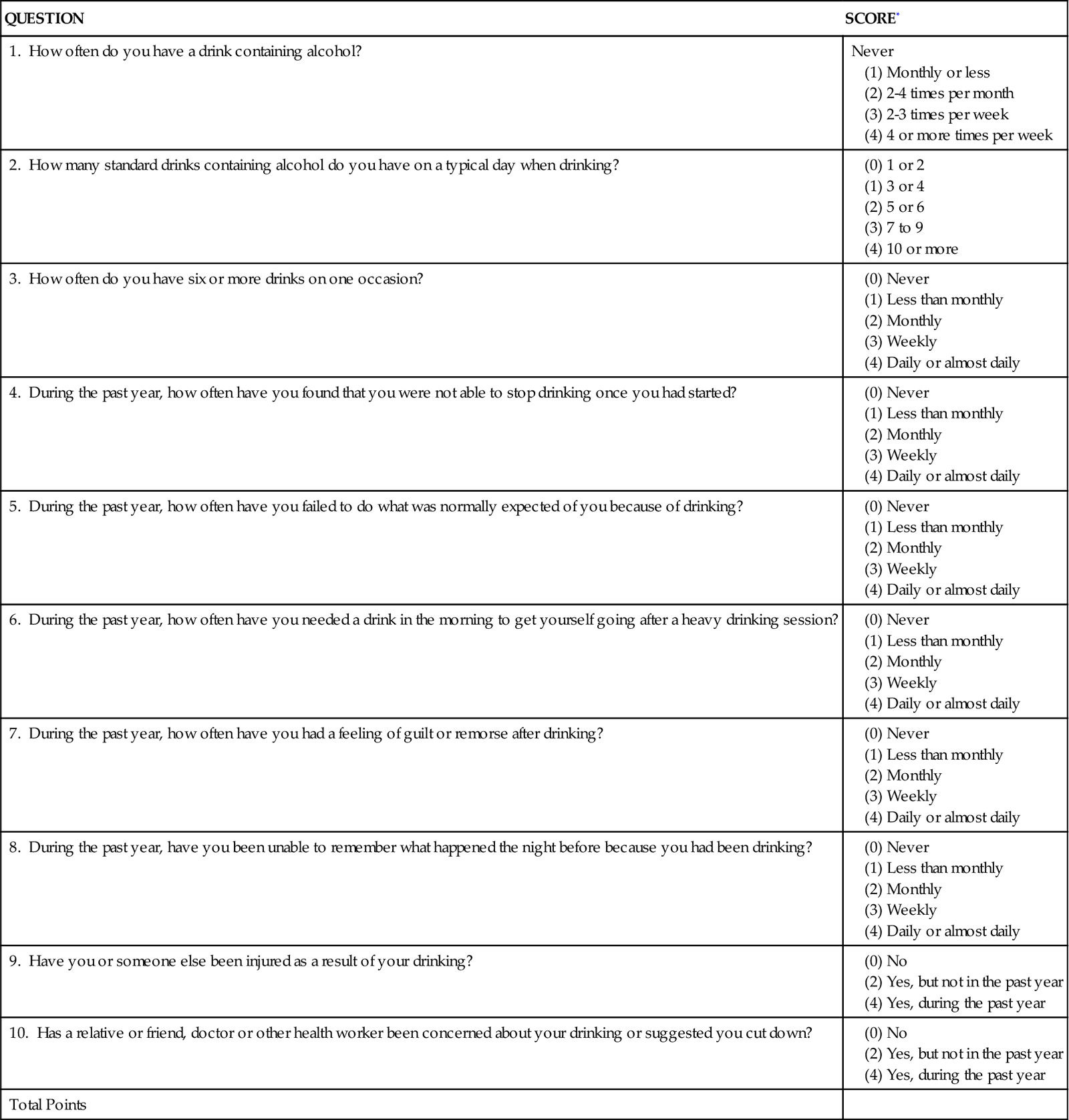
*Scores for each question range from 0 to 4, with the first response for each question (never) scoring 0, the second (less than monthly) scoring 1, the third (monthly) scoring 2, the fourth (weekly) scoring 3, and the fifth response (daily or almost daily) scoring 4. For the last two questions, which only have three responses, the scoring is 0, 2, and 4. A score of 8 or more is associated with harmful or hazardous drinking, and a score of 13 or more by women or 15 or more by men is likely to indicate alcohol dependence.
A patient who screens positive is recommended to undergo “brief interventions” for alcohol use. Brief interventions are by their very name short and are based upon motivational interviewing. Once a rapport is built with the patient, the following motivational-style interview questions may be used:3 1) What is a typical day like for you on a day when you drink? 2) How important is it to you to make a change in your drinking? 3) How confident are you that you can make a change? 4) What do you like and dislike about your drinking habits? 5) How would your life be different if you were to change your drinking? 6) What are some of the most important things to you? These questions serve to help the patient dichotomize the impact of drinking, both positively and negatively.
Major advances have been made in the management of patients with traumatic injuries. This chapter reviews selected critical care nursing management of patients with traumatic injuries.
Mechanisms of Injury
Trauma occurs when an external force of energy impacts the body and causes structural or physiological alterations, or injuries. External forces can be radiation, electrical, thermal, chemical, or mechanical forms of energy. This chapter focuses on trauma from mechanical energy. Mechanical energy can produce blunt or penetrating traumatic injuries. Understanding the mechanism of injury helps health care providers anticipate and predict potential internal injuries.
Blunt Trauma
Blunt trauma is seen most often with MVCs, contact sports, blunt force injuries (e.g., trauma caused by a baseball bat), or falls. Injuries occur because of the forces sustained during a rapid change in velocity (deceleration). To estimate the amount of force sustained in an MVC, multiply the person’s weight by the miles per hour (speed) the vehicle was traveling. A 130-pound woman in a vehicle traveling at 60 miles per hour that hits a brick wall, for example, would sustain 7800 pounds of force within milliseconds. As the body stops suddenly, tissues and organs continue to move forward. This sudden change in velocity causes injuries that result in lacerations or crush injuries of internal body structures.
Penetrating Trauma
Penetrating injuries occur with stabbings, firearms, or impalement—injuries that penetrate the skin and result in damage to internal structures. Damage is created along the path of penetration. Penetrating injuries can be misleading inasmuch as the condition of the outside of the wound does not determine the extent of internal injury. Bullets can create internal cavities 5 to 30 times larger than the diameter of the bullet.
Several factors determine the extent of damage sustained as a result of penetrating trauma. Different weapons cause different types of injuries. The severity of a gunshot wound depends on the type of gun, type of ammunition used, and the distance and angle from which the gun was fired. At close range, shotgun pellets expand on impact and cause multiple injuries to internal structures. From a distance, shotgun pellets cause only minor injuries. Handgun bullets usually damage what is directly in the bullet’s path. Inside the body, the bullet can ricochet off bone and create further damage along its pathway. With penetrating stab wounds, factors that determine the extent of injury include the type and length of object used and the angle of insertion.
Phases of Trauma Care
Care of trauma victims during wartime enhanced principles of triage and rapid transport of the injured to medical facilities. The military experience has demonstrated that decreasing the time from injury to definitive care saves more lives. It also has enhanced incentives and models for improvements in civilian trauma care, such as emergency medical service (EMS) systems and trauma care centers. The goal with critically injured patients is to minimize the time from initial insult to definitive care and to optimize prehospital care so that the patient arrives at the hospital alive.
Nursing management of the patient with traumatic injuries begins the moment a call for help is received and continues until the patient’s death or return to the community. Care of the trauma patient is seen as a continuum that includes six phases: prehospital resuscitation, hospital resuscitation, definitive care and operative phase, critical care, intermediate care, and rehabilitation.
Prehospital Resuscitation
The goal of prehospital care is immediate stabilization and transportation. This is achieved through airway maintenance, control of external bleeding and shock, immobilization of the patient, and immediate transport (ground or air) to the closest appropriate medical facility.4 Prehospital personnel should communicate information needed for triage at the hospital. Advanced planning for the injured patient is essential.
Emergency Department Resuscitation
The American College of Surgeons developed Advanced Trauma Life Support (ATLS) guidelines for rapid assessment, resuscitation, and definitive care for trauma patients in the emergency department.4 The ATLS guidelines delineate a systematic approach to care of the trauma patient: rapid primary survey, resuscitation of vital functions, more detailed secondary survey, and initiation of definitive care. This process constitutes the ABCDEs of trauma care and assists in identifying injuries.
Primary Survey
On arrival of the trauma patient in the emergency department, the primary survey is initiated. During this assessment, life-threatening injuries are discovered and treated. The five steps in the trauma primary survey are performed in ABCDE sequence (Table 25-2):
Airway maintenance with cervical spine protection
Circulation with hemorrhage control
TABLE 25-2
PRIMARY SURVEY OF THE TRAUMA PATIENT
| SURVEY COMPONENT | NURSING DIAGNOSIS | NURSING ASSESSMENT, CARE |
| Airway | Ineffective Airway Clearance related to obstruction or actual injury | Immobilize cervical spine.
• Is there obvious airway trauma, tachypnea, accessory muscle use, tracheal shift? • Stridor, hyperresonance, dullness to percussion? • For air exchange over the mouth; insert finger sweep to clear foreign bodies. Secure airway. |
| Breathing | Ineffective Breathing Pattern related to actual injury Impaired Gas Exchange related to actual injury or disrupted tissue perfusion |
Assess for:
For absent breathing: If breathing, but ineffective: |
| Circulation | Decreased Cardiac Output related to actual injury Alteration in Tissue Perfusion related to actual injury or shock Deficient Fluid Volume related to actual loss of circulating volume |
Assess pulse quality and rate. Use ECG monitoring. If no pulse: If pulse, but ineffective Initiate two large-bore IVs or central catheter; obtain serum samples for laboratory tests. |
| Disability | Ineffective Cerebral Tissue Perfusion Risk for Injury related to actual injury of brain or spinal cord |
Determine Glasgow Coma Scale score. Assess pupil size and reactivity. |
| Exposure or environmental control | Risk for Imbalanced Body Temperature | Remove all clothing to inspect all body regions. Prevent hypothermia. |
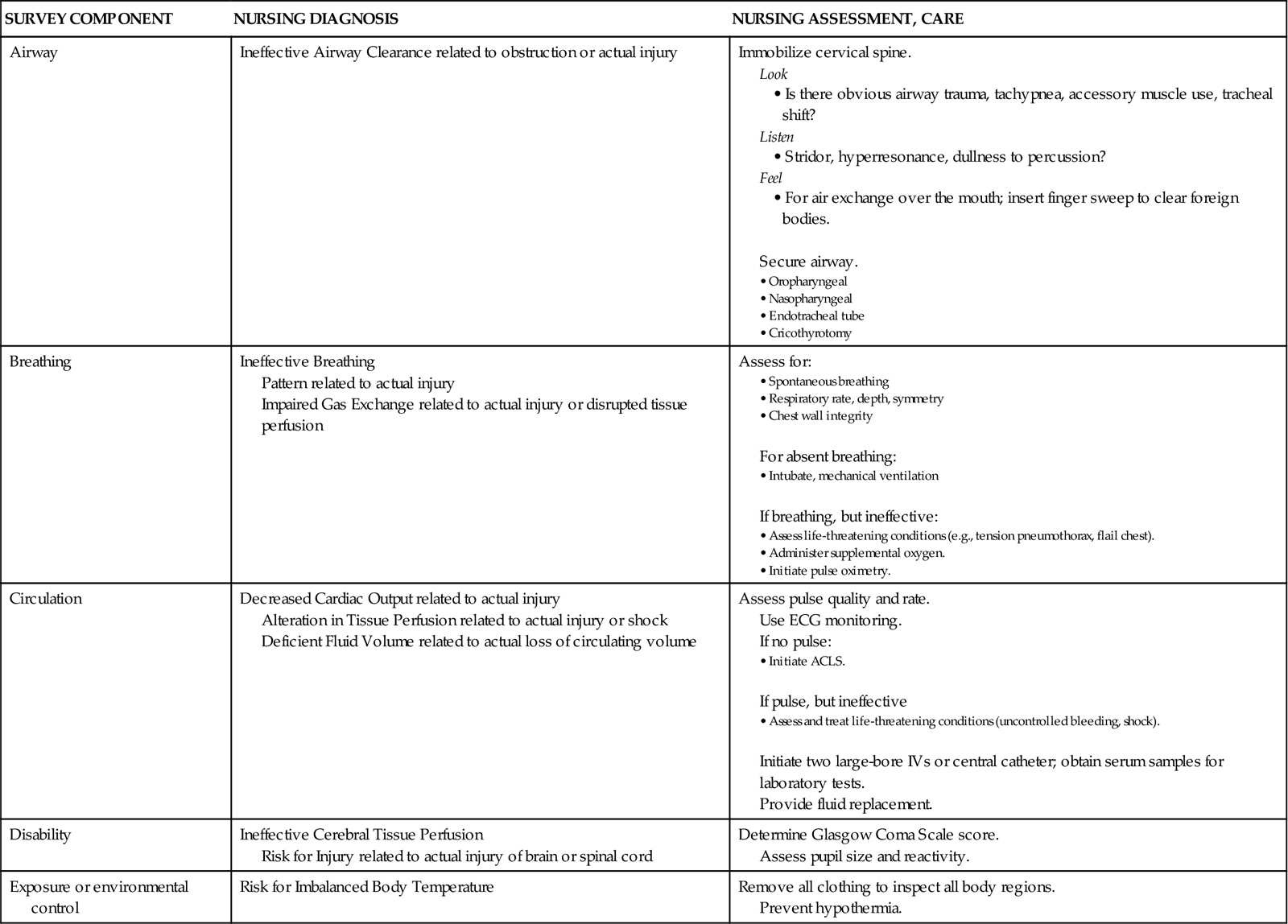
ACLS, advanced cardiac life support; ECG, electrocardiogram; IV, intravenous line.
Resuscitation Phase
Concurrent with the primary survey is the resuscitation phase. Hypovolemic shock is the most common type of shock that occurs in trauma patients.4 Hemorrhage must be identified and treated rapidly. Two large-bore (14- to 16-gauge) peripheral intravenous catheters, a central venous catheter or intraosseous access is inserted. Management of hemorrhagic shock starts with IV fluids followed quickly by O-negative blood or type-specific blood.4 Many trauma centers have developed massive transfusion protocols to make sure adequate blood products are available as needed. Fluid warmers are used to prevent hypothermia. Blood samples are also drawn (Box 25-2).
Placement of urinary and gastric catheters is part of the resuscitation phase. An indwelling urinary catheter can help evaluate urine output as an indicator of volume status and kidney perfusion. A gastric tube is inserted to reduce gastric distention and lower the risk of aspiration.
The resuscitation phase begins in the emergency department and may continue well into the critical care phase. During resuscitation from traumatic hemorrhagic shock, normalization of standard clinical parameters such as blood pressure, heart rate, and urine output are important but do not represent the end-point.5 Optimal resuscitation goals are to improve oxygen tissue delivery and normalize base deficit, lactate, or gastric pH during the first 24 hours after injury.6
Secondary Survey
The secondary survey begins when the primary survey is completed, resuscitation is well established, and the patient is hemodynamically stable. During the secondary survey, a head-to-toe approach is used to thoroughly examine each body region. The history is one of the most important aspects of the secondary survey. Prehospital providers can usually provide vital information pertaining to the unintentional injury. Specific information that must be elicited about mechanism of injury is summarized in Box 25-3. The patient’s pertinent past history can be assessed by use of the mnemonic AMPLE:
During the secondary survey, the nurse ensures the completion of special procedures, such as an electrocardiogram (ECG), radiographic studies, and the FAST exam (Focused Assessment Sonography for Trauma). Throughout the secondary survey, the nurse continuously monitors the patient’s vital signs and response to medical therapies. Emotional support to the patient and family is imperative.
Definitive Care and Operative Phase
After the secondary survey has been completed, specific injuries are diagnosed. Trauma is often referred to as a “surgical disease” because the nature and extent of injuries may require operative management. After surgery, depending on the patient’s status, transfer to a critical care unit may be indicated.
Critical Care Phase
Critically ill trauma patients are admitted into the intensive care unit (ICU) as direct transfers from the emergency department or operating room. Information the ICU nurse must obtain from the emergency department, operating room nurse, or both, is summarized using the SBAR method: Situation, Background, Assessment, and Recommendation (Box 25-4). This information must be obtained before the patient’s admission to the ICU to ensure availability of needed personnel, equipment, and supplies. This information also helps the nurse to assess the impact of trauma resuscitation on the patient’s presentation and course. Table 25-3 summarizes the prehospital, emergency department, and operating room resuscitative measures that can affect the trauma patient’s care in the ICU.
TABLE 25-3
EFFECTS OF TRAUMA RESUSCITATION
| ASPECT OF INJURY OR RESUSCITATION | EFFECT ON ICU COURSE |
| Prolonged extrication time | Gives an indication of length of time patient may have been hypotensive and/or hypothermic before medical care |
| Period of respiratory or cardiac arrest | Effects of loss of perfusion to brain (anoxic injury), kidneys, and other vital organs |
| Time on backboard | Potentiates risk of sacral or occipital breakdown |
| Number of units of blood; whether any were not fully cross-matched; packed cells versus whole blood used | Potentiates risk of ARDS, MODS |
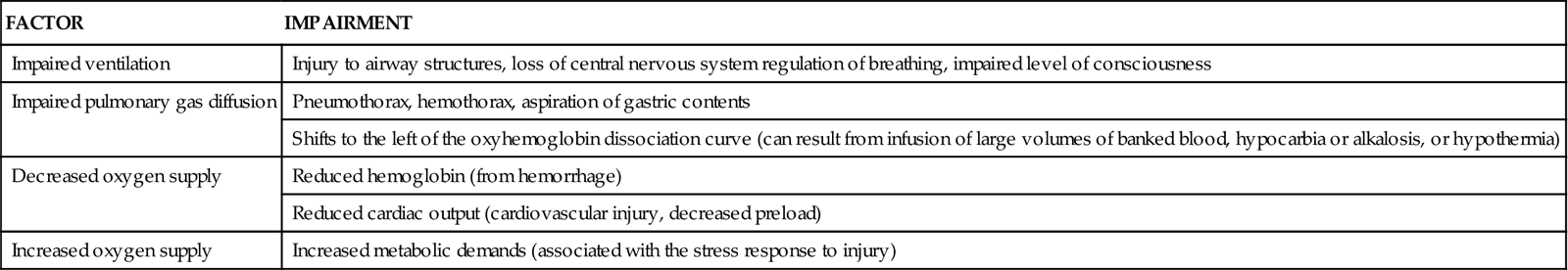
One of the most important nursing roles is assessment of the balance between oxygen delivery and oxygen demand. Oxygen delivery must be optimized to prevent further system damage. Assessment of circulatory status includes the use of noninvasive and invasive techniques. The trauma patient is at high risk for impaired oxygenation as a result of a variety of factors (Table 25-4). These risk factors must be promptly identified and treated to prevent life-threatening sequelae. Prevention and treatment of hypoxemia depend on accurate assessment of the adequacy of pulmonary gas exchange, oxygen delivery, and oxygen consumption.
TABLE 25-4
FACTORS PREDISPOSING THE TRAUMA PATIENT TO IMPAIRED OXYGENATION
| FACTOR | IMPAIRMENT |
| Impaired ventilation | Injury to airway structures, loss of central nervous system regulation of breathing, impaired level of consciousness |
| Impaired pulmonary gas diffusion | Pneumothorax, hemothorax, aspiration of gastric contents |
| Shifts to the left of the oxyhemoglobin dissociation curve (can result from infusion of large volumes of banked blood, hypocarbia or alkalosis, or hypothermia) | |
| Decreased oxygen supply | Reduced hemoglobin (from hemorrhage) |
| Reduced cardiac output (cardiovascular injury, decreased preload) | |
| Increased oxygen supply | Increased metabolic demands (associated with the stress response to injury) |
Frequent and thorough nursing assessments of all body systems are important because these assessments are the cornerstone of the medical and nursing management of the critically ill trauma patient. The nurse can detect subtle changes and facilitate the implementation of timely therapeutic interventions to prevent complications often associated with trauma. The nurse must be knowledgeable about specific organ injuries and their associated sequelae.
Trauma Injuries
Traumatic Brain Injuries
More than 1.7 million traumatic brain injuries (TBIs) occur annually, with approximately 275,000 patients hospitalized as a result of their injuries. Approximately 52,000 Americans die each year of TBI.7 At least 5.3 million Americans are living with disabilities resulting from TBI.8
Mechanism of Injury
TBIs occur when mechanical forces are transmitted to brain tissue. Mechanisms of injury include penetrating or blunt trauma to the head. The leading causes of TBI include falls (35.2%), MVCs (17.3%), struck by or against events (16.5%), and assaults (10%).7 Penetrating trauma can result from the penetration of a foreign object such as a bullet, which causes direct damage to cerebral tissue. Blunt trauma can be the result of deceleration, acceleration, or rotational forces. Deceleration causes the brain to crash against the skull after it has hit something such as the dashboard of a car. Acceleration injuries occur when the brain has been forcefully hit, such as with a baseball bat. Brian injury occurs when the brain moves toward the point of impact (acceleration) and then as the brain reverses direction (deceleration) it hits the other side of the skull. These injuries are described as coup and contrecoup; this injury is shown in Figure 25-1.
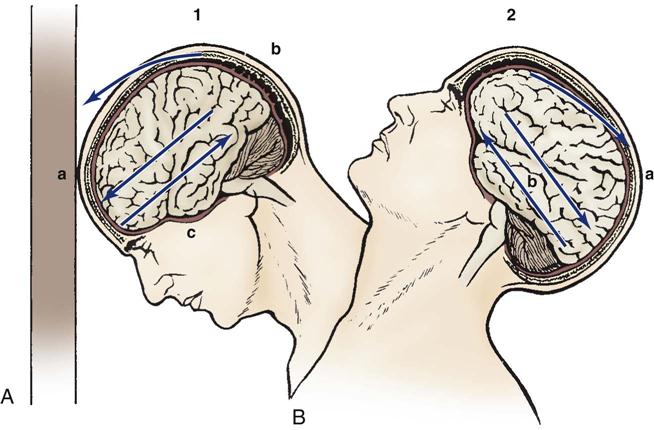
A, Coup injury: impact against object, showing the site of impact and direct trauma to brain (a), shearing of subdural veins (b), and trauma to the base of the brain (c). B, Contrecoup injury: impact within skull, showing the site of impact from brain hitting opposite side of skull (a) and shearing forces throughout brain (b). These injuries occur in one continuous motion; the head strikes the wall (coup) and then rebounds (contrecoup).
Pathophysiology
Review of the pathophysiology of a TBI can be divided into two categories: primary injury and secondary injury. The critical care nurse uses knowledge of this pathophysiology to provide interventions that reduce morbidity and mortality from secondary injury.
Primary Injury.
The primary injury occurs at the moment of impact as a result of mechanical forces to the head. The extent of and recovery from injury are related to whether the primary injury was localized to an area or whether it was diffuse or widespread throughout the brain. Primary injuries may include direct damage to the parenchyma or injury to the vessels that causes hemorrhage, compressing nearby structures. Examples of primary injuries include contusion, laceration, shearing injuries, and hemorrhage. Primary injury may be mild, with little or no neurological damage, or severe, with major tissue damage. Immediately after the injury, a cascade of neural and vascular processes is activated.
Secondary Injury.
Secondary injury is the biochemical and cellular response to the initial trauma that can exacerbate the primary injury and cause loss of brain tissue not originally damaged. Secondary injury can be caused by ischemia, hypercapnia, hypotension, cerebral edema, sustained hypertension, calcium toxicity, or metabolic derangements. Hypoxia or hypotension, the best known culprits for secondary injury, typically are the result of extracranial trauma.9 A detrimental cycle may develop causing a focal primary injury to expand as a result of uncontrolled, refractory secondary injury.
Tissue ischemia occurs in areas of poor cerebral perfusion as a result of hypotension or hypoxia. The cells in ischemic areas become edematous. Extreme vasodilation of the cerebral vasculature occurs in an attempt to supply oxygen to the cerebral tissue. This increase in blood volume increases intracranial volume and raises intracranial pressure (ICP).
Significant hypotension causes inadequate perfusion to neural tissue. Hypotension rarely is associated with TBI. Hypotension typically is not caused by brain injury unless terminal medullary failure occurs.10 If a trauma patient is unconscious and hypotensive, a detailed assessment of the chest, abdomen, and pelvis is performed to rule out internal injuries.
Hypercapnia (elevated CO2) is a powerful vasodilator. Most often caused by hypoventilation in an unconscious patient, hypercapnia results in cerebral vasodilation and increased cerebral blood volume and ICP.
Cerebral edema occurs as a result of the changes in the cellular environment caused by contusion, loss of autoregulation, and increased permeability of the blood-brain barrier. Cerebral edema can be focal as it localizes around the area of contusion, or diffuse as a result of hypotension or hypoxia. Optimizing other aspects of secondary injury, such as oxygenation, ventilation, and perfusion can limit the extent of cerebral edema.
Initial hypertension in the patient with severe TBI is common. Because of the loss of autoregulation, increased blood pressure results in increased intracranial blood volume and ICP. The effects of increased ICP may be varied. As pressure increases inside the closed skull vault, cerebral perfusion decreases, which further compromises the brain. The combined effects of increasing pressure and decreasing perfusion precipitate a downward spiral of events.
Classification
Injuries of the brain are described by the functional changes or losses that occur. Some of the major functional abnormalities seen in head injury are described here.
Skull Fracture.
Skull fractures are common, but they do not by themselves cause neurological deficits. Skull fractures can be classified as open (dura is torn) or closed (dura is not torn), or they can be classified as those of the vault or those of the base. Common vault fractures occur in the parietal and temporal regions. Basilar skull fractures usually are not visible on conventional skull films and a computed tomography (CT) scan is typically required. Assessment findings may include cerebral spinal fluid rhinorrhea (from nose) or otorrhea (from ear), Battle’s sign (ecchymosis overlying the mastoid process behind the ear), “raccoon eyes” (subconjunctival and periorbital ecchymosis), or palsy of the seventh cranial nerve.
The significance of a skull fracture is that it identifies the patient with a higher probability of having or developing an intracranial hematoma. Open skull fractures require surgical intervention to remove bony fragments and to close the dura. The major complications of basilar skull fractures are cranial nerve injury and leakage of cerebrospinal fluid (CSF). CSF leakage may result in a fistula, which increases the possibility of bacterial contamination and resultant meningitis. Because fistula formation may be delayed, patients with a basilar skull fracture are admitted to the hospital for observation and possible surgical intervention.
Concussion.
A concussion is a brain injury accompanied by a brief loss of neurological function, especially loss of consciousness. When loss of consciousness occurs, it may last for seconds to an hour. The neurological dysfunctions include confusion, disorientation, and sometimes a period of antegrade or retrograde amnesia. Other clinical manifestations that occur after concussion are headache, dizziness, nausea, irritability, inability to concentrate, impaired memory, and fatigue. The diagnosis of concussion is based on the loss of consciousness inasmuch as the brain remains structurally intact despite functional impairment.
Contusion.
Contusion, or bruising of the brain, usually is related to acceleration-deceleration injuries, which result in hemorrhage into the superficial parenchyma, often the frontal and temporal lobes. Frontal or temporal contusions are most common and can be seen in a coup-contrecoup mechanism of injury (see Figure 25-1). Coup injury affects the cerebral tissue directly under the point of impact. Contrecoup injury occurs in a line directly opposite the point of impact.
The clinical manifestations of contusion are related to the location of the contusion, the degree of contusion, and the presence of associated lesions. Contusions can be small, in which localized areas of dysfunction result in a focal neurological deficit. Larger contusions can evolve over 2 to 3 days after injury as a result of edema and further hemorrhaging. A large contusion can produce a mass effect that can cause a significant increase in ICP.
Contusions of the tips of the temporal lobe are a common occurrence and are of particular concern. Because the inner aspects of the temporal lobe surround the opening in the tentorium where the midbrain enters the cerebrum, edema in this area can cause rapid deterioration of the patient’s condition and can lead to herniation. Because of the location, this deterioration can occur with little or no warning at a deceptively low ICP.
Medical management of cerebral contusions may consist of medical or surgical therapies. Because a contusion can progress over 3 to 5 days after injury, secondary injury may occur. If contusions are small, focal, or multiple, they are treated medically with serial neurological assessments and possibly with ICP monitoring. Larger contusions that produce considerable mass effect require surgical intervention to prevent the increased edema and ICP as the contusion matures. Outcome of cerebral contusion varies, depending on the location and the degree of contusion.
Cerebral Hematomas.
Extravasation of blood creates a space-occupying lesion within the cranial vault that can lead to increased ICP. Three types of hematomas are discussed here (Figure 25-2). The first two, epidural and subdural hematomas, are extraparenchymal (outside of brain tissue) and produce injury by pressure effect and displacement of intracranial contents. The third type, intracerebral hematoma, directly damages neural tissue and can produce further injury as a result of pressure and displacement of intracranial contents.
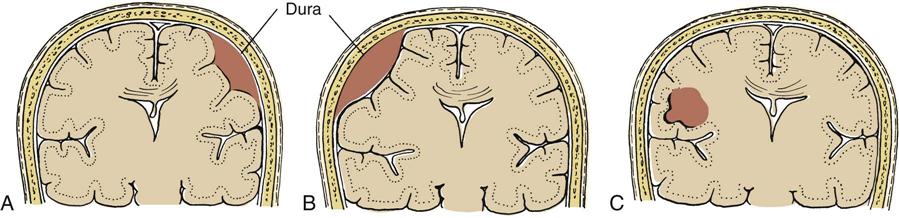
A, Subdural hematoma. B, Epidural hematoma. C, Intracerebral hematoma.
Epidural Hematoma.
Epidural hematoma (EDH) is a collection of blood between the inner table of the skull and the outermost layer of the dura. EDHs are most often associated with patients with skull fractures and middle meningeal artery lacerations (two thirds of patients) or skull fractures with venous bleeding.4 A blow to the head that causes a linear skull fracture on the lateral surface of the head may tear the middle meningeal artery. As the artery bleeds, it pulls the dura away from the skull, creating a pouch that expands into the intracranial space.
The incidence of EDH is relatively low. EDH can occur as a result of low-impact injuries (e.g., falls) or high-impact injuries (e.g., MVCs). EDH occurs from trauma to the skull and meninges rather than from the acceleration-deceleration forces seen in other types of head trauma.
The classic clinical manifestations of EDH include brief loss of consciousness followed by a period of lucidity. Rapid deterioration in the level of consciousness should be anticipated because arterial bleeding into the epidural space can occur quickly. A dilated and fixed pupil on the same side as the impact area is a hallmark of EDH.4 The patient may complain of a severe, localized headache and may be sleepy. Diagnosis of EDH is based on clinical symptoms and evidence of a collection of epidural blood identified on the CT scan. Treatment of EDH involves surgical intervention to remove the blood and to cauterize the bleeding vessels.
Subdural Hematoma.
Subdural hematoma (SDH), which is the accumulation of blood between the dura and the underlying arachnoid membrane, most often is related to a rupture in the bridging veins between the cerebral cortex and the dura. Acceleration-deceleration and rotational forces are the major causes of SDH, which often is associated with cerebral contusions and intracerebral hemorrhage. SDH is common, representing about 30% of severe head injuries. The three types of SDH—acute, subacute, and chronic—are based on the time frame from injury to clinical symptoms.
Acute Subdural Hematoma.
Acute SDHs are hematomas that occur after a severe blow to the head. The clinical presentation of acute SDH is determined by the severity of injury to the underlying brain at the time of impact and the rate of blood accumulation in the subdural space. In other situations, the patient has a lucid period before deterioration. Careful observation for deterioration of the level of consciousness or lateralizing signs, such as inequality of pupils or motor movements, is essential. Rapid surgical intervention—including craniectomy, craniotomy, or burr hole evacuation—and aggressive intervention can reduce mortality.
Subacute Subdural Hematoma.
Subacute SDHs are hematomas that develop symptomatically 2 days to 2 weeks after trauma. In subacute SDHs, the expansion of the hematoma occurs at a rate slower than that in acute SDH, and it takes longer for symptoms to become obvious. Clinical deterioration of the patient with a subacute SDH is slower than with an acute SDH, but treatment by surgical intervention, when appropriate, is identical.
Chronic Subdural Hematoma.
Chronic SDH is diagnosed when symptoms appear days or months after injury. Most patients with chronic SDH are older or in late middle age. Patients at risk for chronic SDH include those with coordination or balance disturbances, older adults, and those receiving anticoagulation therapy. Clinical manifestations of chronic SDH are insidious. The patient may report a variety of symptoms, such as lethargy, absent-mindedness, headache, vomiting, stiff neck, and photophobia and may show signs of transient ischemic attack, seizures, pupillary changes, or hemiparesis. Because a history of trauma often is not significant enough to be recalled, chronic SDH seldom is seen as an initial diagnosis. CT evaluation can confirm the diagnosis of chronic SDH.
If surgical intervention is required, evacuation of the chronic SDH may occur by craniotomy, burr holes, or catheter drainage. Evacuation by burr hole involves drilling a hole in the skull over the site of the chronic SDH and draining the fluid. Drains or catheters are left in place for at least 24 hours to facilitate total drainage. Outcome after chronic SDH evacuation varies. Return of neurological status often depends on the degree of neurological dysfunction before removal. Because this condition is most common in older or debilitated patients, recovery is a slow process. Recurrence of chronic SDH is not infrequent.
Intracerebral Hematoma.
Intracerebral hematoma (ICH) results when bleeding occurs within cerebral tissue. Traumatic causes of ICH include depressed skull fractures, penetrating injuries (bullet, knife), or sudden acceleration-deceleration motion. The ICH can act as a rapidly expanding lesion; late ICH into the necrotic center of a contused area also is possible. Sudden clinical deterioration of a patient 6 to 10 days after trauma may be the result of ICH.
Medical management of ICH may include surgical or nonsurgical treatment. Hemorrhages that do not cause significant ICP elevation are treated without surgery. Over time, the hemorrhage may be reabsorbed. If significant problems with ICH mass effect occur, surgical removal is necessary. The outcome of a patient with an ICH depends greatly on the location of the hemorrhage. Size, mass effect, and displacement of other intracranial structures also affect the outcome.
Missile Injuries.
Missile injuries penetrate the skull and produce significant focal damage, but little acceleration-deceleration or rotational injury. The injury may be depressed, penetrating, or perforating (Figure 25-3). Depressed injuries are caused by fractures of the skull, with penetration of bone into cerebral tissue. A low-velocity penetrating injury (knife) may involve only focal damage and no loss of consciousness. A high-velocity missile (bullet) can produce shock waves that are transmitted throughout the brain in addition to the injury caused by the bullet. Perforating injuries are missile injuries that enter and then exit the brain. Perforating injuries have much less ricochet effect but are still responsible for significant injury.
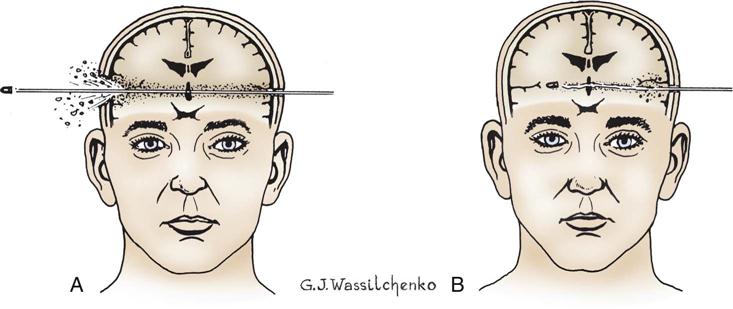
A bullet wound or other penetrating missile wounds cause an open (compound) skull fracture and damage to brain tissue. Shock wave effects are transmitted throughout the brain. A, Perforating injury. B, Penetrating injury.
Risk of infection and cerebral abscess is a concern in cases of missile injuries. If fragments of the missile are embedded within the brain, careful consideration of the location and risk of increasing neurological deficit is weighed against the risk of abscess or infection. The outcome after missile injury is based on the degree of penetration, the location of the injury, and the velocity of the missile.
Diffuse Axonal Injury.
Diffuse axonal injury (DAI) is a term used to describe prolonged posttraumatic coma that is typically not caused by a mass lesion. DAI covers a wide range of brain dysfunction typically caused by acceleration-deceleration and rotational forces. DAI occurs as a result of damage to the axons or disruption of axonal transmission of the neural impulses.
The pathophysiology of DAI is related to the stretching and tearing of axons as a result of movement of the brain inside the cranium at the time of impact. The stretching and tearing of axons result in microscopic lesions throughout the brain, but especially deep within cerebral tissue and the base of the cerebrum. Disruption of axonal transmission of impulses results in loss of consciousness. Unless surrounding tissue areas are significantly injured, causing small hemorrhages, DAI may not be visible on CT or magnetic resonance imaging (MRI). DAI can be classified as one of three grades based on the extent of lesions: mild, moderate, or severe. The patient with mild DAI may be in a coma for 24 hours and may exhibit periods of decorticate and decerebrate posturing. Patients with moderate DAI may be in a coma for longer than 24 hours and exhibit periods of decorticate and decerebrate posturing. Severe DAI usually manifests as a prolonged, deep coma with periods of hypertension, hyperthermia, and excessive sweating. Treatment of DAI includes support of vital functions and maintenance of ICP within normal limits. The outcome after severe DAI is poor because of the extensive dysfunction of cerebral pathways.
Neurological Assessment of Traumatic Brain Injury
The neurological assessment is the most important tool for evaluating the patient with a severe TBI, because it can indicate the severity of injury, provide prognostic information, and dictate the speed with which further evaluation and treatment must proceed. The cornerstone of the neurological assessment is the Glasgow Coma Scale (GCS),4 although it is not a complete neurological examination. Pupils and motor strength assessment must be incorporated into the early and ongoing assessments. After injuries are specifically identified, a more thorough, focused neurological assessment, such as examination of the cranial nerves, is warranted. To assist with the initial assessment, TBIs are divided into three descriptive categories—mild, moderate, or severe—on the basis of the patient’s GCS score and duration of the unconscious state.
Degree of Injury
Mild Injury.
Mild TBI is described as a GCS score of 13 to 15, with a loss of consciousness that lasts up to 15 minutes. Patients with mild injury often are seen in the emergency department and discharged home with a family member who is instructed to evaluate the patient routinely and to bring the patient back to the hospital if any further neurological symptoms appear.
Moderate Injury.
Moderate TBI is described as a GCS score of 9 to 12, with a loss of consciousness for up to 6 hours. Patients with this type of TBI usually are hospitalized. They are at high risk for deterioration from increasing cerebral edema and ICP, and serial clinical assessments are an important function of the nurse. Hemodynamic and ICP monitoring and ventilatory support may not be required for these patients unless other systemic injuries make them necessary. A CT scan usually is obtained on admission. Repeat CT scans are indicated if the patient’s neurological status deteriorates.
Severe Injury.
Patients with a GCS score of 8 or less after resuscitation or those who deteriorate to that level within 48 hours of admission have a severe TBI. Patients with severe TBI often receive ventilatory support along with ICP and hemodynamic monitoring. A CT scan is performed to rule out mass lesions that can be surgically removed. Patients are placed in a critical care setting for continual assessment, monitoring, and management.
Nursing Assessment of the Patient with Traumatic Brain Injury
As in all traumatic injuries, evaluation of the airway, breathing, and circulation (ABCs) is the first step in the assessment of the patient with TBI in the ICU. After stabilization of the ABCs is ensured, a neurological assessment is performed.
Level of consciousness, motor movements, pupillary response, respiratory function, and vital signs are all part of a complete neurological assessment in the patient with a TBI. Level of consciousness to assess wakefulness is elicited by obtaining the patient’s response to verbal and painful stimuli. Determination of orientation to person, place, and time assesses mental alertness. Pupils are assessed for size, shape, equality, and reactivity. Pupil asymmetry must be reported immediately. Pupils are also assessed for constriction to a light source (parasympathetic innervation) or dilation (sympathetic innervation). A dilated or “blown” pupil can be caused by compression of the third ocular nerve or by transtentorial herniation.
Neurological assessments are ongoing as part of the initial shift assessment and as part of ongoing assessments to detect subtle deterioration. Serial assessments include hemodynamic status and ICP monitoring. The use of muscle relaxants and sedation for ICP control can mask neurological signs in the patient with a severe head injury. In these situations, observations for changes in pupils and vital signs become extremely important. Sedatives with a very short half-life, such as propofol, can be turned off, and within minutes, a neurological examination can be performed.
Diagnostic Procedures
The cornerstone of diagnostic procedures for evaluation of TBI is the CT scan. CT is a rapid, noninvasive procedure that can provide invaluable information about the presence of mass lesions and cerebral edema. Serial CT scans may be used over a period of several days to assess areas of contusion and ischemia and to detect delayed hematomas. A nurse must always remain with a TBI patient during a CT scan to provide continued observation and monitoring and during transport to and from the scanner. Transporting the patient, moving the patient from the bed to the CT table, and positioning the head flat during the CT scan are all stressful events and can cause severe increases in ICP. Continuous monitoring enables rapid intervention.
Medical Management
Surgical Management.
If a lesion identified on CT is causing a shift of intracranial contents or increasing ICP, surgical intervention is necessary. Space-occupying hematomas such as EDH or SDH are removed via craniotomy. To alleviate excessive intracranial pressure and prevent herniation, a part of the skull may be removed (decompressive craniectomy). Patients who have had penetrating head trauma have an increased incidence of posttraumatic seizures, and may receive anticonvulsants.
Nonsurgical Management.
Most of the TBI management occurs in the ICU. Nonsurgical management includes management of ICP, maintenance of adequate cerebral perfusion pressure and oxygenation, and treatment of any complications (e.g., pneumonia, infection). ICP monitoring may be required for patients with a GCS score less than 8 and abnormal findings on a head CT scan.9
Nursing Management
Nursing priorities in management of traumatic brain injury focus on (1) stabilizing vital signs, (2) preventing further injury, and (3) reducing increased ICP and maintaining adequate CPP.
Nursing diagnoses for the patient with TBI are listed in the Nursing Diagnosis Priorities Box on Traumatic Brain Injury. Ongoing nursing assessments are the cornerstone of the care of patients with TBI. These assessments are the primary mechanism for determining secondary brain injury from cerebral edema and increased ICP.
Hemodynamic and fluid management are vital. Arterial blood pressure should be monitored because hypotension in a patient with TBI is rare and may indicate additional injuries. Cerebral perfusion pressure (CPP) should be maintained at a minimum of 60 mm Hg.9 If secondary injury is to be prevented, the critical care nurse must respond immediately to hypotensive events and, in collaboration with physicians, maximize cerebral perfusion pressure through reduction of ICP and restoration of mean arterial pressure.9
Aggressive pulmonary care must be instituted. However, endotracheal suctioning can elevate ICP. Techniques to eliminate elevation in ICP with suctioning are outlined in Box 25-5. Cerebral oxygen consumption is increased during periods of increased body temperature, and therefore normothermia (36° to 37° C) is achieved with use of antipyretics and cooling measures.
In the early postinjury phase, the patient’s environment must be controlled. Stimuli that produce pain, agitation, or discomfort can increase ICP. Analgesics and sedatives are administered, and patients are given rest periods.
After ICP stabilization, stimulation programs for patients in a coma may be employed. These programs provide stimulation to the tactile, kinesthetic, olfactory, gustatory, auditory, and visual senses. Several methods have been used to stimulate coma patients with various degrees of intensity:
• Formalized Not-Intensive Stimulation Program: cycles of stimulation of 10 to 60 minutes twice daily
Whatever program is used, a stimulation schedule should be established. Accurate documentation of the stimulus and response is essential. Coma stimulation programs should be individualized and family members encouraged to participate.
Spinal Cord Injuries
Approximately 12,000 new spinal cord injuries (SCIs) occur annually. Of the new cases of SCI each year, about 4000 patients will die before arrival to the hospital, and 1000 patients will die of complications of their SCI during hospitalization.11 The diagnosis of SCI begins with a detailed history of events surrounding the incident, precise evaluation of sensory and motor function, and radiographic studies of the spine.
Mechanism of Injury
The type of primary injury sustained depends on the mechanism of injury. Mechanisms of injury can include hyperflexion, hyperextension, rotation, axial loading (vertical compression), and missile or penetrating injuries.
Hyperflexion.
Hyperflexion injury most often is seen in the cervical area, especially at the level of C5 to C6, because this is the most mobile portion of the cervical spine. This type of injury most often is caused by sudden deceleration motion, as in head-on collisions. Injury occurs from compression of the cord as a result of fracture fragments or dislocation of the vertebral bodies. Instability of the spinal column occurs because of the rupture or tearing of the posterior muscles and ligaments.
Hyperextension.
Hyperextension injuries involve backward and downward motion of the head. With this injury, often seen in rear-end collisions or MVCs, the spinal cord is stretched and distorted. Neurological deficits associated with this injury are often caused by contusion and ischemia of the cord without significant bony involvement. A mild form of hyperextension is the whiplash injury.
Rotation.
Rotation injuries often occur in conjunction with a flexion or extension injury. Severe rotation of the neck or body results in tearing of the posterior ligaments and displacement (rotation) of the spinal column.
Axial Loading.
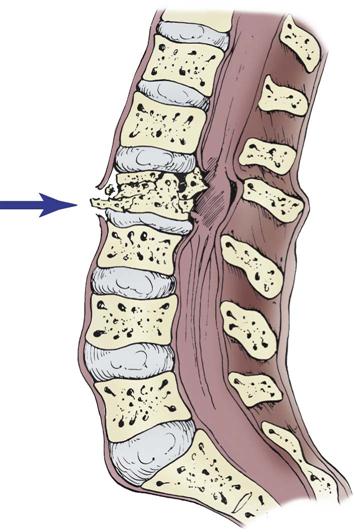
Axial loading—or vertical compression—injuries occur from vertical force along the spinal cord. This most commonly is seen in a fall from a height in which the person lands on the feet or buttocks. Compression injuries cause burst fractures of the vertebral body that often send bony fragments into the spinal canal or directly into the spinal cord (Figure 25-4).
Penetrating Injuries.
Injury to the spinal cord can result from a bullet, a knife, or any other object that penetrates the cord. These types of injury cause permanent damage by transection of the spinal cord.
Pathophysiology
SCIs are the result of a mechanical force that disrupts neurological tissue or its vascular supply, or both. Much like the pathophysiology of TBI, the injury process includes primary and secondary injury mechanisms. Primary injury is the neurological damage that occurs at the moment of impact. Secondary injury refers to the complex biochemical processes affecting cellular function. Secondary injury can occur within minutes of injury and can last for days to weeks.11
Several events after an SCI lead to spinal cord ischemia and loss of neurological function. A cascade of events is initiated that includes systemic and local vascular changes, electrolyte and biochemical changes, neurotransmitter accumulation, and local edema (Box 25-6). Collectively, these pathophysiological events result in worsening of the injury, potentially extending the level of functional deficit and worsening long-term outcome.11 Knowledge of the pathophysiology of secondary processes has led to the development of new drugs that target the cellular changes contributing to injury.11 Despite ongoing research efforts at repairing the primary injury, minimizing damage by reducing secondary injury has shown the most promise.
Functional Injury of the Spinal Cord
Functional injury of the spinal cord refers to the degree of disruption of normal spinal cord function. This depends on what specific sensory and motor structures within the cord are damaged. SCIs are classified as complete or incomplete. SCI cannot be classified until spinal shock has resolved.
Complete Injury.
Complete SCI results in a total loss of sensory and motor function below the level of injury. Regardless of the mechanism of injury, the result is a complete dissection of the spinal cord and its neurochemical pathways, resulting in one of two conditions: tetraplegia or paraplegia.
Tetraplegia.
With tetraplegia, the injury occurs from the C1 to T1 level. Residual muscle function depends on the specific cervical segments involved. The potential functional status resulting from different neurological levels of injury is described in Table 25-5.
TABLE 25-5
QUADRIPLEGIA/TETRAPLEGIA FUNCTIONAL STATUS
| NEUROLOGICAL LEVEL (VERTEBRAE) OF COMPLETE INJURY | FUNCTIONAL ABILITY |
| C1-C4 | Requires electric wheelchair with breath, head, or shoulder controls |
| C5 | Needs electric wheelchair with hand control and/or manual wheelchair with rim projections; may require adaptive devices to assist with ADLs |
| C6 | Independent in manual wheelchair on level surface; may need hand controls; adaptive devices may be needed for ADLs |
| C7 | Requires manual wheelchair on most surfaces |
| C8-T1 | May need adaptive devices |
Paraplegia.
With paraplegia, the injury occurs in the thoracolumbar region (T2 to L1). Patients with injuries in this area may have full use of the arms but need a wheelchair. Thoracic L1 and L2 injuries produce paraplegia with variable innervation to intercostal and abdominal muscles.
Incomplete Injury.
Incomplete SCI results in a mixed loss of voluntary motor activity and sensation below the level of the lesion. Incomplete SCI exists if any function remains below the level of injury. Incomplete injuries can result in a variety of syndromes, which are classified according to the degree of motor and sensory loss below the level of injury.
Spinal Shock.
Spinal shock is a condition that can occur shortly after traumatic injury to the spinal cord. Spinal shock is the complete loss of all muscle tone and normal reflex activity below the level of injury.4 Patients with spinal shock may appear completely without function below the area of the injury, although all of the area may not necessarily be destroyed.
Neurogenic Shock.
Neurogenic shock results from injury to the descending sympathetic pathways in the spinal cord. This results from loss of vasomotor tone and sympathetic innervation to the heart. A relative hypovolemia and hypovolemic shock ensues, causing hypotension and decreased systemic vascular resistance. Patients with SCI at T6 or above may have profound neurogenic shock as a result of interruption of the sympathetic nervous system and loss of vasoconstrictor response below the level of the injury. Blood vessels cannot constrict, and the heart rate is slow, which results in hypotension, venous pooling, and decreased cardiac output. Cellular oxygenation is threatened as cardiac output declines because of a decrease in stroke volume (hypovolemia) and heart rate (bradycardia). The duration of this shock state can persist for up to 1 month after injury. Blood pressure support may be required with the use of sympathomimetic drugs (medications that mimic the actions of the sympathetic nervous system). Orthostatic blood pressure changes, leading to hypotension, can occur during change in head-of-bed position, or repositioning in bed.
Autonomic Dysreflexia.
Autonomic dysreflexia is a life-threatening complication that may occur with SCI. This condition is caused by a massive sympathetic response to any noxious stimuli (e.g., full bladder, line insertions, fecal impaction), which results in bradycardia, hypertension, facial flushing, and headache. Immediate intervention is needed to prevent cerebral hemorrhage, seizures, and acute pulmonary edema. Treatment is aimed at alleviating the noxious stimuli. A clinical algorithm for treatment of autonomic dysreflexia is provided in Box 25-7.12 If symptoms persist, antihypertensive agents can be administered to reduce blood pressure. Prevention of autonomic dysreflexia is imperative and can be accomplished through the use of a comprehensive bowel and bladder program.
Assessment
On admission to the ICU, attention to the ABCs is imperative in the patient with known or suspected SCI. Stabilization of the spinal cord is mandatory to prevent further injury, and spinal precautions are maintained until the spine is cleared of injury. Stabilization in the ICU may include the use of bed rest with log-rolling maneuvers and a hard cervical collar until definitive stabilization is achieved. After the ABCs have been evaluated and interventions for life-threatening complications have been initiated, a full physical assessment is made to determine the extent of injury.
Airway.
Assessment of ABCs is essential to ensure optimal oxygenation and perfusion to all vital organs, including the spinal cord. Complete cardiovascular and respiratory assessments are essential to the patient’s survival and prognosis. The primary assessment begins with an evaluation of airway clearance. In an unresponsive person, an oral airway is inserted while the patient’s neck is maintained in a neutral position. The patient must undergo intubation before severe hypoxia can occur, which could further damage the spinal cord.
Breathing.
Assessment of breathing patterns and gas exchange is made after an airway has been secured. The level of injury dictates the degree of altered breathing patterns and gas exchange (Table 25-6). Because complete injuries above the C3 level result in paralysis of the diaphragm, patients with these injuries require mechanical ventilatory support.
TABLE 25-6
EFFECTS OF SPINAL CORD INJURY ON VENTILATORY FUNCTIONS
| NEUROLOGICAL LEVEL (VERTEBRAE) OF COMPLETE INJURY | RESPIRATORY FUNCTION | COMMENT |
| C1-C2 | Paralysis of diaphragm | Ventilator dependent |
| C3-C5 | Various degrees of diaphragm paralysis | Some diaphragm control; may need ventilatory support; weaning depends on preinjury pulmonary status |
| C6-T11 | Various degrees of impaired intercostal muscles and abdominal muscles | Compromised respiratory function; reduced inspiratory ability; paradoxical breathing patterns; ineffective cough, sneeze |
Modified from Moore EE, et al: Organ injury scaling, Surg Clin North Am 75(2):293, 1995.
Circulation.
Assessment of cardiac output and tissue perfusion is imperative to detect life-threatening injuries and promote recovery of injured spinal cord tissue. The patient with SCI is at high risk for developing alterations in cardiac output and tissue perfusion because the cardiovascular system is subjected to a variety of serious and potential physiological alterations, including dysrhythmias, cardiac arrest, orthostatic hypotension, emboli, and thrombophlebitis.
The patient with an SCI is assessed for adequate tissue perfusion by means of invasive and noninvasive hemodynamic monitoring techniques. Cardiac monitoring is required to detect bradycardia and other dysrhythmias that occur in response to reflex vagus activity mediated by the dominant parasympathetic nervous system, as well as changes in heart rhythm as a result of hypothermia or hypoxia.
Neurological Assessment for Spinal Cord Injury.
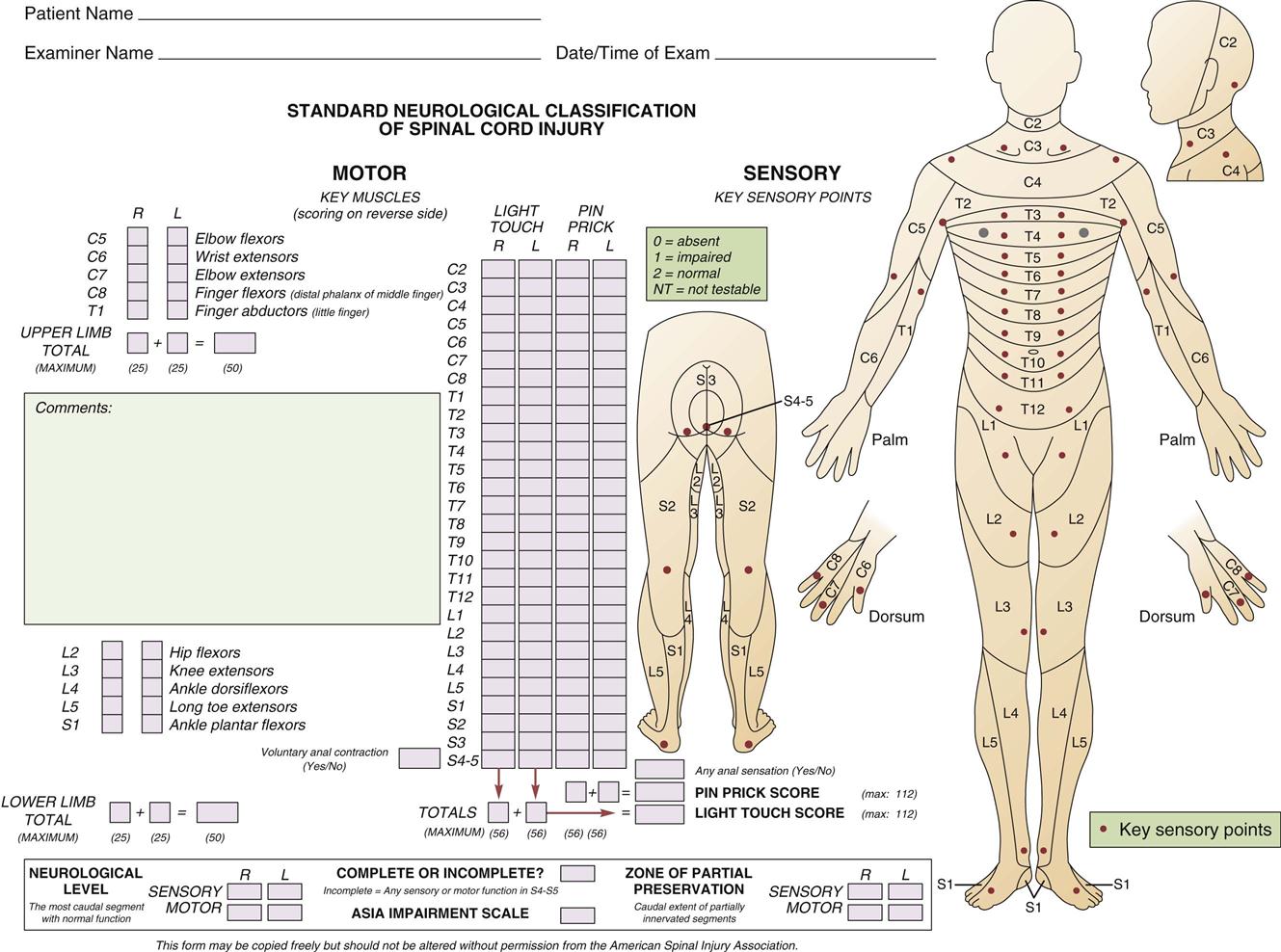
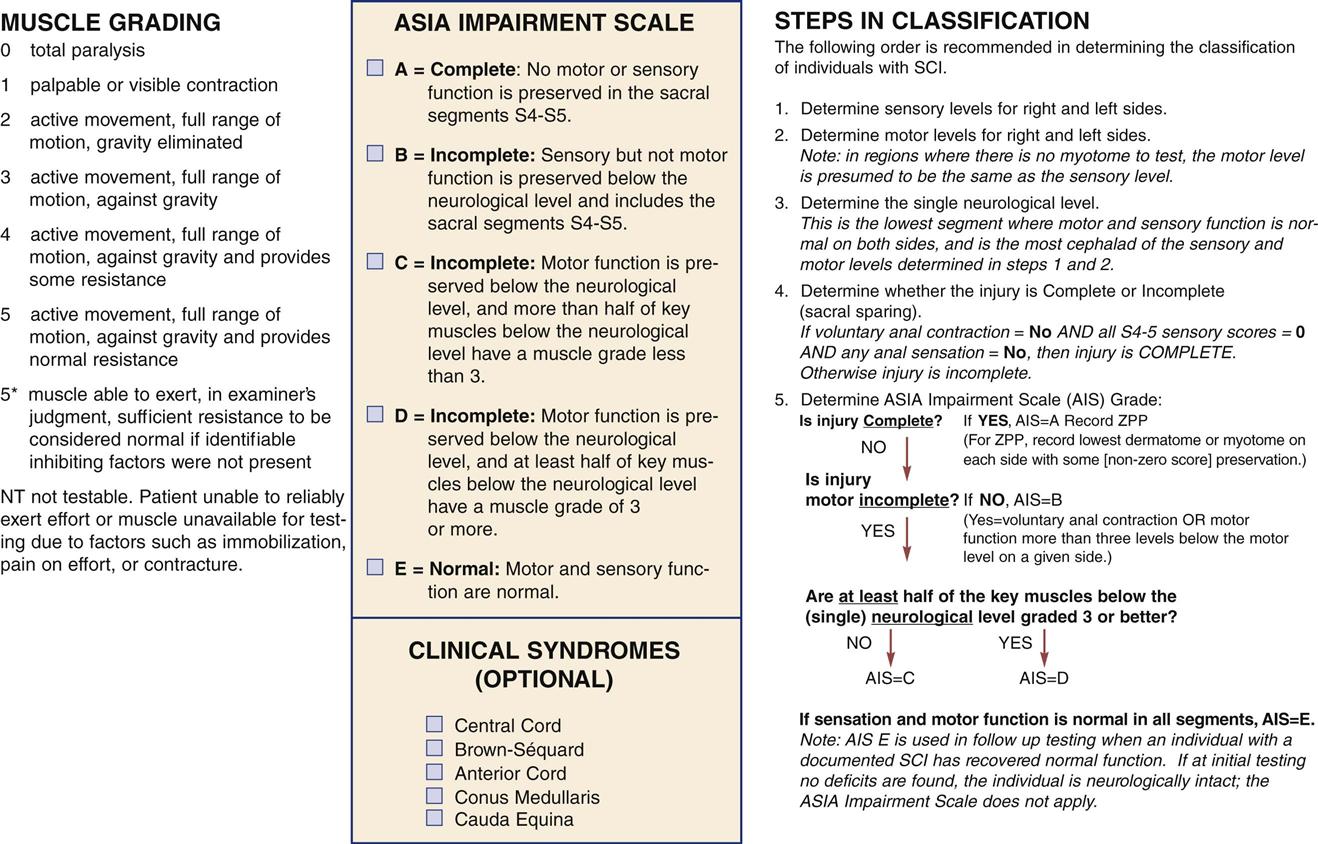
The initial neurological assessment may not be an accurate indication of eventual motor and sensory loss. It focuses on the rapid and accurate identification of present, absent, or impaired functioning of the motor, sensory, and reflex systems that coordinate and regulate vital functions. A detailed motor and sensory examination includes the assessment of all 32 spinal nerves for evidence of dysfunction. Carefully mapped pathways for the sensory portion of the spinal nerves, called dermatomes, can assist in localizing the functional sensory level of injury. Motor function may be graded on a 6-point scale (Table 25-7). Initial assessment must be performed correctly and findings thoroughly documented in detail so that subsequent serial assessments can rapidly identify deterioration. The American Spinal Injury Association (ASIA) has developed a form that outlines the required assessments for initial and ongoing classification of SCIs (Figure 25-5). Ongoing spinal cord assessments must be documented during the critical care phase.
TABLE 25-7
| ASSESSMENT FINDING | GRADE OF STRENGTH |
| Active movement against maximal resistance | 5 |
| Active movement through range of motion against resistance | 4 |
| Active movement through range of motion against gravity | 3 |
| Active movement through range of motion with gravity eliminated | 2 |
| Flicker or trace of contraction | 1 |
| No contraction; total paralysis | 0 |
Diagnostic Procedures.
Diagnostic radiographic evaluations can identify the severity of damage to the spinal cord. Initial evaluation includes anteroposterior and lateral views for all areas of the spinal cord. A CT scan of all seven cervical vertebrae and the top of T1 must be obtained to rule out cervicothoracic junction injury. Flexion and extension views can identify subtle ligament injuries. Tomography, myelography, and MRI also may be used.
Screening for Spinal Cord Injury.
About 15% of trauma patients with an SCI have a cervical spine injury.10 Screening of the spinal cord for injury becomes an integral part of the assessment for all trauma patients. The degree of trauma, alteration in mentation, intoxication, and distracting injuries dictate the type and extent of examination required to clear the cervical spine. The Eastern Association of Surgeons in Trauma (EAST) developed guidelines for the clearance of the cervical spine (Table 25-8). In these guidelines, CT scan has replaced plain radiography as the principal modality for cervical spine assessment following trauma. On admission, the spine is palpated for obvious deformity, and the patient is assessed for the subjective response of pain to palpation. If the patient is intoxicated, has distracting injuries such as rib fractures, or has received analgesics, examination of the spinal cord may be deferred.10 MRI may be warranted to definitively diagnose an SCI when the patient is stabilized.
TABLE 25-8
EAST GUIDELINES FOR CERVICAL SPINE CLEARANCE
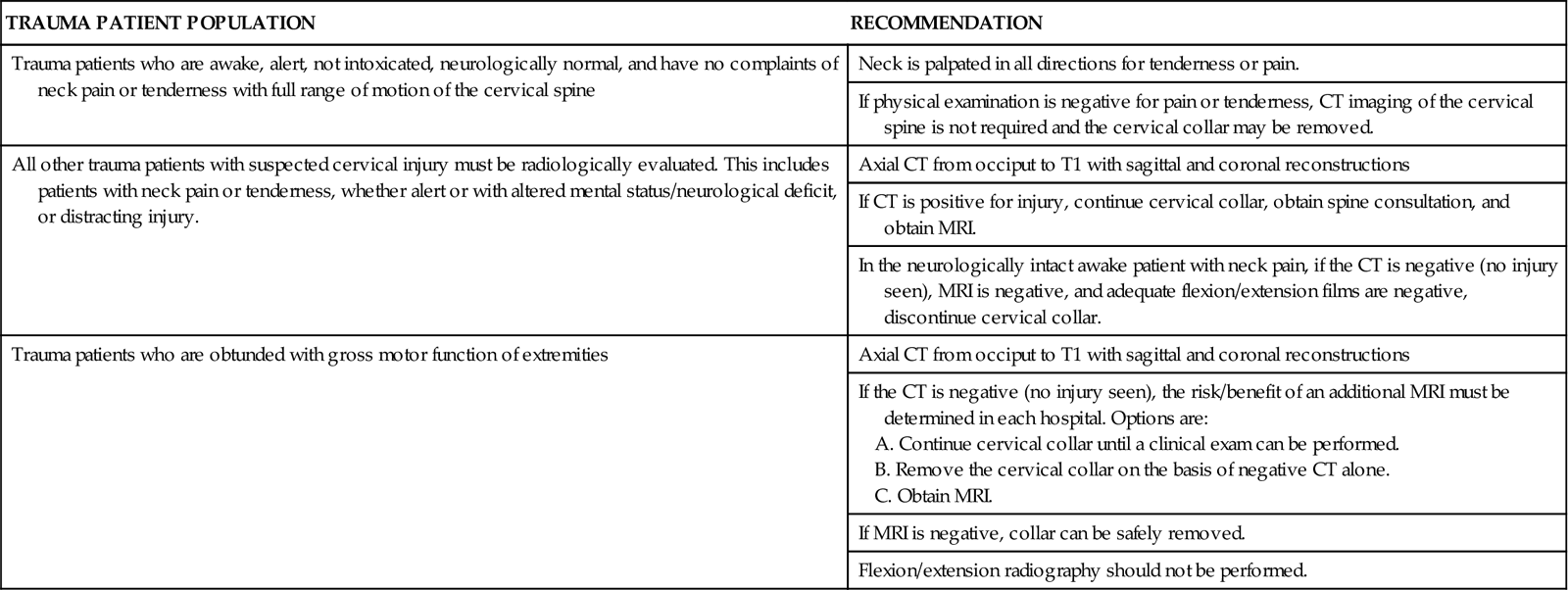
From Eastern Association for the Surgery of Trauma: EAST guidelines: determination of cervical spine stability in trauma patients, Chicago, 2000, Eastern Association for the Surgery of Trauma. Available at www.east.org/tpg/cspine2009.pdf. Accessed October 2010.
Medical Management
After assessment and diagnosis of the SCI, medical management begins. The primary treatment goal is to preserve remaining neurological function with pharmacological, surgical, and nonsurgical interventions.
Pharmacological Management.
Methylprednisolone can improve neurological outcome after SCI, although it has been called into question because of the infection risk in these patients.12 Current guidelines cite the use of methylprednisolone as an option for the management of acute cervical spine injury.13 When it is used, patients receive a methylprednisolone bolus followed by a continuous infusion for at least 24 hours (preferably 48 hours) if their treatment began 3 to 8 hours after their injury.13 Although the exact mechanism is not completely understood, it is thought that methylprednisolone directly affects the changes that occur within the spinal cord after injury, primarily by preventing posttraumatic spinal cord ischemia, improving energy metabolism, restoring extracellular calcium, and improving nerve impulse conduction.
Surgical Management.
Surgical intervention provides spinal column stability in the presence of an unstable injury. Unstable injuries include disrupted ligaments and tendons and a vertebral column that cannot maintain normal alignment. Identification and immobilization of unstable injuries are particularly important for the patient with incomplete neurological deficit. Without adequate stabilization, movement and dislocation of the vertebral column may cause a complete neurological deficit. A variety of surgical procedures may be performed to achieve decompression and stabilization. The question of when surgery should be performed remains controversial.
Nonsurgical Management.
If the injury to the spinal cord is stable, nonsurgical management is the treatment of choice. Nonsurgical management for cervical and thoracolumbar injuries is discussed in the following sections.
Cervical Injury.
Management of cervical injuries involves the immobilization of the fracture site and realignment of any dislocation. This is accomplished through skeletal traction that involves the use of two-point tongs, which are inserted into the skull through shallow burr holes and are connected to traction weights. Several types of cervical tongs are used. Gardner-Wells and Crutchfield tongs are the most common. These tongs can be applied at the bedside with the use of a local anesthetic.
After the procedure, the patient can be immobilized on a kinetic therapy bed or a regular bed. The kinetic therapy bed is the most popular method used for cervical immobilization because it maintains spinal column alignment while providing constant turning motion to reduce pulmonary and skin breakdown. Use of cervical skeletal traction on a regular bed makes it difficult to provide adequate care to the pulmonary system and skin because of the extensive degree of immobility.
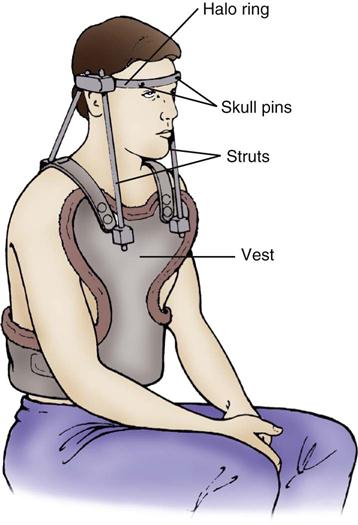
After the spinal column has been adequately realigned by means of skeletal traction, a halo traction brace often is applied. The halo vest consists of a metal ring secured to the skull with two occipital and two temporal screws. Steel bars anchor the screws to the vest to provide cervical immobilization (Figure 25-6). The halo traction brace immobilizes the cervical spine, which allows the patient to ambulate and participate in self-care.
Thoracolumbar Injury.
Nonsurgical management of the patient with a thoracolumbar injury also involves immobilization. Skeletal traction may be used in high thoracic injury. For the most part, misalignment of the spinal canal does not occur in stable injuries of the thoracolumbar spine. Immobilization to allow fractures to heal is accomplished by bed rest with the bed in the reverse Trendelenburg position. A plastic or fiberglass jacket, a body cast, or a brace may also be used.
Nursing Management
Nursing priorities for the patient with SCI are aimed at (1) preventing secondary damage to the spinal cord, (2) managing cardiovascular and pulmonary complications, and (3) coaching the patient to overcome the psychosocial challenges associated with severe neurological deficit.
Nursing diagnoses and management for the patient with SCI are summarized in the Nursing Diagnosis Priorities Box on Spinal Cord Injury. The goal during the critical care phase is to prevent life-threatening complications while maximizing the function of all organ systems. Nursing interventions are aimed at preventing secondary damage to the spinal cord and managing the complications of the neurological deficit. Because almost all body systems are affected by SCI, nursing management must include interventions that optimize nutrition, elimination, skin integrity, and mobility. Patients with SCIs have complex psychosocial needs that require a great deal of emotional support from the critical care nurse.
Thoracic Injuries
Thoracic injuries involve trauma to the chest wall, lungs, heart, great vessels, and esophagus. Thoracic trauma most commonly is the result of a violent crime or an MVC.
Mechanism of Injury
Blunt Thoracic Trauma.
Blunt trauma to the chest most often is caused by MVCs or falls; thoracic injuries account for 20% of trauma deaths. The underlying mechanism of injury tends to be a combination of acceleration-deceleration injury and direct transfer mechanics, as in a crush injury. Various mechanisms of blunt trauma are associated with specific injury patterns. After head-on collisions, drivers have a higher frequency of injury than do backseat passengers because the driver comes in contact with the steering assembly. Severe thoracic injuries often are seen in patients who are unrestrained.14,15 Falls from greater than 20 feet are typically associated with thoracic injury.
Penetrating Thoracic Injuries.
The penetrating object involved determines the damage sustained from penetrating thoracic trauma. Low-velocity weapons (e.g., .22-caliber gun, knife) usually damage only what is in the weapon’s direct path. Of particular concern, however, are stab wounds that involve the anterior chest wall between the midclavicular lines, the angle of Louis, and the epigastric region because of the proximity of the heart and great vessels.
Specific Thoracic Traumatic Injuries
Chest Wall Injuries.
Rib Fractures.
Fractures of certain ribs or multiple rib fractures can be serious, even life-threatening, particularly when associated with additional injuries and occurring in older patients.16 Fractures of the first and second ribs are associated with intrathoracic vascular injuries (e.g., brachial plexus, great vessels), and because they are protected by the scapula, clavicle, humerus, and muscles, they signify a very high degree of force applied to the thorax. Fractures to the lower ribs (7th to 12th) may be associated with abdominal injuries, such as spleen and liver injuries. Fractures to the middle ribs may be associated with lung injury, including pulmonary contusion and pneumothorax. Lack of bone calcification in pediatric trauma patients results in a more compliant chest wall, and rib fractures need not have occurred for a tremendous amount of force to be absorbed, causing injury to the underlying thoracic structures.
The pain associated with rib fractures can be aggravated by respiratory excursion. The patient often splints, takes shallow breaths, and refuses to cough, which can result in atelectasis and pneumonia. Localized pain that increases with respiration or that is elicited by rib compression may indicate rib fractures. A definitive diagnosis can be made with a chest radiograph. Interventions include aggressive pulmonary physiotherapy and pain control to improve chest expansion efforts and gas exchange. Pain management interventions must be tailored to the patient’s response to therapy. The primary goal of pain management in patients with rib fractures is prevention of pulmonary complications and patient comfort. Nonsteroidal antiinflammatory drugs (NSAIDs), intercostal nerve blocks, thoracic epidural analgesia, and opiates may be considered to assist with pain control.17 Epidural analgesia can help increase the functional residual capacity, dynamic lung compliance, and vital capacity; decrease the airway resistance; and increase PaO2.17 The patient’s preexisting pulmonary status and age may dictate the course of recovery.16
Flail Chest.
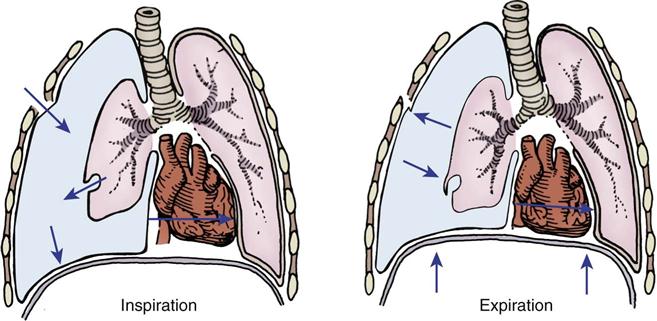
Flail chest, which is caused by blunt trauma, disrupts the continuity of chest wall structures. A flail chest occurs when two or more ribs are fractured in two or more places and are no longer attached to the thoracic cage, producing a free-floating segment of the chest wall. The segment moves independently from the rest of the thorax and causes paradoxical chest wall movement during the respiratory cycle (Figure 25-7). During inspiration, the intact portion of the chest wall expands while the injured part is sucked in. During expiration, the chest wall moves in, and the flail segment moves out. Although the flail segment increases the work of breathing, the main cause of hypoxemia is the underlying pulmonary contusion. The physiological effects of the impaired chest wall motion of a flail chest include decreased tidal volume and vital capacity and impaired cough, which lead to hypoventilation and atelectasis.
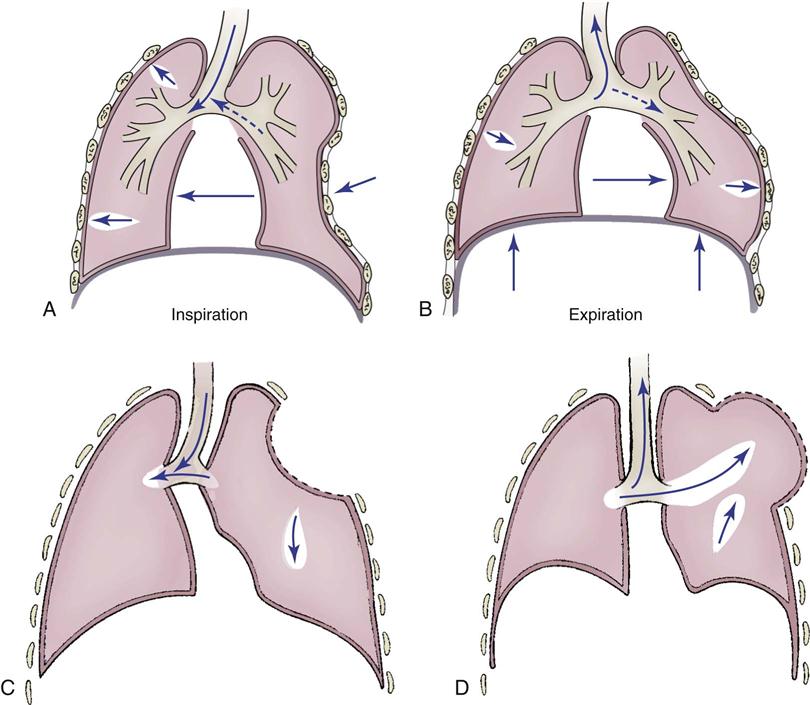
A, Normal inspiration. B, Normal expiration. C, The area of lung underlying the unstable chest wall sucks in on inspiration. D, The same area balloons out on expiration. Notice the movement of mediastinum toward opposite lung on inspiration.
Inspection of the chest reveals paradoxical movement. Palpation of the chest may indicate crepitus and tenderness near fractured ribs. A chest radiograph that reveals multiple rib fractures and evidence of hypoxia demonstrated by ABG values aids in the diagnosis.
Interventions focus on ensuring adequate oxygenation, judicious administration of fluids, and analgesia to improve ventilation. Intubation and mechanical ventilation may be required to prevent further hypoxia.
Diaphragmatic Injury.
Diagnosis of a diaphragmatic injury is often missed in trauma patients because of the subtle and nonspecific symptoms this injury produces. The mechanism of injury appears to be a rapid rise in intraabdominal pressure as a result of compression force applied to the lower part of the chest or upper region of the abdomen. This injury can occur when a person is thrown forward over the edge of the steering wheel in a high-speed MVC involving deceleration forces. The diaphragm, which offers little resistance to the force, can rupture or tear. Abdominal viscera then can gradually enter the thoracic cavity, moving from the positive pressure of the abdomen to the negative pressure in the thorax. Diaphragmatic injury can be a life-threatening event. Massive herniation of abdominal contents into the thoracic cavity can compress the lungs and mediastinum, which hampers venous return and decreases cardiac output. Herniated bowel can become strangulated and perforate.
Diaphragmatic herniation may produce significant compromise and changes in respiratory effort. Auscultation of bowel sounds in the chest or unilateral breath sounds may indicate a ruptured diaphragm. The patient may complain of shoulder pain, shortness of breath, or abdominal tenderness. A chest radiograph may reveal the tip of a nasogastric tube above the diaphragm, a unilaterally elevated hemidiaphragm, a hollow or solid mass above the diaphragm, and a shift of the mediastinum away from the affected side. Treatment of a ruptured diaphragm includes its immediate surgical repair.
Pulmonary Injuries
Pulmonary Contusion.
A pulmonary contusion is fundamentally a bruise of the lung. Pulmonary contusion often is associated with blunt trauma and other chest injuries, such as rib fractures and flail chest. Pulmonary contusions can occur unilaterally or bilaterally. A contusion manifests initially as a hemorrhage, followed by alveolar and interstitial edema. The edema can remain rather localized in the contused area or can spread to other lung areas. Inflammation affects alveolar-capillary units. This results in a ventilation-perfusion imbalance, progressive hypoxemia and poor ventilation over a 24- to 48-hour period.
Clinical manifestations of pulmonary contusion may take up to 24 to 48 hours to develop. Inspection of the chest wall may reveal ecchymosis at the site of impact. Moist crackles may be auscultated in the contused lung. The patient may have a cough and blood-tinged sputum. Abnormal lung function can manifest as systemic arterial hypoxemia. The diagnosis is made primarily by chest x-ray studies consistent with pulmonary infiltrate corresponding to the area of external chest impact that manifests within 12 to 24 hours of injury. Pulmonary contusions may worsen over a 24- to 48-hour period and then slowly resolve unless complications such as sepsis or acute lung injury occur.
Aggressive respiratory care is the cornerstone for care of non-intubated patients with pulmonary contusion. Interventions include ambulation, deep-breathing exercises, turning, and incentive spirometry. Aggressive removal of airway secretions is important to avoid infection and to improve ventilation. Patients with unilateral contusions and significant hypoxia are placed with the injured side up and uninjured side down (“down with the good lung”). This positioning maximizes the match between pulmonary ventilation and perfusion. Patients with severe contusions may continue to show decompensation despite aggressive nursing management. Respiratory acidosis, increases in peak airway and plateau pressures, and increased work of breathing may require endotracheal intubation and mechanical ventilation with positive end-expiratory pressure (PEEP). Adequate pain control is accomplished with administration of NSAIDs, opiates, intercostal nerve blocks, or thoracic epidural analgesia.
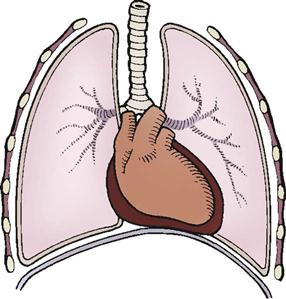
Tension Pneumothorax.
A tension pneumothorax usually is caused by an injury that perforates the chest wall or pleural space. Air flows into the pleural space with inspiration and becomes trapped. As pressure in the pleural space increases, the lung on the injured side collapses and causes the mediastinum to shift to the opposite side (Figure 25-8). As pressure continues to build, the shift exerts pressure on the heart and thoracic aorta, which results in decreased venous return and decreased cardiac output. Tissue perfusion with oxygenated blood is further hampered because the collapsed lung cannot participate in gas exchange.
Clinical manifestations of a tension pneumothorax include dyspnea, tachycardia, hypotension, and sudden chest pain extending to the shoulders. Tracheal deviation can be observed as the trachea shifts away from the injured side. On the injured side, breath sounds may be decreased or absent. Percussion of the chest reveals a hyperresonant sound over the affected side. Diagnosis of a tension pneumothorax is made by clinical assessment.
There is no time for a chest radiograph because this potentially lethal condition must be treated immediately.4 A large-bore (14-gauge) needle or chest tube is inserted into the affected hemithorax in the second intercostal space, midclavicular line. This procedure allows immediate release of air from the pleural space. A hissing sound is heard as the tension pneumothorax is converted to a simple pneumothorax and a chest tube is then inserted.
Open Pneumothorax.
An open pneumothorax (“sucking chest wound”) usually is caused by penetrating trauma. Open communication between the atmosphere and intrathoracic pressure results in immediate lung deflation. Air moves in and out of the hole in the chest, producing a sucking sound heard on inspiration. An open pneumothorax produces the same symptoms as a tension pneumothorax. Subcutaneous emphysema may be palpated around the wound.
Initial management of an open pneumothorax is accomplished by promptly closing the wound at end expiration with a sterile occlusive dressing (plastic wrap or petroleum gauze) large enough to overlap the wound’s edges.4 The dressing should be taped securely on three sides. As the patient breathes in, the dressing gets sucked in to occlude the wound and prevent air from entering. A chest tube is placed as soon as possible. Surgical intervention may be required to close the wound.
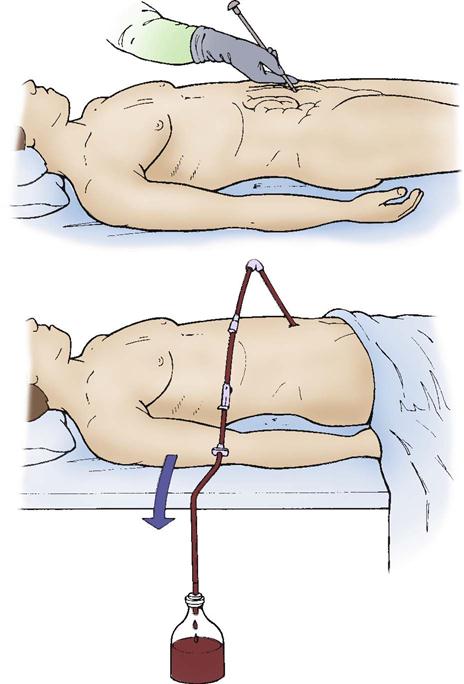
Hemothorax.
Blunt or penetrating thoracic trauma can cause bleeding into the pleural space, resulting in a hemothorax (Figure 25-9). A massive hemothorax results from the accumulation of more than 1500 mL of blood in the chest cavity. The source of bleeding may be the intercostal or internal mammary arteries, lungs, heart, or great vessels. Lacerations to the lung parenchyma are low-pressure bleeds and typically stop bleeding spontaneously. Arterial bleeding from hilar vessels usually requires immediate surgical intervention.4 In either case, increasing intrapleural pressure results in a decrease in vital capacity. Increasing vascular blood loss into the pleural space causes decreased venous return and decreased cardiac output.
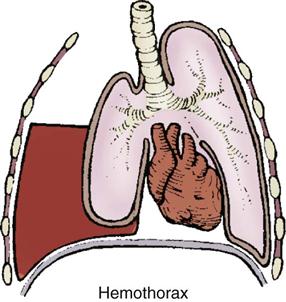
Assessment findings for patients with a hemothorax include hypovolemic shock. Breath sounds may be diminished or absent over the affected lung. With hemothorax, the neck veins are collapsed, and the trachea is at midline. Massive hemothorax can be diagnosed on the basis of clinical manifestations of hypotension associated with the absence of breath sounds or dullness to percussion on one side of the chest.4
This life-threatening condition must be treated immediately. Resuscitation with intravenous fluids is initiated to treat the hypovolemic shock. A chest tube is placed on the affected side to allow drainage of blood. An autotransfusion device can be attached to the chest tube collection chamber. Thoracotomy may be necessary for patients who require persistent blood transfusions or who have significant bleeding (200 mL/hr for 2 to 4 hours or more than 1500 mL on initial tube insertion) or when there are injuries to major cardiovascular structures.
Cardiac and Vascular Injuries
Penetrating Cardiac Injuries.
Penetrating cardiac trauma can occur from mechanical injuries as a result of bullets, knives, or impalements. The chest wall offers little protection to the heart from penetrating trauma. The most common site of injury is the right ventricle because of its anterior position. The mortality rate from penetrating trauma to the heart is high. The prehospital mortality rate for penetrating cardiac injuries is very high, and most deaths occur within minutes after injury as a result of exsanguination or tamponade.
Cardiac Tamponade.
Cardiac tamponade is the progressive accumulation of blood in the pericardial sac (Figure 25-10). With cardiac tamponade, progressive accumulation of 120 to 150 mL of blood increases the intracardiac pressure and compresses the atria and ventricles. An increase in intracardiac pressure leads to decreased venous return and decreased filling pressure, which leads to decreased cardiac output, myocardial hypoxia, cardiac failure, and cardiogenic shock.
Classic assessment findings associated with cardiac tamponade are called Beck’s triad—presence of elevated central venous pressure with neck vein distention, muffled heart sounds, and hypotension. Pulsus paradoxus may occur. Pulseless electrical activity (PEA) in the absence of hypovolemia and tension pneumothorax suggests cardiac tamponade.4 Bedside ultrasound is used to diagnose tamponade.
Immediate treatment is required to remove the accumulation of fluid in the pericardial sac. Pericardiocentesis involves aspiration of fluid from the pericardium by use of a large-bore needle. The inherent risk in this procedure is potential laceration of the coronary artery. Other approaches include surgical procedures such as thoracotomy or median sternotomy. The goal of these procedures is to locate and control the source of bleeding.
Blunt Cardiac Injuries.
The most common causes of blunt cardiac trauma include high-speed MVCs, direct blows to the chest, and falls. Because of its mobility and its location between the sternum and thoracic vertebrae, the heart is susceptible to blunt traumatic injury. Sudden acceleration (as from contact with a steering wheel) can cause the heart to be thrown against the sternum (Figure 25-11). Sudden deceleration can cause the heart to be thrown against the thoracic vertebrae by a direct blow to the chest, such as blows caused by a baseball, animal kick, or fall.
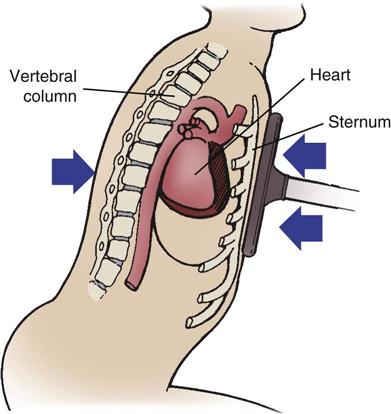
Sudden acceleration (as from contact with the steering wheel) can cause the heart to be thrown against the sternum.
Blunt cardiac injury (BCI), formerly called myocardial contusion, covers the spectrum of myocardial contusion, concussion, and rupture. Few clinical signs and symptoms are specific for BCI. Evidence of external chest trauma, such as steering wheel imprint or sternal fractures, should raise the suspicion for blunt cardiac injury. However, the presence of a sternal fracture does not predict the incidence of BCI. The patient may complain of chest pain that is similar to angina, but the pain is not relieved with nitroglycerin.18 The EAST guidelines for screening of BCI are listed in Box 25-8. The 12-lead ECG may reveal dysrhythmias, ST changes, heart block, or unexplained sinus tachycardia.
Nursing Management
Nursing priorities for the patient with traumatic chest or pulmonary injury emphasize delivery of adequate (1) oxygenation, (2) ventilation, (3) pain management, and (4) prevention of complications. Assistance with intubation and monitoring of mechanical ventilation may be required to prevent tissue hypoxia and provide adequate ventilatory support. Judicious administration of IV fluids and analgesia is important to improve patient comfort. Aggressive removal of airway secretions is important to avoid infection and improve ventilation. Hypoxemia is prevented by continuous pulse oximetry monitoring of arterial oxygen saturation (SpO2) and ABG analysis. See the Nursing Diagnosis Priorities Box on Thoracic Injuries.
Abdominal Injuries
Abdominal injuries often are associated with multisystem trauma. Abdominal injuries are the third leading cause of traumatic death. Injuries to the abdomen are the result of blunt or penetrating trauma. Two major life-threatening conditions that occur after abdominal trauma are hemorrhage and hollow viscus perforation with associated peritonitis. Death occurring after 48 hours following injury is the result of sepsis and its complications. The critical care nurse must pay particular attention to complication-prevention strategies throughout the trauma cycle.
Mechanism of Injury
Blunt Trauma.
Blunt abdominal injuries are common. They result most often from MVCs, falls, and assaults. In MVCs, abdominal injury is more likely to occur when a vehicle is struck from the side. In the passenger position of the front seat, hepatic injury is likely when the point of impact is on the same side as the passenger. A driver is likely to sustain injury to the spleen when the impact is on the driver’s side. Pedestrians hit by motor vehicles are at risk for serious abdominal injuries. Blunt trauma to the thorax can produce injuries to the liver, spleen, and diaphragm. Deceleration and direct forces can produce retroperitoneal hematomas. Blunt abdominal injuries often are hidden, requiring careful assessment and reassessment. Unrecognized abdominal trauma is a common cause of preventable deaths, and blunt abdominal injury deaths are more likely to be fatal than are penetrating abdominal injuries.
Penetrating Trauma.
Penetrating abdominal trauma is often caused by knives or bullets. The danger of penetrating abdominal trauma is that the outside appearance of the wound does not reflect the extent of internal injury. Commonly injured organs from knife wounds are the colon, liver, spleen, and diaphragm. Gunshot wounds to the abdomen usually are more serious than are stab wounds. A bullet destroys tissue along its path. Inside the abdomen, a bullet can travel in erratic paths and ricochet off bone. Death from penetrating injuries depends on the injury to major vascular structures and resultant intraabdominal hemorrhage.
Assessment
The initial assessment of the trauma patient, whether in the emergency department or the critical care unit, follows the primary and secondary survey techniques as outlined by ATLS guidelines.4 The initial physical assessment may be unreliable given the confounding influences of alcohol, illicit drugs, analgesics, and an altered level of consciousness. Specific assessment findings associated with abdominal trauma are reviewed here.
Physical Assessment.
The location of entry and exit sites associated with penetrating trauma are assessed and documented. Inspection of the patient’s abdomen may reveal purplish discoloration of the flanks or umbilicus (Cullen’s sign), which indicates blood in the abdominal wall. Ecchymosis in the flank area (Turner’s sign) may indicate retroperitoneal bleeding or a possible fracture of the pancreas. A hematoma in the flank area suggests kidney injury. A distended abdomen may indicate the accumulation of blood, fluid, or gas resulting from a perforated organ or ruptured blood vessel. Auscultation of the abdomen may reveal friction rubs over the liver or spleen and may indicate rupture. The abdomen is assessed for rebound tenderness and rigidity. These assessment findings indicate peritoneal inflammation. Referred pain to the left shoulder (Kehr’s sign) may indicate a ruptured spleen or irritation of the diaphragm from bile or other material in the peritoneum. Subcutaneous emphysema palpated on the abdomen suggests free air as a result of a ruptured bowel.
Diagnostic Procedures.
Insertion of a nasogastric tube and urinary catheter serves as a useful diagnostic and therapeutic aid. A nasogastric tube can decompress the stomach, and the contents can be checked for blood. Urine obtained from the urinary catheter can also be tested for the presence of blood, though placement of a urinary catheter is contraindicated if there is noticeable blood at the urethral meatus on initial inspection.
Serial laboratory test results may be nonspecific for the patient with abdominal trauma. A serum amylase determination can detect pancreatic injuries. Because of hemoconcentration, hemoglobin and hematocrit results may not reflect actual values. Serial values are more valuable in diagnosing abdominal injuries.
Because of the unreliability of physical examination alone in the patient suspected of having abdominal trauma, diagnostic testing may occur simultaneously during the primary and secondary surveys. Noninvasive tests include bedside ultrasound, CT, and chest and abdominal radiographs.
The bedside ultrasound, called the focused assessment with sonography for trauma (FAST) examination is done at most trauma centers to evaluate the patient for the presence of intraabdominal blood.19 This is a quick and noninvasive means of rapid assessment, but success depends on the skill level of the operator.19 Bedside ultrasonography is used widely in the United States for the detection of abdominal free fluid and hemoperitoneum. Typically, the right and left upper abdominal quadrant areas are examined: the right upper quadrant (Morrison’s pouch), the left upper quadrant splenorenal area, the pericardial sac, and the pelvis (Douglas’ pouch). The primary disadvantage of FAST is the need for free intraperitoneal fluid to produce a positive study result.4 An initial negative FAST result may be followed by serial ultrasound examinations or abdominal CT.19
Although the FAST test has had good sensitivity and specificity, it is not intended to replace a CT scan. Obese abdomens and patients with ascites may have erroneous results, and further workup for these patients is warranted.20 Ultrasound is also limited in its ability to diagnose diaphragmatic, intestinal, or pancreas injuries. Abdominal CT provides information about specific organ injury, pelvic injury, and retroperitoneal hemorrhage.
Invasive tests such as the diagnostic peritoneal lavage (DPL) can exclude or confirm the presence of intraabdominal injury.4 DPL is used only when the FAST exam or a CT scan is inconclusive. After the patient’s bladder has been emptied, a small incision is made in the abdomen through the skin and into the peritoneum. A small catheter is inserted (Figure 25-12). If frank blood is encountered, intraabdominal injury is evident, and the patient is taken immediately to the operating room. If gross blood is not initially encountered, a liter of fluid (lactated Ringer’s or 0.9% normal saline) is infused through the catheter into the abdomen. The intravenous bag is then placed in a dependent position, and abdominal fluid is allowed to drain into the intravenous bag. The drainage fluid is sent to the laboratory for analysis. Positive DPL results signal intraabdominal trauma and usually necessitate surgical intervention (Box 25-9). DPL is invasive, has been associated with complications, and cannot exclude retroperitoneal injuries.
Combined Abdominal Organ Injuries
Patients with multiple visceral injuries may require surgical intervention that uses somewhat nontraditional techniques (“damage control” resuscitation). The three phases of this treatment strategy are the initial operation, ICU resuscitation, and definitive reoperation (Box 25-10).21 The duration of the initial operation is kept to a minimum. The decision to abbreviate the initial operation is made early during surgery. Factors that may lead the surgeon to choose an abbreviated laparotomy include hypothermia and coagulopathy in a patient who is hemodynamically unstable, an inability to control bleeding by direct pressure, and an inability to close the abdomen because of massive abdominal edema.21 Hypothermia induced by an open visceral cavity in conjunction with massive blood transfusion can lead to coagulopathy and continued bleeding, which results in shock and metabolic acidosis. The triad of hypothermia, coagulopathy, and acidosis creates a self-propagating cycle that can eventually lead to an irreversible physiological insult.21 The initial operation must be completed quickly to terminate this self-propagating cycle. Reconstruction and formal closure of the wound may not be completed at this time. The patient is transferred to the ICU.
The goal of the critical care phase of this strategy is to continue resuscitation and correct hypothermia, coagulopathy, and acidosis. Rewarming techniques, described in Box 25-11, are used to correct hypothermia. Coagulation factors and platelets may be administered to correct coagulopathy. Serial lactate and base deficit measurements, as well as a mixed venous oxygen saturation pulmonary artery catheter, may be used to guide fluid resuscitation, inotropic support, and oxygenation to treat and prevent acidosis.
Abdominal Compartment Syndrome.
The patient is assessed for additional complications, including ongoing hemorrhage, intraabdominal hypertension, and abdominal compartment syndrome. Abdominal compartment syndrome is defined as end-organ dysfunction caused by intraabdominal hypertension.22 Increased intraabdominal pressure can result from internal bleeding, visceral edema, or a noncompliant abdominal wall. Increased abdominal cavity pressure can impinge on diaphragmatic excursion and can affect ventilation. Clinical manifestations of abdominal compartment syndrome include decreased cardiac output, increased pulmonary vascular resistance, increased peak pulmonary pressures, increased ICP, decreased urine output, and hypoxia.22 Intraabdominal pressure can be measured through a bladder catheter after the injection of 25 mL of sterile saline.22
Surgical decompression of the abdomen may be required for abdominal pressures greater than 20 to 25 mm Hg that are associated with other assessment findings such as decreased cardiac output, hypotension, elevated peak inspiratory pressures, and decreased urine output.23 Surgical decompression involves opening the abdomen and then temporarily closing the abdomen with a sterile perforated plastic sheet, clips, vacuum-assisted techniques, and many other options.23 The open abdomen is then covered with towels or dressings, and closed suction drains are placed over the top and brought out through a plastic drape over the entire wound. The wound is closed permanently several weeks later, or it is allowed to heal by second intention and eventual skin grafting.
After the patient is hemodynamically stable and the triad of hypothermia, coagulopathy, and acidosis has been corrected, the patient is taken back to the operating room for the definitive surgery, if required. During this phase, definitive repairs and wound closure are performed. Postoperatively, the patient is transported back to the ICU for continued care.
Specific Organ Injuries
Diagnostic studies such as FAST, CT scans, and DPL aid in the detection of specific abdominal organ injuries. The medical and nursing management vary by affected organ: liver, spleen, and bowel injuries, which are seen more commonly, are discussed here.
Liver Injuries.
The liver is the primary organ injured in penetrating trauma and the second most often injured organ in blunt trauma. Abdominal CT is considered to be the most reliable diagnostic tool to identify and assess the severity of the injury to the liver.24 The severity of liver injuries is graded to provide a mechanism for determining the amount of trauma sustained by that organ, the care needed, and the possible outcomes (Table 25-9). Nonoperative management is considered the standard of care for hemodynamically stable patients with liver injury.24 Patients are admitted to the ICU or a step-down unit and are monitored for signs of hemorrhage. Serial serum hematocrit and hemoglobin levels and vital signs are monitored over several days.
TABLE 25-9
| GRADE* | INJURY | DESCRIPTION |
| I | Hematoma Laceration |
Subcapsular, <10% surface area Capsular tear, <1 cm parenchymal depth |
| II | Hematoma Laceration |
Subcapsular, 10%-50% surface area; intraparenchymal <10 cm in diameter Capsular tear, 1-3 cm parenchymal depth, <10 cm long |
| III | Hematoma Laceration |
Subcapsular, >50% surface area or expanding; ruptured subcapsular or parenchymal hematoma; intraparenchymal hematoma >10 cm or expanding >3 cm parenchymal depth |
| IV | Laceration | Parenchymal disruption involving 25%-75% of hepatic lobe or 1-3 Couinaud’s segments within a single lobe |
| V | Laceration Vascular |
Parenchymal disruption involving >75% of hepatic lobe or >3 Couinaud’s segments within a single lobe Juxtahepatic venous injuries (retrohepatic vena cava, central major hepatic veins) |
| VI | Vascular | Hepatic avulsion |
*Advance one grade for multiple injuries up to grade II.
Modified from Trunkey DD: Hepatic trauma: contemporary management, Surg Clin North Am 84(2):437, 2004.
Patients with penetrating or blunt liver trauma who are hemodynamically unstable may require surgical intervention to correct the defect. Resection of the devitalized tissue is required for massive injuries. Hemorrhage is common with liver injuries, and ligation of the hepatic arteries or veins may be required to control hemorrhage. Drains may be placed intraoperatively to drain areas of blood and to prevent hematomas.
Care of the patient with severe liver injuries can be challenging for the critical care nurse. Lack of hemodynamic stability can result from hemorrhage and hypovolemic shock, leading to fluid volume deficit, decreased cardiac output, and decreased tissue perfusion. Combinations of crystalloid and colloid intravenous solutions may be used to correct hypovolemia. Fresh-frozen plasma, platelets, and cryoprecipitate may be administered to correct coagulopathies. A crucial nursing responsibility is to monitor the patient’s response to medical therapies. Continued hemodynamic instability (e.g., hypotension, decreased cardiac output) despite aggressive medical intervention may indicate continued hemorrhage, in which case an exploratory laparotomy may be required to determine and correct the source of bleeding. The patient’s postoperative ICU course may be complicated by coagulopathy, acidosis, and hypothermia. Jaundice may occur as a sign of hepatic dysfunction, but it may also be caused by reabsorption of hematomas or breakdown of transfused blood.
Spleen Injuries.
The spleen is the organ most commonly injured by blunt abdominal trauma and is second to the liver as a source of life-threatening hemorrhage. Spleen injuries, like liver injuries, are graded for the purpose of determining the amount of trauma sustained, the care needed, and the possible outcomes (Table 25-10). Hemodynamically stable patients may be monitored in the critical care unit by means of serial hematocrit values and vital signs. Progressive deterioration may indicate the need for operative management.24
TABLE 25-10
| GRADE* | INJURY | DESCRIPTION |
| I | Hematoma | Subcapsular, <10% surface area |
| Laceration | Capsular tear, <1 cm, parenchymal depth | |
| II | Hematoma | Subcapsular, 10%-50% surface area; intraparenchymal <5 cm in diameter |
| Laceration | Capsular tear: 1-3 cm parenchymal depth, which does not involve a trabecular vessel | |
| III | Hematoma | Subcapsular, >50% surface area or expanding; ruptured subcapsular or parenchymal hematoma; intraparenchymal hematoma >5 cm or expanding |
| IV | Laceration | >3 cm parenchymal depth or involving trabecular vessels |
| Laceration | Laceration involving segmental or hilar vessels producing major devascularization (>25% of spleen) | |
| V | Laceration | Completely shattered spleen |
| Vascular | Hilar vascular injury that devascularizes spleen |
Patients who exhibit hemodynamic instability require operative intervention with splenectomy, partial splenectomy, or splenorrhaphy. Patients who have had a splenectomy are at risk for the development of overwhelming postsplenectomy sepsis with streptococcal pneumonia. These patients require polyvalent pneumococcal vaccine (Pneumovax) to help promote immunity against most pneumococcal bacteria. Patients with isolated spleen injuries that require surgical intervention rarely are admitted to the critical care unit. Complications after splenic trauma include wound infection, sepsis, subdiaphragmatic abscess, and fistulas of the colon, pancreas, and stomach.
Intestinal Injuries.
Intestinal injuries can result from blunt or penetrating trauma. The diagnosis of small intestinal injuries is difficult. Surgical intervention is usually required in the presence of multiple CT findings (e.g., unexplained free fluid, pneumoperitoneum, bowel wall thickening, mesenteric fat streaking, mesenteric hematoma, intravenous contrast extravasation).25 Regardless of the mechanism of injury, intestinal contents (e.g., bile, stool, enzymes, bacteria) leak into the peritoneum and cause peritonitis. Surgical resection and repair are required. The patient’s postoperative course is dictated by the amount of spillage of intestinal contents. The patient is observed for signs of sepsis and for abscess or fistula formation.
Genitourinary Injuries
Trauma to the genitourinary tract seldom occurs as an isolated injury. A genitourinary injury must be suspected in any patient with penetrating trauma to the torso; pelvic fracture; blunt trauma to the lower chest or flank; contusions, hematoma, tenderness over the flank, lower abdomen, or perineum; genital swelling or discoloration; blood at the urethral meatus; hematuria after Foley catheter placement; or difficulty with micturition.4
Mechanism of Injury
Genitourinary injuries, like all other traumatic injuries, can result from blunt or penetrating trauma.
Assessment
Evaluation of genitourinary trauma begins after the primary survey has been conducted and immediate life-threatening conditions have been effectively managed. The conscious patient may complain of flank pain or colic pain. Rebound tenderness can be elicited if intraperitoneal extravasation of urine has occurred. Inspection may reveal blood at the urethral meatus. Bluish discoloration of the flanks may indicate retroperitoneal bleeding, whereas perineal discoloration may indicate a pelvic fracture and possible bladder or urethral injury. Hematuria is the most common assessment finding with genitourinary trauma.9,26
Specific Genitourinary Injuries
Kidney Trauma.
Most renal trauma is caused by blunt trauma, resulting in contusions or lacerations without urinary extravasation. Injury to the kidneys may be reflected by flank ecchymosis and fracture of inferior ribs or spinous processes. Gross or microscopic hematuria may be present; however, the extent of kidney damage is often incongruous with the degree of hematuria.26 Gross hematuria can exist with minor injuries and usually clears within a few hours. CT is the most accurate modality available for diagnosing kidney injury because it can assess the extent of parenchymal laceration, urine extravasation, hemorrhage, and the presence of vascular injury.26
Contusions and minor lacerations can usually be treated with observation. The success of nonoperative management may be enhanced by using angiographic embolization in those who are hemodynamically stable.27 Operative interventions may be performed in patients with kidney injuries with a devascularized segment of the kidney. Postoperative and postinjury complications can include infection, hemorrhage, infarction, extravasation, calcification, acute tubular necrosis, and hypertension.
Bladder Trauma.
A large percentage of bladder injuries result from pelvic fractures.26 Physical findings may include lower abdominal bruising, distention, and pain. More definitive findings include difficulty voiding or incomplete recovery of irrigation fluids from catheterized patients.26 Bladder injuries are classified as contusions, extraperitoneal ruptures, intraperitoneal ruptures, or combined injuries. The type of injury depends on the location and strength of the blunt force and volume of urine in the bladder at the time of injury. Extraperitoneal rupture of the bladder may be managed conservatively with catheterization and antibiotics for 7 to 10 days.27 Unresolved extravasation may require surgical intervention.
Nursing Management
Nursing priorities for the patient with genitourinary trauma include (1) assessment for hemorrhage, (2) maintenance of fluid and electrolyte balance, and (3) maintaining patency of drains and tubes.
After the patient is admitted to the critical care unit, the nurse makes an assessment according to the ATLS guidelines. After the patient’s condition has stabilized, nursing management of postoperative kidney trauma is similar to that for genitourinary surgery. The primary nursing interventions include assessment for hemorrhage,28 maintenance of fluid and electrolyte balance, and maintenance of patency of drains and tubes. Measurement of urinary output includes drainage from the urinary catheter and the nephrostomy or suprapubic tubes. Drainage from these areas is recorded separately. Urine output is measured frequently until bloody drainage and clots have cleared. Gentle irrigation of drainage tubes may be required to clear clots and maintain the patency of the tubes.
Complications of Trauma
Ongoing nursing assessments are imperative for early detection of complications associated with traumatic injuries. A single complication can increase hospital length of stay and the associated costs of treating the complication.
Hypermetabolism
Nutritional support is an essential component in the care of critically ill trauma patients. Within 24 to 48 hours after traumatic injury, a predictable hypermetabolic response occurs. The metabolic response to injury mobilizes amino acids and accelerates protein synthesis to support wound healing and the immunological response to invading organisms. Stress hypermetabolism occurs after any major injury and is characterized by increases in metabolic rate and oxygen consumption. Energy requirements accelerate to promote immune function and tissue repair. The goal of early aggressive nutrition is to maintain host defenses by supporting this hypermetabolism and to preserve lean body mass.29
Most nutrition experts advocate beginning enteral nutrition as early as possible. Current guidelines recommend enteral feedings be initiated within 72 hours for patients with blunt and penetrating abdominal injuries and those with severe head injuries.29 Enteral feeding sites can include the gastric route or any site beyond the pylorus of the stomach, including the duodenum and jejunum. Prompt feeding tube placement by the critical care nurse must be a priority, unless contraindicated. Diminished or absent bowel sounds do not mean the small bowel is not working. Small bowel function and the ability to absorb nutrients remain intact, despite the presence of gastroparesis and absent bowel sounds. Because access to the stomach can be obtained more quickly and easily than the duodenum, early gastric feeding is possible.29 Patients at risk for pulmonary aspiration due to gastric retention or gastroesophageal reflux should receive enteral feedings into the jejunum.29 If enteral feeding is not successful, parenteral nutrition should be initiated by day 7.29
Infection
Infection remains a major source of mortality and morbidity in critical care units. The trauma patient is at risk for infection because of contaminated wounds, invasive therapeutic and diagnostic catheters, intubation and mechanical ventilation, host susceptibility, and the critical care environment. The patient with multiple trauma injuries is at risk for infection because of host susceptibility (including preexisting medical conditions) and the adverse effect of trauma on the immune system.
Wound contamination poses an infection risk for the trauma patient, especially with injuries resulting from deep or penetrating trauma. Exogenous bacteria (from the external environment) can enter through open wounds. Exogenous bacteria can be introduced by dirt, grass, and debris inoculated into the wound at the time of injury. Endogenous bacteria (from the internal environment) can be released as a result of gastrointestinal or genitourinary perforation, which spills bacteria into the internal environment.
Meticulous wound care is essential. The goals of wound care include minimizing infection risks, removing dead and devitalized tissue, allowing for wound drainage, and promoting wound epithelialization and contraction.
Standard interventions for the prevention of ventilator-associated pneumonia and catheter-related bloodstream infection apply to the trauma patient. Proper hand hygiene, invasive catheter care, patient positioning, sterile technique for all invasive procedures, and tight glucose control are paramount to optimal to patient outcome.
Sepsis
The patient with multiple injuries is at risk for overwhelming infections and sepsis. The source of sepsis in the trauma patient can be invasive therapeutic and diagnostic catheters or wound contamination with exogenous or endogenous bacteria. The source of the sepsis must be promptly identified and treated. Gram stain and cultures of blood, urine, sputum, invasive catheters, and wounds are obtained.
Pulmonary Complications
Respiratory Failure
Posttraumatic respiratory failure often leads to the development of ARDS.30 ARDS can be caused by direct injury to the lungs or indirect injury (see “Acute Respiratory Failure” in Chapter 15).30 Primary direct injuries in the trauma patient can include aspiration, inhalation, and pulmonary contusion. The indirect injuries include sepsis, massive transfusion, fat emboli, and missed injury. ARDS in the trauma patient can develop 24 to 72 hours after initial injury. The patient receiving multiple blood products, particularly fresh-frozen plasma, must also be monitored for transfusion-related acute lung injury (TRALI).31 Signs of TRALI are similar to those of ARDS, although there is a temporal relationship between the new onset of respiratory distress and the transfusion of blood products.31
Fat Embolism Syndrome
Fat embolism syndrome can occur as a complication of orthopedic trauma. The clinical onset of fat embolism syndrome ranges from 12 to 72 hours after injury, although 90% of patients develop it within 24 hours after injury.32 Fat embolism syndrome appears to develop as a result of fat droplets that leak from fractured bone and embolize to the lungs. The droplets are broken down into free fatty acids that are toxic to the pulmonary microvascular membranes. Pulmonary fat emboli alter pulmonary hemodynamics and pulmonary vascular permeability. The lung becomes highly edematous and hemorrhagic. The clinical presentation is indistinguishable from that of ARDS. Early stabilization of unstable extremity fractures may limit the seeding of fat droplets into the pulmonary system.32
Pain
Pain in the ICU may come from many sources, including surgery, procedures, and trauma. Trauma may contribute to cellular death and inflammation that leads to pain. Relief of pain is a major component in the care of trauma patients.
An issue that often complicates pain management is the high incidence of substance abuse among patients who sustain traumatic injury. The Society of Critical Care Medicine has published guidelines for the optimal use of analgesia and sedatives33 (see Chapters 8 and 9).
Kidney Complications
Acute Kidney Injury
Assessment and ongoing monitoring of kidney function is critical to the survival of the trauma patient. The cause of posttraumatic renal failure is complex and may involve a variety of factors, as listed in Box 25-12.
Prevention of kidney failure is the best treatment, and it begins with ensuring adequate renal perfusion. Serial assessments of blood urea nitrogen (BUN) and creatinine levels commonly are used to evaluate kidney function. Progressive kidney failure requires prompt diagnosis and treatment (see “Acute Kidney Injury” in Chapter 20).
Myoglobinuria
Patients with a crush injury are susceptible to the development of myoglobinuria, with subsequent secondary kidney failure. Crush injuries can compromise blood flow. Loss of arterial blood flow, particularly to the extremities, results in the loss of oxygen transport to distal tissues and ischemia. This initiates a cascade of events that leads to the necrosis of skeletal muscle cells. As cells die, intracellular contents—particularly potassium and myoglobin—are released. Myoglobin, a muscular pigment, is a large molecule. Dark tea-colored urine suggests myoglobinuria. After myoglobinuria is diagnosed, treatment is aimed at kidney preservation by aggressive administration of intravenous fluids. Nursing management is directed toward achievement of fluid and electrolyte balance. The patient should be assessed for hypernatremia, hyperosmolarity, and volume overload.
Vascular Complications
Compartment Syndrome
Compartment syndrome is a condition in which increased pressure within a limited space compromises circulation, resulting in ischemia and necrosis of tissues within that space. Among those at high risk for the development of compartment syndrome are patients with lower extremity trauma, including fractures, penetrating trauma, vascular ruptures, massive tissue injuries, or venous obstruction. Clinical manifestations of compartment syndrome include obvious swelling and tightness of an extremity, paresis, and pain of the affected extremity. Diminished pulses and decreased capillary refill do not reliably identify compartment syndrome because they may be intact until after irreversible changes have occurred. Elevated compartment pressures confirm the diagnosis. The treatment can consist of simple interventions, such as removing an occlusive dressing to fasciotomy.
Venous Thromboembolism
Despite improvements in the care of the trauma patient, venous thromboembolism (VTE) and the attendant risk of pulmonary embolism remain important causes of morbidity and mortality in the multiply injured trauma patient. The factors that form the basis of VTE pathophysiology are blood stasis, injury to the intimal surface of the vessel, and hypercoagulopathy. Trauma patients are at risk for VTE because of endothelial injury, coagulopathy, and immobility.
Trauma patients are at the greatest risk for developing thromboembolism early in their hospitalization. Prevention is key. Practice guidelines for the prevention and management of thromboembolism recommend that trauma patients at high risk for VTE receive sequential compression devices for prophylaxis against VTE.34 High-risk patients include those with an SCI, lower extremity or pelvic fractures, need for a surgical procedure, increasing age, central venous catheters or venous injury, and prolonged immobility or hospital stay. For patients in whom the lower leg is inaccessible, foot pumps may act as an effective alternative to lower the rate of VTE. Low-molecular-weight heparin (e.g., enoxaparin) is recommended for VTE prophylaxis in trauma patients with the following injury patterns:
• Pelvic fractures requiring operative fixation or prolonged bed rest (longer than 5 days)
• Complex lower extremity fractures requiring operative fixation or prolonged bed rest
The selection of VTE prophylaxis for trauma patients is often challenging because of the need to achieve balance between VTE risk and bleeding risk. For high-risk trauma patients who cannot receive anticoagulation because of their risk of bleeding, the prophylactic placement of an inferior vena cava filter is considered when the following patterns of injury are present:34
The purpose of inferior vena cava filter placement is to reduce the risk of fatal pulmonary embolism. Filter placement does not negate the requirement to use sequential compression devices and pharmacological prophylaxis against VTE.
Missed Injury
Nursing assessment of the multiply injured patient in the critical care unit may reveal missed diseases or missed injuries. Missed disorders may include preexisting undiagnosed medical illnesses, such as endocrine disorders (diabetes, hypothyroidism), myocardial infarction, hypertension, decreased respiratory reserve, undiagnosed kidney failure, or malnutrition.
Occasionally, injuries may not be diagnosed in the pre-critical care phases. Missed injuries are commonly discovered in the first 24 to 48 hours of the hospital stay during the routine assessments of the trauma tertiary survey. Injuries are missed for a variety of reasons as summarized in Box 25-13. In the critical care unit, a missed injury may be suspected if the patient fails to show appropriate response to medical or surgical intervention. Nurses play a key role in identifying missed injuries, particularly when patients regain consciousness and begin to increase their activity.
Multiple Organ Dysfunction Syndrome
MODS is a clinical syndrome of progressive dysfunction of organ systems. Trauma patients are at high risk for systemic inflammatory response syndrome (SIRS) and MODS. Organ dysfunction can be the result of primary MODS, which is caused by direct traumatic injury, as may occur with acute lung dysfunction because of pulmonary contusion. Secondary MODS—organ dysfunction that occurs later in the trauma patient’s ICU course—results from uncontrolled systemic inflammation with resultant organ dysfunction. Treatment is aimed at controlling or eliminating the source of inflammation, maintenance of oxygen delivery and consumption, and nutritional and metabolic support for individual organs.
Special Considerations
Meeting the Needs of Family Members and Significant Others
The impact of traumatic injury can be devastating for patients and for family members and significant others. They are faced with a crisis situation for which they have had little time to prepare. Trauma can precipitate a crisis within the family. Families may exhibit physical and sociocultural reactions and a combination of emotional reactions, including anger, fear, powerlessness, confusion, and mistrust. Recovery from traumatic injury can be long and frustrating for families. There may be many peaks and valleys of good days and bad days. During this time, the family may exhaust its social and financial support systems. Nurses should recognize this and facilitate supportive relationships for families. Regardless of the specific system of care delivery, the nurse ensures the family is supported during all aspects of care.
A valuable intervention is to bring families of trauma patients together in support groups. Trauma-related family support groups can offer sharing of experiences, expression of emotions, mutual support, sharing of coping strategies, and education about hospital and community services.
Trauma in Older Adults
Trauma affects people of all ages. Older patients are predisposed to traumatic injuries because of the inevitable consequences of aging. The ability to react to or avoid environmental hazards is impaired because of age-related deterioration of the senses and changes in motor strength, postural stability, balance, and coordination (see Chapter 14).
Older persons experience most of the falls that result in injuries, and these falls are likely to occur from level surfaces or steps.35 Factors that predispose older persons to falls are summarized in Box 25-14.35 Because many of the falls may be caused by an underlying medical condition (e.g., syncope, myocardial infarction, dysrhythmias), management of the older patient who has fallen must include an evaluation of events and conditions immediately preceding the fall.
The exposure of older adults to MVC trauma is a consequence of the increasing growth of the older population and the growing number of older drivers and occupants of motor vehicles. Factors that predispose older adults to MVCs are summarized in Box 25-15.35 A pedestrian struck by a motor vehicle receives one of the most devastating injuries. Many deaths of older individuals occur in crosswalks. Physiological deterioration of cerebral and motor skills and alterations in visual and auditory acuity cause older pedestrians to walk directly into the path of oncoming vehicles.
Trauma in older adults is associated with higher mortality rates, even when the injuries are less severe. Older adults have a higher complication rate and a higher mortality rate, starting at age 40, because of preexisting medical conditions, decreased physiological reserves, and decreased ability to compensate for severe injury. Older patients who do survive traumatic injury are often faced with changes in their preinjury functional status. Relatively minor trauma can be the event that changes the lifestyle of an older person from one of relative independence to one that requires prolonged rehabilitation or skilled nursing care. Discharge planning early in the patient’s hospitalization is necessary.
The concept of limited physiological reserve in the older trauma patient highlights the key difference between the average younger trauma patient with normal physiological reserve and the older patient with underlying physiological derangements. Age-related changes that occur in virtually every organ system may not produce evidence of organ dysfunction in the resting state. However, the ability of organs to augment function in response to traumatic stress may be greatly compromised. Fluid resuscitation is an integral part of trauma resuscitation. Patients on chronic diuretic therapy may require more volume and potassium supplementation as a result of chronic volume and potassium depletion. The assessment and management of hypovolemic shock is more complex in the older trauma patient. Older adults have limited ability to increase their heart rate in response to blood loss, obscuring one of the earliest signs of hypovolemia—tachycardia.4 Loss of physiological reserve and the presence of preexisting medical conditions are likely to produce further conflicting hemodynamic data. The older patient’s lack of physiological reserve makes it imperative that early nutritional support is initiated.
Trauma protocols are well established for the management of young patients after injury. Clinicians increasingly are recognizing that these protocols must be individualized for the older trauma patient. The best outcomes for this patient population have been achieved through early, appropriate, aggressive trauma care, including early hemodynamic monitoring in high-risk older trauma patients (those with a high-risk mechanism of injury, unknown cardiovascular status, or preexisting heart or kidney disease).36