Transverse Colectomy
Surgical Principles
Anatomy for Transverse Colectomy
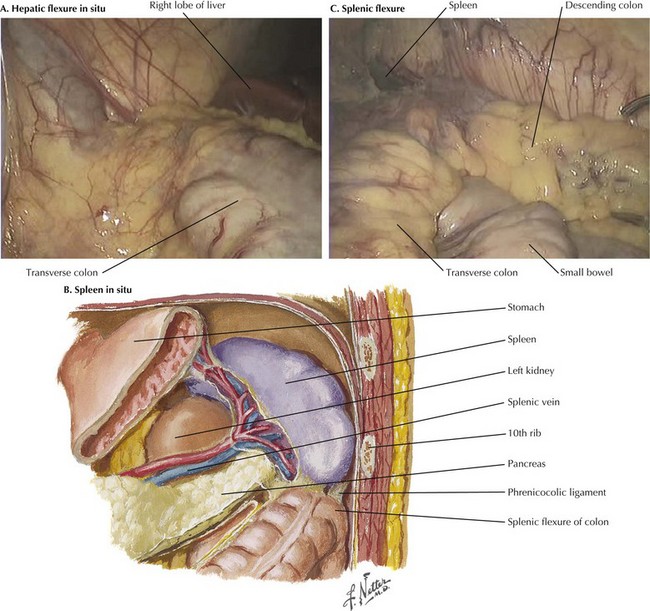
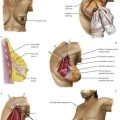
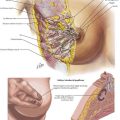
The intraoperative photograph demonstrates the hepatic flexure in situ (Fig, 23-1, A). The liver is cephalad and the right kidney is posterior. The ascending colon is mobilized from its lateral attachments at the white line of Toldt. As the ascending colon is mobilized medially, the dissection is complete when the duodenum is identified and preserved posteriorly with the retroperitoneum. Aggressive traction near the end of the mobilization may cause avulsion injury to the middle colic vein, which results in difficult-to-control hemorrhage.
Splenic Flexure
The splenic (or left) flexure of the colon is the bend in the bowel where the distal transverse colon transitions to the descending colon. To mobilize the splenic flexure, the avascular lateral attachments of the descending colon to the retroperitoneum must be divided along the white line of Toldt. The splenocolic (lienocolic) ligament is the superior extension of this and forms connective bands connecting the apex of the splenic flexure to the inferior aspect of the splenic capsule. The anatomic relations of the splenic flexure in situ are shown in Figure 23-1, B and C.
Relationships of Greater Omentum to Transverse Colon and Stomach
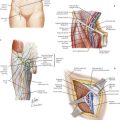
The omentum originates from the greater curvature of the stomach and continues anteriorly and caudad over the top of the transverse colon. If the omentum is to be resected, the gastroepiploic artery, which parallels the greater curve of the stomach, is preserved while the blood vessels of the omentum itself require ligation. The stomach is reflected cephalad to provide entry to the lesser sac (Fig. 23-2, A). If the omentum is to be preserved, the surgeon must dissect it off of the colon in the anatomic plane and reflected cephalad, making sure the colon is not injured in the process. A thin, filmy plane without vascular supply is noted between the transverse colon and the omentum and should be entered and can be followed into the lesser sac.
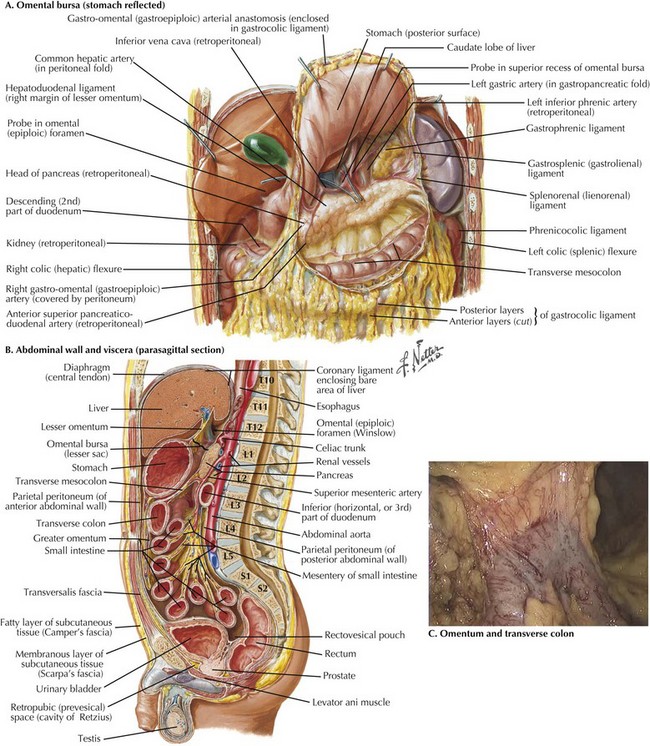
FIGURE 23–2 Greater omental and stomach in transverse colectomy.
Vertebrae: L1, 1st lumbar; S1, 1st sacral; T10, 10th thoracic.
The plane of dissection in sagittal view is illustrated in Figure 23-2, B, which clearly shows the transverse colon mesentery originating off the anterior border of the pancreas. The dissection plane to separate the greater omentum off the transverse colon is shown in Figure 23-2, C. This mobilization is complete when the posterior wall of the stomach is clearly visualized and isolated from the mesentery of the transverse colon.
Middle Colic Artery and Vein
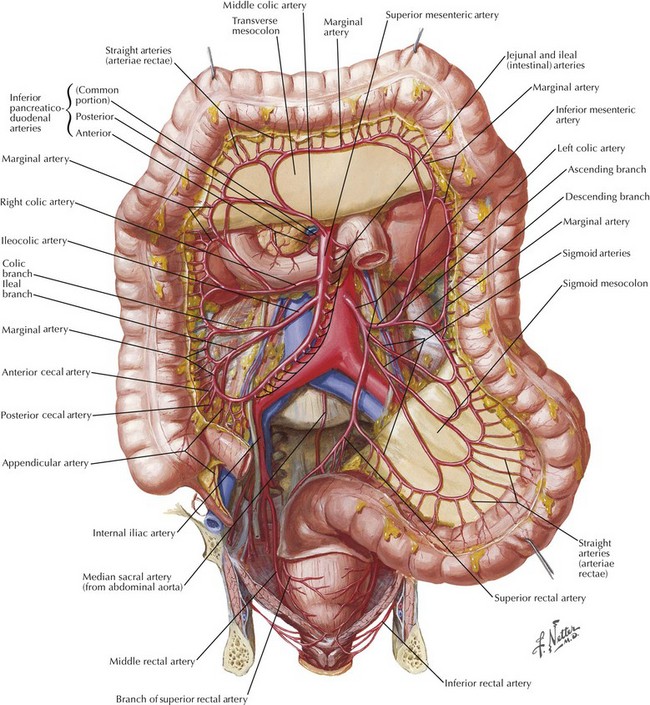
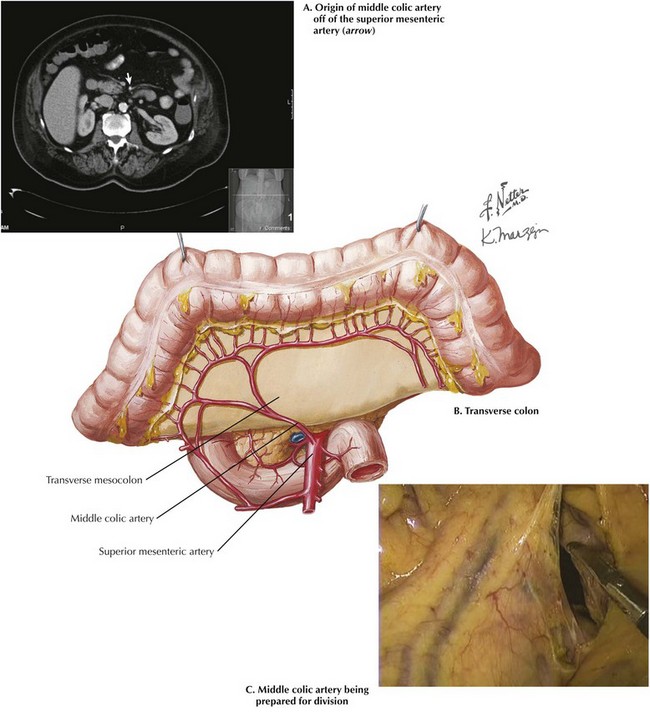
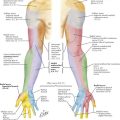
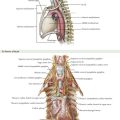
Division of the middle colic artery and vein are the most difficult aspect of a transverse colectomy because of the potential for rapid and difficult-to-control hemorrhage. The middle colic artery is the second branch of the superior mesenteric artery (SMA), after the inferior pancreaticoduodenal artery. The middle colic artery originates from the right lateral aspect of the SMA and classically bifurcates into right and left branches, although as many as five different branches are often noted. The classic arrangement of arteries to the large intestine is shown in Figure 23-3. Although called the middle colic artery, it is generally oriented more to the patient’s right than the middle (Fig. 23-4, A).
A number of anatomic variations of the middle colic arteries are possible, and a variation from normal is often seen. Several cadaver dissection studies have provided insight into the frequency of these anomalies. Some uncommon variations of the middle colic arteries within the SMA system are outlined in Chapter 21 (see Fig. 21-3). The middle colic is a short artery, with a mean length of 32 mm, sometimes presenting difficulties in transection. The view of the transverse colon with flexures and mesenteric vessels is best seen with a laparoscope (Fig. 23-4, B).
As previously stated, the middle colic artery and vein may be transected after the greater omentum has been dissected from the transverse colon or the omental arteries ligated and the lesser sac opened. A clear space to the patient’s left of the middle colic artery is often used for retrocolic Roux limbs, such as those used for gastric bypass surgery (see Chapter 11). This space has been opened to facilitate subsequent division of the middle colic arteries (Fig. 23-4, C).
Chitra, R. Clinically relevant variations of the coeliac trunk. Singapore Med J. 2010;51(3):216–219.
Garcia-Ruiz, A, Milsom, JW, Ludwig, KA, Marchesa, P. Right colonic arterial anatomy: implications for laparoscopic surgery. Dis Colon Rectum. 1996;39(8):906–911.
Gerber, SA, Rybalko, VY, Bigelow, CE, et al. Preferential attachment of peritoneal tumor metastases to omental immune aggregates and possible role of a unique vascular microenvironment in metastatic survival and growth. Am J Pathol. 2006;169(5):1739–1752.
Lawrance, RJ, Loizidou, M, Cooper, AJ, Alexander, P, Taylor, I. Importance of the omentum in the development of intraabdominal metastasis. Br J Surg. 1991;78(1):117–119.
McGory, ML, Zingmond, DS, Sekeris, E, Ko, CK. The significance of inadvertent splenectomy during colorectal cancer resection. Arch Surgery. 2007;142(7):668–674.