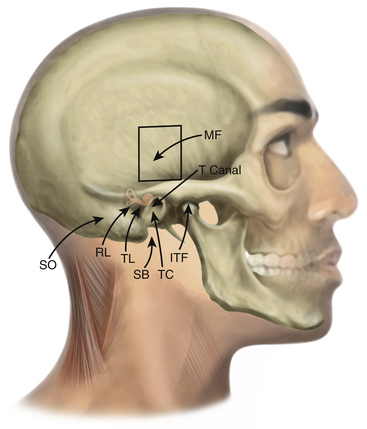
Chapter 47 Transtemporal Approaches to Posterior Cranial Fossa
Traditional approaches to the posterior cranial fossa do not permit direct access to complex lesions of the lateral skull base, cerebellopontine angle (CPA), or clivus. To circumvent brain retraction and allow for complete resection, approaches have been developed that position the dissection both lateral and anterior to the brain stem and cerebellum. All of these skull base approaches are combinations and variations of transtemporal bone routes (Table 47-1 and Fig. 47-1). Unlike craniotomies performed elsewhere, entry to the posterior fossa through the temporal bone poses special problems for the surgeon if the internal carotid artery (ICA), sigmoid sinus (SS), cranial nerves VII and VIII, and specialized structures for hearing and balance are to be preserved. Despite the widely varied nomenclature, often only subtle differences exist among these approaches. It is imperative that the location, type, and extent of the lesion dictate the type of the approach. Tailored approaches to the lesion instead of standard ones are recommended for minimal disruption of normal structures. In this respect, transtemporal approaches represent an anatomic continuum of temporal bone dissection, with frequent overlaps and minor discrepancies or differences among the various approaches. The complicated nomenclature has arisen because skull base tumors tend to extend into different anatomic compartments, often necessitating combined approaches. Because of the overlap of neurosurgery and otology in this area, collaboration of neurosurgeons and otologists is mandatory.
TABLE 47-1 Temporal Bone Approaches
| Anterior Approaches |
| Middle cranial fossa |
| Extended middle cranial fossa |
| Middle cranial fossa transtentorial |
| Posterior Approaches |
| Retrolabyrinthine—presigmoid |
| Retrolabyrinthine—trans-sigmoid |
| Retrolabyrinthine—retrosigmoid |
| Translabyrinthine |
| Transotic |
| Transcochlear |
| Infralabyrinthine |
| Transcanal–infracochlear |
| Combined Approaches |
| Petrosal |
| Infratemporal fossa |
| Endoscopic Approaches |
| Endonasal endoscopic approach |
Anterior Transpetrosal Approaches
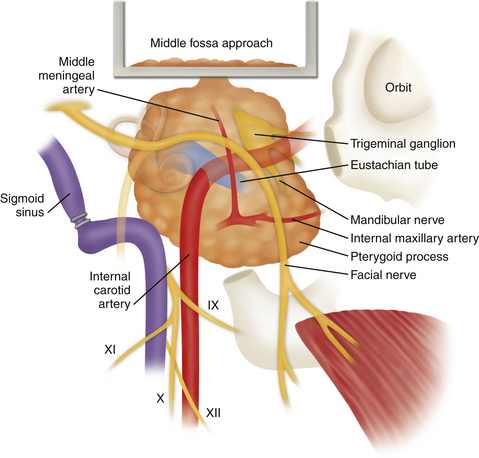
Middle Fossa Approach
House developed the middle fossa access to the IAC and adjacent structures in 1961 in an effort to remove foci of labyrinthine otosclerosis.1–3 Although he used this approach briefly for the removal of acoustic tumors, he soon directed his efforts to a translabyrinthine approach. Fisch and Mattox further refined the middle fossa approach.4,5
Several techniques for localization of the IAC have been described. House’s technique identifies the greater superficial petrosal nerve (GSPN), follows it to the geniculate ganglion (GG), and then proceeds along the labyrinthine segment of the facial nerve to the IAC.2 Another popular method described by Fisch and Mattox uses the superior semicircular canal (SSC) as the primary landmark.5 Using this method, once the SSC is identified, the meatal plane overlying the IAC is located in a 60-degree plane centered over the SSC ampulla. Garcia-Ibanez and Garcia-Ibanez6 advocate a technique beginning at the bisection of the angle between the GSPN and the arcuate eminence. Lan and Shiao, in a cadaveric study, described the technique of finding the IAC by drilling a point that is 9.9 mm medial from the GG on a line angled with the GSPN by 96 degrees.7 Because the medially IAC dura enlarges threefold, and there are no neural structures anterior to it, the medial or proximal “safe” part of the IAC is first identified and is followed laterally: once the IAC dura is exposed, it can be distinguished from the posterior fossa dura by its “pinkish” color in contrast to the “white” posterior fossa dura.8 Other techniques involve drilling to expose the semicircular canals and exposure of the ossicles in the middle ear as a reference point.9 Because of the wide variations in anatomy4,6 and small working space, no single technique can ensure avoidance of injury to important structures. Careful dissection and a detailed understanding of the regional anatomy are important for success. Image-guided navigation through the temporal bone has been introduced as an accurate alternative method to localize the IAC without the need to expose the GG or the SSC.10,11
Indications
The middle fossa approach is best suited for lesions situated lateral within the IAC that have limited extension into the CPA (less than 1 cm) and where hearing preservation is the goal.2,12 It is especially useful when preoperative computed tomography (CT) of the temporal bone demonstrates the proximity of the posterior semicircular canal (PSC), common crus, or vestibule to the posterior lip of the IAC. In those circumstances, retrosigmoid approaches are less preferable and the middle fossa approach becomes the hearing-preservation approach of choice. Tumors that are medial in position and do not extend to the fundus of the IAC are best approached by posterior approaches (e.g., retrosigmoid approach).
The middle fossa approach provides access to the labyrinthine segment of the facial nerve without sacrificing hearing. Thus, decompression of the facial nerve in trauma or Bell’s palsy, or resection of facial nerve tumors can be accomplished. During vestibular schwannoma removal, early identification of the facial nerve allows better functional preservation. In a recent retrospective literature review that included 296 studies and more than 25,000 patients, facial nerve preservation was highest in patients treated with middle fossa (85%) approach compared to translabyrinthine (81%) and retrosigmoid (78%) approaches.13 However, the main factor contributing to the facial nerve preservation was found to be tumor size, which is consistent with other studies.14 Therefore, bias can be a contributing factor, because most schwannomas treated with the middle fossa approach are small or medium sized. This approach also permits selective sectioning of the vestibular nerve fibers for Ménière’s disease. In theory, it could also be used to expose the horizontal portion of the ICA, eustachian tube, and temporomandibular joint (TMJ). Other indications include advanced otosclerosis, nerve section for tinnitus, facial nerve repair and facial nerve neuroma, repair of middle fossa encephaloceles, and cerebrospinal fluid (CSF) leakage through the tegmen.2
Surgical Approach
The patient is positioned supine on the operating table with the head turned opposite the side of the tumor.2,4,6,12,15 Facial and auditory nerve monitoring is used. An incision is planned that begins at the level of the zygoma just anterior to the tragus and extends superiorly to approximately the superior temporal line. We prefer an S-shaped incision curving first anteriorly and then posteriorly to allow for greater spreading of the soft tissues.
The temporalis muscle is divided and reflected anteriorly. A 4- by 5-cm bone flap is planned approximately two thirds anterior and one third posterior to the EAC. The inferior margin should be placed as close to the middle fossa floor as possible. A subtemporal craniectomy is performed. The dura is then elevated from the middle fossa floor in a posterior to anterior direction. This direction of dissection helps avoid inadvertent elevation of the GSPN and subsequent traction injury to the GG and facial nerve. Injury to the GSPN can produce a dry eye secondary to loss of lacrimal gland innervation. In approximately 16% of cases, the GG is not covered by bone and inadvertent injury can occur.4 To maintain a visible plane of dissection, a self-retaining brain retractor is used. Some retractors are limited to only 4 to 5 cm of spread; thus, too wide a craniotomy can impair the capability of the retractor to elevate the dura adequately.
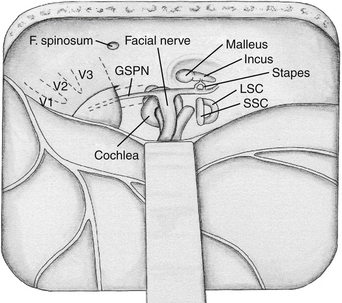
Several landmarks must be identified before bone removal can begin over the IAC: (1) the middle meningeal artery, (2) the arcuate eminence, (3) the GSPN, and (4) the facial hiatus (Fig. 47-2). The first landmark is typically the middle meningeal artery at the foramen spinosum. This can be obliterated and divided if necessary. The foramen may be, albeit rarely, duplicated or absent. It marks the anterior limit of the dural elevation. As dural elevation continues, the arcuate eminence, a rounded elevation of the petrous bone, can be identified. It is usually produced by the underlying SSC. Lateral to the arcuate eminence is the tegmen tympani, a thin lamina of bone that forms the roof of the tympanic cavity. The GSPN originates from the GG, exits through the petrous bone at the facial hiatus, and runs extradurally in an anteromedial direction toward the trigeminal ganglion. The GSPN serves as a landmark for the lateral margin of the horizontal segment of the petrous carotid artery. Care should be taken at this stage not to apply force to the floor of the middle fossa, because the bone over the carotid artery and the area of the tegmen can be quite thin and even dehiscent in up to 20% of cases.4 The arcuate eminence is drilled until the dense bone of the SSC is encountered. The SSC is visualized with a bluish hue through the bone, the so-called blue line. In approximately 15% of cases, however, the arcuate eminence is absent; in 50% of cases where it is present, it is rotated in relationship to the SSC.16
Once the key landmarks have been identified, the IAC can be localized using two key angles. Traditionally, the GSPN-SSC angle (120 degrees)6 and the SSC-IAC angle (60 degrees)17 have been used. Unfortunately, these angles have been shown to be quite variable,15 and in one study the GSPN-SSC angle ranged from 90 to 135 degrees and the SSC-IAC angle from 34 to 75 degrees.4 Thus, simply relying on angles can lead to inadvertent injury to the SSC and hence loss of hearing. House’s technique places the facial nerve at risk, because this structure is first identified and then followed to the IAC.2 Fisch’s technique places the SSC at risk. Orientation can be aided by removal of the tegmen and identification of the head of the malleus.9 This can help predict the location of the GG and IAC because these structures are usually collinear, at the expense of risking a CSF leak through the middle ear.2 None of these techniques is failsafe, and the best method of localizing the IAC is what the surgeon finds most comfortable in his or her own experience.
Once the IAC has been identified, exposure proceeds from lateral to medial until the entire IAC has been exposed along its superior surface. A safer technique is to begin medially, expose the porus acousticus first, and then work laterally, taking advantage of the larger margin for error in the region of the porus.8,12,15 At the fundus of the IAC, the vertical crest (Bill’s bar), a bone spicule that separates the superior vestibular nerve from the facial nerve, is identified. Finally, the dura over the IAC is opened, first posteriorly over the superior vestibular nerve to avoid facial nerve injury.
In the case of vestibular schwannomas, the tumor can be removed by separating the superior vestibular nerve and tumor from the facial nerve. The tumor is usually delivered posteriorly away from the facial nerve. Small hooks are necessary to palpate the limits of the IAC and gently free the tumor, particularly from its inferolateral portion, where the view is especially obscured. The approach carries a higher risk to the facial and cochlear nerves when the tumor less commonly arises from the inferior vestibular nerve. A rigid 0- or 30-degree endoscope can be used at the end of the procedure to inspect for residual tumor, facial and cochlear nerve integrity, and opened air cells.18 Closure of the IAC defect is accomplished with a small free graft or temporalis muscle.
Complications and Disadvantages
The middle fossa approach requires retraction of the temporal lobe;19 therefore, injury can potentially occur (e.g., aphasia, hemiparesis, or seizure). There is potential for CSF leakage through unwaxed air cells or through the middle ear, if the tegmen is opened. Careful hemostasis and tenting sutures can lessen the risk of postoperative hematoma (e.g., epidural hematoma).
Extended Middle Fossa Approach (Anterior Petrosectomy)
The traditional middle fossa approach works well for lesions in the IAC;2,3 however, the exposure is limited. For that reason, extensions of the middle fossa approach have been developed to permit wider access to the petrous apex, clivus, and posterior fossa. The extended middle fossa approach involves a petrous apex resection, in addition to the temporal craniotomy described for the middle fossa approach.20 The horizontal segment of the carotid artery and the cochlea limit the inferior exposure to the level of the inferior petrosal sinus. The TMJ, ossicles, and petrous carotid artery laterally; the trigeminal ganglion anteriorly; and the SSC and vestibule posteriorly represent the limits of the extended middle fossa approach.
Indications
The anterior petrosectomy provides access to the petrous apex and superior clival region. It provides access to the posterior fossa past the carotid artery and trigeminal and facial nerves. Although the extended middle fossa approach is considered a hearing-preservation approach, it can provide additional exposure in the posterior fossa by sacrificing the SSC and labyrinth—and thus hearing.20,21 Sacrifice of the cochlea improves visualization of the lateral extreme of the IAC, the medial wall of the tympanic cavity, and the jugular foramen if needed.
The extended middle fossa approach permits resection of vestibular schwannomas that extend more medially in the CPA. Meningiomas and chordomas of the anterior CPA and clivus can be resected through this approach, as well as lesions of the petrous apex (i.e., cholesteatomas and petrositis). The middle fossa approach is not recommended for cholesterol granulomas because of the absence of permanent aeration and the need for temporal lobe retraction.22 Large lesions may be difficult to resect by this approach, and the angled endoscope can be used to deal with blind spots.23 Lesions of the posterior cavernous sinus, basilar artery, and anterior brain stem up to the level of pontobulbar junction can also be approached.24 The petrous carotid artery can be also approached for bypass anastomosis.25
Surgical Approach
A temporal craniotomy is performed, similar to the middle fossa approach. Occasionally, a zygomatic osteotomy is added for exposure of the middle fossa floor. The middle meningeal artery, foramen ovale, GSPN, and arcuate eminence are identified. The dural sheath of the trigeminal ganglion and its continuation as V3 forms the superior limit of this approach.24 The petrous carotid artery can be exposed at this point by drilling bone along the course of the GSPN (see Fig. 47-2). This area is also known as the posterolateral or Glasscock’s triangle.26 The boundaries of Glasscock’s triangle are a line extending from the foramen spinosum to the arcuate eminence laterally, the GSPN medially, and the third division of the trigeminal nerve (V3) at the base. Medial to this triangle is Kawase’s, or the posteromedial, triangle, which consists of the bone in the area of the petrous apex. Drilling this bone provides access to the clivus and the infratentorial compartment.27 Kawase’s triangle is defined laterally by the GSPN, medially by the petrous ridge, and at the base by the arcuate eminence. Usually the GSPN is sacrificed to avoid traction to the GG and subsequent facial paralysis. Arcuate eminence is absent in significant number of the patients. Therefore, foramen ovale and spinosum can be used alternatively as landmarks to start drilling.24 The cochlea represents the posterolateral limit of exposure within Kawase’s triangle. Infracochlear lesions are hard to access with the conventional approach, and an endoscope can be helpful.23
The IAC is identified by one of the means described in the previous section. Once the IAC is identified, bone is removed, exposing the canal widely and the dura of the posterior fossa. The otic capsule bone is particularly dense and lighter in color than the remaining bone of the petrous apex. To identify the cochlea, Miller et al.28 advocate drilling bone along an imaginary line extending from the tip of the vertical crest to the junction of the petrous carotid artery and cranial nerve V3 until the cochlea is identified. With this exposure, the dura along the medial temporal lobe and infratentorially to the level of the inferior petrosal sinus can be exposed. The superior petrosal sinus (SPS) can be clipped and divided. A dural incision can be extended across the SPS and then inferiorly into the posterior fossa. Occasionally, the dura over Meckel’s cave is divided to mobilize the trigeminal nerve anteriorly and increase exposure of the petroclival region.
The approach can be extended into the infratemporal fossa by removing the bone from lateral to medial toward the foramen ovale. This maneuver eventually connects the temporal and infratemporal fossae, allowing tumor removal, especially in the case of trigeminal schwannomas, with extension along V3.19
Middle Fossa Transtentorial Approach
The middle fossa transtentorial approach was first reported by Kawase et al.27 in 1985 for approaching aneurysms of the midbasilar artery through the petrous pyramid. In 1991, Kawase et al.29 applied this approach for resection of petroclival meningiomas that extended into the parasellar region (sphenopetroclival meningiomas). This approach uses a combination of the extended middle fossa approach with the addition of intradural resection of the tentorium to allow wider posterior fossa exposure. It is in many ways similar to the subtemporal transtentorial approach, with the added advantage of drilling the anterior petrous ridge.
Indications
The middle fossa transtentorial exposure can be accomplished with an anterior or posterior petrosectomy or a combined petrosal approach.20,29–31 It is suitable for meningiomas extending along the superior and middle clivus or along the posterior wall of the petrous ridge, which can have long dural attachments. Hearing is preserved with this approach, as with all middle fossa approaches. It also permits resection of tumors that extend to the parasellar region and posterior cavernous sinus. It is particularly attractive for small tumors in the petroclival region, laterally located pontine lesions (cavernous malformations and gliomas), or basilar trunk aneurysms. Compared with the extended middle fossa approach, it provides wider posterior fossa exposure because of sectioning of the tentorium.
Surgical Approach
The extent of petrous pyramid resection is similar to that obtained by the extended middle fossa approach. The dura is opened above and below the SPS. Cranial nerves IV and V are identified. The SPS is clipped between cranial nerves V and VII, with care taken to avoid sacrificing the petrosal vein. Alternatively, the SPS can be embolized preoperatively.24 The tentorium is cut until the tentorial notch is seen. The tentorium can then be tented open with retention sutures, exposing the petroclival region from cranial nerves III through VII.30 The inferomedial triangle of the cavernous sinus can be visualized, mobilization of the trigeminal nerve can be accomplished by opening Meckel’s cave, and Dorello’s canal can be seen through this exposure.
Posterior Transpetrosal Approaches
Retrolabyrinthine Approaches
Presigmoid Approach
The retrolabyrinthine presigmoid approach was first described by Hitselberger and Pulec32 in 1972, and was popularized by Norrell and Silverstein33 in 1977 and by House et al.34 in 1984. It is performed through the mastoid air cells, with elevation of a dural flap between the labyrinth and the SS. The concept of this procedure is based on its allowing entry into the CPA anterior to the SS, thus lessening the need for cerebellar retraction. It was originally described as being useful for partial sectioning of the fibers of the sensory roots of cranial nerve V for trigeminal neuralgia. It has been used for selective sectioning of the vestibular division of cranial nerve VIII for treatment of vertigo and for endolymphatic duct surgery. It can occasionally be used to remove small acoustic tumors when preservation of hearing is desirable. The major advantage of this approach is that it provides direct access to the CPA without sacrificing hearing and without extensive cerebellar retraction. Its major disadvantage is the limited exposure, which can be compromised even further by a large dominant SS or when the mastoid air space is contracted (“crowded mastoid”).
Trans-sigmoid Approach
The trans-sigmoid approach can be used as part of any posterior transpetrosal approach. Exposure is increased by ligating the SS, usually between the superior and the inferior petrosal sinuses or between the inferior anastomotic vein (vein of Labbé) and the SPS. Thus, the inferior anastomotic vein drains into the transverse sinus and retrogradely into the opposite jugular system. A preoperative angiogram or magnetic resonance venogram is essential to ensure patency of the torcular. A nondominant sinus in the presence of a patent torcular can be sacrificed in selected cases. Temporary clipping across the SS is recommended to assess for the presence of temporal lobe or cerebellar swelling. The SS can be opened and packed with Surgicel and its lumen sutured, or it can be ligated and clipped. Uyar et al.35 described an original technique for sinus closure without opening the dura or the sinus itself. Posterior fossa and presigmoid dura are exposed first, and parallel suture is passed in front and back of the sinus. The muscle graft is placed between and sutures are tied, resulting in bending and obliteration or the lumen. Cadaver and angiographic studies show that the incidence of unilateral transverse sinus is rather infrequent (2.5%) and absence of any communication at the torcular is even rarer.36,37 Despite this, given the catastrophic results of ligating a unilateral SS, a preoperative arteriogram or magnetic resonance venogram is recommended. This approach is advantageous in cases of tumor growth into the SS with spontaneous obstruction. Jugular foramen tumors spreading into the jugular bulb and SS can be managed by ligation of the internal jugular vein in the neck followed by opening the SS, packing, and tumor removal.38
Retrosigmoid (Suboccipital) Approach
The retrosigmoid approach, also known as the lateral suboccipital approach, is not a true transtemporal approach. This approach is most familiar to neurosurgeons and has been the traditional exposure used for resection of tumors of the CPA.39,40 This approach provides wide entry into the posterior fossa, with maximal exposure for tumors such as vestibular schwannomas. Using this approach, the neurovascular structures of the temporal bone are avoided at the expense of cerebellar retraction. The development of monitoring techniques using evoked response methods has greatly increased the practicality of this approach.
Surgical Approach
The retrosigmoid approach can be done with the patient sitting, lateral, or three fourths prone. The head position is carefully adapted to the procedure type that is performed. With upper complex exposure (cranial nerve V and SCA) the vertex should be tilted downward. If the procedure involves the middle complex (cranial nerves VII and VIII, anteroinferior cerebellar artery) or the lower complex (cranial nerves IX-XI, posteroinferior cerebellar artery), the degree of tilt decreases or is reversed. An incision is made approximately 2 cm posterior to the mastoid tip, extending cephalad to slightly above the transverse sinus and caudally into the suboccipital musculature. The asterion has been used as a useful landmark for the junction of the transverse sinus and SS. However, a study showed that it is a quite unreliable landmark and that placing a bur hole over it can potentially lead to sinus damage.41 Neuronavigation alternatively has been shown to locate the position of the transverse sinus–SS junction more accurately.42 Craniectomy or “silver dollar” craniotomy can be performed such that the edges of the transverse sinus and SS and their junction are clearly identified. In difficult reoperative cases, a line drawn from the root of the zygoma to the inion can reliably locate the course of the transverse sinus.43 Bone removal can include the posterior lip of the foramen magnum, as well as the upper cervical lamina. The arachnoid at the foramen magnum should be opened first to permit CSF egress and facilitate cerebellar retraction.
The “extended retrosigmoid approach” involves removing of the bone over the SS to expose its entire length from the transverse sinus to the jugular bulb. This allows some degree of sinus retraction when the dura is reflected anteriorly, lessening the degree of cerebellar retraction.44,45 However, venous flow can be impaired intra- or postoperatively. so frequent reassessment should be performed.44
In the case of vestibular schwannoma resection, the tumor is immediately visualized. It can be explored initially with a rigid endoscope to verify the position of neurovascular structures to the tumor and extent of the tumor to the IAC.18 The facial nerve usually lies anterior to the tumor. For tumors that extend into the IAC, the porus is drilled until the dura over the IAC is seen. In a cadaver study, the amount of posterior IAC that can be safely unroofed averaged 5.9 mm (range 4-8 mm). The best available way to avoid critical labyrinthine structures during the suboccipital approach is to use preoperative high-resolution CT. A line is drawn on axial CT images from the medial aspect of the SS to the fundus of the IAC. If this line crosses any labyrinthine structures, the risk of injury and hearing loss during drilling of the IAC significantly increases.46 In addition, air cells can be encountered during drilling with possibility of postoperative CSF leakage. Alternatively to drilling of the IAC a 70-degree rigid endoscope can be used to visualize the canal and remove tumor remnants.47
Complications and Disadvantages
This exposure requires a certain amount of cerebellar retraction; thus, cerebellar edema or hematoma can occur. This is especially true for large tumors requiring lengthy surgery. If the surgeon maintains gentle retraction (1-2 cm), these types of complications can usually be avoided. In cases of small or medium-sized tumors, an endoscope alone can be used for tumor removal to reduce the amount of cerebellar retraction.48 The extended retrosigmoid approach also reduces the degree of cerebellar retraction. However, there is risk of postoperative sinus thrombosis.44 The facial nerve lies on the anterior surface of the tumor somewhere between the superior and the inferior poles. Therefore, some amount of tumor removal is required to visualize the nerve. This is one of the disadvantages, because manipulation alone can cause nerve traction injury. A possible way to avoid injury to the facial nerve is to start medial to the tumor close to the brain stem or lateral in the region of IAC, where the nerve relation to tumor is relatively constant. After identification of the nerve, tumor removal can be performed safely. Other complications include CSF leakage and postoperative incisional pain attributed to adherence of the suboccipital muscles to the dura. The incisional pain can be lessened by either replacing the bone flap, in the case of a craniotomy, or performing a cranioplasty to fill the bone defect. The incidence of CSF leakage may be lessened by identifying and waxing small air cells in the IAC with the use of an endoscope. In 9% of cases, a high jugular bulb may make drilling of the meatus through the retrosigmoid approach impossible and is associated with increased risk of bleeding and air embolism.49 As previously mentioned, loss of hearing can occur during drilling of the posterior aspect of the IAC.
Translabyrinthine Approach
The first report of a translabyrinthine approach was in 1904 by Panse,50 who advocated this approach for its shortest distance to the CPA. In 1961, House3 described the middle fossa approach for resection of vestibular schwannomas located laterally in the IAC with minimal CPA extension. Because of limited exposure, incidence of facial paresis, need for temporal lobe retraction, and limited control of vascular structures, House and Hitselberger51 introduced the translabyrinthine approach for resection of vestibular schwannomas. This was a more lateral approach and gave direct control of the facial nerve by drilling through the labyrinth. They emphasized the vertical crest at the lateral end of the IAC as a landmark. Differentiation between the superior and the inferior vestibular nerves was more readily apparent with this approach.
Variations of the translabyrinthine approach have been described for more extensive exposure of the SS, with mobilization of the jugular bulb, as well as drilling out of the infralabyrinthine air cells to increase access for large tumors.52 Furthermore, there is evidence that in rare cases hearing preservation may be possible with the translabyrinthine approach, such that in one case ablation of all three semicircular canals with preservation of the cochlea and saccule left the patient with useful, though decreased, hearing.53 Another variation of the translabyrinthine approach is the addition of a suboccipital approach with a partial labyrinthectomy, such that only those elements that are required for visualization of the vertical crest and the lateral IAC are removed.54
Surgical Approach
The patient is positioned supine with the head turned opposite to the side of the tumor. A retroauricular C-shaped skin incision is made, and an anteriorly based periosteal flap is elevated and preserved.55
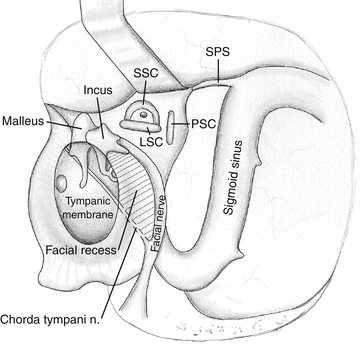
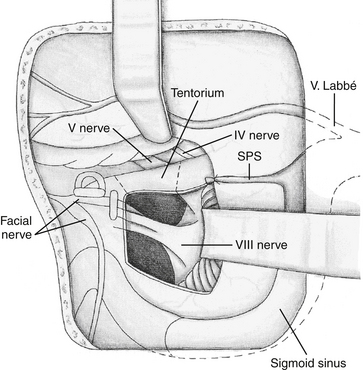
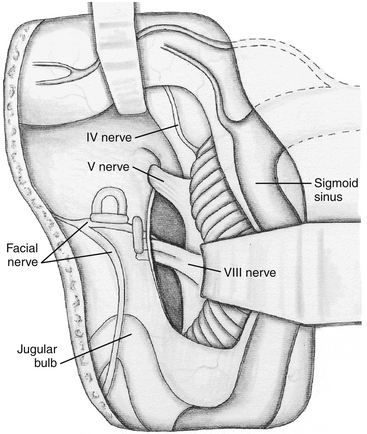
The mastoid antrum is then entered, and the short process of the incus in the fossa incudis is identified. This provides a useful landmark for the lateral semicircular canal (LSC), which is found immediately below. The solid angle is located medial to the mastoid antrum and houses the three semicircular canals (Fig. 47-3). The air cells are then removed inferiorly to the level of the digastric groove. The air cells posterior to the LSC are removed, and the PSC, located between the LSC and the posterior fossa plate, is exposed. The vertical segment of the facial nerve is then located within the fallopian canal by removing the remaining inferior mastoid and retrofacial air cells. The facial nerve passes from the external genu at the inferior surface of the LSC to the stylomastoid foramen, located just anterior to the digastric ridge. The facial nerve is skeletonized only to facilitate exposure of the jugular bulb and foramen; it is otherwise left within the dense bone of the fallopian canal. It is important to stay parallel to the facial nerve course during drilling of the bone around the fallopian canal. This technique avoids inadvertent penetration of the canal if the direction of drilling is perpendicular to the facial nerve.56 The SSC is identified by following the sinodural angle through the supralabyrinthine air cells. The final area of posterior exposure is removal of the middle, posterior, and sigmoid plates, completing the subtotal petrosectomy. The roughly triangular area of bone bounded by the SS, SPS, and bony labyrinth (i.e., solid angle) is known as Trautmann’s triangle. This bone is drilled to expose the presigmoid posterior fossa dura.
The LSC, PSC, and SSC are removed, as well as the bone over the posterior fossa, revealing the endolymphatic sac. Next, the vestibule is opened and completely exposed beneath the facial nerve. Bone is removed over the medial and posterior vestibule until the nerves to the superior, lateral, and inferior ampullas are exposed. These nerves define the superior and inferior limits of the IAC. Bone removal continues over the IAC until a transparent shell remains. The transverse crest separating the superior and inferior division of the vestibular nerves can be seen through the thinned bone.
Because this approach is limited in comparison with the retrosigmoid approach for large vestibular schwannomas, several modifications have been added to House’s classic description. Sanna introduced the enlarged translabyrinthine approach in 2003 with transapical extension as an extension for dealing with vestibular schwannomas greater than 4 cm.57–60 The principle of transapical extension is to increase bone removal anterior and posterior to SS, along the middle fossa, and around IAC up to 320 degrees (type I) or 360 degrees (type II). To increase vertical extension of the exposure in cases with a high jugular bulb (which is one of the limitations of the classic translabyrinthine approach), the bulb is depressed inferiorly with Surgicel and bone wax.57
The wound is closed by placing a free muscle graft over the malleus, incus, and attic. To gain access to the middle ear, the facial recess is sometimes opened. The facial recess is a triangular area defined superiorly by the fossa incudis, medially by the facial nerve, and inferiorly by the chorda tympani (see Fig. 47-3). In this situation, a muscle plug is placed to close the opening of the eustachian tube. The cavity is filled with fat graft, and the periosteal flap is closed. The skin is closed tightly, and a pressure dressing is applied.
Complications and Disadvantages
The main complication with this approach seems to be a high incidence of CSF leakage (up to 30% in some series but usually in the 10% to 20% range).61 Access to the eustachian tube through the middle ear (facial recess) or the epitympanum can lessen the risk of CSF leakage but involves additional exposure and drilling. Placing a free muscle graft over the attic, filling the operative cavity with fat graft, and closing with a periosteal graft decrease the chance of CSF leakage. In a series of 110 patients treated with the expanded translabyrinthine approach closed with abdominal fat, the rate of CSF leakage was 1.8%.57 Other complications include posterior fossa subdural hematoma, brain stem hematoma, cerebellar and cerebral swelling, and lower cranial nerve palsies. In cases of highly pneumatized temporal bone, preventive closure of the eustachian tube can be performed. This is accomplished by “cul de sac” closure of the EAC, removal of the ossicular chain, and additional drilling of hypotympanic air cells with exposure of eustachian tube, which is plugged with a piece of muscle.62
Transotic Approach
The transotic approach was developed by Fisch and Mattox 61 in response to the limitations of the translabyrinthine approach. Unlike the posteriorly directed translabyrinthine approach, the transotic approach permits more extensive temporal bone resection and positions the dissection both anterior and posterior to the facial nerve, giving excellent visualization of the anterior CPA and petrous apex. The early description included transposition of the facial nerve and had similarities with the transcochlear approach of House and Hitselberger.63 Unfortunately, the rate of facial nerve paralysis was unacceptable, and modifications followed such that the facial nerve is not transposed but left in situ in the fallopian canal.61,64
Indications
The indication for the transotic approach is essentially identical to that for the translabyrinthine approach. It was designed for vestibular schwannomas of up to 2.5 cm,61,65 although it can certainly be used for larger schwannomas and other lesions, such as meningiomas, hemangiomas, arachnoid cysts, and mucosal cysts, involving the IAC.66 In contrast to the translabyrinthine approach, the transotic approach circumvents the problem of a high jugular bulb because of the anterior exposure obtained. Because of additional anterior exposure, this approach can be used for treating some petrous apex cholesteatomas.67
Surgical Approach
The surgical approach for the transotic approach is similar to that for the transcochlear approach, with the important difference that the facial nerve is not mobilized (Fig. 47-4). It involves blind sac closure of the EAC, exenteration of the otic capsule including the cochlea, and exposure of the jugular bulb by drilling infralabyrinthine air cells and petrous carotid artery. The additional exposure is obtained by drilling the bone anterior to the tympanic and mastoid segments of the facial nerve. By the end of the exposure, the facial nerve from its entrance into the IAC to its exit at the stylomastoid foramen is exposed in the middle of approach yet remains within bone, thus reducing the potential risk for injury.61,66,68
Complications and Disadvantages
The original transotic approach was associated with a high incidence of facial nerve paralysis secondary to transposition of the facial nerve.65 The modification of leaving the facial nerve within the fallopian canal reduced this risk.64 In Fisch and Mattox’s61 series of 73 patients, for tumors less than 1.4 cm, all patients had normal facial nerve function at 2 years after surgery. For tumors measuring 1.5 to 2.5 cm, facial nerve function was normal in 61% at 2 years. CSF leakage was contained subcutaneously in 4% and was transient in 3%, and no patient required revision. Removal of all of the middle ear mucosa and pneumatic air cells related to the middle ear space and obliteration of the eustachian tube orifice, combined with dural closure and filling of the defect with fat, lessen the chances for CSF leakage.66 In their series, meningitis occurred in 1%, and death occurred in 1%. Disadvantages of this approach are that it adds operative time compared with other procedures (e.g., translabyrinthine approach) and that the presence of facial nerve in the middle of approach not only limits surgeon maneuverability but also exposes the nerve to inadvertent injury.69
Transcochlear Approach
In 1976, House and Hitselberger63 described the transcochlear approach. This approach is a forward extension of the translabyrinthine approach in which the facial nerve is mobilized and the cochlea removed. This exposure essentially removes the entire petrous bone, giving maximal transpetrosal exposure. The transcochlear approach involves the same extent of petrous bone drilling as the transotic approach, but during a transcochlear approach the facial nerve is mobilized posteriorly, giving the surgeon additional working space and maneuverability. Both approaches give access to the CPA anterior to the IAC, but the transotic approach can be converted to a transcochlear approach if wider exposure is required.70
The operative field given by the transcochlear approach is limited by the EAC wall and middle ear and, although more anterior than the translabyrinthine approach, remains posteriorly directed. The modified transcochlear approach was developed to give additional anterior exposure by removing the EAC and the tympanic membrane.65 It also offered more extensive exposure and circumferential control of the petrous ICA.71–75 Some authors also include resection of the glenoid fossa, joint capsule, and meniscus and partial resection of the posterior aspect of the zygomatic arch.74 Others combine it with a neck dissection,76 and some describe extended exposures, including tentorial section for supratentorial exposure.77
Indications
The transcochlear approach was designed for large tumors in the CPA extending anterior to the IAC along the superior two thirds of the clivus, as well as for aneurysms of the middle and lower basilar artery. It is also suitable for complex petrous apex cholesteatomas encasing vital structures.78 Its main advantage is the broadness of the exposure, giving the surgeon a parallel triangular view of the middle clivus. In addition, contralateral cranial nerves and the CPA are exposed.70 The addition of the modified transcochlear approach gives the surgeon a more direct and most laterally directed approach to the CPA, as well as circumferential exposure of the petrous ICA. This is important for selected cases in which a carotid bypass is required. It can be combined with resection of the mandibular condyle, closure of the EAC, zygomatic osteotomy, and drilling of the floor of the middle cranial fossa for tumors extending into the infratemporal fossa and nasopharynx.
Surgical Approach
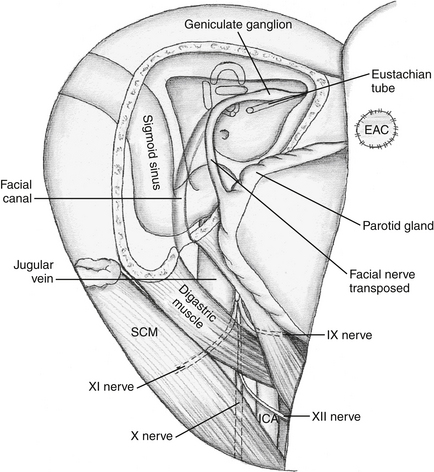
The initial exposure for the transcochlear approach is similar to that for the translabyrinthine approach. A curvilinear C-shaped retroauricular skin incision is made, and the skin of the cartilaginous EAC is everted and sewn shut (see Fig. 47-4). A musculoperiosteal flap from the mastoid process is used medially as a second layer of closure.74 The skin of the bony EAC and tympanic membrane were initially left in place, but removal of this bone improves access to the midline without increasing morbidity. The entire osseous EAC can be removed without affecting the function of the mandibular condyle in the glenoid fossa.
A mastoidectomy is performed exposing the canal wall inferiorly. The middle and posterior fossa plates, along with the SS, are then skeletonized. The facial nerve is skeletonized from its entrance into the IAC to its exit from the stylomastoid foramen. The facial recess is opened. The middle ear space and epitympanum are entered, and the ossicles can then be removed. The chorda tympani is then sectioned inferiorly at its origin from the descending portion of the facial nerve. Drilling is continued into the retrofacial air cells. The GSPN is then divided just anterior to its origin at the GG. The facial nerve can then be mobilized from its bony canal and transposed posteriorly. This invariably results in facial nerve paralysis, because it disrupts the blood supply to the GG. It is best to leave the facial nerve within the canal, if possible. If transposition is necessary, then anterior transposition by mobilizing the mastoid segment and the nerve exiting at the stylomastoid foramen is safer.
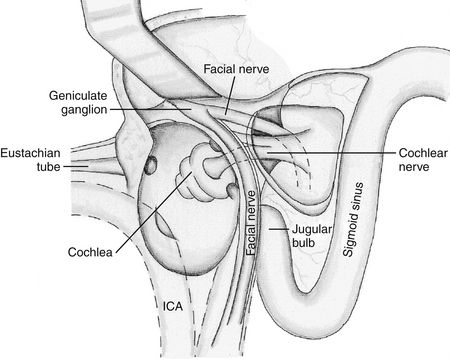
Next, the cochlea is removed, including the bony septum between the basal turn and the ICA (see Fig. 47-6). The jugular bulb is also exposed completely. Care should be taken not to injure the underlying neurovascular structures, namely, cranial nerves IX through XI near the jugular bulb and foramen, as well as the facial nerve in the dura of the IAC.
Complications and Disadvantage
The main disadvantage of the transcochlear approach is that hearing is sacrificed. The extensive mobilization of the facial nerve places it at risk, and all patients have significant facial nerve paralysis.79 Although it usually improves after surgery, facial nerve function infrequently exceeds grade III on the House-Brackmann grading scale,80 and the nerve often is permanently impaired. Section of the GSPN can result in an ipsilateral dry eye. There is also a risk of CSF leakage and meningitis. Resection of the mandibular condyle can result in TMJ dysfunction.
Given the high frequency of facial nerve paralysis and time-consuming exposure, some groups advocated the use of relatively simple retrosigmoid approach in combination with an orbitozygomatic approach for petroclival meningiomas.79
Infralabyrinthine Approach
Indications
In 1985, Gherini et al.81 advocated the infralabyrinthine approach for the surgical management of cholesterol granulomas of the petrous apex and CPA. The purpose of this approach is to permit access to that portion of the petrous apex that is inferior to the labyrinth. As such, it is valuable in decompression of a cholesterol granuloma of the petrous apex.82 The advantages of this approach for cholesterol granulomas include absence of ossicular chain work and risk of ICA injury, as well as easiness of revision surgery. It can also be useful in conjunction with the suboccipital approach for resection of meningiomas of the petrous ridge with extension into the temporal bone, but without involvement of the labyrinth76,83 and jugular paragangliomas.84
Complications and Disadvantages
If the jugular bulb is in a high position, access below the labyrinth and above the bulb can be limited. A careful preoperative evaluation using high-resolution CT is useful.46,49 Measuring the distance between the labyrinth and the jugular bulb on coronal images can be particularly useful; a distance of less than 1 cm was found to be inadequate for satisfactory drainage of cholesterol granulomas.85 In those instances, another approach is recommended (e.g., the transcanal–infracochlear approach, described later).
Transcanal–Infracochlear Approach
The transcanal part of the transcanal–infracochlear approach was first described by Farrior85,86 in 1984.
Indications
The transcanal–infracochlear approach is used for access to the petrous apex cholesterol granulomas when hearing preservation is a goal and the jugular bulb is positioned high, limiting exposure through an infralabyrinthine approach. Because this approach is directed cephalad, it provides dependent drainage for cholesterol granulomas of the petrous apex. Additional advantages are drainage to a well-aerated region near the eustachian tube and easy reexploration through an inferior myringotomy.22 Because of these advantages over other approaches, the transcanal–infracochlear approach is considered the approach of choice for cholesterol granulomas of the petrous apex.22,85,87
Surgical Approach
The transcanal–infracochlear approach uses a C-shaped retroauricular skin incision similar to that used for the translabyrinthine and infralabyrinthine approaches. The soft tissues are reflected forward, and the ear canal is transected just medial to the bony cartilaginous junction. The anterior, inferior, and posterior portions of the ear canal skin are lifted superiorly to the level of the umbo. Bone is removed from over the anterior, inferior, and posterior portions of the bony canal wall, effectively achieving near-total removal of the tympanic bone and enlargement of the canal. The thin bone over the TMJ is preserved. The carotid artery is then skeletonized anterior and inferior to the eustachian tube orifice. Bone is then removed between the ICA and the internal jugular vein without actually exposing the jugular bulb. The region of the facial nerve is identified using continuous electrical monitoring, but the nerve is not exposed. Inferiorly, the cholesterol granuloma sac is identified, opened for drainage, and irrigated. Essentially, the silicone catheter should be positioned to maintain the patency of fenestration.22 Finally, the tympanomeatal flap is repositioned and the resultant bone defect is filled with bone plate.
Complications and Disadvantages
The complications of the transcanal–infracochlear approach are similar to those with the translabyrinthine approach except that injury to the cochlea, carotid artery, and jugular bulb is possible. This approach provides only limited exposure of the petrous apex and therefore is useful only in the specific indications of drainage of a cholesterol granuloma or petrous apicitis. Mattox reported use of a combination of microscope and endoscope for cholesterol granulomas and cholesteatomas.88 An endoscope facilitates the exposure of cystic cavity and allows the surgeon to clean any sediment, as well as fenestrate all cysts into a single cavity.89,90
Combined Approaches
Petrosal Approach
The petrosal approach is also referred to as the combined suprainfratentorial approach because it combines both supratentorial and infratentorial exposures to give wide anterior access to the CPA and ventral brain stem. The first reported transtentorial exposure was in 1896 by Stieglitz et al.,91 in which a CPA tumor was approached through a supramastoid–suboccipital exposure. Several authors followed with modifications of the occipital flap with a suboccipital craniectomy, including ligation of the SS for wider exposure,92 combined occipitotemporal craniotomy with or without ligation of the lateral sinus,93 and reapproximation of the lateral sinus,94 as well as other approaches,95,96 including the addition of a mastoidectomy.97,98
The petrosal approach was popularized by Malis,98 who described ligation of the SS between its junction with the superior anastomotic vein and the SPS for increased exposure. Spetzler et al.99 operated on 83 patients with the petrosal approach and sacrificed the SS in 50% of cases. Al-Mefty et al.100 described the petrosal approach in detail, emphasizing an extensive petrous resection and directing the approach more laterally, thus lessening the operative distance to the clivus. They also stressed the importance of preserving the venous sinuses.
Indications
The petrosal approach includes a combined temporal craniotomy with a posterior fossa craniectomy–craniotomy for supratentorial and infratentorial exposure. Crucial to this approach is sectioning of the tentorium. With the addition of an extensive petrous resection, the anterior surface of the brain stem can be approached to the level of the inferior one third of the clivus. The lowest portion of the clivus is often obscured by the jugular tubercle. The petrosal approach provides access to lesions in the CPA and petroclival junction (upper two thirds of the clivus), such as meningiomas, trigeminal schwannomas, epidermoids, or chondrosarcomas. Vascular pathologies such as aneurysms of the middle third of the basilar artery can be accessed.101 For lesions of the lower third of the clivus and the foramen magnum, the far lateral transcondylar approach provides better access.
Not only the posterior fossa structures but also the supratentorial ventrolateral brain stem can be exposed through this approach. Hakuba et al.102 first used this route for retrochiasmatic craniopharyngiomas. The main advantage of this approach in comparison with pterional or orbitozygomatic approaches is the ability to attack the tumor from an inferior to a superior direction. This is especially important for tumors with significant superior extension.103,104
Surgical Approach
A mastoidectomy is first accomplished with preservation of the labyrinth and exposure of the mastoid segment of the facial nerve. A combined temporo-occipital bone flap is then raised (Fig. 47-5). The transverse sinus and SS were previously identified during the mastoidectomy. This gives exposure along the middle fossa floor, transverse sinus, SS, and suboccipital dura. The dura can then be opened on the inferior aspect of the temporal lobe and anterior or posterior to the SS. After opening the dura over the temporal lobe and the presigmoid dura, the SPS is clipped and divided. The tentorium can be divided in three directions (Fig. 47-6). The first cut is done posteriorly along the transverse sinus to allow for retraction of the transverse sinus–SS junction posteriorly, thus enlarging the presigmoid corridor. The second cut is aimed medially toward the free edge to identify and protect the trochlear nerve. The third cut is parallel to the petrous pyramid and the SPS. This allows resection of the lateral tentorial leaflet and a view of the supratentorial and infratentorial compartments. Ipsilateral cranial nerves IV through X are well visualized (Fig. 47-7). A retractor can be placed to retract the temporal lobe superiorly, and another one can be placed to retract the transverse sinus and SS posteriorly. If the surgeon decides to work through the retrosigmoid corridor, a cerebellar retractor can be placed. Care must be taken to avoid injury to the inferior anastomotic vein. In certain cases, the presigmoid avenue is limited and ligation and division of the SS can provide maximum anterior exposure of the CPA.
The surgical technique for resection of retrochiasmatic craniopharyngiomas with the petrosal approach has several nuances.102–105 Exposure is performed as described earlier, except that the presigmoid dura is exposed only enough for opening and closure. The temporal dura is opened along the floor of the middle fossa, the SPS is clipped and cut, and the incision is carried along the SS. Care is taken to avoid damage to the vein of Labbé. The tentorium is cut to the free edge, which enables retraction of the SS posteriorly, creating a presigmoid working corridor. The approach is then directed toward the crural and ambient cisterns. Opening the arachnoid exposes the tumor anterior and superior to the basilar artery, inferior to chiasm, and medial to cranial nerves III and IV. As mentioned previously, this approach allows retrochiasmatic tumor resection with minimal brain retraction.
Infratemporal Fossa Approach
The infratemporal fossa approach, developed by Fisch106 in 1977, is a craniotemporocervical approach for exposure of the lateral inferior skull base. This approach is divided into three exposures, types A, B, and C, depending on the amount of anterior exposure required. The type A approach is similar to the combined lateral skull base approach reported by Gardner et al.107 in 1977. With the type A exposure, a subtotal petrosectomy with transposition of the facial nerve is accomplished for exposure of the apical and infralabyrinthine temporal bone, as well as the mandibular fossa and posterior infratemporal fossa. The type B exposure gives additional anterior exposure of the clivus and horizontal segment of the ICA. The anterioposterior limits are from the foramen ovale to the SS. The type C approach is an anterior extension of the type B approach, giving exposure of the infratemporal fossa, pterygopalatine fossa, parasellar region, and nasopharynx. The exposure is obtained by removal of middle cranial fossa bone. With most indications for the type C approach, the surgeon can use a more anterior, preauricular pterional type of incision with a zygomatic osteotomy and subtemporal craniectomy.108
Indications
According to Fisch,106 the type A infratemporal fossa approach is useful for lesions involving the jugular foramen (e.g., class C and D glomus jugulare tumors), lesions of the petrous apex, lower cranial nerve schwannomas, high cervical and petrous carotid artery lesions, and certain infratemporal fossa lesions. Modifications of the approach can be made according to the therapeutic goal. Deveze et al.109 reported a modified type A approach for vascular lesions of high cervical and petrous carotid artery with subsequent reconstruction of EAC and ossicular chain. Despite the aggressiveness of the approach, patients had satisfactory facial and hearing preservation.
Surgical Approach
The details of the types of infratemporal fossa approaches were well described by Fisch and Mattox5 and are summarized here. The skin incision is an extension of the standard C-shaped retroauricular incision. The skin and periosteal flap is reflected anteriorly, with transection and closure of the EAC. Facial nerve is exposed distal to the stylomastoid foramen in the parotid gland. Next, the great vessels (carotid artery and jugular vein) and nerves of the neck (glossopharyngeal, vagus, spinal accessory, and hypoglossal) are exposed (Fig. 47-8). The posterior belly of the digastric muscle is divided near its insertion at the mastoid process. The external carotid artery and its branches are ligated and transected above the lingual artery. The ICA is followed to the carotid foramen. Next, a subtotal petrosectomy is done by exposing the temporal bone and reflecting the sternocleidomastoid muscle away from the mastoid tip. The operation proceeds with removal of the EAC, mastoidectomy with complete mobilization of the facial nerve for anterior transposition, exposure and possible ligation of the SS, removal of the styloid process for exposure of the ICA, obliteration of the eustachian tube, and exposure of the infratemporal fossa. This includes anterior translocation of the mandible. The exposure obtained with this approach spans from the middle ear to the mastoid and upper neck, exposing the posterior portion of the infratemporal fossa.
The type C approach adds anterior exposure to the type B approach. The nasopharynx can be entered by removing the lateral pharyngeal wall behind the medial pterygoid process. Exposure here gives visualization of the vomer, opposite inferior turbinate, and pharyngeal end of the opposite eustachian tube. The pterygopalatine fossa is exposed by removing the pterygoid process. To expose the parasellar region, the zygoma and basal portion of the sphenoid are removed. The ipsilateral sphenoid and maxillary sinus are opened. For complete exposure of the cavernous sinus, the maxillary nerve is divided and the bone at the floor of the middle fossa is removed for extradural elevation of the temporal lobe.
We have used a combination of middle fossa and infratemporal fossa approaches for treating en plaque meningiomas of the temporal bone (Fig. 47-9). This particular combination of approaches is a logical extension of either individual approach when both areas are involved pathologically. This approach could be of value in providing wider access to the petrous carotid artery.
Complications and Disadvantages
According to Fisch and Mattox,5 transposition of the facial nerve always results in some paresis, but the average recovery of function (to House-Brackmann grade II) was 80%. A conductive hearing loss is common in all infratemporal fossa approaches because of removal of the tympanic membrane and ossicles. Tachycardia can occur after removal of glomus jugulare tumors. Preoperative laboratory evaluation of suspected glomus tumors should include blood vanillylmandelic acid levels and possible use of alpha-adrenergic blockers. With the additional exposure of the eustachian tube, ascending infection can occur even with subsequent primary closure of the eustachian tube. CSF leakage and meningitis can obviously occur. Fisch and Mattox5 recommend obliteration of the wound with muscle rather than free fat graft to aid in closure of the eustachian tube. For the type C approach, the major risks include hearing loss, which occurs in most, and loss of mandibular function as a result of translocation of the mandibular condyle and resection of the articular disc and glenoid fossa during exposure. Initially, there may be limitation in jaw opening, but this eventually resolves if the mandibular condyle is preserved. Resection of cranial nerves V2 and V3 usually results in facial and tongue anesthesia; this generally improves over 9 months.5
Endonasal Endoscopic Approach
The endoscopic approach to the cranial base is not truly a transtemporal approach. The first report of a trans-sphenoidal approach was reported by Montgomery,110 who used an open access through the medial canthus. Fucci et al.111 first used an endoscope to drain a giant cholesterol granuloma of the petrous apex. However, it was not until recently that technological advances popularized endoscopic skull base surgery. The advantages of this approach are less invasiveness, better cosmetic results, hearing and vestibular function preservation, and shorter operative time.112,113 In addition, as in the case of cholesterol granuloma, stent patency can be easily assessed and the stent can be removed during office follow-up.114 The endonasal endoscopic approach is considered appropriate for patients with benign cystic lesions abutting, prolapsing, or invading the sphenoid sinus, such as cholesterol granulomas or cholesteatomas,112,115,116 yet solid lesions can also be resected.114
Indications
The endoscopic endonasal approach provides excellent visualization of midline structures from the dorsum sella to C2. Therefore, it is suitable for midline and paramedian lesions, such as chordomas, chondrosarcomas, osteosarcomas, cholesterol granulomas, and cholesteatomas. The carotid arteries laterally are the main limitation of the exposure. Therefore, petrous apex lesions lying medial to the carotid artery can be exposed. An additional advantage for treating a cholesterol granuloma is the presence of the sphenoid sinus, which can be used for aeration of the cavity. Unique to the endoscopic approach is the ability to visualize blind spots with angled rigid 30- and 70-degree scopes. This allows the surgeon to reexplore the surgical field for tumor remnants. Image guidance systems allow early identification of the carotid arteries, as well as an entry point into the petrous apex.117,118 One of the advantages of image guidance in skull base surgery is the absence of the shift seen during intracranial procedures. Generally, intradural lesions are not considered for endoscopic treatment because of the high risk of postoperative CSF leakage and meningitis.
Surgical Approach
The endoscopic endonasal approach is centered over the sphenoid sinus. Usually a right-sided approach is preferred for right-handed surgeons. However, for paramedian structures, a contralateral approach allows a slightly oblique view—more in line with the axis of the lesion. Depending on the lesion size, a unilateral or bilateral approach can be selected. For lesions requiring less manipulation, a unilateral approach through an enlarged sphenoid ostium can be performed. We prefer to use an anterior trans-sphenoidal approach with lateralization of the posterior nasal septum and opening of both sphenoid ostia, because this method provides better visualization of the sinus cavity. Septations in the sinus are removed carefully to avoid injury to the carotid arteries. Usually both carotid and optic protuberances can be visualized. If not visualized adequately, the position of carotid artery can be identified with a Doppler probe119 or image guidance.117,118 Midline tumors are identified in the clival recess. Paramedian lesions protruding into the sinus—so-called sphenopetroid cholesterol granulomas are visualized at this stage. In those cases, marsupialization can be done.113,114 Usually the petrous apex is entered from a point halfway between the medial surface of the ICA and the midline of the sphenoid sinus at a horizontal level one third of the way up from the posteroinferior aspect of the sphenoid sinus wall. Additional exposure can be done by mobilizing of the carotid artery as described by Zanation et al.114
Complications and Disadvantages
Early experience with the endoscopic approach for petrous apex cholesterol granulomas showed subsequent stenosis.120 However, subsequent studies showed satisfactory results, broadening the applications.114 The main risk during surgery is carotid artery injury. It can be avoided by intraoperative visualization of anatomic landmarks or image guidance. Optic nerves, the vidian nerve, and intercavernous and cavernous sinus structures are also at risk during exposure.
Al-Mefty O., Ayoubi S., et al. The petrosal approach: indications, technique, and results. Acta Neurochir Suppl (Wien). 1991;53:166-170.
Brackmann D.E., Toh E.H. Surgical management of petrous apex cholesterol granulomas. Otol Neurotol. 2002;23(4):529-533.
Deveze A., Alimi Y., et al. Surgical management of lesions of the internal carotid artery using a modified Fisch type A infratemporal approach. Otol Neurotol. 2007;28(1):94-99.
Fisch U., Mattox D.E. Infratemporal fossa approach type B. In: Fisch U., Mattox D.E. Microsurgery of the skull base. New York: Stuttgart; 1988:286-343.
Fisch U., Mattox D.E. Translabyrinthine approach. In: Fisch U., Mattox D.E. Microsurgery of the Skull Base. New York: Stuttgart; 1988:546-576.
Fisch U., Mattox D.E. Transotic approach to the cerebellopontine angle. In: Fisch U., Mattox D.E. Microsurgery of the Skull Base. New York: Stuttgart; 1988:74-127.
Fisch U., Mattox D.E. Transtemporal supralabyrinthine approach. In: Fisch U., Mattox D.E. Microsurgery of the Skull Base. New York: Stuttgart; 1988:418-454.
Horn K.L., Hankinson H.L., et al. The modified transcochlear approach to the cerebellopontine angle. Otolaryngol Head Neck Surg. 1991;104(1):37-41.
House W. Surgical exposure of the internal auditory canal and its contents through the middle, cranial fossa. The Laryngoscope. 1961;71(11):1363-1385.
House W.F. Middle Cranial Fossa Approach to the Petrous Pyramid. Report of 50 Cases. Arch Otolaryngol. 1963;78:460-469.
Kabil M.S., Shahinian H.K. A series of 112 fully endoscopic resections of vestibular schwannomas. Minim Invasive Neurosurg. 2006;49(6):362-368.
Kawase T., Shiobara R., et al. Middle fossa transpetrosal–transtentorial approaches for petroclival meningiomas. Selective pyramid resection and radicality. Acta Neurochir (Wien). 1994;129(3-4):113-120.
Mann W.J., Amedee R.G., et al. Transsigmoid approach for tumors of the jugular foramen. Skull Base Surg. 1991;1(3):137-141.
Mattox D.E. Endoscopy-assisted surgery of the petrous apex. Otolaryngol Head Neck Surg. 2004;130(2):229-241.
Oyama K., Ikezono T., et al. Petrous apex cholesterol granuloma treated via the endoscopic transsphenoidal approach. Acta Neurochir (Wien). 2007;149(3):299-302. discussion 302
Quinones-Hinojosa A., Chang E.F., et al. The extended retrosigmoid approach: an alternative to radical cranial base approaches for posterior fossa lesions. Neurosurgery. 2006;58(4 suppl 2):ONS-208-214. discussion ONS-214
Sanna M. Lateral Skull Base Surgery. Atlas of Temporal Bone and Lateral Skull Base Surgery. New York: Stuttgart; 1995. 37-50
Sanna M. Transotic approach. The Temporal Bone: A Manual for Dissection and Surgical Approaches. New York. 2006:98-105
Sanna M. The Translabyrinthine Approaches. In: Sanna M, et al, eds. Atlas of Microsurgery of the Lateral Skull Base. New York: Thieme. 2008:34-77
Sanna M., Dispenza F., et al. Otoneurological management of petrous apex cholesterol granuloma. Am J Otolaryngol. 2009;30(6):407-414.
Shiobara R., Ohira T., et al. A modified extended middle cranial fossa approach for acoustic nerve tumors. Results of 125 operations. J Neurosurg. 1988;68(3):358-365.
Vrionis F.D., Cano W.G., et al. Microsurgical anatomy of the infratemporal fossa as viewed laterally and superiorly. Neurosurgery. 1996;39(4):777-785. discussion 785–776
Vrionis F.D., Foley K.T., et al. Use of cranial surface anatomic fiducials for interactive image-guided navigation in the temporal bone: a cadaveric study. Neurosurgery. 1997;40(4):755-763. discussion 763–754
Vrionis F.D., Robertson J.H., et al. Image-interactive orientation in the middle cranial fossa approach to the internal auditory canal: an experimental study. Comput Aided Surg. 1997;2(1):34-41.
Vrionis F.D., Robertson J.H., et al. Asterion meningiomas. Skull Base Surg. 1998;8(3):153-161.
1. Parry R. A case of tinnitus and vertigo treated by division of the auditory nerve. The Journal of Laryngology and Otology. 1904;19(08):402-406.
2. House W.F. Middle cranial fossa approach to the petrous pyramid. Report of 50 cases. Arch Otolaryngol. 1963;78:460-469.
3. House W. Surgical exposure of the internal auditory canal and its contents through the middle, cranial fossa. The Laryngoscope. 1961;71(11):1363-1385.
4. Arìstegui M., et al. Surgical anatomy of the extended middle cranial fossa approach. Skull base surgery. 1994;4(4):181-188.
5. Fisch U., Mattox D.E. Infratemporal fossa approach type B. In: Fisch U., Mattox D.E. Microsurgery of the Skull Base. New York: Stuttgart; 1988:286-343.
6. Garcia-Ibanez E., Garcia-Ibanez J.L. Middle fossa vestibular neurectomy: a report of 373 cases. Otolaryngol Head Neck Surg. 1980;88(4):486-490.
7. Lan M.Y., Shiao J.Y. Using greater superficial petrosal nerve and geniculate ganglion as the only two landmarks for identifying internal auditory canal in middle fossa approach. Eur Arch Otorhinolaryngol. 2010;267(12):1867-1871.
8. Cokkeser Y., et al. Identification of internal acoustic canal in the middle cranial fossa approach: a safe technique. Otolaryngol Head Neck Surg. 2001;124(1):94-98.
9. Catalano P.J., Eden A.R. An external reference to identify the internal auditory canal in middle fossa surgery. Otolaryngol Head Neck Surg. 1993;108(2):111-116.
10. Vrionis F.D., et al. Image-interactive orientation in the middle cranial fossa approach to the internal auditory canal: an experimental study. Comput Aided Surg. 1997;2(1):34-41.
11. Vrionis F.D., et al. Use of cranial surface anatomic fiducials for interactive image-guided navigation in the temporal bone: a cadaveric study. Neurosurgery. 1997;40(4):755-763. discussion 763-764
12. Brackmann D.E. Middle fossa approach for acoustic tumor removal. Clin Neurosurg. 1992;38:603-618.
13. Sughrue M.E., et al. Preservation of facial nerve function after resection of vestibular schwannoma. Br J Neurosurg. 2010;24(6):666-671.
14. Bloch O., et al. Factors associated with preservation of facial nerve function after surgical resection of vestibular schwannoma. J Neurooncol. 2011;102(2):281-286.
15. Parisier S.C. The middle cranial fossa approach to the internal auditory canal—an anatomical study stressing critical distances between surgical landmarks. Laryngoscope. 1977;87(4 Pt 2 suppl 4):1-20.
16. Kartush J.M., Kemink J.L., Graham M.D. The arcuate eminence. Topographic orientation in middle cranial fossa surgery. Ann Otol Rhinol Laryngol. 1985;94(1 Pt 1):25-28.
17. Fisch U., Mattox D.E. Transtemporal supralabyrinthine approach. In: Fisch U., Mattox D.E. Microsurgery of the Skull Base. New York: Stuttgart; 1988:418-454.
18. Wackym P.A., et al. Adjunctive use of endoscopy during acoustic neuroma surgery. Laryngoscope. 1999;109(8):1193-1201.
19. Chung J.C., et al. Surgery for a case of three-compartment trigeminal schwannoma: technical aspects. J Korean Neurosurg Soc. 2010;48(4):383-387.
20. Shiobara R., et al. A modified extended middle cranial fossa approach for acoustic nerve tumors. Results of 125 operations. J Neurosurg. 1988;68(3):358-365.
21. King T.T. Combined translabyrinthine–transtentorial approach to acoustic nerve tumours. Proc R Soc Med. 1970;63(8):780-782.
22. Brackmann D.E., Toh E.H. Surgical management of petrous apex cholesterol granulomas. Otol Neurotol. 2002;23(4):529-533.
23. Kojima H., et al. Endoscope-assisted surgery via the middle cranial fossa approach for a petrous cholesteatoma. Auris Nasus Larynx. 2008;35(4):469-474.
24. François P., et al. Anterior transpetrosal and subtemporal transtentorial approaches for pontine cavernomas. Acta Neurochirurgica. 2010;152(8):1321-1329.
25. Andrews J.C., et al. Middle cranial fossa transtemporal approach to the intrapetrous internal carotid artery. Skull Base Surg. 1991;1(3):142-146.
26. Glasscock M.E.3rd. Middle fossa approach to the temporal bone. An otologic frontier. Arch Otolaryngol. 1969;90(1):15-27.
27. Kawase T., et al. Transpetrosal approach for aneurysms of the lower basilar artery. J Neurosurg. 1985;63(6):857-861.
28. Miller C.G., et al. Transpetrosal approach: surgical anatomy and technique. Neurosurgery. 1993;33(3):461-469.
29. Kawase T., Shiobara R., Toya S. Anterior transpetrosal–transtentorial approach for sphenopetroclival meningiomas: surgical method and results in 10 patients. Neurosurgery. 1991;28(6):869-875. discussion 875-876
30. Kawase T., Shiobara R., Toya S. Middle fossa transpetrosal–transtentorial approaches for petroclival meningiomas. Selective pyramid resection and radicality. Acta Neurochir (Wien). 1994;129(3-4):113-120.
31. Megerian C.A., et al. The subtemporal–transpetrous approach for excision of petroclival tumors. Am J Otol. 1996;17(5):773-779.
32. Hitselberger W.E., Pulec J.L. Trigeminal nerve (posterior root) retrolabyrinthine selective section. Operative procedure for intractable pain. Arch Otolaryngol. 1972;96(5):412-415.
33. Norrell H., Silverstein H. Neurological Surgery of the Ear. Birmingham, Ala: Aescuplapius Pub. Co; 1977. 318–340
34. House J.W., et al. Retrolabyrinthine section of the vestibular nerve. Otolaryngol Head Neck Surg. 1984;92(2):212-215.
35. Uyar Y., Ulku C.H., Erongun U. A different technique used for the closure of sigmoid sinus during infratemporal approach. Auris Nasus Larynx. 2002;29(4):367-370.
36. Vrionis F.D., et al. Asterion meningiomas. Skull Base Surg. 1998;8(3):153-161.
37. Durgun B., et al. Evaluation by angiography of the lateral dominance of the drainage of the dural venous sinuses. Surg Radiol Anat. 1993;15(2):125-130.
38. Mann W.J., et al. Transsigmoid approach for tumors of the jugular foramen. Skull Base Surg. 1991;1(3):137-141.
39. Cushing H. Tumors of the Nervus Acusticus and the Syndrome of the Cerebellopontile Angle. New York: Hafner; 1963.
40. Dandy W. An operation for the total extirpation of tumors in the cerebello-pontine angle. A preliminary report. Bull Johns Hopkins Hosp. 1922;33:344-345.
41. Day J.D., Tschabitscher M. Anatomic position of the asterion. Neurosurgery. 1998;42(1):198-199.
42. da Silva E.B.Jr., et al. Image-guided surgical planning using anatomical landmarks in the retrosigmoid approach. Acta Neurochir (Wien). 2010;152(5):905-910.
43. Day J.D., et al. Surface and superficial surgical anatomy of the posterolateral cranial base: significance for surgical planning and approach. Neurosurgery. 1996;38(6):1079-1083.
44. Raza S.M., Quinones-Hinojosa A. The extended retrosigmoid approach for neoplastic lesions in the posterior fossa: technique modification. Neurosurg Rev. 2011;34(1):123-129.
45. Quinones-Hinojosa A., Chang E.F., Lawton M.T.. The extended retrosigmoid approach: an alternative to radical cranial base approaches for posterior fossa lesions. Neurosurgery, 2006;4 suppl 2:58 ONS-208-214; discussion ONS-214
46. Yokoyama T., et al. Surgical approach to the internal auditory meatus in acoustic neuroma surgery: significance of preoperative high-resolution computed tomography. Neurosurgery. 1996;39(5):965-969. discussion 969-970
47. Hori T., et al. Endoscope-controlled removal of intrameatal vestibular schwannomas. Minim Invasive Neurosurg. 2006;49(1):25-29.
48. Kabil M.S., Shahinian H.K. A series of 112 fully endoscopic resections of vestibular schwannomas. Minim Invasive Neurosurg. 2006;49(6):362-368.
49. Shao K.N., Tatagiba M., Samii M. Surgical management of high jugular bulb in acoustic neurinoma via retrosigmoid approach. Neurosurgery. 1993;32(1):32-36. discussion 36-37
50. Panse R. Ein gliom des Akustikus. Arch Ohrenheilk. 1904;61:251-255.
51. House W.F., Hitselberger W.E. Transtemporal bone microsurgical removal of acoustic neuromas. Morbidity and mortality of acoustic neuromas. Arch Otolaryngol. 1964;80:599-756.
52. Naguib M.B., et al. The enlarged translabyrinthine approach for removal of large vestibular schwannomas. J Laryngol Otol. 1994;108(7):545-550.
53. McElveen J.T.Jr., et al. Modifying the translabyrinthine approach to preserve hearing during acoustic tumour surgery. J Laryngol Otol. 1991;105(1):34-37.
54. Feghali J.G., Kantrowitz A.B. Transcranial translabyrinthine approach to vestibular schwannomas. J Laryngol Otol. 1993;107(2):111-114.
55. Fisch U., Mattox D.E. Translabyrinthine approach. In: Fisch U., Mattox D.E. Microsurgery of the Skull Base. New York: Stuttgart; 1988:546-576.
56. Sanna M. The translabyrinthine approaches. In: Sanna M., et al. Atlas of Microsurgery of the Lateral Skull Base. New York: Thieme; 2008:34-77.
57. Angeli R.D., et al. Enlarged translabyrinthine approach with transapical extension in the management of giant vestibular schwannomas: personal experience and review of literature. Otol Neurotol. 2011;32(1):125-131.
58. Sanna M., et al. Transapical extension in difficult cerebellopontine angle tumours: preliminary report. J Laryngol Otol. 2003;117(10):788-792.
59. Sanna M., et al. Transapical extension in difficult cerebellopontine angle tumors. Ann Otol Rhinol Laryngol. 2004;113(8):676-682.
60. Sanna M., et al. Enlarged translabyrinthine approach for the management of large and giant acoustic neuromas: a report of 175 consecutive cases. Ann Otol Rhinol Laryngol. 2004;113(4):319-328.
61. Fisch U., Mattox D.E. Transotic approach to the cerebellopontine angle. In: Fisch U., Mattox D.E. Microsurgery of the Skull Base. New York: Stuttgart; 1988:74-127.
62. Sanna M. Lateral skull base surgery. In: Sanna M., editor. Atlas of Temporal Bone and Lateral Skull Base Surgery. New York: Stuttgart; 1995:37-50.
63. House W.F., Hitselberger W.E. The transcochlear approach to the skull base. Arch Otolaryngol. 1976;102(6):334-342.
64. Gantz B.J., Fisch U. Modified transotic approach to the cerebellopontile angle. Arch Otolaryngol. 1983;109(4):252-256.
65. Briggs R.J., et al. Translabyrinthine removal of large acoustic neuromas. Neurosurgery. 1994;34(5):785-790.
66. Browne J.D., Fisch U. Transotic approach to the cerebellopontine angle. Otolaryngol Clin North Am. 1992;25(2):331-346.
67. Sanna M., et al. Otoneurological management of petrous apex cholesterol granuloma. Am J Otolaryngol. 2009;30(6):407-414.
68. Sanna, M. Transotic approach. In Sanna M, et al., eds. The Temporal Bone: A Manual for Dissection and Surgical Approaches. New York. 2006:98-105.
69. Sanna M. Transcochlear approaches. In: Sanna M., editor. Atlas of temporal bone and lateral skull base surgery. New York: Stuttgart; 1995:51-61.
70. De la Cruz A., Teufert K.B. Transcochlear approach to cerebellopontine angle and clivus lesions: indications, results, and complications. Otol Neurotol. 2009;30(3):373-380.
71. Sanna M., et al. Lateral approaches to the median skull base through the petrous bone: the system of the modified transcochlear approach. J Laryngol Otol. 1994;108(12):1036-1044.
72. De La Cruz A. The transcochlear approach to meningiomas and cholesteatomas of the cerebellopontine angle. In: Brackmann D.E., editor. Neurological Surgery of the Ear and Skull Base. New York: Raven Press; 1982:353-360.
73. Sekhar L.N., Estonillo R. Transtemporal approach to the skull base: an anatomical study. Neurosurgery. 1986;19(5):799-808.
74. Horn K.L., et al. The modified transcochlear approach to the cerebellopontine angle. Otolaryngol Head Neck Surg. 1991;104(1):37-41.
75. Sanna M., Mazzoni A., Gamoletti R. The system of the modified transcochlear approaches to the petroclival area and the prepontine cistern. Skull Base Surg. 1996;6(4):237-248.
76. Gardner G., Robertson J.H., Clark W.C.. Transtemporal approaches to the cranial cavity. Am J Otol, 1985;suppl:114-120
77. Thedinger B.A., Glasscock M.E.3rd, Cueva R.A. Transcochlear transtentorial approach for removal of large cerebellopontine angle meningiomas. Am J Otol. 1992;13(5):408-415.
78. Pandya Y., et al. Management of complex cases of petrous bone cholesteatoma. Ann Otol Rhinol Laryngol. 2010;119(8):514-525.
79. Bambakidis N.C., et al. Evolution of surgical approaches in the treatment of petroclival meningiomas: a retrospective review. Neurosurgery. 2007;61(5 suppl. 2):202-209. discussion 209-211
80. House J.W., Brackmann D.E. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2):146-147.
81. Gherini S.G., et al. Cholesterol granuloma of the petrous apex. Laryngoscope. 1985;95(6):659-664.
82. Sanna M., et al. Otoneurological management of petrous apex cholesterol granuloma. American Journal of Otolaryngology. 2009;30(6):407-414.
83. Brodkey J.A., et al. Cholesterol granulomas of the petrous apex: combined neurosurgical and otological management. J Neurosurg. 1996;85(4):625-633.
84. Gjuric M., Bilic M. Transmastoid–infralabyrinthine tailored surgery of jugular paragangliomas. Skull Base. 2009;19(1):75-82.
85. Giddings N.A., Brackmann D.E., Kwartler J.A. Transcanal infracochlear approach to the petrous apex. Otolaryngol Head Neck Surg. 1991;104(1):29-36.
86. Farrior J.B. Anterior hypotympanic approach for glomus tumor of the infratemporal fossa. Laryngoscope. 1984;94(8):1016-1021.
87. Muckle R.P., De la Cruz A., Lo W.M. Petrous apex lesions. Am J Otol. 1998;19(2):219-225.
88. Mattox D.E. Endoscopy-assisted surgery of the petrous apex. Otolaryngol Head Neck Surg. 2004;130(2):229-241.
89. El-Meselaty K., et al. Endoscope affects decision making in cholesteatoma surgery. Otolaryngol Head Neck Surg. 2003;129(5):490-496.
90. Axon P.R., et al. Petrosal cholesteatoma: management considerations for minimizing morbidity. Am J Otol. 1999;20(4):505-510.
91. Stieglitz L., Gerster A.G., Lilienthal H. A study of three cases of tumor of the brain in which operation was performed—one recovery, two deaths. The American Journal of the Medical Sciences. 1896;111(5):509-530.
92. Naffziger H. Brain surgery with special reference to exposure of the brain stem and posterior fossa; the principle of intracranial decompression, and the relief of impactions in the posterior fossa. Surg Gynecol Obstet. 1928;46:241-248.
93. Fay T. The management of tumors of the posterior fossa by a transtentorial approach. Surg Clin North Am. 1930;10:1427-1459.
94. Bailey P. Concerning the technique of operation for acoustic neurinoma. Zentralbl Neurochir. 1939;4:1-5.
95. Samii M., et al. Surgery of petroclival meningiomas: report of 24 cases. Neurosurgery. 1989;24(1):12-17.
96. Tarlov E. Surgical management of tumors of the tentorium and clivus. In: Schmidek H.H., Sweet W.H. Operative Neurosurgical Techniques: Indications, Methods, and Results. New York: Grune & Stratton; 1977:381-388.
97. Malis L. Surgical resection of tumors of the skull base. Neurosurgery. 1985;1:1011-1021. New York: McGraw-Hill
98. Malis L.I. The petrosal approach. Clin Neurosurg. 1991;37:528-540.
99. Spetzler R.F., Hamilton M.G., Daspit C.P. Petroclival lesions. Clin Neurosurg. 1994;41:62-82.
100. Al-Mefty O., Fox J.L., Smith R.R. Petrosal approach for petroclival meningiomas. Neurosurgery. 1988;22(3):510-517.
101. Origitano T.C., et al. Skull base approaches to complex cerebral aneurysms. Surg Neurol. 1993;40(4):339-346.
102. Hakuba A., Nishimura S., Inoue Y. Transpetrosal–transtentorial approach and its application in the therapy of retrochiasmatic craniopharyngiomas. Surg Neurol. 1985;24(4):405-415.
103. Al-Mefty O., Ayoubi S., Kadri P.A. The petrosal approach for the resection of retrochiasmatic craniopharyngiomas. Neurosurgery. 62(5 suppl 2), 2008. ONS331-5; discussion ONS335-6
104. Al-Mefty O., Ayoubi S., Smith R.R. The petrosal approach: indications, technique, and results. Acta Neurochir Suppl (Wien). 1991;53:166-170.
105. Al-Mefty O., Ayoubi S., Kadri P.A. The petrosal approach for the total removal of giant retrochiasmatic craniopharyngiomas in children. J Neurosurg. 2007;106(2 suppl):87-92.
106. Fisch U. Infratemporal fossa approach to tumours of the temporal bone and base of the skull. J Laryngol Otol. 1978;92(11):949-967.
107. Gardner G., et al. Combined approach surgery for removal of glomus jugulare tumors. Laryngoscope. 1977;87(5 Pt 1):665-688.
108. Vrionis F.D., Cano W.G., Heilman C.B. Microsurgical anatomy of the infratemporal fossa as viewed laterally and superiorly. Neurosurgery. 1996;39(4):777-785. discussion 785-786
109. Deveze A., et al. Surgical management of lesions of the internal carotid artery using a modified Fisch type A infratemporal approach. Otol Neurotol. 2007;28(1):94-99.
110. Montgomery W.W. Cystic lesions of the petrous apex: transsphenoid approach. Ann Otol Rhinol Laryngol. 1977;86(4 Pt 1):429-435.
111. Fucci M.J., et al. Endoscopic management of a giant cholesterol cyst of the petrous apex. Skull Base Surg. 1994;4(1):52-58.
112. Oyama K., et al. Petrous apex cholesterol granuloma treated via the endoscopic transsphenoidal approach. Acta Neurochir (Wien). 2007;149(3):299-302. discussion 302
113. Georgalas C., et al. Endoscopic transsphenoidal surgery for cholesterol granulomas involving the petrous apex. Clin Otolaryngol. 2008;33(1):38-42.
114. Zanation A.M., et al. Endoscopic endonasal surgery for petrous apex lesions. Laryngoscope. 2009;119(1):19-25.
115. Samadian M., et al. Endoscopic transrostral–transsphenoidal approach to petrous apex cholesterol granuloma: case report. Turk Neurosurg. 2009;19(1):106-111.
116. Presutti L., Villari D., Marchioni D. Petrous apex cholesterol granuloma: transsphenoid endoscopic approach. J Laryngol Otol. 2006;120(6):e20.
117. Chatrath P., et al. Endonasal endoscopic approach to the petrous apex: an image-guided quantitative anatomical study. Clin Otolaryngol. 2007;32(4):255-260.
118. DiNardo L.J., Pippin G.W., Sismanis A. Image-guided endoscopic transsphenoidal drainage of select petrous apex cholesterol granulomas. Otol Neurotol. 2003;24(6):939-941.
119. Dusick J.R., et al. Avoidance of carotid artery injuries in transsphenoidal surgery with the Doppler probe and micro-hook blades. Neurosurgery. 2007;60(4 suppl 2):322-328. discussion 328-329
120. Griffith A.J., Terrell J.E. Transsphenoid endoscopic management of petrous apex cholesterol granuloma. Otolaryngol Head Neck Surg. 1996;114(1):91-94.