18 Transposition of the Great Arteries
I. CASE
A. Fetal echocardiography findings
1. The fetal echo reveals situs solitus of the atria, levocardia, left aortic arch, and heart rate of 142 bpm.
2. The four-chamber view is normal, with equal-sized ventricles and a normal cardiac axis, position, and size (cardiothoracic ratio = 0.3).
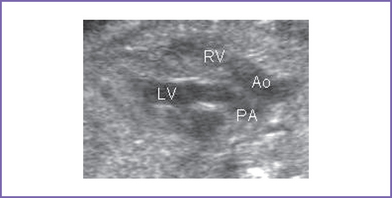
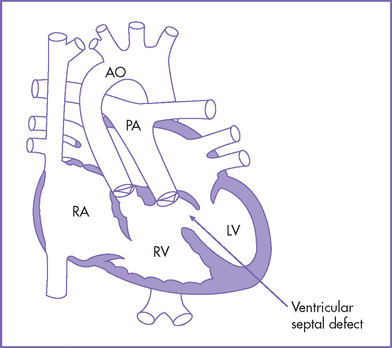
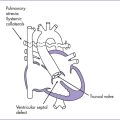
3. The outflow assessment reveals transposition of the great arteries (TGA) (aorta anterior and to the right of the pulmonary artery), with mild asymmetry. Aorta–to–pulmonary artery diameter ratio is 1:0.7 (Fig. 18-1).
4. The pulmonary valve is bicuspid and domes mildly in systole. There is moderate flow acceleration through it by Doppler (1.5 m/s).
5. There are good-sized branch pulmonary arteries. The right pulmonary artery is 2.2 mm and the left pulmonary artery is 2.2 mm.
6. The aortic annulus is of normal size and there is normal Doppler velocity through it.
7. The aortic arch is leftward and the ductal and aortic arches are of similar size. Both arches have antegrade flow.
8. There is a large subpulmonary perimembranous VSD.
9. Flow through the foramen ovale is unrestricted, with a bidirectional shunt.
10. The pulmonary venous flow pattern is normal.
11. The RV and LV Tei indices (myocardial performance index) are normal.
D. Fetal management and counseling
1. Amniocentesis was not offered. The risks from amniocentesis are probably higher than the risk of abnormal karyotype in simple transposition.
2. Follow-up includes serial antenatal studies at 4- to 6-week intervals.
a. The size of the ventricles should be compared. RV hypoplasia is a rare finding but one that can alter the surgical management to a single-ventricle repair.
b. Pulmonary outflow should be monitored for progressive pulmonary outflow tract obstruction as well as progressive main and branch pulmonary arteries and change in direction of ductus arteriosus flow.
c. Progressive foramen ovale restriction can occur (Maeno et al, 1999).
d. Progressive ductus arteriosus constriction can occur (Maeno et al, 1999).
E. Delivery
1. In TGA with pulmonary stenosis, delivery should be at term or as close as possible to term in a tertiary care center given the risk of foramen ovale restriction and ductus arteriosus constriction.
2. In addition, the true degree of pulmonary stenosis after birth and after ductus arteriosus closure may be difficult to fully predict.
F. Neonatal management
a. Prostaglandin E1 (PGE1) infusion to keep the ductus open to increase pulmonary blood flow and raise arterial oxygen saturation.
b. Administration of oxygen can increase oxygen saturation by decreasing the pulmonary vascular resistance and increasing blood flow.
c. Therapeutic balloon atrial septostomy may be performed if the infant is very cyanotic, particularly with metabolic acidosis (a PaO2 level of 25 mm Hg and a pH of less than 7.15).
d. At times, volume and inotropic support may be indicated to improve ventricular function and atrial-level mixing.
G. Follow-up
1. Subacute bacterial endocarditis (SBE) prophylaxis is required for life.
2. If there are residual hemodynamic abnormalities, anticongestive medications and some restriction of activities may be required.
3. The patient should have one or two yearly outpatient follow-up visits with 12-lead electrocardiogram (ECG) and echocardiogram.
4. If there is any history of unexplained palpitations or abnormal heart rhythm, a metabolic stress test with 24-hour Holter monitoring is recommended.
H. Risk of recurrence
Because there is a previous sibling with VSD, the recurrence risk is 3% to 5%.
I. Outcome of this case
1. The baby was born at term with good Apgar scores and good weight.
2. Moderate cyanosis was noted at birth. Pulse oximeter reading was 77%.
3. Postnatal echocardiography confirmed the diagnosis with large subpulmonary VSD with posterior deviation of the outlet septum causing valvar and subvalvar pulmonary stenosis.
4. The pulmonary arteries were of good size, with a transpulmonary Doppler velocity of 4.5 m/s.
5. The foramen ovale and ductus arteriosus were good sized and patent, with bidirectional shunting. PGE1 infusion was started.
6. The baby had a palliative aortopulmonary shunt at 1 week of age followed by a successful Rastelli operation at 12 months of life.
II. YOUR HANDY REFERENCE
A. Prevalence
1. TGA accounts for 5% to 7% of all congenital heart disease and is more common in boys than girls (10:3).
2. The incidence of TGA plus VSD plus pulmonary stenosis is 10% to 20% of fetuses with TGA.
3. More than 80% of cases of TGA have simple transposition with no associated lesions or with a small VSD.
4. About 10% to 20% of cases have a significant VSD with left ventricular outflow tract (LVOT) obstruction, pulmonary stenosis, or coarctation of the aorta.
5. Less than 5% of cases have complex associated lesions such as straddling of the atrioventricular (AV) valve, AV septal defect (AVSD), or hypoplasia of one ventricle.
6. In about 25% to 30% of infants, the atrial septum is restrictive at birth.
7. TGA tends to be underrepresented in prenatal series.
a. It constitutes less than 3% of the cases of congenital heart disease identified in Allan and Sharland’s (1994) series.
b. In France, prenatal diagnosis is greater than 25% and in some reports up to 67%.
C. Clues to fetal sonographic diagnosis
a. Normal four-chamber view including cardiac axis (normal venous atrial and AV connections).
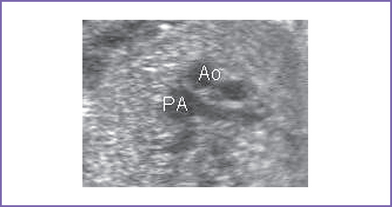
b. Abnormal three-vessel view (Fig. 18-2).
c. No crossover of the great vessels (parallel arrangements of the great vessels).
d. Posteriorly branching great artery (pulmonary artery) arising from the LV.
e. Superiorly branching great artery (aorta) from the RV, identifying the aortic arch with the brachiocephalic arteries.
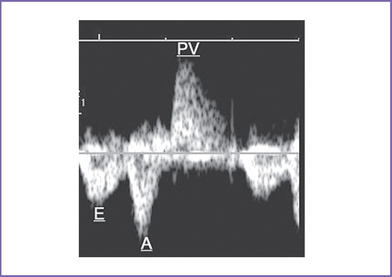
f. Doppler of the LV outflow tract shows a rapid upstroke of the pulmonary artery with the mitral valve inflow (Fig. 18-3).
2. Three-vessel view (Fig. 18-4).
a. The three-vessel view is an orthogonal transverse view of the upper mediastinum, where normally the oblique section of the main pulmonary artery and cross section of ascending aorta and SVC are arranged in a straight line with a decreasing order of their size (pulmonary artery, aorta, SVC).
b. This could be helpful in evaluating the spatial relationship and size of the great arteries (aorta and pulmonary).
c. This view is abnormal in almost all cases of outflow tract abnormalities (discrepancy in size, such as pulmonary atresia or aortic atresia) or abnormal spatial relationship (e.g., TGA).
D. Cardiovascular profile score
1. The score is 10/10 if there are no abnormal signs and reflects 2 points for each of five categories: hydrops, venous Doppler, heart size, cardiac function, and arterial Doppler.
2. Respondek and colleagues (1994) have reported that 20% of 100 consecutive fetuses with congenital heart disease have intrauterine growth restriction. This observation justifies serial prenatal follow-up with the cardiovascular profile for fetuses without structural heart defect who could be at risk for congestive heart failure (CHF).
E. Progression in utero
1. VSD size and site (its relationship to the great arteries).
a. In the context of a large VSD there is potential for a redistribution of flow through the VSD.
b. This can make a significant gradient through the pulmonary outflow less likely (rarely >1.5 m/s).
2. Reexamine for coronary anomalies with advanced gestation (better views).
a. The coronary arteries can be extremely difficult to assess before birth. This is one of the limitations that must be discussed with the parents.
b. Difficult coronary artery anatomy (e.g., intramural course) can contribute to the risk of the surgery if an arterial switch is in order.
a. In simple TGA the great arteries should be normally related in size, with the pulmonary trunk slightly larger than the aorta, particularly later in gestation.
b. In TGA with pulmonary outflow obstruction, the pulmonary artery is smaller than the aorta (depending on the degree of stenosis).
c. The pulmonary artery confluence and the size of the branch pulmonary arteries should be serially measured, especially close to the end of pregnancy, in case a shunt procedure is necessary in the neonate.
d. The site of the aortic arch is important in planning the site of the aortopulmonary shunt.
4. The subpulmonary and subaortic area.
a. These should be checked for the development of obstruction.
b. LVOT obstruction can result from:
a. The subaortic obstruction is less common. It may be due to anterior malalignment of the outlet septum.
b. The obstruction is usually dynamic. When the conal septum is anteriorly malaligned with a subaortic obstruction, one should assess the aortic arch for coarctation of the aorta because this is a typical coexisting lesion (size of the transverse aortic arch should be compared to the duct size).
c. Coarctation can contribute to the surgical mortality for repair.
6. Progressive RV hypoplasia, which rarely occurs in this setting.
7. Size and direction of flow, especially in late pregnancy, in the foramen ovale and the ductus arteriosus (aorta to pulmonary in severe pulmonary stenosis or critical aortic stenosis).
a. In simple transposition in particular, there is a risk of progressive foramen ovale restriction and ductus arteriosus constriction that can be fatal to the neonate if not recognized.
b. In such a state, immediate balloon atrial septostomy is critical and may be considered as an exit procedure.
8. Amplitude of diastolic ductal flow (for any evidence of ductal constriction).
F. Immediate postnatal management
1. For patients without prenatal diagnosis of TGA:
a. Check pulse oximeter readings.
b. Check four-limb blood pressure.
c. Transfer the patient to the neonatal or pediatric (cardiac if available) intensive care unit (ICU) for further assessment and management.
2. For other complex forms of TGA: Corrective surgery.
a. Definitive surgeries consist of switching the right- and left-sided structures at the atrial level (Senning operation), at the ventricular level (Rastelli operation), or at the great artery level (arterial switch operation).
b. Pulmonary artery banding for TGA with multiple VSDs or large VSD with hypoplastic RV or aortic valve abnormalities.
c. For TGA plus VSD plus subaortic stenosis, more often subaortic stenosis is mild or dealt with minimally through a patch or muscle resection with an arterial switch operation and arch repair. Rarely, the Damus–Kaye– Stansel operation is used in TGA.
G. Pathophysiology
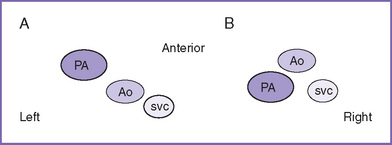
1. The classic complete TGA is often referred to as D-transposition (DTGA), in which the aorta is located anterior to and to the right of the pulmonary artery (Fig. 18-5). In corrected transposition or L-transposition (LTGA) the aorta is located anterior to and to the left of the pulmonary artery.
2. The aorta arises anteriorly from the RV, and the pulmonary artery arises posteriorly from the LV.
a. The result is parallel systemic and pulmonary circulations, with deoxygenated blood circulating in the body and returning to the RV only to be ejected again to the body, and the oxygenated blood returning from the lungs to the LV only to be ejected back to the lungs.
b. Defects that permit mixing of the two circulations—including ASD and VSD—are necessary for survival postnatally. The ductus arteriosus does not really allow mixing directly. Rather, it increases venous return to the LA and thus increases LA pressures, which leads to more LA-to-RA shunting.
3. The diagnostic view fails to show the circle-and-sausage pattern of the normal great arteries in the parasternal short-axis view.
a. The view shows two circular structures (aorta and pulmonary artery) either parallel or transposed.
b. The great arteries are seen together both in their long axis or both in their short axis.
4. Neonates with poor mixing of the two parallel circulations despite PGE1 infusion.
a. CHF can develop in the first week of life, but in many patients with this condition, mainly those who have simple transposition or who have TGA with large VSD or ductus arteriosus, this is usually not a major issue.
b. Mortality is estimated to be around 4%, because progressive hypoxia can result in progressive metabolic acidosis and death.
5. Antenatal restriction of the foramen ovale and/or the ductus arteriosus predicts significant neonatal morbidity and mortality.
6. According to Maeno and colleagues (1999):
a. The foramen ovale was considered at risk for postnatal early restriction if:
b. For the ductus arteriosus, the diameter was measured at the narrowest portion, typically at the pulmonary end.
c. The Doppler flow pattern was considered abnormal if it was either antegrade and continuous or bidirectional.
III. TAKE-HOME MESSAGE
A. Diagnosis
1. Simple or complete TGA is synonymous with the term discordant ventriculoarterial connection.
2. In most cases of simple transposition there is normal situs with levocardia.
3. TGA is a common diagnosis of infants, yet the condition could be easily missed in the prenatal ultrasound. This could happen if the evaluation of the outflow tract is not added to the four-chamber view in the routine prenatal ultrasound.
4. The aortic arch in complete transposition is shaped like a hockey stick and can be mistaken for a ductal arch. However, the aortic arch gives rise to the head and neck vessels.
5. In complete transposition, subaortic obstruction is less common than subpulmonary obstruction.
6. VSD occurs in about 40% of transposition of great arteries. It can occupy any part of the ventricular septum but is more common in the outlet part with either posterior or anterior malalignment of the outlet septum.
7. The incidence of dominant right coronary arteries in TGA is higher than in the normal population and in tetralogy of Fallot, although the reason for this is not clear.
8. Prenatal diagnosis of TGA has reduced neonatal mortality and morbidity.
B. Treatment
1. Because the surgical mortality for the arterial switch operation is as low as 2% in many institutions, the preoperative mortality is a major issue in the management and outcome of infants with TGA.
2. Data on long-term follow-up are limited for the arterial switch and the Rastelli corrective surgeries.
Allan LD, Sharland GK, Milburn A, et al. Prospective diagnosis of 1,006 consecutive cases of congenital heart disease in the fetus. J Am Coll Cardiol. 1994;23(6):1452-1458.
Bader R, Perrin D, Yoo SJ. Congenitally corrected transposition of the great arteries with Ebstein malformation and hypoplasia of the aortic arch in a fetus. Fetal Pediatr Pathol. 2004;23(4):257-263.
Bartlett JM, Wypij D, Bellinger DC, et al. Effect of prenatal diagnosis on outcomes in D-transposition of the great arteries. Pediatrics. 2004;113(4):e335-e340.
Chaoui R. The four-chamber view: Four reasons why it seems to fail in screening for cardiac abnormalities and suggestions to improve detection rate. Ultrasound Obstet Gynecol. 2003;22(1):3-10.
DeVore GR, Polanco B, Sklansky MS, Platt LD. The “spin” technique: A new method for examination of the fetal outflow tracts using three-dimensional ultrasound. Ultrasound Obstet Gynecol. 2004;24(1):72-82.
Huhta JC. Evaluating the fetus with transposition. Cardiol Young. 2005;15(Suppl 1):88-92.
Jouannic JM, Gavard L, Fermont L, et al. Sensitivity and specificity of prenatal features of physiological shunts to predict neonatal clinical status in transposition of the great arteries. Circulation. 2004;110(13):1743-1746.
Li J, Tulloh RM, Cook A, et al. Coronary arterial origins in transposition of the great arteries: Factors that affect outcome. A morphological and clinical study. Heart. 2000;83(3):320-325.
Maeno YV, Kamenir SA, Sinclair B, et al. Prenatal features of ductus arteriosus constriction and restrictive foramen ovale in D-transposition of the great arteries. Circulation. 1999;99(9):1209-1214.
Respondek ML, Binotto CN, Smith S, et al. Extracardiac anomalies, aneuploidy and growth retardation in 100 consecutive fetal congenital heart defects. Ultrasound Obstet Gynecol. 1994;4(4):272-278.
Sidi D, Bonnet D. Sensitivity and specificity of prenatal features of physiological shunts to predict neonatal clinical status in transposition of the great arteries. Circulation. 2004;110(13):1743-1746.
Yoo SJ, Lee YH, Kim ES, et al. Three-vessel view of the fetal upper mediastinum: An easy means of detecting abnormalities of the ventricular outflow tracts and great arteries during obstetric screening. Ultrasound Obstet Gynecol. 1997;9(3):173-182.