25 Transplantation surgery
Introduction
For many patients, the optimal treatment of their end-stage renal failure (ESRF) is kidney transplantation because it not only improves quality of life but may also confer survival benefits (EBM 25.1). Liver, heart and lung transplantation can be truly life-saving, as often no alternative treatments are available. The two main obstacles to transplantation are overcoming the recipient’s immune response and a shortage of donor organs.
Transplant immunology
The recipient’s immune response to the donor organ
Early events
Inflammation lies at the heart of the rejection process and is activated through early events around the time of transplantation. Brain-stem death and retrieval of organs, as well as cold ischaemic time (while the organ is stored on ice) and a period of warm ischaemia (while the vascular anastomoses are completed) stimulate an early inflammatory response to the transplanted organ. Reperfusion is associated with endothelial activation and the infiltration of inflammatory cells, particularly macrophages. The importance of this early ischaemia reperfusion injury (IRI) in shaping the patient’s subsequent course is illustrated by the superior outcome observed following living donor transplantation, despite more significant major histocompatibility (MHC) mismatching, and the adverse impact of a more prolonged cold ischaemic time on graft outcome. Indeed, the severity of this inflammatory injury modulates the subsequent alloimmune response, generating a ‘danger signal’ which primes the immune response to the transplanted organ. Thus, IRI impacts upon long-term outcome: it leads to a delay in primary graft function, increases acute rejection rates and reduces long-term graft survival (EBM 25.2). The crucial role of IRI in mediating transplant-associated injury has become more apparent as acute rejection rates fall with the introduction of new and highly effective immunosuppressive agents, and has led to an increasing interest in how IRI may be reduced through preconditioning strategies.
Antigen presentation
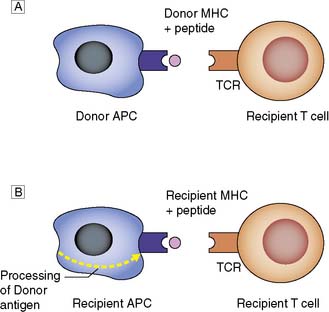
Donor MHC antigens are recognized as foreign (allo- recognition) by recipient T cells following presentation upon donor (direct) or recipient (indirect) antigen-presenting cells (APCs) (Fig. 25.1).
Patterns of allograft rejection
Acute rejection
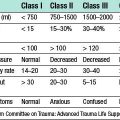
This occurs in up to 50% of grafts, usually in the first 6 months. Acute rejection is diagnosed on renal transplant biopsy and is classified according to Banff 07 diagnostic criteria (Table 25.1).
Table 25.1 Banff 07 diagnostic criteria for renal allograft biopsies
Immunosuppression
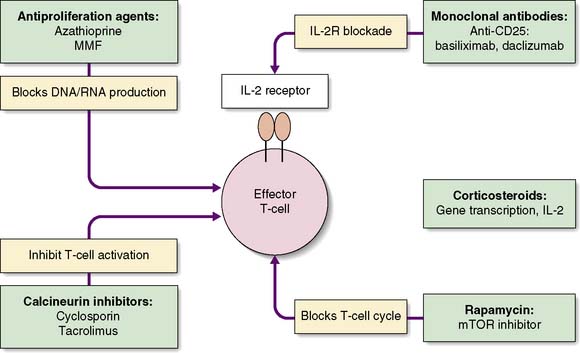
The challenge is to minimize the risk of graft rejection with as few side effects as possible. Various strategies are adopted: induction therapy, maintenance immunosuppression and treatment of rejection. The mechanisms of action of the common immunosuppressive drugs are outlined on Figure 25.2.
Immunosuppressive drugs
Steroids
Corticosteroids play an important role in induction and maintenance and are the first-line treatment for acute rejection. The side effects of steroids are numerous and are responsible for many of the long-term complications of immunosuppressive therapy (Table 25.2). This has led to attempts to withdraw steroid therapy some time after transplant, or to minimize their use.
Calcineurin inhibitors (CNIs)
Tacrolimus
Tacrolimus, the second CNI to be introduced into clinical practice, resulted in significantly improved one-year outcome in liver transplant patients (EBM 25.3), and this has been borne out in studies in renal transplantation. Tacrolimus now forms the mainstay of many immunosuppressive regimens. It too carries the risk of nephrotoxicity, and serum levels must be monitored closely. Other side effects include neurotoxicity, diabetes and alopecia.
The future of immunosuppression
Summary Box 25.1 The immune response
• The immune response to a transplanted organ is largely mediated by T cells, and these are the target for immunosuppressive therapy
• Acute rejection is seen in up to 50% of grafts and episodes are treated with high-dose steroids
• Chronic rejection has a multifactorial aetiology and results in significant graft loss over the months and years following transplantation.
Organ donation
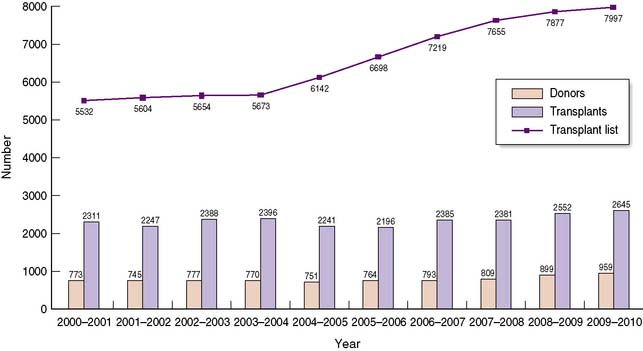
The shortage of organs for transplantation remains a major challenge to the transplant community, with demand consistently outstripping supply over many years (Fig. 25.3). Such a shortage has led to significant changes in practice over the last decade, with an increasing number of patients undergoing transplants from living donors, and from marginal or extended criteria deceased donors. These will be discussed in this section.
Deceased donation
Few absolute contraindications for organ donation exist; those that do are directed against the avoidance of disease transmission from donor to recipient (Table 25.3).
Donor management
Specific criteria must be met in order to make the diagnosis of brain-stem death (Table 25.4). Events leading up to brain death may impact upon the quality of the retrieved organs. Initially, at the point of brainstem death, compression associated with coning results in hypertension and bradycardia, known as the Cushing reflex. An autonomic storm ensues, characterized by the massive release of catecholamines, with resultant hypertension and hypoperfusion. The effect on cardiac function is the deterioration of ventricular systolic function, the liver and kidneys are affected by hypoperfusion, and it is likely that these changes contribute to non-specific endothelial cell damage, which increases the immunogenicity of the organs.
| Preconditions |
Multiorgan retrieval
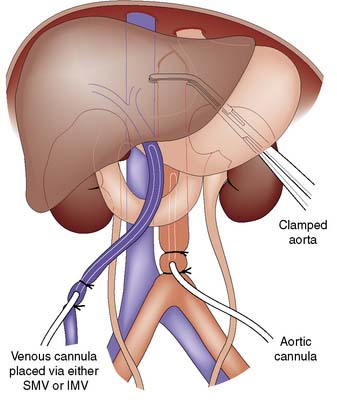
Organ retrieval begins with opening the abdomen (midline incision) and the chest (sternotomy). Once other pathology has been excluded and the liver mobilized, exposure of the inferior mesenteric vein and the aorta allows insertion of the perfusion cannulae (Fig. 25.4). Cold perfusion is commenced and the vena cava is divided in the mediastinum. Crushed ice is placed throughout the peritoneal cavity, with particular emphasis on the organs to be retrieved. The heart and lungs are excised. The pancreas is usually retrieved in conjunction with the liver. The kidneys are excised with ureters and the renal arteries on aortic patches. A portion of spleen and mesenteric lymph nodes are excised for the purpose of tissue typing and cross-matching, and the iliac vessels are excised and preserved for formation of conduits if necessary. The operation is completed by careful wound closure, application of dressings and patient cleaning. The procedure must be carefully documented in the patient’s notes.
Donation after circulatory death
Recent years have seen the development of DCD programmes (previously known as non-heart beating donors) and this has resulted in the cincrease in numbers of deceased donors. Donors after circulatory death are categorized according to the Maastricht criteria (Table 25.5). The majority of such donors in the UK are category III donors, with successful outcomes for renal, liver, pancreas and lung transplant patients.
Table 25.5 Maastricht criteria for donation after circulatory death
| Category 1: Dead on arrival at hospital. The moment of sudden death must be witnessed and the time documented. |
| Category 2: Unsuccessful resuscitation. Usually in the Accident and Emergency department. |
| Category 3: Awaiting cardiac arrest. Patients in whom cardiac arrest is inevitable, but they do not fulfil criteria for brain-stem death testing. |
| Category 4: Cardiac arrest in a brain-stem dead individual. |
| Category 5: Unexpected death in a patient in ITU or critical care unit. |
Renal transplantation following donation after circulatory death is associated with increased rates of delayed graft function because of longer warm ischaemic periods, but the long-term outcome is comparable to DBD (EBM 25.3). An increasing number of liver transplants are being performed following DCD, and whilst concerns regarding increased risk of primary non-function have not been borne out, with careful selection of donors, the risk of biliary complications is higher than in DBD.
Living donation
Living donor kidney transplantation
Donor selection
Each potential donor must go through a rigorous assessment process, always bearing in mind that the major operation they are planning to undergo will have no direct benefit to the individual, and so great care must be taken to minimize risk. The assessment is outlined in Table 25.6, and evidence of significant co-morbidity should halt the work-up. Uncontrolled hypertension or diabetes should be considered absolute contraindications to living donation, because of the risk of deterioration in donor renal function following nephrectomy. Obesity is not an absolute contraindication, but it does make the operation more technically challenging, and it increases the risk of postoperative complications such as atelectasis, pneumonia, venous thromboemobolic disease and wound infections. For these reasons, most units within the United Kingdom set a maximum body mass index limit of 30 kg/m2.
Immunology
Procedure-related morbidity and mortality
Summary Box 25.2 Organ donation
• Shortage of organs for donation remains a significant challenge to the transplant community
• Strategies aimed at increasing use of organs for donation include use of marginal donors and donors without a heart beat
• Well-established programmes for living donor kidney transplants exist within the UK, with an increasing drive to establish living donor liver transplantation.
Renal transplantation
Indications and patient assessment
Absolute contraindications to renal transplantation are active infection and malignancy; relative contraindications include advanced age, severe cardiovascular disease and likelihood of non-compliance with immunosuppressive therapy. In addition, heed must be paid to the likelihood of the underlying disease causing problems within the transplanted kidney (e.g. diabetes mellitus, glomerulo- sclerosis, amyloidosis and hypertension). Potential recipients need to undergo rigorous medical (Table 25.7), psychological and social evaluation. An important component of this is the education of the patient and their family about the benefits and risks of transplantation and of immunosuppression, so that fully informed written consent can be obtained.
Table 25.7 Assessment of the potential recipient for renal transplantation
| General assessment |
Patient listing for transplantation
Once a decision has been made to list an individual for transplant, their blood group and tissue typing must be carefully documented, and this information is given to the central UK office, which has responsibility for kidney allocation. Several factors are taken into account in the national allocation scheme (EBM 25.4), but priority is given to HLA matching, as this has been shown to have a significant impact on outcome. Once the kidney has been allocated, a final cross-match test is undertaken in the majority of cases for anti-HLA antibodies that may have developed in response to blood transfusion, pregnancy or a previous transplant. An increasing number of transplants in which the risk of a positive cross-match is low are being performed prior to receiving the cross-match results. Patients that are eligible for this virtual cross-matching are carefully selected, and this is undertaken in an attempt to reduce the cold ischaemic time, which is a main contributor to delayed graft function.
The operative procedure
Recipient operation
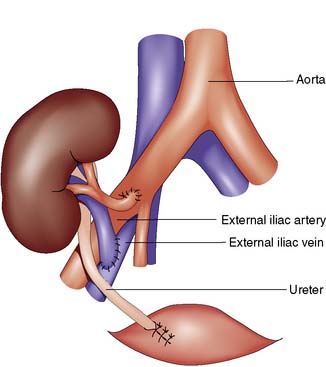
The Rutherford Morris incision is made in the iliac fossa, and the iliac vessels are exposed by retroperitoneal dissection. Lymphatics overlying the iliac vessels must be ligated and divided with care, to avoid the development of a postoperative lymphocoele. Following careful preparation of the recipient vessels, the anastomosis is performed between donor renal vein and recipient external iliac vein. The arterial anastomosis may be performed onto the recipient’s internal or external iliac artery, depending on the calibre of the donor renal artery and the positioning of the arteries (Fig. 25.5). If the internal iliac artery has been used on the contralateral side, this must not be used. The kidney is then reperfused, and the ureteric anastomosis performed between donor ureter and recipient bladder, over a double J stent. Insertion of the ureteric stent has been shown to reduce the risk of early ureteric complications such as urinary leak.
Liver transplantation
Indications and patient assessment
The most effective therapy for end stage liver failure is liver transplantation. Patients with chronic liver failure who show signs of hepatic decompensation despite optimal medical management, those with certain forms of liver tumours or patients with acute liver failure should be referred to specialist liver transplant units. Signs of decompensation in chronic liver failure include oesophageal varices, ascites, infection including spontaneous bacterial peritonitis, all of which may be combined with poor synthetic liver function as demonstrated by hypoalbuminaemia, hyperbilirubinaemia and prolonged clotting times where the prothrombin time is prolonged over control. Indications for liver transplant are demonstrated in Table 25.8.
| Common indications in adults |
Patient assessment must be undertaken by a multidisciplinary team comprising surgeons, hepatologists, specialist anaesthetists and, where the patient demonstrates addictive behaviour, a specialist psychiatrist. In general patients should be expected to have at least a 50% chance of surviving 5 years post-transplantation. Table 25.9 demonstrates the criteria used to decide whether someone should receive a liver transplant in fulminant hepatic failure. To help assess priority for organ allocation, the model for end stage liver disease (MELD) was developed in the United States. This was originally developed to assess the prognosis of cirrhotic patients who underwent treatment for portal hypertension. It has also been shown to predict short-term outcome in patients awaiting liver transplantation and therefore is used both in the US and, sometimes with modification, in other countries for identifying those patients most in need of liver transplant (Table 25.10).
Table 25.9 Criteria for liver transplantation in acute liver failure
| Paracetamol toxicity |
| Non-paracetamol toxicity |
Table 25.10 Equation for determining MELD score
| MELD score = |
The operative procedure
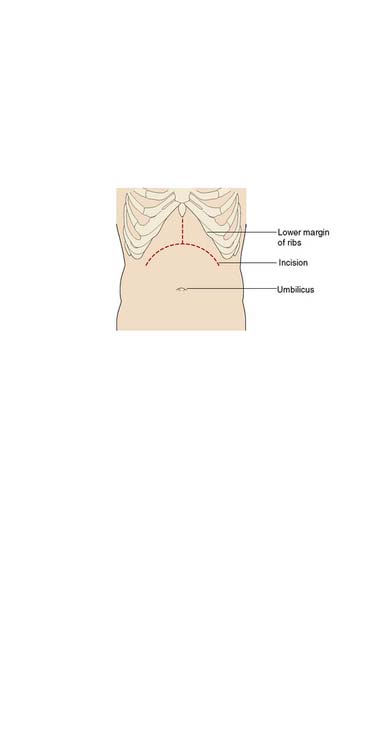
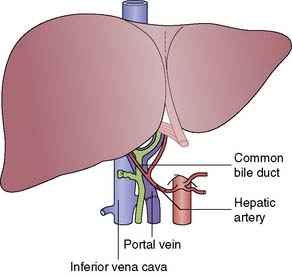
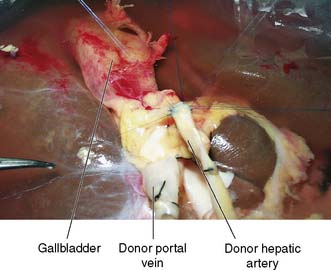
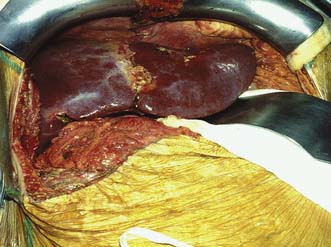
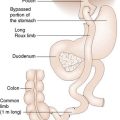
The recipient’s abdomen is opened either through a ‘Mercedes-Benz’ incision or through a midline incision which is curved to the right following the line of the costal margin (Fig. 25.6). There are many variations to the liver transplant operation and in the most common (Fig. 25.7) the liver from the recipient is removed but leaving the inferior vena cava intact. The new liver is attached to the recipient by joining together the inferior vena cava of the donor liver with the inferior vena cava of the recipient. This is called a ‘piggyback procedure’ and accomplished by performing a side-to-side anastomosis between the two vena cava. This is then followed by portal venous and hepatic arterial anastomoses (Fig. 25.8). The liver is reperfused with recipient blood and haemostasis carried out carefully. The donor gallbladder is then removed and an end-to-end common bile duct to common bile duct anastomosis performed unless there is a specific indication to perform a roux-en-Y hepaticojejunostomy (re-transplantation or primary sclerosing cholangitis in the recipient). Figure 25.9 shows a well-perfused liver following completion of the anastomoses.
Pancreas transplantation
The operative procedure
Back table preparation
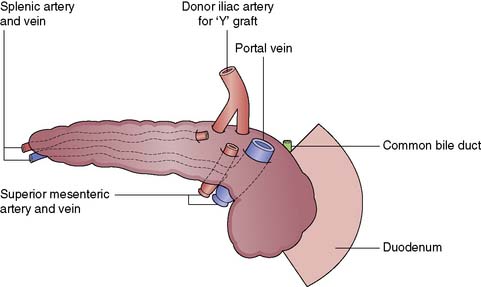
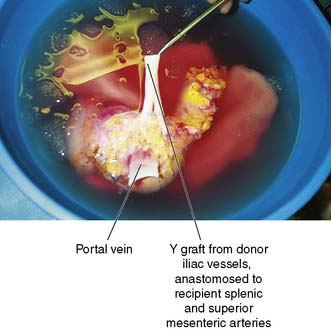
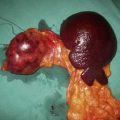
The pancreas is retrieved en bloc with the spleen, and this is carefully excised on the back table, paying careful attention to the ligation and division of the vessels. The c of duodenum is carefully reduced and the ends both stapled and oversewn to avoid leakage. An arterial conduit is created: a Y graft is formed using donor iliac vessels onto the donor splenic and superior mesenteric vessels (Fig. 25.10). Figure 25.11 illustrates a pancreas graft just before implantation and shows the vascular reconstruction that is performed on the back table.
Postoperative management and complications
Pancreas transplantation is associated with a higher incidence and a greater range of complications than kidney transplant, due to a requirement for greater immunosuppression, in a high-risk diabetic population who have impaired infection resistance, poor healing and high levels of co-morbidity. Between one-fifth and one-quarter of patients require relaparotomy in the early postoperative period. Complications of pancreas transplant are outlined in Table 25.11. Risk factors for complications include increasing donor age, prolonged preservation time and donor and recipient obesity.
| Infective complications |
| Systemic infection (opportunistic infections) |
| Local infections (peritonitis, localized collections, fistulae) |
| Vascular complications |
| Haemorrhage: early or late |
| Thrombosis: arterial or venous thrmobosis |
| Allograft pancreatitis |
| Ischaemia-reperfusion injury |
| Reflux pancreatitis |
Outcome
Summary Box 25.5 Pancreas transplantation
• Combined kidney and pancreas transplantation is an excellent treatment for a carefully selected group of patients with type 1 diabetes mellitus
• Patients must undergo rigorous medical evaluation prior to being listed, as they have complicated diabetic disease
• Pancreas rejection is difficult to diagnose and this may lead to delay in initiating therapy for such episodes
• Despite this, combined kidney-pancreas transplant is associated with a 5-year survival of 70%.
Heart and lung transplantation
Indications and patient assessment
Heart
Contraindications to heart transplant include:
• factors that increase perioperative mortality, such as irreversible pulmonary hypertension, active sepsis, severe obesity
• factors affecting long-term prognosis, such as age > 65 years, severe renal impairment, active or recent malignancy, other major co-morbidity
• factors that impair compliance such as active mental illness, recent drug abuse refractory to treatment.
Summary
Summary Box 25.6 Heart-lung transplantation
• Ischaemic heart disease and cardiomyopathy are the most common indications for heart transplant
• Both heart and lung transplantation require short cold ischaemic times in comparison with intra-abdominal organs
• Outcomes following heart and lung transplantation are similar to those seen following transplantation of intra-abdominal organs.