8 Transmitters and receptors
Electrical Synapses
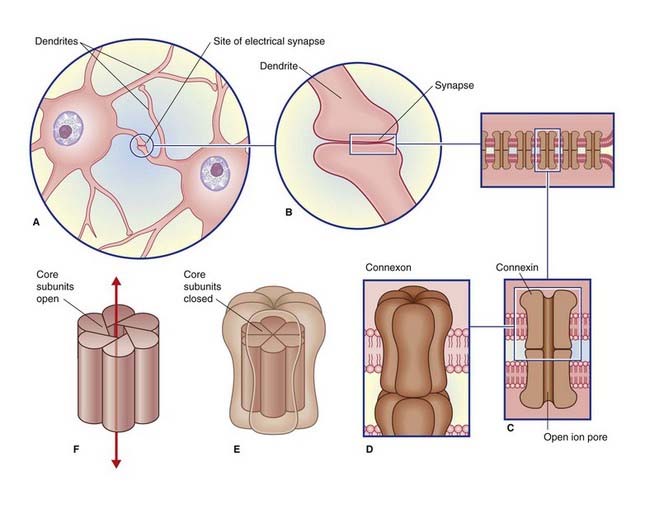
Electrical synapses are scarce in the mammalian nervous system. As seen in Figure 8.1, they consist of gap junctions (nexuses) between dendrites or somas of contiguous neurons, where there is cytoplasmic continuity through 1.5-nm channels. No transmitter is involved, and there is no synaptic delay.
Chemical Synapses
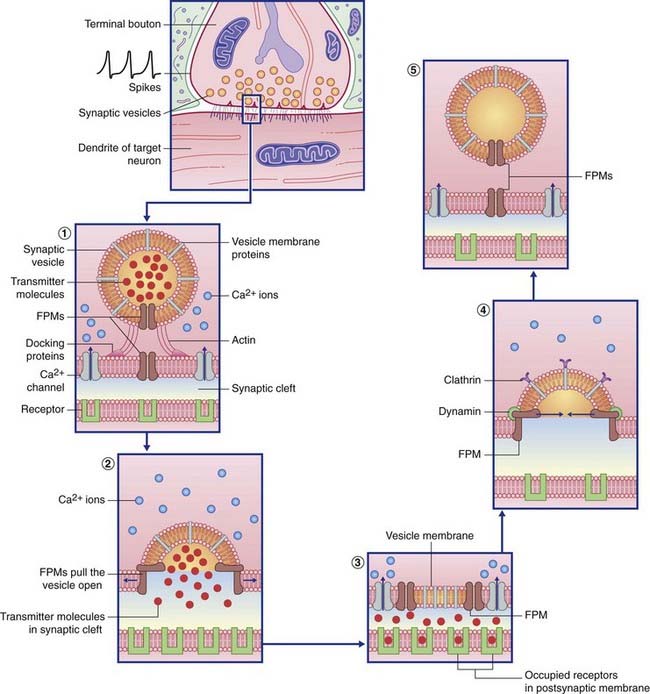
Transmitter liberation (Table 8.1 and Figure 8.2)
Table 8.1 Some named proteins involved in transmitter transport and vesicle recycling
| Named protein | Function |
|---|---|
| Actin | Brings vesicle into contact with presynaptic membrane |
| Synaptophysin | Creates the membrane fusion pore |
| Calmodulin | Expels vesicle content into synaptic cleft |
| Clathrin | Withdraws vesicle membrane from synaptic cleft |
| Dynamin | Pinches the neck of the developing vesicle to complete its separation |
| Ligand | Receptor protein that binds with the transmitter molecule |
Target cell receptor binding
Ionotropic receptors
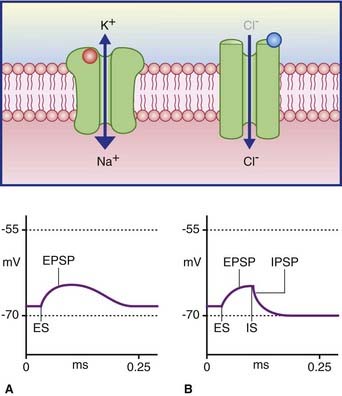
Ionotropic receptors are characterized by the presence of an ion channel within each receptor macromolecule (Figure 8.2). The transmitter binds with its specific receptor facing the synaptic cleft, causing it to change its conformation so as to open or close its ion pore. Ionotropic receptor channels are said to be transmitter-gated, or ligand-gated (from the Latin ligandum, ‘binding’), signifying their capacity to bind a transmitter molecule or a drug substitute.
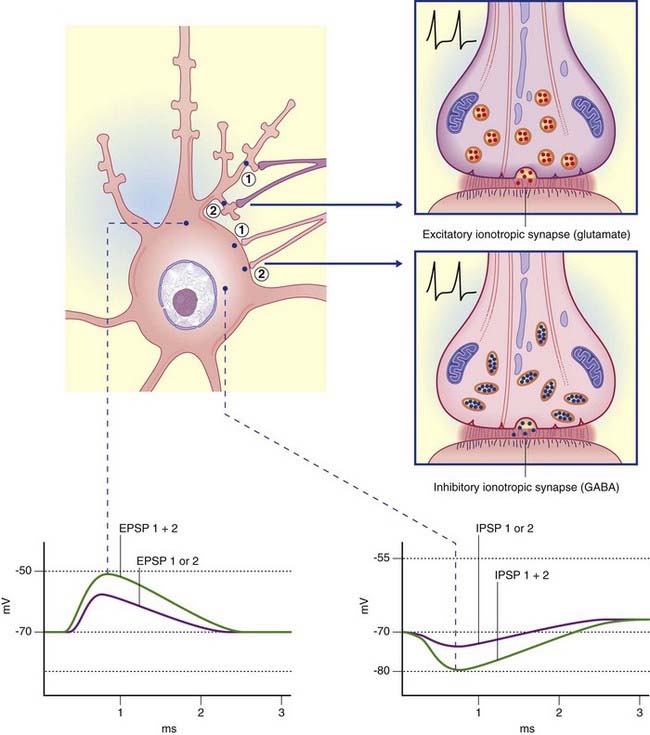
In Figure 8.3A, the excitatory channel has been opened by the transmitter, causing a major influx of sodium and a minor efflux of potassium; the excitatory postsynaptic potential (EPSP) has depolarized the cell membrane almost to firing point. In Figure 8.3B, the membrane can be hyperpolarized only to −70 mV, the chloride equilibrium potential. A greater level of depolarization requires a second messenger system (see below).
Metabotropic receptors
Three second messenger systems are well recognized.
Cyclic AMP system
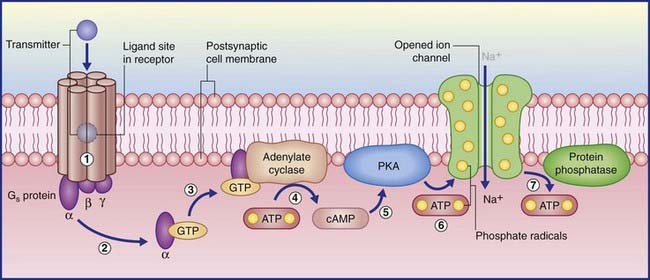
(The abbreviation cyclic AMP represents cyclic adenosine monophosphate.) In the examples shown in Figure 8.4, transmitter–receptor binding releases the alpha subunit of a Gs protein, leaving it free to link with GTP, which in turn facilitates adenosine cyclase to convert ATP to cyclic AMP. Protein kinase A in the membrane is stimulated by cyclic AMP to transfer phosphate ions from ATP to an ion channel, causing its pore to admit sodium ions, initiating depolarization of the target neuron. When the Gs protein is switched off, the membrane-attached enzyme protein phosphatase catalyzes extraction of the phosphate ions, resulting in pore closure.
Phosphoinositol system
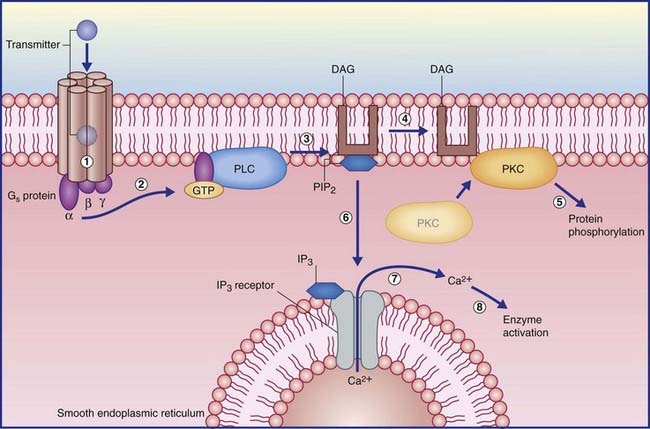
In the example shown in Figure 8.5, activation of another kind of Gs protein alpha subunit causes the effector enzyme phospholipase C to split a membrane phospholipid (PIP2) into a pair of second messengers: diacylglycerol (DAG) and inositol phosphate (IP3). DAG activates protein kinase C, which initiates protein phosphorylation. IP3 diffuses into the cytosol, where it opens calcium-gated channels, mainly in nearby membranes of smooth endoplasmic reticulum. The Ca2+ ions activate certain calcium-dependent enzymes downstream, and may open calcium-gated K+ (outward) and Cl− (inward) channels with excitatory effect.
Transmitters and Modulators
Several criteria should be fulfilled for a substance to be accepted as a neurotransmitter.
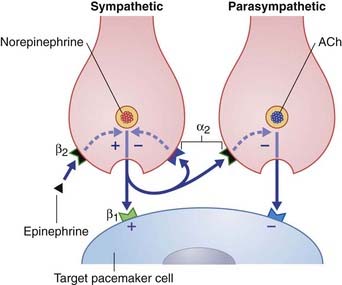
The term neuromodulator (L. modulare, to regulate) has been subject to several interpretations. The most satisfactory appears to derive from the terms amplitude modulation and frequency modulation in electrical engineering, signifying superimposition of one wave or signal onto another. Figure 8.6 represents a sympathetic and a parasympathetic nerve ending, close to a pacemaker cell (modified cardiac myocyte). This neighborly arrangement of nerve endings is common in the heart, and allows the respective transmitters to modulate each other’s activity. The sympathetic nerve ending liberates norepinephrine (noradrenaline), which has a stimulatory effect. The three modulators shown exert their effects via second messenger systems.
Fate of neurotransmitters
The principal transmitters and modulators are shown in Table 8.2. Respective receptor types are in Table 8.3.
Table 8.2 Main types of transmitters and modulators, with examples of eacha The neuropeptides are modulators
| Type | Example(s) |
|---|---|
| Amino acids | Glutamate |
| γ-Aminobutyric acid (GABA) | |
| Glycine | |
| Biogenic amines | Acetylcholine |
| Monoamines | |
| Catecholamines (dopamine, norepinephrine [noradrenaline], epinephrine [adrenaline]) | |
| Serotonin | |
| Histamine | |
| Neuropeptides | Vasoactive intestinal polypeptide |
| Substance P | |
| Enkephalin | |
| Endorphins | |
| Many others | |
| Adenosine | — |
| Gaseous | Nitric oxide |
a The five monoamines contain a single amine group. Catecholamines also contain a catechol nucleus.
Table 8.3 Receptor types activated by different neurotransmitters
| Ionotropic receptors | Metabotropic receptors |
|---|---|
| Glutamate (AMPA/K) | Glutamate (mGluR) |
| GABAA | GABAA |
| Acetylcholine (nicotinic) | Acetylcholine (muscarinic) |
| Glycine | Dopamine (D1, D2) |
| Serotonin (5-HT3) | Serotonin (5-HT1, 5-HT2) |
| Norepinephrine (noradrenaline) (α1, α2), epinephrine (adrenaline) | |
| Histamine (H1, H2, H3) | |
| All neuropeptides | |
| Adenosine |
Amino acid transmitters
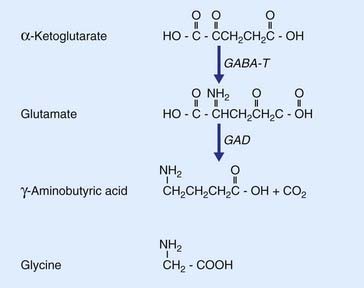
The most prevalent excitatory transmitter in the brain and spinal cord is the amino acid l-glutamate (Figure 8.7). As an important example, all neurons projecting into the white matter from the cerebral cortex, regardless of their destinations in other areas of cortex or brainstem or spinal cord, are excitatory and use glutamate as transmitter. Glutamate is derived from α-ketoglutarate; it also provides the substrate for formation of the most common inhibitory transmitter, GABA.
GABA is synthesized from glutamate by the enzyme glutamic acid decarboxylase.
Glutamate
Ionotropic glutamate receptors
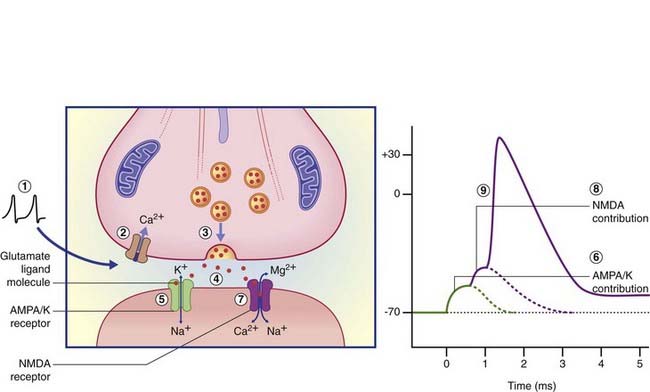
Activation of AMPA–K receptor channels in the postsynaptic membrane allows an immediate inrush of Na+ together with a small outward movement of K+ ions (Figure 8.8), generating the early component of the EPSP in the target neuron. Should this component depolarize the target cell membrane from −65 mV to −50 mV, this will suffice to generate electrostatic repulsion of Mg2+ cations that plug the NMDA receptor ion pore at rest. Na+ ions enter via the pore and generate action potentials. Significantly, Ca2+ ions also enter, and the extended period of depolarization (up to 500 ms from a single action potential) allows activation of calcium-dependent enzymes with knock-on capacity to modify the structure and even the number of synaptic contacts in the target cell. The phenomenon of activity-dependent synaptic modification is especially detectable in experimental studies of cultured slices of rat hippocampus, and is likely to be important in the generation of short-term memory traces. (For example, the anesthetic drug ketamine, which blocks the NMDA channel, also blocks memory formation.) A characteristic effect of repetitive activation of the NMDA receptor is long-term potentiation, represented by above-normal EPSP responses even some days after ‘training’. (See long-term depression, later.)
GABA
Two major classes of GABA receptor are recognized, one being ionotropic and the other metabotropic.
Ionotropic GABA receptors
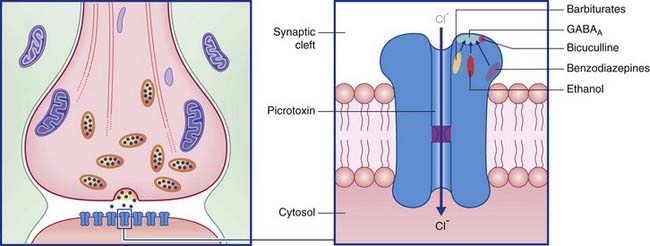
Termed GABAA, these are especially abundant in the limbic lobe of the brain. Each is directly linked to a chloride ion channel (Figure 8.9). Following activation of GABAA receptors, channel pores are opened and Cl− ions diffuse down their concentration gradient from synaptic cleft to cytosol. Hyperpolarization up to −80 mV or more is brought about by summation of successive inhibitory postsynaptic potentials (IPSPs) (Figure 8.10).
Metabotropic GABA receptors
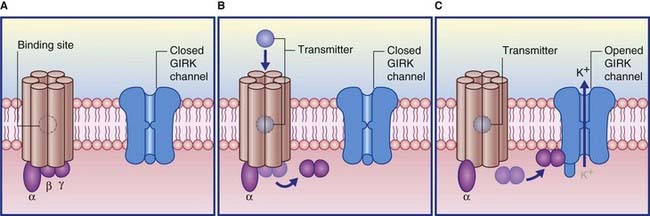
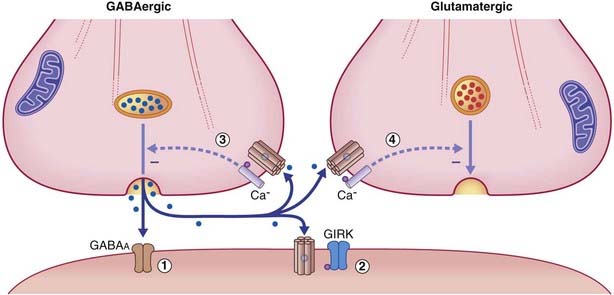
Termed GABAB, these are relatively uniformly distributed throughout the brain. They are also found within peripheral autonomic nerve plexuses. Although most of their G proteins operate via second messengers, a significant number act directly, on a special class of postsynaptic K+ channels known as GIRK channels (G protein inwardly rectifying K+ channels). As shown in Figure 8.11, transmitter-binding releases the βγ subunit, which expels K+ ions through the GIRK channel, thereby producing an IPSP.
The response properties of the target neuronal receptors are slower and weaker than those of GABAA ionophores, requiring higher-frequency stimulation to be activated. This has led to the belief that they may be extrasynaptic in position rather than facing the synaptic cleft. This is indicated in Figure 8.12, where the belief is supported by the existence of another type of G-direct channel in extrasynaptic locations. This is a calcium channel that is also voltage-gated, and therefore participates in provision of the calcium ions needed to draw synaptic vesicles against the presynaptic membrane. Activation of a G–Ca2+ ligand site closes Ca2+ channels, thereby reducing the effectiveness of action potentials, with inhibitory effect on the parent neuron and on any nearby glutamatergic neurons.
In clinical disorders that involve excessive tonic muscle reflex responses (the state of spasticity, Ch. 16), the muscle relaxant baclofen is sometimes injected into the subarachnoid space surrounding the spinal cord. The drug seeps into the cord and inhibits release of glutamate from the terminals of muscle afferents, mainly by diminishing the massive calcium entry associated with excessively frequent action potentials.
Recycling of glutamate and GABA
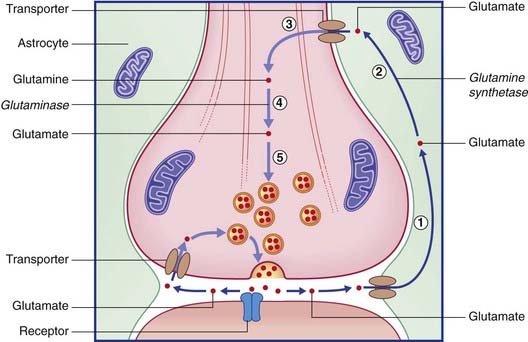
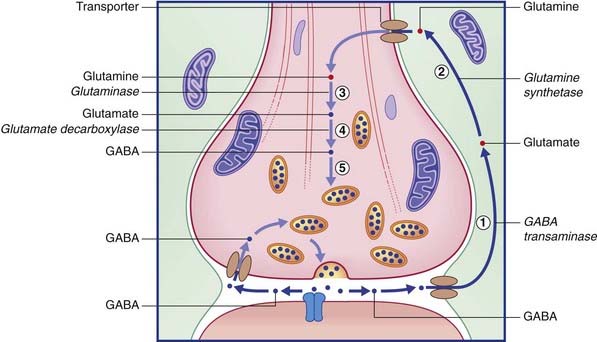
The two routes for recycling are indicated for glutamate in Figure 8.13 and for GABA in Figure 8.14. On the left of each diagram, some transmitter molecules are retrieved from the synaptic cleft by a membrane transporter protein and reincorporated into a synaptic vesicle. On the right, transmitter molecules are being recycled through an adjacent astrocyte. Glutamate is converted to glutamine by glutamine synthetase during transit through astrocytes. Following intercellular transport into the bouton, glutamate is reassembled by glutaminase and then repacked into a synaptic vesicle. GABA is converted to glutamate by GABA transaminase during transit. Following return to the bouton, glutamate is converted (by glutamate decarboxylase) to GABA prior to storage in vesicles.
The remarkable autoimmune disorder known as stiff person syndrome, caused by blockade of glutamate decarboxylase, is described in Chapter 29.
Glycine
Glycine is synthesized from glucose via serine. Its main function as a transmitter is to provide tonic negative feedback on to motor neurons in the brainstem and spinal cord. Inactivation of glycine, for example by strychnine poisoning, results in agonizing convulsions (Clinical Panel 8.1).
Clinical Panel 8.1 Strychnine poisoning
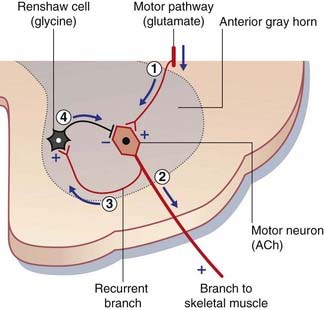
Strychnine is a glycine receptor blocker. The victim of strychnine poisoning suffers agonizing convulsions because of liberation of α motor neurons from the tonic inhibitory control of Renshaw cells (Figure CP 8.1.1). The convulsions resemble those induced by the tetanus toxin, described in Chapter 6. This is no surprise, because tetanus toxin prevents the release of glycine from Renshaw cells. Postmortem studies of normal human brain, using radiolabeled strychnine, have shown glycine receptors to be especially abundant on internuncial neurons in the nucleus of the trigeminal nerve supplying the jaw muscles, and in the nucleus of the facial nerve supplying the muscles of facial expression. These two muscle groups are especially affected in both types of convulsive attack.
Biogenic amine transmitters
Acetylcholine
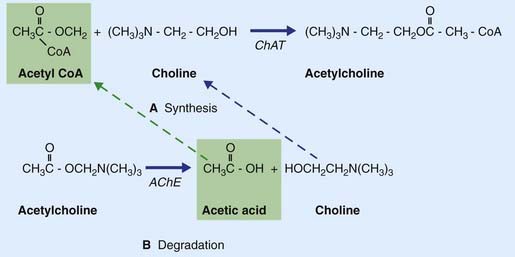
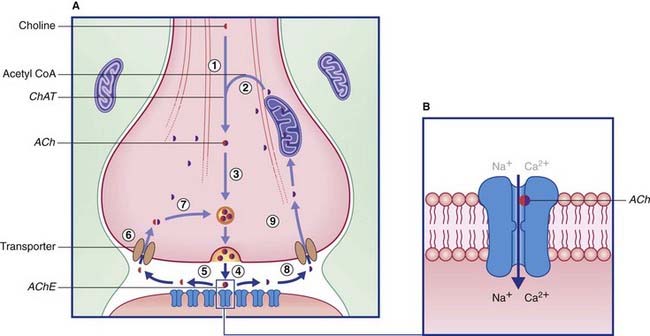
Acetylcholine is formed when an acetyl group is transferred to choline from acetyl coenzyme A (acetyl CoA) by the enzyme choline acetyltransferase (Figure 8.15), which is unique to cholinergic neurons. The choline is actively transported into the neuron from the extracellular space. Acetyl CoA is synthesized in mitochondria that are concentrated in the nerve terminal and also provide the enzyme. Following release, ACh is degraded in the synaptic cleft by acetylcholinesterase (AChE), yielding choline and acetic acid. These molecules are largely recaptured and recycled to form fresh transmitter.
Some steps in synthesis, degradation, and recycling of ACh are also shown in Figure 8.16.
Nicotinic receptors
The nicotinic receptor is considered further in relation to the innervation of skeletal muscle (Ch. 9).
Monoamines
Catecholamines
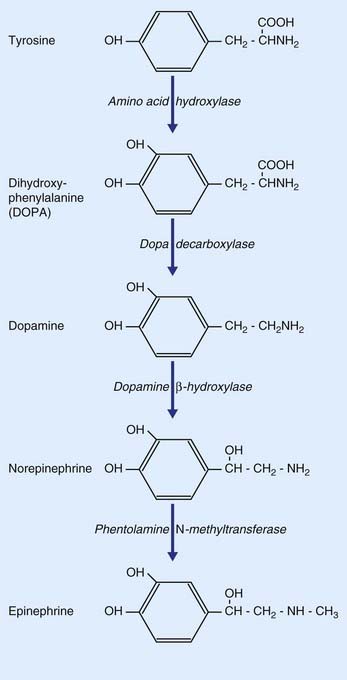
As indicated in Table 8.2, the catecholamines comprise dopamine, norepinephrine, and epinephrine (adrenaline). As shown in Figure 8.17, all three are derived from the amino acid tyrosine.
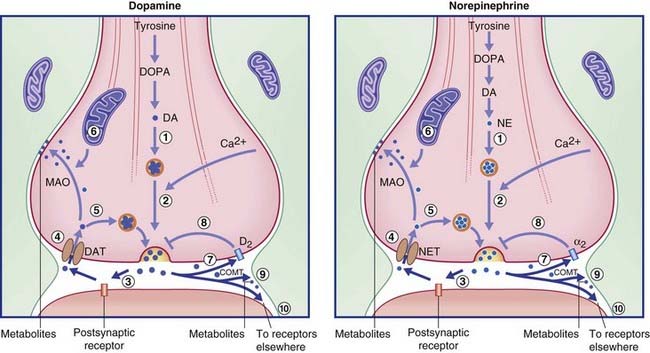
The transmitters are synthesized in the nerve terminals, the requisite tyrosine and enzymes having been sent there by rapid transport. Newly synthesized transmitter must be packaged immediately into a synaptic vesicle by a monoamine transporter protein lodged in the vesicular membrane, because the catabolic enzyme monoamine oxidase (MAO) permeates the cytosol. On release, most of the transmitter binds with one or more specific receptors in the postsynaptic membrane and (where present) with an autoreceptor in the presynaptic membrane. Of the remainder, some is inactivated by catechol-O-methyl transferase (COMT), an enzyme liberated from the postsynaptic membrane into the synaptic cleft (Figure 8.18). The rest is taken up by a specific uptake transporter, and is either collected by a vesicular protein transporter or is inactivated by MAO.
Dopamine
Dopamine is of particular interest in the clinical contexts of Parkinson disease, drug addiction, and schizophrenia. It is synthesized from tyrosine in two steps (Figure 8.17), being converted to DOPA (dihydroxyphenylalanine) by amino acid hydroxylase, and from DOPA to dopamine by dopa decarboxylase, an enzyme restricted to catecholaminergic neurons. The two main sets of dopaminergic neurons are located in the midbrain. They are the substantia nigra and the ventral part of the tegmentum called the ventral tegmental area (VTA).
The substantia nigra belongs functionally to the basal ganglia (Ch. 28). A dopaminergic nigrostrial pathway projects from substantia nigra to striatum (caudate nucleus and putamen). This pathway controls a motor loop of neurons feeding forward to the motor cortex. Degeneration of neurons in the substantia nigra is a classic feature of Parkinson disease, in which normal movements are disrupted by rigidity of the musculature and/or tremor.
The VTA projects groups of dopaminergic neurons into the forebrain. One group, called mesocortical, projects to the prefrontal cortex; overactivity of this system has been invoked to explain some clinical features of schizophrenia (Ch. 29). The other, called mesolimbic, projects to several limbic nuclei including the nucleus accumbens (bedded in the ventral striatum); dopamine liberation within the nucleus accumbens appears to be the basis of the dopamine rush, or dopamine high, associated with several kinds of drug addiction (Ch. 34).
Receptors
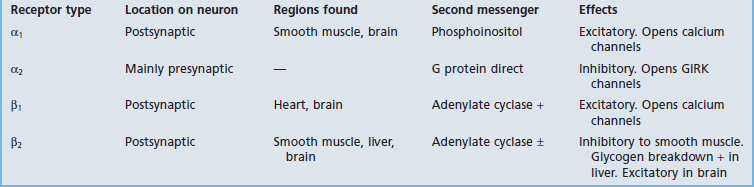
Dopamine receptors are all G protein-coupled. D1 and D2 receptors are recognized, each having more than one subtype. D1 receptors activate Gs proteins and are excitatory, activating adenylate cyclase with consequent receptor phosphorylation. D2 receptors activate Gi proteins and are inhibitory; they may inactivate adenylate cyclase, and may also promote hyperpolarization by opening GIRK ion channels and/or inhibiting voltage-gated Ca2+ channels. Both kinds are numerous in the striatum, where they are required for the proper execution of learned motor programs including locomotion (Ch. 28).
Norepinephrine
In the CNS, noradrenergic neurons are concentrated in the cerulean nucleus (locus ceruleus) in the floor of the fourth ventricle. From here, they project to all parts of the gray matter of the brain and spinal cord (see Figure 24.4). These neurons are important for regulation of the sleep–wake cycle and the control of mood.
Catecholamine recycling
Recycling of dopamine and norepinephrine occurs via specific reuptake transporters, as indicated in Figure 8.18. The figure indicates that not all the molecules are recycled into synaptic vesicles within the parent neuron. Any of three other fates are possible: some are metabolized in or near the synaptic cleft by the enzyme COMT; others are carried for up to 100 µm by the extracellular fluid, perhaps bonding to isolated specific membrane heteroreceptors on other neurons as depicted in Figure 6.6 (‘volume transmission’); and others achieve reuptake only to be metabolized by the enzyme MAO liberated by nearby mitochondria.
Epinephrine
Neuronal production of epinephrine in the CNS appears to be confined to a group of cells in the upper lateral part of the medulla oblongata. Only these contain the enzyme (phentolamine N-methyltransferase) that provides the final link in the catecholamine chain (Figure 8.17). Some of these neurons project upward to the hypothalamus, others to the lateral gray horn of the spinal cord. Their functions are not yet clear.
In the PNS, the chromaffin cells of the adrenal medulla liberate epinephrine as a hormone into the capillary bed. The epinephrine augments sympathetic effects on the circulatory and other systems during the alarm response to danger. As shown in Figure 13.6, the chromaffin cells are modified sympathetic ganglion cells receiving synaptic contacts from preganglionic cholinergic neurons. One function of circulating epinephrine, illustrated in Figure 13.5, is to boost norepinephrine output at sympathetic nerve terminals by activating β2 heteroreceptors there.
Serotonin
As indicated in Figures 21.2 and 21.5 Figure 21.2 Figure 21.5, serotonergic cell bodies occupy the midregion or raphe (seam) of the brainstem. Their axonal ramifications are quite prodigious, penetrating to every region of the gray matter of brain and spinal cord.
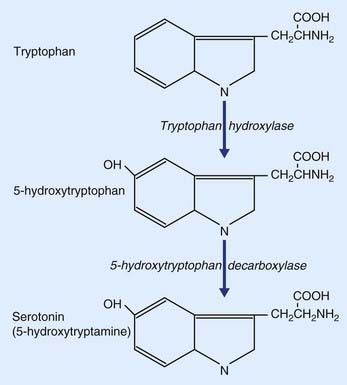
Serotonin, commonly referred to as 5-HT because it is 5-hydroxytryptamine, is derived from the dietary amino acid tryptophan, present in the circulation. It is actively transported across the blood–brain barrier into the brain extracellular fluid, then transported into serotonergic neurons. Formation of serotonin from tryptophan is a two-step process (Figure 8.19). Tryptophan is converted to 5-hydroxytryptophan by the enzyme tryptophan hydroxylase, and this is converted to serotonin by 5-hydroxytrytophan decarboxylase.
Receptors
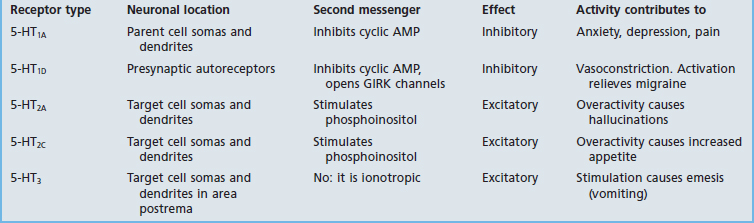
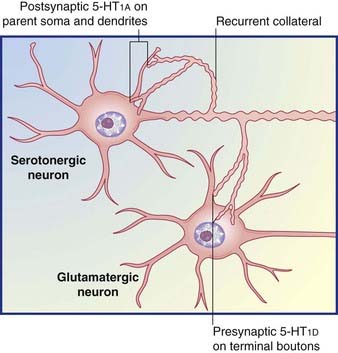
Table 8.5 provides some details for a selected shortlist of serotonin receptors. The table includes a reference to receptors on the somas and dendrites of the parent cell. These are targets of recurrent axon collaterals, as indicated in Figure 8.20.
Recycling
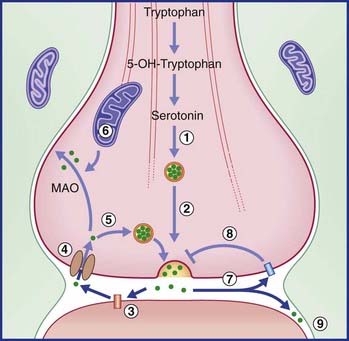
Recycling follows the same general pattern as for the catecholamines. Here again, the final step of transmitter synthesis takes place within the terminal bouton, and the released molecules may activate either presynaptic autoreceptors on the parent neuron or isolated heteroreceptors on other neurons nearby. There appears to be no degradatory enzyme in or near the synaptic cleft comparable with COMT, but MAO is present within the parent bouton (as shown in Figure 8.21).
Monoamines and abnormal emotional or behavioral states
Abnormal monoamine function has been implicated in a great variety of abnormal emotional or behavioral states, including depression, insomnia, anxiety disorders, panic attacks, and specific phobias. Brain areas involved are discussed in Chapters 23 and 29.
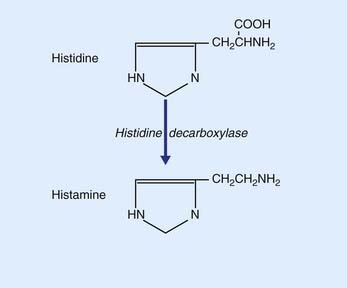
Histamine
Histamine is synthesized from histidine by histidine decaboxylase, as shown in Figure 8.22. The somas of histaminergic neurons appear to be confined to the posterior part of the hypothalamus, where they occupy the small tuberomammillary nucleus shown in Figure 26.1. However, their axons extend widely, mainly to all parts of the cerebral cortex. The main function of histaminergic neurons is to participate with cholinergic and serotonergic neurons in maintaining the awake state. These neurons are active in the awake state and silent during sleep (see Ch. 24). Activation is a function of the peptide orexin locally produced by lateral hypothalamic neurons. The compulsive daytime sleep disorder known as narcolepsy (Ch. 30) appears to result from failure of orexin production.
Neuropeptides
More than 50 neuropeptides have been isolated. All of them are linear chains of amino acids linked by peptide bonds. Peptide precursor chains (called propeptides) are passed through the Golgi complex and budded off in large, dense-cored vesicles that are rapidly transported to the nerve endings, where peptide formation is completed. As previously illustrated in Figure 6.5, peptides undergo non-synaptic release and may travel some distance to reach their receptors.
Receptors
These are all G protein-coupled. In general, they are cotransmitters, and their function is to modulate the effect of principal, small-molecule transmitters such as glutamate or ACh. Calcium channels are relatively scarce outside the synaptic cleft, and peptide liberation characteristically requires relatively high-frequency action potentials. An example is mentioned in Chapter 13: sweat glands are supplied by cholinergic neurons having vasoactive intestinal polypeptide (VIP) as cotransmitter. At low-frequency stimulation, ACh alone is sufficient to provide routine ‘insensible perspiration’, which is also invisible. Sweating for any length of time requires local vasodilatation in addition to an abundance of ACh, and this is provided by VIP, a potent dilator of arterioles.
Within the CNS, naturally occurring opioid (opium-like) peptides, called endorphins, are highly significant in relation to the control of pain perception, as discussed in Chapter 24.
Nitric oxide
Nitric oxide is not a ‘classical’ transmitter, but is a lipid- and water-soluble gaseous radical that diffuses briefly and rapidly across cell membranes, including those of neurons. It is synthesized from arginine by the enzyme nitric oxide synthase in response to Ca2+ entry following depolarization; it activates guanylate cyclase and increases cyclic AMP in target cells, thereby enabling cyclic AMP to modulate the activity of conventional neurotransmitters. In the autonomic nervous system, it is a powerful smooth muscle relaxant (Ch. 13). In the brain, it appears to be especially relevant to memory formation by eliciting long-term potentiation in glutamatergic neurons in the hippocampus (Ch. 34).
An S, Seong D. Regulation of exocytosis in neurons and neuroglial cells. Curr Opin Neurobiol. 2004;14:522-530.
The glutamate receptors, Gereau RW, Swanson G, Humana Press, Totowa, NJ, 2008.
Leonard BE. Fundamentals of psychopharmacology, ed 3. Chichester: Wiley; 2003.
Mayer ML. Glutamate receptor ion channels. Curr Opin Neurobiol. 2005;15:282-288.
Nestler EJ, Hyman SE, Malenka RC. Molecular pharmacology: a foundation for clinical neuroscience. New York: McGraw-Hill; 2001.
Stevens CF. Presynaptic function. Curr Rev Neurobiol. 2004;14:341-345.