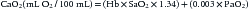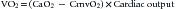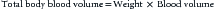Chapter 82 Transfusion Medicine
Red Blood Cells
RBCs contain hemoglobin (Hb), which binds and carries oxygen (O2) to cells, thus facilitating efficient adenosine triphosphate (ATP) production via cellular respiration. Because energy expenditure is high in critically ill patients, it would seem rational to maintain their Hb level in the normal range. Anemia is observed in 74% of critically ill children.1 RBC transfusion is the only effective way to rapidly increase the Hb level. However, the safety of RBC transfusion has been questioned in recent years. Infections transmitted by blood products were the most important concern in the 1980s. In the 1990s, nosocomial infections and multiple organ dysfunction syndrome (MODS) observed in critically ill adults who received an RBC transfusion have become a cause of concern.2 Also transfusion-related immune modulation (TRIM),3 and transfusion reactions like transfusion-related acute lung injury (TRALI) and transfusion-associated cardiac overload (TACO)4 have become significant concerns. There are few data on these adverse events in PICU. Actually, the impact of RBC transfusion on risk/benefit and the cost/benefit ratios among critically ill children is not well characterized.
Red Blood Cell Transfusion: Why and Why Not
Anemia and O2 Delivery
O2 Delivery in the Critically Ill
In this formula, the Hb level is expressed in grams per deciliter, arterial O2 saturation (SaO2) is expressed as a fraction rather than a percentage, and PaO2 is expressed in mm Hg or torr. Because global DO2 is directly linked to the Hb concentration, the most rapid and effective way of increasing DO2 (within minutes) is by increasing the Hb concentration, and this represents the most common rationale underlying RBC transfusion in critically ill patients. More modest augmentation in global DO2 can be attained by increasing cardiac output and/or SaO2. Indeed, a prospective study conducted in 2005 involving 30 North American PICUs showed that about 50% of critically ill children received at least one transfusion of packed RBC unit during their PICU stay.1
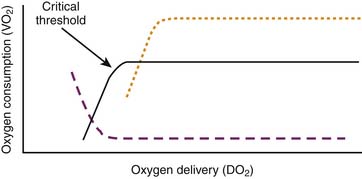
in which CmvO2 is the mixed venous O2 concentration. VO2 depends on substrate availability and on metabolic demands; it can be amplified by increasing cellular O2 extraction rate (O2ER), or by increasing DO2 if there is VO2/DO2 dependence (Figure 82-1).
The relationship between O2 delivery and consumption is characterized by two phases: a directly linear relationship between VO2 and DO2 up to a “critical threshold” (often referred to as the critical DO2), and a flat section above this threshold (see Figure 82-1). Below the threshold, VO2 diminishes if DO2 decreases. Above this threshold, a fall in DO2 does not cause a drop in VO2 because it is compensated by mechanisms such as an increase in O2ER; these mechanisms are limited though, which explains why there is a critical threshold of DO2 under which O2ER cannot increase any further, and under which VO2 begins to fall.
Adaptive Mechanisms to Anemia
Anemia significantly decreases blood O2 carrying capacity. However, in the normal host, the amount of O2 delivered to tissues exceeds resting O2 requirements by a two- to fourfold factor.5 When the Hb concentration falls below 10 g/dL, several adaptive processes maintain VO2. These processes include (1) increased O2ER; (2) increased heart rate and stroke volume, which increase cardiac output; (3) redistribution of blood flow from nonvital organs toward the heart and brain, at the expense of O2 delivery to less vital vascular beds, such as the splanchnic vasculature; and (4) rightward shift of the oxy-Hb-dissociation curve, which decreases affinity between Hb and O2, thereby increasing the amount of O2 released to cells.
O2 Kinetics in the Critically Ill
Tissue hypoxia from low DO2 may be due to low Hb concentration (anemic hypoxia), low cardiac output (stagnant hypoxia) or low Hb saturation (hypoxic hypoxia).5 A significant number of intensivists use RBC transfusion to increase DO2 in critically ill children.6 RBC transfusion indeed increases DO2, but there is no clear evidence that RBC transfusion improves tissue VO2 in ICU patients.2 Many mechanisms may explain why VO2 does not always increase in such instances. Mitochondrial dysfunction is frequent in critically ill patients; this may prevent O2 utilization (see Chapter 74).7 Moreover, O2 delivery to tissue is impaired in ICU patients, and there is some evidence that RBC transfusion may worsen that problem.
Regulation by Red Blood Cells of DO2 to Tissue
Although RBC transfusion certainly increases systemic DO2 in the central circulation, it does not mean that local O2 delivery to tissues is improved. There is indeed evidence that RBC transfusion may disturb local DO2. For example, Kiraly et al.8 showed that tissue SO2 (StO2) in critically ill patients who received a transfusion of RBC units stored more than three weeks declined from about 89% to 81%, whereas this did not happen in controls. However, data on local DO2 are inconsistent, their clinical significance remains to be determined, and the mechanisms are not well characterized; blood viscosity, microcirculatory flow, local DO2, and cellular respiration may be involved.
Activation of white blood cells (WBC) in packed RBC units and cytokine generation in the supernatant of transfused RBC units may also have a microcirculatory effect: some cytokines can mediate vasoconstriction or thrombosis of small vessels and cause local ischemia.9 However, most packed RBC units are now prestorage leukocyte-reduced, which significantly decreases cytokine levels in the supernatant.10 The clinical impact of cytokines in the supernatant of prestorage leukocyte-reduced packed RBC units remains to be determined.
There is evidence that RBC transfusion can cause vasoconstriction of small blood vessels via a mechanism involving an interaction between the Hb in RBC and the nitric oxide released by small vessels. With local tissue hypoxia, Hb in the microvasculature releases nitric oxide and triggers local vasodilatation; conversely, if there is sufficient O2 in the microvasculature, Hb binds nitric oxide resulting in vasoconstriction. This regulatory mechanism is almost immediately lost once RBCs are stored: it has been shown that in vitro exposure of blood vessels to RBC units stored 3 hours or more causes vasoconstriction.11 However, the level of nitric oxide increases rapidly after transfusion.12 Although the clinical significance of these observations is not clear yet, these findings nonetheless suggest that local DO2 can be disturbed by RBC transfusion.
Packed RBC units undergo several changes during storage, which are generally referred to as “storage lesion.”13 For example, the level of 2,3-diphospho- glycerate (2,3-DPG) in stored RBC decreases over time and can induce a leftward shift in the oxy-Hb dissociation curve, which impedes O2 release to tissues even if DO2 is increased. In addition, RBC deformability decreases after 2 or 3 weeks of storage, which may alter their capacity to pass through the capillary bed. Furthermore, hemolysis in older packed RBC units releases substantial amounts of free Hb ranging from 0.5 mg/dL in a 1-day-old RBC unit to 250 mg/dL in a 25 day-old unit14; free intravascular Hb has the ability to bind nitric oxide and therefore likely mediates vasoconstriction.15
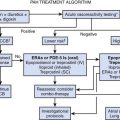
Transfusion of Red Blood Cells: Indications (When)
Blood is clearly indicated for the treatment of hemorrhagic shock.2 In such instances, the decision to prescribe RBC transfusions should be based on the physiologic state, the estimated amount of blood loss, and risk of ongoing hemorrhage, not on the Hb concentration.
RBC transfusion is more questionable if the hemorrhage is not clinically significant or if there is no hemorrhage. Pediatric intensivists have stated in two surveys that their decision to prescribe a RBC transfusion would be based on reasons such as a low DO2 or VO2, cardiovascular insufficiency, respiratory failure, or use of certain specific technologies such as extracorporeal membrane oxygenation (ECMO), hemodialysis, hemofiltration, plasmapheresis, or exchange transfusion; nonetheless, the most frequent reason to transfuse RBCs was reported to be a low Hb concentration.1,6 The Hb level that should prompt a pediatric intensivist to prescribe RBC transfusion remains a matter of debate, but there is some evidence in the medical literature that can guide practitioners.
Evidence-Based Medicine: Clinical Studies
Four case series are applicable to critically ill children: two in children with septic shock,16,17 another in postoperative cardiac surgery patients,18 and the last in children with cyanotic heart disease undergoing elective cardiac catheterization.19 These studies assessed hemodynamic parameters before and after a 0 to 20 mL/kg packed RBC transfusion; all reported a significantly increased Hb level as well as a greater DO2 after transfusion, but only one study reported an increase in VO2.16 These findings support the hypothesis that RBC transfusion increases systemic DO2, but does not necessarily increase VO2.
There is evidence that severe anemia increases mortality and morbidity among severely ill children. In two studies involving patients who refused blood products for religious reasons, the risk of mortality increased significantly with a postoperative Hb level less than 4 g/dL in healthy adults20 and less than 10 g/dL in adults with heart disease.21 There are three prospective descriptive studies addressing this issue in pediatrics. Lackritz et al.22 followed 2433 African anemic children younger than 12 years among whom 20% received a RBC transfusion. They reported that RBC transfusion was beneficial if the Hb level was below 4.7 g/dL and if the patient presented some respiratory distress. Lackritz et al.23 subsequently published another prospective study examining 1223 consecutively hospitalized children in Kenya. Local guidelines suggested that an RBC transfusion should be given to all children with an Hb level less than 5 g/dL. The Hb level was less than 5 g/dL in 303 cases; 116 (38%) did not receive a transfusion, mostly because packed RBC units were not available. Each child with severe anemia was paired with the next child hospitalized with an Hb greater than 5 g/dL. The death rates were: 19.5% in 303 patients with Hb greater than 5 g/dL who were not transfused, 21.4% in 187 patients with Hb less than 5 g/dL who were transfused, and 41.4%% in 116 patients with Hb less than 5 g/dL who were not transfused. English et al.24 completed a prospective cohort study of 1269 children with malaria hospitalized in Kenya; they reported that RBC transfusion decreased mortality if anemia was severe (Hb level <4 g/dL), or if a Hb level less than 5 g/dL was associated with dyspnea. These three studies suggest that the risk of mortality increases significantly in severely ill children requiring hospitalization if their Hb concentration is lower than 5 g/dL particularly if respiratory symptoms are present.
Two randomized clinical trials evaluated RBC transfusion strategies in children. The first trial was performed in Africa in 106 children with malaria crisis (hematocrit: 12% to 17%); RBC transfusion did not improve mortality rate (1/53 vs. 2/53) in patients without respiratory or cardiovascular compromise.25 In the TRIPICU study, a large multicenter international randomized clinical trial involving 637 stable critically ill children with Hb level lower than 9.5 g/dL, 320 patients were allocated to a RBC transfusion threshold of 7 g/dL of Hb (restrictive group) and 317 to a threshold of 9.5 g/dL (liberal group). A statistically significant noninferiority was found: 38 and 39 patients respectively developed new or progressive MODS, and there were 14 deaths in both strategy groups within 28 days postrandomization. The conclusion of this study was that a restrictive strategy is as safe as a liberal strategy in stable critically ill children. Moreover, given that 174 patients (54%) in the restrictive group received no RBC transfusion compared to 7 (2%) in the liberal group (P <.0001) and that patients in the restrictive group received 54% fewer RBC transfusions, the findings supported adopting a restrictive transfusion strategy for stabilized critically ill children.26 Subgroup analyses were subsequently undertaken in patients with sepsis27 and those having undergone noncardiac surgery28: both showed trends very similar to those reported in the original TRIPICU study.
Red Blood Cell Transfusion: Current Recommendations
Guidelines from many organizations emphasize that the decision to administer RBC should not be determined solely by an Hb value, but should be based on sound clinical judgment.2,29,30 RBC transfusion in PICU is indeed associated not only with a low Hb level, but also with admission for cardiac disease (odds ratio [OR], 8.07; 95% confidence interval [CI], 5.14 to 14.65), higher severity of illness (PRISM score >10: OR, 4.83; CI, 2.33 to 10.04), and presence of MODS (OR, 2.06; CI, 1.18 to 3.57).31 In a survey,6 pediatric intensivists declared that they might consider prescribing a RBC transfusion based on the following markers: Hb concentration, low SaO2, low PaO2, low cardiac output (poor DO2), high blood lactate level, low ScvO2 or SmvO2, poor VO2, high severity of illness, active bleeding, and emergency surgery. However, how these determinants of RBC transfusion interacted with each other was unclear.
Many physicians advocate “goal-directed transfusion therapy.”32,33 Although it is theoretically rational to base the decision to transfuse RBCs on physiologic need, it is still a matter of debate what parameters best determine that need. It has been suggested that a RBC transfusion is indicated for patients with symptomatic anemia, but most critically ill children are unable to report these symptoms. Some have suggested it would be better to use global markers of oxygenation deficit, such as systemic VO2, VO2/DO2 dependence, blood lactate level, ScvO2, SmvO2, or O2ER.34 Others propose the use of measurements that reflect local, regional, or tissue oxygenation deficit, such as brain tissue O2 pressure (PbtO2),35 gastric tonometry36 StO2 measured by near-infrared spectroscopy8 or digital O2 extraction rate measured by noninvasive devices. Actually, it is presently not known what markers are best suited for this purpose and what cutoff values should be used to determine the need for RBC transfusion in critically ill children. The concept of goal-directed transfusion therapy is laudable, but is presently vaguely defined, and not supported by hard data. Recommendations specific to goal-directed transfusion therapy remain undetermined at the present time.32
In practice, a low Hb concentration remains the most frequent and the primary justification for pediatric intensivists to prescribe a RBC transfusion.1 Therefore it makes sense that the Hb concentration be the first parameter assessed when a RBC transfusion is considered. Given the available evidence, RBC transfusion is recommended for all critically ill children who present with a Hb concentration below 5 g/dL. In stable patients, including septic patients,27 patients having undergone noncardiac surgery,28 and severely burned children,37 it is suggested by experts to consider a RBC transfusion if the Hb concentration is lower than 7 g/dL, but a transfusion is not recommended if the Hb concentration is above this level.2 These thresholds are not so far from current practice: Goodman et al.38 have reported that all critically ill children with a Hb ≤5.3 g/dL and that 93% of those with a Hb of 6.4 g/dL or less received at least one RBC transfusion. However, determinants other than the Hb concentration must be considered including age, severity of illness or evidence of organ dysfunction or O2 dependency, such as a high blood lactate level or low ScvO2. For example, it would seem appropriate to consider a higher threshold and a more aggressive RBC transfusion strategy in unstable patients, but the optimal and safe lower limit of the transfusion threshold has not been established for such patients. Moreover, any recommendations made must also factor in specific considerations for disorders such as sickle cell disease, hemolytic uremic syndrome, and some cardiac diseases.
Many experts in the field of pediatric cardiology and cardiac surgery believe that the optimal Hb concentration for patients in the postoperative phase of cardiac surgery should be significantly higher, and advocate levels as high as 14 to 18 g/dL in cases of uncorrected cyanotic congenital cardiopathy.39,40 Few clinical studies have addressed RBC transfusion in cyanotic heart disease. Experience with bloodless cardiac surgery for congenital heart disease in children whose families refuse transfusion for religious reasons seems to suggest that a lower Hb level may be well tolerated. This is supported by a randomized clinical trial involving 59 children with bidirectional Glenn or Fontan procedures. In this trial, patients were randomized either to a restrictive or liberal transfusion strategy (respective Hb concentration thresholds of 9 or 12 g/dL). The mean postoperative Hb was 11.1 ± 13 and 13.9 ± 0.5 g/dL, and the mean number of RBC transfusions was 0.47 ± 0.6 and 2.03 ± 1.2 per patient in the restrictive and liberal groups. No difference was found with respect to outcomes like peak blood lactate level (3.0 ± 1.5 vs. 3.1 ± 1.3 mmol/L), ventilator or pressor duration, ICU or hospital length of stay, or survival. More data are required before implementing a restrictive transfusion strategy in patients with cyanotic heart disease.41 Beekman’s 1985 statement that “The optimal Hb concentration for children with cyanotic heart disease has yet to be determined” remains true today.19
On the other hand, there is some evidence that a 7 g/dL threshold may be safe in the postoperative care of noncyanotic congenital heart disease in stabilized patients older than 28 days.42 Willems et al.43 analyzed a subgroup of 125 postoperative cardiac patients enrolled in the TRIPICU study after a cardiac surgery. No significant difference was found between the restrictive and liberal groups in new or progressive MODS (12.7% vs. 6.5%; P = .36), PICU length of stay (7.0 ± 5.0 vs. 7.4 ± 6.4 days) or 28-day mortality (two vs. two deaths). The British Society of Haematology30 supports the acceptance of a postoperative hemoglobin level of 7 g/dL in children when there is good postoperative cardiac function unless there is a cyanotic heart lesion persisting. The Society of Thoracic Surgeons makes a similar recommendation for all cardiac surgery patients.44 Data reported by Willems et al.43 support these recommendations.
There is a debate on the usefulness of blood transfusion as a preventive measure. Some evidence suggests that this may be appropriate in critically ill children who have certain forms of congenital anemia (for example, sickle cell disease,45 and in patients who require ECMO (a Hb threshold of 13 to 15 g/dL is suggested46) or surgery.47 However, there are few hard data to support such recommendations in the latter two groups.
Prevention of Red Blood Cell Transfusion
“Bloodless medicine” is a popular concept in many American hospitals; it refers to all the strategies that can be used to provide medical care without allogeneic RBC transfusion, including blood conservation.48 There are indeed many strategies that can prevent and/or significantly decrease the need for RBC transfusions and exposure to a transfusion. Adopting a restrictive RBC transfusion strategy in stable critically ill children is one of them; other possible means range from raising the Hb concentration before an elective surgery to using blood products only when necessary, limiting blood losses and administering the patient’s own blood.
Bloodless medicine begins before surgery. Among the possible strategies in the preoperative period, the use of erythropoietin and iron supplementation to optimize the preoperative Hb level, collection of autologous donations to prevent some allogeneic transfusion,49 avoidance of any medication that increases the risk of bleeding, including herbal medicine (e.g., garlic, ginseng, ginger33), and optimal control of any existing coagulation disorders just prior to surgery should be considered.
During surgery, maximal attention should be given to limiting blood loss50 and ensuring good hemostasis and rapid control of all bleeding. In some instances, desmopressin,51 fibrin sealants, or antifibrinolytic agents such as aprotinin or tranexamic acid48 may be used to stop a hemorrhage. Recombinant activated factor VII (rFVIIa) is also advocated by some practitioners, but it is associated with a significant risk of thrombosis; the cost/benefit ratio of rFVIIa in children is not well evaluated and its use should be limited to situations with uncontrolled bleeding that is life-threatening.52 The safety and cost-effectiveness of intraoperative blood conservation strategies such as normovolemic hemodilution,53 autologous blood cell salvage modalities,54 intraoperative autotransfusion, and deliberate hypotension50 remain to be determined in children.
Postoperative and ICU management of anemia and bleeding is also important. A restrictive transfusion strategy is in line with the concept of “permissive anemia” supported by the British Committee for Standards in Haematology Transfusion Task Force.30 A prospective study reported that 73% of blood loss in PICU is attributable to blood draws.1 The number and the frequency of blood tests must be limited, and the amount of blood collected reduced. Many devices can help to minimize blood loss, including the use of loop sampling, pediatric blood collection tubes, microanalysis techniques requiring small volumes of blood, and in-line measurement of parameters such as blood gases and Hb concentration.55–57 The erythropoietin response to anemia is blunted58 and poorer than expected in critically ill patients.59 In spite of this, there are data suggesting that erythropoietin can prevent anemia in critically ill adults,60 in low-birth-weight preterm infants61–63 and in the postoperative care of neonates.64 In critically ill children, there are no data to support the use of erythropoietin as a preventive measure because most RBC transfusions are administered within 2 or 3 days after PICU admission,1,65 a period of time too short to allow for a response to erythropoietin that generally requires several days.66 The standard use of erythropoietin is presently not recommended in PICU.65,67 In addition, iron supplementation is not indicated because most critically ill patients are not iron depleted.59
An RBC transfusion should be administered only if the anticipated benefit outweighs the potential risk. A threshold Hb concentration of 7 g/dL is adequate in most stable critically ill children.68 The optimal Hb concentration or transfusion threshold above which the benefits outweigh the risks and costs remains to be determined in unstable patients and in patients with congenital heart disease.
Types of Packed Red Blood Cell Units
Standard Packed RBC Units
Storage of RBC units is made possible by refrigeration at about 4° C and by storage in preservative anticoagulant solutions that contains dextrose, sodium citrate, citric acid, and sodium diphosphate. Erythrocytes use dextrose and phosphate to generate ATP, which is essential for their survival. Citrate blocks coagulate by chelating calcium; it is also transformed into bicarbonate, which stabilizes the stored RBC unit pH above 6.4. Citrate-phosphate-dextrose (CPD) solution can be stored up to 28 days. Citrate-phosphate-dextrose-adenine (CPDA-1) contains more dextrose (2 g vs. 1.6 g/63 mL) and more adenine (17.3 mg/63 mL) than CPD; it can be stored up to 35 days because the level of ATP remains normal after 21 days of storage and is about 50% after 35 days. Packed RBC units are prepared by removing 200 to 250 mL of plasma from one unit of whole blood by centrifugation. To support the nutrient needs of RBCs after plasma is removed, additive solutions were developed, such as AS-1 (Adsol), AS-3 (Nutricel), and AS-5 (Optisol), and saline-adenine-glucose (SAG) or SAG-mannitol (SAGM)69; these additive solutions further decrease RBC lysis and allow for storage up to 42 days.70
Other Types of Packed Red Blood Cell Units
Whole Blood
The use of whole blood has been advocated mostly for first-line therapy in hemorrhagic shock as it contains RBCs, coagulation factors, and platelets. However, it is generally not available. Additionally, refrigeration in storage solution for even several hours results in decreased levels of coagulation factors, oxygen-carrying capacity, as well as platelet function. In practice, component therapy is standard practice and it is usually easier to give one or more packed RBC units diluted with normal saline along with plasma and/or platelets. Whole blood units are also used by some for neonatal exchange transfusions, and in small children when priming for cardiac bypass procedures or continuous hemoperfusion. Whole blood is not recommended in normovolemic patients because it can cause a cardiac overload. The volume of a typical whole blood unit is about 450 mL.
Leukocyte-Reduced Packed Red Blood Cell Units
PackedRBC units contain some nonviable platelets, small amounts of coagulation factors, and WBCs that can release proinflammatory and antiinflammatory mediators during storage. Prestorage leukocyte reduction is a standard procedure for all blood components in many countries, such as Australia, Canada, and the United Kingdom; it can decrease the number of WBCs in packed RBC units from 1 × 109 to less 1 × 106 per product, and it decreases the concentration of cytokines in the supernatant, as well as some T cells that regulate immunomodulation.71 In 2005, 88% of packed RBC units given in American PICUs were leukocyte-reduced at collection.1 Transmission of intracellular viruses such as CMV and herpes is less frequent if there are fewer WBCs.
Irradiated Packed Red Blood Cell Units
Some WBCs remain in RBC and platelets units, even in prestorage leukocyte-reduced units. The objective of irradiation is to induce enough DNA damage to prevent leukocyte proliferation.72 Irradiation destroys the ability of transfused lymphocytes to divide and therefore to respond to host foreign antigens, thereby decreasing the risk of developing transfusion-associated graft versus host disease (TAGVH) in susceptible recipients. However, irradiation is not without some drawbacks. For example, it can damage the RBC membrane causing the release of significant amount of free Hb and potassium. Moreover, the shelf life of irradiated RBC units is reduced from 42 to 28 days.73
Cytomegalovirus-Negative Packed Red Blood Cell Units
A large proportion (30% to 70%) of blood donors are CMV positive. Most CMV infections are of little clinical consequence, but CMV can be fatal in patients with immunodeficiency. Fresh frozen plasma is not known to transmit CMV infection, but RBC units are. Although it would be ideal to administer only CMV-negative RBC units to CMV negative patients, the high prevalence of CMV infection among donors does not permit this.74 Nevertheless, prestorage leukocyte reduction of blood products decreases transmission of CMV to 1% to 2% (similar to the rate of infection following the transfusion of CMV negative units) compared to standard products for which transmission is 13% to 37%. Administration of a CMV positive RBC unit is generally not an issue for immunocompetent patients. Established indications for CMV-negative units include CMV-negative recipients of organ or bone marrow transplants from CMV-negative donors, CMV-negative bone marrow transplant recipients, and intrauterine transfusions. Less well-established indications include CMV-negative patients who are potential candidates for autologous or allogeneic bone marrow transplant, CMV-negative patients undergoing splenectomy, potential seronegative donors for bone marrow transplant and CMV-negative patients with HIV.
Directed Packed Red Blood Cell Units
Directed blood is donated by family members or friends. Parents frequently believe that giving their own blood decreases the risks of transfusion, which, in practice, is not the case. A small increase of transfusion-transmitted infectious diseases has been reported.46 Moreover, the risk of contracting a TAGVH is increased even in immunocompetent patients. In spite of this, directed blood donation remains popular; good clinical studies are warranted to better estimate the risk/benefit ratio of this practice. All directed RBC units must be irradiated pretransfusion.
Autologous Packed Red Blood Cell Units
Older healthy children can give their own blood a few weeks before elective surgery. It is frequently believed that autologous RBC units are free of risk, but this is untrue. These units are usually quite old by the time transfusion is required, which raises significant concerns with respect to RBC unit length of storage.75 Moreover, autologous RBC units are not leukocyte-reduced, at least in Canada. The risk/benefit ratio of autologous RBC units remains to be determined.
RBC substitutes and other alternatives to RBC transfusion
Hemoglobin-based oxygen carrier solutions (semisynthetic or synthetic preparations of Hb) and perfluorocarbon derivatives can carry O2.76,77 Both were developed as alternatives to RBCs, but there are serious concerns about their safety and usefulness. None can be recommended presently.
Transfusion of Packed Red Blood Cells: How
Erythrocyte transfusion is the best way to rapidly increase the Hb concentration. The practitioner must address a few questions after a decision is made to prescribe a RBC transfusion: what type of RBC unit (see the previous section), what blood type, how much (volume), how the unit is infused and what monitoring must be performed.
Blood Types
Table 82-1 describes the compatibility of different blood products. A completed cross-match is mandatory before any transfusion is given, with few exceptions. Transfusion of group O Rh negative RBC and/or group AB Rh positive plasma can be lifesaving, but this must be reserved for very severe and acute situations. It takes 15 to 20 minutes to complete ABO and Rh typing of a patient. If there are no RBC antibodies, fully compatible blood or immediate spin cross-match may be issued quickly. In the case of RBC antibodies or other anomalies, a full serologic cross-match is required, which will take more time. The risk of severe reaction to typed, but not cross-matched blood RBC units, is about 1 in 1000 if the patient has never received a transfusion; the risk is decreased by tenfold if a cross-match is done. If the patient has received at least one transfusion, the risks are respectively 1 in 100 with no cross-match and 1 in 1000 if a cross-match is done. It was recommended that similar units be used until patient recovery if a patient receives more than 20% of his blood volume with uncross-matched packed RBC units. However, this practice is a little outdated, as there is very little plasma in AS-3 and SAGM RBC units. If anti-A/B antibodies are detected on blood typing then antigen negative blood should be provided; otherwise ABO-identical units should be used when they become available. Repeat verification that the correct blood unit has been delivered to a given patient is essential because blood mismatch is the most important cause of severe transfusion reaction.
| Blood Product | Receiver | Donor |
|---|---|---|
| Packed RBC unit and whole blood | A, O | |
| B | B, O | |
| O | O | |
| AB | AB, A, B, O | |
| Rh+ | Rh+ or Rh− | |
| Rh− | Rh− | |
| Plasma or platelets | A, AB | |
| B | B, AB | |
| AB | AB | |
| Platelets | Rh+ | or Rh− |
| Rh− | Rh− or Rh+∗ |
∗ Give as an anti-D vaccine (Win Rho SDF) if the receiver is Rh− and the platelet concentration is Rh+.
Volume and Number of Units
where Hbtargeted is the Hb concentration targeted posttransfusion (e.g., 10 g/dL), Hbobserved is the most recently measured Hb concentration of the patient (g/dL), and HbRBC unit is the average Hb concentration in the packed RBC units (g/dL) delivered by the blood bank. Hb concentration in RBC units may vary from one center to another and with different preservative solutions. For example, the hematocrit of RBC units with AS-3 (Nutricel) is approximately 0.55, and HbRBC unit concentration is about 19.5 g/dL (range, 18-21 g/dL); the hematocrit of CPDA-1 is 0.75 to 0.65, and the HbRBC unit is about 25 g/dL. The blood volume can be calculated according to the following formula:
where weight is expressed in kilograms, and blood volume is in liters per kilogram (0.08 L/kg for a child younger than 2 years, 0.07 L/kg for child aged 2 to 14 years). For example, if the Hbobserved in a 2-week-old baby weighing 3 kg is 6.5 g/dL, his blood volume is 0.24 L (0.08 L/kg × 3 kg), and the HbRBC unit is 19.5 g/dL (AS-3), and if the physician targets a Hb concentration (Hbtargeted) of 10 g/dL, the volume of RBC unit to be transfused would be:
If the volume needed to reach the Hbtargeted is greater than the volume of one unit of packed RBCs, blood should be transfused one unit at a time to minimize exposure to multiple donors. Before the administration of additional packed RBCs, the Hb concentration should be measured after allowing at least 30 minutes posttransfusion for Hb and hematocrit values to equilibrate.78 The transfusion can be completed with another unit or partial unit if a reasonable Hb level is not attained.
If the volume of packed RBCs required is less than one unit, a partial unit can be given. Whole packed RBC units can be subdivided in half (standard division) or in small pediatric 75 mL transfer packs (Pedi-Pak). Partial units should be prepared sterilely where possible. Partial units prepared nonsterilely expire 24 hours after the preparation. On the other hand, partial units prepared sterilely can be kept as long as the original unit (up to 42 days for AS-3). A small volume of packed RBC unit placed in a syringe must always be administered within 24 hours.
Length of Storage
Regulatory agencies and scientific societies such as the Food and Drug Administration and the American Association of Blood Banks mandate that packed RBC units can be stored up to 42 days based on the premise that at least 75% of transfused RBC will be alive 24 hours posttransfusion.46,79 However, a “storage lesion” occurs over time, which raises many concerns.13 These changes are associated with a number of biochemical and biomechanical changes including RBC ATP depletion, low 2,3-DPG levels, membrane phospholipid vesiculation and loss, protein oxidation, lipid peroxidation of RBC membranes, and RBC loss of deformability.
Prolonged storage changes the supernatant as well as the RBCs themselves. In the storage medium, studies have noted the generation of cytokines and other bioreactive substances,80 including histamine,81 complement activators, O2 free radicals, lyso-phosphatidyl-choline species, and microvesicles containing lipids shed by RBCs. These bioactive substances may stimulate pro-inflammatory pathways and perhaps change flow patterns in the microcirculation.11 The latter observation might explain the effect of RBC transfusion reported by Kiraly et al.8 on StO2 in critically ill trauma adults. A significant decline in StO2 was observed in 17 patients who received RBC units stored since more than 21 days in the posttransfusion period compared to baseline. This was not observed in 15 patients who received fresher blood, nor in 16 patients who were not transfused.
Other well-documented time dependent changes in the storage medium are described, including a progressive fall in pH, an increase in plasma potassium and release of free Hb from lysed RBCs. On the other hand, fresh blood products stored for less than a week are not without risk and can be associated with transmission of certain intracellular viruses as well as TAGVH.82,83
There is presently controversy regarding the impact of RBC storage time in critically ill patients, both adult and pediatric. Some descriptive studies suggest that the outcome in critically ill adults is better if they receive fresher RBC units, while other studies do not support this.84 Two prospective descriptive studies undertaken in critically ill children suggest that outcome is less favorable with transfusion of packed RBC units stored for more than 2 or 3 weeks.85,86 On the other hand, a large retrospective study conducted in neonates undergoing cardiac surgery suggests that fresh blood may be more detrimental.87
The societal impact of implementing a “fresh RBC strategy” can be enormous: presently, RBC unit wastage is less than 1%; it is estimated that wastage would approach 30% if the allowable length of storage is decreased from 42 to 21 days.79 Thus it is unethical to implement a fresh RBC transfusion strategy without strong evidence to support its usefulness. Whether the length of storage of RBC units really affects outcome in critically ill children remains to be determined by randomized clinical trials. The ABLE study (ISRCTN44878718) is such a trial and has been enrolling adults since 2009, whereas a pediatric trial is in preparation. Until hard evidence is available, the use of fresh rather than “old” blood cannot be recommended for PICU patients.
Perfusion, Warming, and Filtration
A RBC transfusion must be completed within 4 hours after the unit is delivered by the hospital blood bank. A packed RBC unit is usually given over 1 to 3 hours, but it might be given more slowly (up to 4 hours) or divided in two transfusions if there is some risk of cardiac overload.46
The viscosity of packed RBC units is high, which implies it is preferable to use larger bore needles to administer them.88 No drugs should be given in the line used for packed RBC unit perfusion. It is also inappropriate to mix RBC units with dextrose or hypotonic solutions (risk of hemolysis), with Ringers lactate (risk of coagulation), or calcium.
Plasma
Plasma is separated from the RBC after collection of whole blood or it is collected using an apheresis machine; it is then frozen for storage to preserve the levels of coagulation factors. It is named “fresh frozen plasma” if the unit is refrigerated within 8 hours of collection, and “frozen plasma” within 24 hours of collection. There is a slight reduction in factor VIII levels in frozen plasma, but in clinical practice these two types of plasma are essentially interchangeable.89 The acronym FP (frozen plasma) is used in this section to designate both of them.
FP units are systematically leukocyte-reduced by filtration before storage in many countries, but not in the United States. FP volume is about 200 to 250 mL/unit,46 whereas the volume of SD plasma is about 200 mL/unit. On average, FP contains 1 unit/mL of all coagulation factors, but there is significant variability among individual units, which is attributable to biological variation in factor levels among individual donors, and differences in processing, storage and preparation for transfusion.90 The levels of coagulation factors in SD plasma are more standard with little variation among units as it is a pooled plasma product. FP is stored at −18° C up to one year after collection. Solvent detergent plasma can be stored for up to 48 months.
Transfusion of Plasma: Indication (When)
Generally, FP is transfused to correct multiple coagulation factor deficiencies (or single-factor deficiencies when no recombinant or plasma-derived coagulation factor concentrates are available) to patients with active bleeding or before invasive procedures when no alternative therapies are available or appropriate. Common coagulopathies for which FP may be given include liver disease and symptomatic disseminated intravascular coagulation (DIC). However, the use of plasma to treat disseminated intravascular coagulation is controversial because thrombosis is frequently a component of this disorder.46 Frozen plasma can also be given for the emergency reversal of warfarin or vitamin K deficiency when prothrombin complex concentrates are not available.91 Guidelines suggest transfusing FP only when the international normalized ratio (INR), the prothrombin time (PT), or the activated partial thromboplastin time (aPTT) is more than 1.5 times normal as coagulation factors are generally adequate for hemostasis below this level. However, some data suggest that FP is not very effective at normalizing mild abnormalities of coagulation tests, like an INR below 1.85,92 and the potential clinical benefit of FP transfusion seems minimal when the INR is less than 1.7.93
Frozen plasma may also be administered during massive transfusion of packed RBC units. Some experts advocate early replacement of FP in a 1:1 ratio with RBC units in trauma patients with massive transfusion, whereas others suggest that FP transfusions should be guided by the presence of abnormal coagulation test results as previously listed or of whole blood: it should be administered in patients who have received more than 1.5 their circulating blood volume, even if there is no bleeding, or in patients who have received more than one circulating blood volume and who have clinical evidence of oozing or microvascular bleeding.29,91,94
Other indications for FP transfusion include plasma exchange for thrombotic thrombocytopenic purpura (TTP).91 Some physicians advocate transfusion of plasma to treat cases of hemolytic uremic syndrome and to restore the blood volume of patients in shock, but there is no hard evidence to support such uses.
Transfusion of Plasma: How
One milliliter of FP contains about 1 unit of each of the coagulation factors. For most deficiencies, 30% of normal factor activity is enough to ensure hemostasis. Surgical hemostasis may be achieved with factor II levels 5% to 50% of normal, factor V approximately 30% of normal, factor VII 25% of normal, and factor VIII 30% to 60% of normal.33 Practitioners usually administer 10 to 20 mL/kg of plasma initially; this should increase the level of the individual coagulation factors above 30%. As the levels of coagulation factors vary among units, the response to FP is not consistent. Therefore, the effectiveness of FP transfusion should be estimated by clinical judgment of ongoing bleeding and, if necessary, repeat coagulation testing. Additional doses of FP may be required for ongoing bleeding with persistently elevated coagulation tests. However, normalization of coagulation tests often does not occur and, therefore, should not be used as the only guide for additional FP transfusions.95
Platelets
The prevalence of thrombocytopenia, defined by a platelet count less than 150,000/μL (<150 × 109/L), is 17.3% on admission into PICU; 25.3% of children are thrombocytopenic at some point during their PICU stay.96 Thrombocytopenia arises from decreased platelet production, increased platelet destruction, and dilutional or distributional causes.97 In PICU, most thrombocytopenia is caused by sepsis, disseminated intravascular coagulation, MODS, or hemolytic uremic syndrome; however, heparin-induced thrombocytopenia,98 massive transfusion,99 and reactive hemophagocytic syndrome are not so rare.100 In critically ill children, thrombocytopenia at PICU entry is associated with increased mortality (17.6% vs 2.5%)96, bleeding complications, thrombosis, and prolonged PICU and hospital length of stay.
Platelet dysfunction is also observed quite frequently in PICU. Rarely platelet dysfunction can be caused by hereditary disease (e.g., Bernard-Soulier disease, etc.), but, more commonly, it is caused by specific treatments (hypothermia, pentastarch, and hetastarch,101 etc.) or antiplatelet drugs (e.g., low-dose aspirin. nonsteroidal antiinflammatory drugs).
Standard Platelet Concentrates
Different methods can be used to obtain platelet concentrates: they may be whole blood derived platelets, either by the platelet-rich plasma (United States and United Kingdom) or the buffy-coat method (Europe and Canada) or by apheresis (single-donor) platelets (United States, Europe, and Canada). For whole blood derived platelets, platelet concentrates are often pooled (up to 6 units) for a single platelet transfusion.
Maximum platelet lifespan is 10.5 days.102 Each platelet unit contains about 55 × 109 platelets. When stored at 20° C to 24° C and gently agitated in a continuous manner, platelets can be used up to 5 days after they were collected, but will become active only 4 hours after transfusion to the recipient. If they are stored at 4° C, they are active immediately, but cannot be stored for more than 48 hours. In practice, most platelets units are stored at 20° C.
Special Platelet Concentrates
Leukocyte-Reduced Platelets
A platelet concentrate must contain les than 8.3 × 105 WBCs to be labeled leukocyte reduced.46 Prestorage leukocyte reduction is a standard procedure in many countries. Bedside leukocyte reduction filter should not be used when prestorage leukocyte reduction was done because it is useless and it can decrease the number of platelets.
Irradiated Platelets
The risk of TAGVH is increased in patients who receive HLA-compatible platelets. Therefore pretransfusion irradiation is mandatory for all human leukocyte antigen (HLA)-compatible platelet concentrates. Irradiation is also recommended for intrauterine transfusion and infants at risk of TAGVH.46
Transfusion of Platelets: Indication (When)
Over 1.5 million platelet products are transfused in the United States each year.103 Platelet transfusions are indicated for the prevention or treatment of bleeding in patients with thrombocytopenia or platelet dysfunction. As platelet transfusions will only result in modest elevations for 1 to 3 days in patients with persistent thrombocytopenia, the purpose of platelet therapy is not to eliminate all bleeding, but to prevent or stop major hemorrhagic events.
Therapeutic platelet transfusions are given to treat clinically significant bleeding associated with a low platelet count. There is evidence that correction of thrombocytopenia reduces mortality of critically ill patients,96,104 but a platelet transfusion should be considered only if the platelet count in an actively bleeding patient is less than 50,000 to 100,000/μL.
More than 50% of platelets transfusions are given to prevent bleeding, even though the need for prophylactic transfusions has not been conclusively proven. There is insufficient evidence to support a particular threshold for prophylactic platelet transfusion in children. Most recommendations come from guidelines developed for adults, based on expert opinion. For patients with hypoproliferative thrombocytopenia (e.g., chemotherapy induced), a platelet transfusion threshold of 10,000/μL is recommended.46 This is based on clinical trials in adults, which showed no increases in bleeding rates when comparing platelet transfusion thresholds of 10,000 vs. 20,000/μL.102
The platelet count must be monitored closely if a large amount, more than one blood volume, of crystalloids, and/or packed RBC units is given because this can significantly dilute the circulating platelet volume.105
The capacity of platelets to stop a hemorrhage is not only related to their number, but also to their function. Many tests can be used to estimate platelet function: thromboelastogram, Sonoclot coagulation analyzed, Plateletworks analyzer, Hemostatus platelet function test, Platelet function analyzer, VerifyNow (Ultegra) System, and so on.33 However, the results of these tests are not available on an emergency basis in most hospitals. Measurement of platelet mass may be a practical alternative. There is evidence that larger platelets exhibit increased hemostatic activity.106 The results of a randomized clinical trial conducted in a neonatal ICU suggest that using platelet mass (Platelet count × Mean platelet volume) rather than platelet count to trigger a platelet transfusion may reduce the number of transfusions.107 Nevertheless, it must be underlined that the clinical usefulness of all these tests remains undetermined in PICU.
There is significant diversity in the stated practice pattern with respect to platelet transfusions.108 Most guidelines are based on experts’ opinion, and not on hard data.29,30 Significant work needs to be done to better determine when platelets should be administered in critically ill children.
Transfusion of Platelets: How
A simple rule of thumb suggests giving 1 or 2 platelet units per 10 kg, but not more than 6 units per transfusion. For children weighing less than 10 kg, the platelet dose can be 5 to 10 mL/kg of pooled or apheresis platelet unit. Transfusion of one platelet concentrate per m2 of body surface generally increases the platelet count by 7000 to 11,000/μL. By body weight, the administration of one unit per 10 kg should increase the platelet count by 30,000 to 50,000/μL. However, a clinical trial, where platelet dose was estimated by body surface area, evaluated low (1.1 × 1012 platelets/m2), medium (2.2 × 1012 platelets/m2), and high (3.3 × 1012 platelets/m2) dose prophylactic platelet transfusions and did not find any differences in bleeding among adult or pediatric patients.109 Platelet units must be used within 4 hours after they are delivered by the blood bank, but there is some evidence that the platelet count raises more if the unit is given within an hour. A filter with 80 or 170 micron pores must be used to remove aggregates that can form between harvesting and transfusion.
The volume of a platelet-rich unit is about 50 to 70 mL, whereas that of an apheresis platelet unit ranges from 200 to 300 mL; 90% to 95% of this volume is plasma. It is important to note that this plasma is not an adequate source of coagulation factors because their concentration drops rapidly during platelet storage. Platelet units can be volume reduced (removal of plasma) before transfusion, but this process can decrease the platelet count by 15% to 20%, shortens the storage time to 4 hours and delays platelet release from the blood bank by approximately 1 hour. Volume reduction can only be considered if there is a risk of severe cardiac overload, but is not recommended as a standard procedure because it can activate platelets.46
Unlike RBC units, ABO compatibility is not mandatory with platelets. However, it is better to use ABO compatible platelet unit in young patients with small blood volumes. Moreover it is recommended to deliver ABO compatible platelets when inventory and time permit, unless the plasma component has been substantially reduced. On the other hand, Rh compatibility is desired because all platelet units contain some RBCs. Transfusion of a Rh-positive unit to a Rh-negative patient can cause Rh alloimmunization; an anti-D immunoglobulin (Win Rho SDR) should be administered within 48 hours to prevent this complication when Rh positive platelets are given to an Rh-negative patient, particularly in female patients.103
The percent platelet increment (difference between post- and pretransfusion on pretransfusion platelet count) should be higher than 20% if the dose is adequate and if a repeat platelet count is performed 10 to 60 minutes posttransfusion; it should be higher than 10% if measured 18 to 24 hours posttransfusion.103 Platelet refractoriness can occur because of nonimmune factors such as disseminated intravascular coagulation, in which platelet consumption can be high, acquired hemophagocytic syndrome, drugs such as amphotericin or heparin, or immune factors involving antiplatelet antibodies, and so on.100,103,110 Treatment of the underlying problem is mandatory in such instances, for example stopping heparin administration. Patients with anti-IgA antibodies should receive washed platelets or platelets collected from IgA deficient donors.
Transfusion Reactions and Complications
Red Blood Cells, Plasma, and Platelets
Immediate Transfusion Reactions
The incidence of typical immediate transfusion reactions to RBC, FP, and platelets is reported in Table 82-2. Acute transfusion reactions are probably underdiagnosed in critically ill children. In a study conducted in the PICU of Sainte-Justine Hospital, all transfusions between February 2002 and February 2004 were prospectively monitored.111 A total of 2509 transfusions were administered to 305 patients; 40 acute transfusion reactions (1.6%) occurred: 24 nonhemolytic febrile reactions, 6 minor and 1 major (anaphylactic shock) allergic reactions, 4 isolated hypotensive reactions, 3 bacterial contaminations, 1 hemolytic reaction, and 1 TRALI.
The SHOT report on transfusion reactions observed in children in the United Kingdom from 1996 to 2005 estimated the incidence of adverse outcomes to be 18 per 100,000 RBC units issued for children less than 18 years, 37:100,000 for infants less than 12 months, and 13:100,000 for adults.112 Among the 321 adverse reports in children, 82% were instances of incorrect blood component transfused, and 50 cases (14%) were acute transfusion reactions. The most important acute transfusion reactions are described below.
Respiratory System
TRALI is one of the most dangerous transfusion reactions. Two pathophysiologic mechanisms are currently proposed.113,114 (1) According to the antibody hypothesis, TRALI is caused by an antigen-antibody reaction.115 The antibodies (granulocyte antibodies and/or HLA class I or II antibodies) are present in the donor plasma and react with the recipient’s WBC antigens (or rarely vice versa). The administration of such antibodies can directly injure the lung or can activate neutrophils, monocytes, and complement, creating an inflammatory reaction that may result in pulmonary damage.115 (2) According to the “two-hit” or “neutrophil priming” hypothesis, recipients must first have a predisposing factor that “primes” their neutrophils, such as a septic state; then, the recipient’s neutrophils are activated by donor plasma that contains leukocyte antibody or pro-inflammatory molecules like cytokines and bioactive lipids.116
The diagnosis of TRALI is made on the basis of clinical signs and symptoms, chest X-ray suggestive of pulmonary edema, and time relationship with transfusion (onset per-transfusion or within 6 hours posttransfusion). Causes of pulmonary edema other than TRALI should also be excluded, such as fluid overload or cardiac dysfunction. A panel of experts suggested a consensus definition of TRALI in 2004117; the list of diagnostic criteria advocated by these experts is detailed in Box 82-1. The experts defined TRALI as a new ALI for which no other risk factor than the transfusion can be found. They suggested to use the term “possible TRALI” if another risk factor can be temporally related to the ALI. TRALI is a clinical syndrome, and no laboratory test is pathognomonic of TRALI, but the presence of HLA and/or neutrophil antibodies in the donor plasma is highly suggestive113; however, the absence of such antibodies does not exclude a typical case of TRALI.
The diagnostic criteria advocated by the panel of experts in 2004 exclude the possibility that a TRALI appears in a patient who already presents an ALI or an ARDS, a frequent occurrence in PICU. There is indeed some evidence that a TRALI should also be considered in some patients with ALI/ARDS before a transfusion if their respiratory dysfunction deteriorates significantly during or after a transfusion. Marik at al.118 suggested expanding the definition of TRALI in ICU to ALI/ARDS observed within 72 hours after the transfusion of a blood product: they reported that such “delayed TRALI syndrome” occurred in up to 25% of critically ill adults receiving a blood transfusion. Church et al.119 also reported an association between the transfusion of plasma and/or packed RBC units and ALI/ARDS. The bioactive substances contained in packed RBC and plasma units can cause or add to the severity of cases of ALI/ARDS.119,120 Further investigation is required to better characterize the epidemiology, the mechanisms and the clinical impact of transfusion-related ALI/ARDS in PICU.
All blood products that contain plasma, even in minute quantities, can cause a TRALI. When such reaction occurs, the transfusion must be stopped immediately and supportive treatment administered with oxygen (in 100% of the cases) and mechanical ventilation (in 70% of the cases). The associated hypotension may be unresponsive to fluid administration and may require use of inotropes/vasopressors.121 Diuretics are not useful; they are contraindicated in hypotensive patients.122 All suspected TRALI reactions must be reported to the blood bank, because the donor’s other blood products have to be withdrawn.
The prognosis of cases of TRALI is usually good if the patient survives, but mortality rate of 6% is reported. In survivors, resolution is usually rapid (within 96 hours) and there are no long-term sequelae.122
Cardiovascular System
TACO figures among the most frequent potentially severe adverse events attributable to RBC transfusion.123 Its incidence in PICU is not well characterized. It is most commonly associated with a rapid or massive transfusion that causes pulmonary edema secondary to heart failure.124 Reduced cardiac reserve, chronic and severe anemia (Hb <5 g/dL), and age (infants and elderly patients) are risk factors. The main symptoms are respiratory distress, hypoxemia, tachycardia, and hypertension. When a TACO is suspected, the transfusion must be stopped and supportive treatment with oxygen and diuretics administered. Slow transfusion (≤1 mL/kg/h) in at-risk patients can prevent TACO.
Isolated hypotensive reactions are increasingly recognized,125 but their etiology remains uncertain. They are probably attributable to bradykinin generation, which can happen when a blood product is exposed to negatively charged surfaces (e.g., filters). The risk of hypotensive reaction is increased in patients receiving angiotensin-converting enzyme inhibitors or with diminished bradykinin metabolism.126 Hypotensive reactions are more frequently associated with platelets than with RBC and plasma. The hypotension may happen alone or with some flushing; it occurs rapidly after the transfusion begins. The treatment is straightforward: the transfusion must be stopped and supportive treatment (i.e., fluid bolus) should be undertaken.
Other Acute Transfusion Reactions
Nonhemolytic febrile reaction is the most frequent and benign acute transfusion reaction. In addition to fever, it can be accompanied by chills, discomfort, headache, nausea, or vomiting.127 The symptoms usually occur toward the end or soon after a transfusion. These reactions are mediated by pyrogenic substances that accumulate during storage, or by recipient’s antibodies that bind with leukocytes from the donated blood, which allow activation of the complement system and production of cytokines.80 Acetaminophen can be used to minimize fever, but premedication with acetaminophen, diphenhydramine, or steroids is not useful.128,129 A decrease in the incidence of these reactions is reported with prestorage leukocyte reduction.130,131
Acute hemolytic reactions may be much more serious. They are caused by lysis or accelerated destruction of RBC from immunological incompatibility between donor and recipient blood.132 The mortality rate associated with transfusion errors is less than 10%.133 ABO mismatch is the most frequent and most severe of the blood group incompatibilities, with hemolysis (1 in 60,000) and death (1 in 600,000) as the results.29 In most instances, the patient received a packed RBC unit that was prepared for another patient. The risk that such error happens is obviously higher in an emergency setting and its prevention warrants careful verification by medical staff of all blood products administered. The reaction is characterized by fever, chills, discomfort, diffuse pain, and hemoglobinuria; hypotension, shock, renal failure, and disseminated intravascular coagulation are also observed in some cases. When such reaction is suspected, the transfusion must be stopped immediately and supportive treatment must be administered. To avoid these hemolytic reactions, the donor and recipient’s compatibility must be thoroughly checked (ABO and Rh types, unit identification number) and the recipient must be properly identified (name and medical record number) when the sample is taken for pretransfusion analyses as well as before the transfusion is started.
Allergic transfusion reactions result from the interaction between donor allergens and recipient antibodies (IgE) that provokes a type I hypersensitivity reaction.134 Other possible mechanisms are: preexisting class-specific anti-IgA in patients with IgA deficiency, preexisting antibodies to polymorphic forms of other serum proteins (e.g., IgG, albumin, haptoglobin) that the patient is lacking, transfusion of allergens to which a patient is presensitized, (drugs, chemicals, etc), and passive transfer of IgE antibodies to transfused patients.134 The reaction can be minor (e.g., isolated urticaria) or major (e.g., hypotension, anaphylactic shock, respiratory distress, digestive disorders). A severe reaction usually occurs quickly, whereas a benign reaction may occur up to 4 hours after the transfusion is completed. When an allergic reaction is suspected, the transfusion must be stopped and supportive treatment undertaken with antihistamines, steroids, and epinephrine if required. Prevention must be considered. Premedication of patients with antihistamines is suggested if they have already presented two minor episodes. In patients with major reactions, premedication can be used with steroids and antihistamines. Washed RBC and platelets units can also be used. Patients with anti-IgA antibodies must receive blood products from donors with IgA deficiency or products that have been washed several times. Leukocyte reduction does not offer any benefit.
Bacterial contamination is more frequent with platelets than with RBC units or plasma since platelet concentrates are stored at 20° C to 24° C. Contamination may be due to unsuspected bacteremia in the donor, skin flora when taking a blood sample from the donor or the environment, and product handling.135 The most common germs are: gram-negative bacteria such as Klebsiella pneumoniae, Serratia marcescens, and Pseudomonas species, and gram-positive such as Staphylococcus aureus, Staphylococcus epidermidis, and Bacillus cereus. The reaction is characterized by fever and chills and may lead to septic shock. Symptoms usually appear during or within 4 hours after transfusion. When a bacterial contamination is suspected, the transfusion must be stopped immediately, wide spectrum antibiotics must be given (third-generation cephalosporin or beta-lactam in combination with aminoglycoside) and supportive treatment administered. The blood bank must be informed immediately, as other blood products from the same donor may need to be withdrawn.
Delayed Transfusion Reactions
Among the 321 adverse events reported in children by SHOT in UK from 1996 to 2005,112 there were five cases of severe delayed transfusion reactions, including two cases of TAGVH disease. Transfusion-associated graft versus host disease is rare but very serious.82,83 It may occur when viable lymphocytes from a donor are infused to a recipient who is unable to reject them because of immuno-suppression or partial HLA matching (closed donor genetic profile). Donor leukocytes can then persist in the recipient; because these lymphocytes recognize the recipient’s HLA antigens as foreign, an immune reaction is triggered. Signs and symptoms (generalized skin rash, diarrhea, abnormal liver function, or fever) appear 8 to 10 days after transfusion.136 Associated complications are aplastic anemia with pancytopenia, which may lead to hemorrhage and severe infections. The mortality rate is 90%. The risk of TAGVH is 1 in 700 after cardiopulmonary bypass in some immunocompetent adult populations.137 The risk is also high in premature babies and if a patient with a congenital or acquired immunodeficiency receives a nonirradiated RBC unit or if the blood was a directed donation collected from a relative. Neoplasia (e.g., leukemia, solid tumors), chemotherapy and transplantation (stem cell, bone marrow, solid organ),138 intrauterine transfusions, and exchange-transfusions are other risk factors. No effective treatment is recognized. Prevention can be accomplished by irradiating blood products that will be transfused to at-risk patients or that result from directed donations.
Delayed (extravascular) hemolytic reactions result from the interaction between recipient irregular alloantibodies and donor RBCs. They involve either antibodies that were present before transfusion, but were missed because they were undetectable by cross-matching, or antibodies that appear after the transfusion. Involved antibodies are usually E, Jka, c, Fya, and K.139 These reactions occur 3 days to 2 weeks after the transfusion. Symptoms include anemia and jaundice. The outcome is usually good except in some patients with sickle cell anemia. There is no specific treatment, but using only appropriate RBC units can prevent cases.
Posttransfusion purpura is characterized by dramatic, sudden, and self-limiting thrombocytopenia. The pathogenesis is unclear, but it is presumably related to the development of platelet-specific antibody following transfusion. The platelet count drops below 10,000/μL 5 to 10 days after a transfusion was given to a patient with a history of sensitization by pregnancy or prior transfusion.140 Purpura and diffuse hemorrhages (mucosal, gastrointestinal, urinary, cerebral) may be observed. The thrombocytopenia is refractory to platelet transfusion. The mortality rate is 8%. Treatment includes steroids, plasmapheresis, and immunoglobulins.
Complications Related to Massive Red Blood Cell Transfusion
Massive transfusion is defined by giving more than one circulating volume of blood within 24 hours, or more than 50% of the circulating blood volume in three hours or less, or 10 RBC units in adults.99 A number of complications may occur: (1) coagulopathy and dilutional thrombocytopenia, which may trigger bleeding141; (2) hypothermia due to a rapid infusion of cold blood products, which can lead to arrhythmias, platelet dysfunction, and cardiac dysfunction; (3) citrate toxicity that may trigger hypocalcemia, hypomagnesemia, and metabolic alkalosis142; and (4) hyperkalemia. These complications can be avoided by implementing the following precautions: use a blood heater if the transfusion rate exceeds 50 mL/kg/h; monitor the coagulation profile and transfuse platelets, plasma or cryoprecipitate to maintain a platelet count greater than 50,000/μL, an INR less than 1.5 to 2.0, and fibrinogen level greater than 0.1 g/dL; monitor hypocalcemia, and administer CaCl2 if necessary.138
Transfusion Transmitted Infections
In the United States, blood donation is tested at least for the following infectious agents: hepatitis A, B and C, human immunodeficiency virus (HIV), human T-cell lymphotropic virus (HTLV), West Nile virus (seasonal), and syphilis.46 These tests and better donor selection leads to a significant decrease in the risk of transfusion-transmitted infectious diseases. However, there will always be some residual risk, attributable to the “window period” (time from the beginning of an infection to the time when tests can detect the infection) and to false-negative results. Table 82-3 lists the risks of contracting specific infections with transfusion.
Table 82–3 Transfusion-Transmitted Nonbacterial Infectious Diseases in Canada
| Infection | Risk per Units of Blood Component∗ |
|---|---|
| HIV (AIDS)† | <1:4 million |
| Hepatitis B† | 1:82,000 to 1:275,000 |
| Hepatitis C† | <1:2.8 million |
| HTLV type I/II† | <1:1 million |
| Parvovirus B19† | 1:5000 to 1:20,000 |
| Cytomegalovirus† | Rare |
| Other infections‡ | Low |
HIV, Human immunodeficiency virus; HTLV, human T-cell lymphotropic virus.
∗ Most transfusion-transmitted infections are attributable to RBCs. However, there is historic evidence that the following infectious agents can be transmitted by plasma-derived products: HIV, hepatitis B and C, HTLV, and parvovirus B19—but not cytomegalovirus or parasites.152
‡ Many other agents can be transmitted by a transfusion, such as West Nile virus, insect-borne zoonoses (e.g., malaria,157 babesiosis,158 Bartonella quintana,159 and variant Creutzfeld-Jacob disease160).
Transfusion-Related Immunomodulation
There is strong evidence that transfusions might generate and/or enhance both anti- and proinflammatory reactions. Clinically important immune suppression is described in recipients of RBC units. For example, transfusions of packed RBC units have been reported to improve renal and cardiac allograft survival. This immune suppression might explain the increased rates of nosocomial infections reported in transfused critically ill adults.143,144
On the other hand, many proinflammatory molecules are found in RBC units, and may initiate, maintain, or enhance an inflammatory process.13 The WBC and these substances may trigger or maintain a systemic inflammatory response syndrome in the recipients of RBC units. Administering a RBC transfusion to a critically ill patient with systemic inflammatory response syndrome may stimulate their inflammatory syndrome and constitute a “second hit,” which can cause additional organ dysfunction, contribute to MODS,145 and perhaps ultimately result in higher mortality rates.143 Some clinical data suggest that these risks decrease significantly if the packed RBC unit is leukocyte-reduced before storage.68,146 However, in vitro data suggest that some inflammatory mediators are active even in leukocyte-reduced packed RBC units.147 There are presently no data on this issue in pediatrics. The clinical impact of RBC transfusion on the immunological responses of critically ill children remains to be determined.
Complications Specific to Plasma Transfusion
Overall, the noninfectious and infectious complications associated with FP are similar to those of RBC transfusions, excluding hemolysis. The most notable risks associated with FP transfusion are TRALI and TACO. There are antibodies and other biologically active substances in plasma, but data suggest that SD plasma may be associated with a reduced incidence of TRALI. Frozen plasma is known to have immunomodulative properties,148 which may explain why plasma is associated with an increased risk of MODS, ALI/ARDS,119,149 and nosocomial infections.150 Additionally the large volumes of FP transfused can result in TACO. Allergic reactions are also relatively common with FP transfusions (1% to 3%).
Complications Specific to Platelet Transfusion
The overall risk of clinically symptomatic adverse transfusion reactions attributed to a platelet transfusion was 10.9 per 100 pooled units in Canada.123 Major allergic reactions and bacterial contamination are the most frequent severe complications associated with platelet transfusion.123 Serious noninfectious complications of platelet transfusion are similar to those reported with plasma and RBC with the exception of hemolytic transfusion reactions. Rarely, hemolytic transfusion reactions can be seen after the transfusion of non-ABO identical platelets, which contain anti-A or anti-B antibodies. Platelet transfusion may also be associated with specific adverse effects including platelet refractoriness due to HLA alloimmunization. Prestorage leukocyte-reduced platelet products are available in most North American blood banks, which significantly decrease the risk of HLA alloimmunization and platelet refractoriness, nonhemolytic febrile reactions, and CMV transmission.
Conclusion
A transfusion can save a life, but it can also cause significant problems. The risk of death attributable to the transfusion of a labile blood product is low: only 1 death over 2,845,459 blood component units was reported in the United Kingdom during year 2008.151 However, serious reactions and severe complications can happen. A transfusion is a serious matter: it should be prescribed only if deemed necessary. Closed monitoring of the recipient is mandatory while a transfusion is given.
References are available online at http://www.expertconsult.com.
1. Bateman S.T., Lacroix J., Boven K., et al. Anemia, blood loss and blood transfusion in North American children in the intensive care unit. Am J Respir Crit Care Med. 2008;178:26-33.
2. Napolitano L.M., Kurek S., Luchette F.A., et al. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med. 2009;37:3124-3157.
3. Vamvakas E.C., Blajchman M.A. Deleterious clinical effects of transfusion-associated immunomodulation: Fact or fiction? Blood. 2001;97:1180-1195.
4. Popovsky M.A. Transfusion reactions, ed 2. Bethesda: AABB Press; 2001.
5. Desmet L., Lacroix J. Transfusion in pediatrics. Crit Care Clin. 2004;20:299-311.
6. Laverdière C., Gauvin F., Hébert P.C., et al. Survey of transfusion practices in pediatric intensive care units. Pediatr Crit Care Med. 2002;3(2):335-340.
7. Brealey D., Brand M., Hargreaves I., et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219-223.
8. Kiraly L.N., Underwood S., Differding J.A., Schreiber M.A. Transfusion of aged packed red blood cells results in decreased tissue oxygenation in critically injured trauma patients. J Trauma. 2009;67:29-32.
9. Shanwell A., Kristiansson M., Remberger M., Ringdén O. Generation of cytokines in red cell concentrates during storage is prevented by prestorage white cell reduction. Transfusion. 1997;37:678-684.
10. Wadhwa M., Seghatchian M.J., Dilger P., et al. Cytokines in WBC-reduced apheresis PCs during storage: A comparison of two WBC-reduction methods. Transfusion. 2000;40:1118-1126.
11. Doctor A., Platt R., Sheram M.L., et al. Hemoglobin conformation couples erythrocyte S-nitrosothiol content to O2 gradients. Proc Natl Acad Sci U S A. 2005;102:5709-5714.
12. Bennett-Guerrero E., Veldman T.H., Doctor A., et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104:17063-17068.
13. Tinmouth A., Fergusson D., Chin-Yee I., Hebert P.C. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014-2027.
14. Nishiyama T., Hanaoka K. Hemolysis in stored red blood cell concentrates: Modulation by haptoglobin or ulinastatin, a protease inhibitor. Crit Care Med. 2001;29:1979-1982.
15. Pagnier J., Marden M., Poyart C. Le point sur les transporteurs d’oxygène à base d’hémoglobine. Médecine/sciences. 1996;12:1342-1350.
16. Lucking S.E., Williams T.M., Chaten F.C. Dependence of oxygen consumption on oxygen delivery in children with hyperdynamic septic shock and low oxygen extraction. Crit Care Med. 1990;18:1316-1319.
17. Mink R.B., Pollack M.M. Effect of blood transfusion on oxygen consumption in pediatric septic shock. Crit Care Med. 1990;18:1087-1091.
18. Seear M., Wensley D., MacNab A. Oxygen consumption-oxygen delivery relationship in children. J Pediatr. 1993;123:208-214.
19. Beekman R.H., Tuuri D.T. Acute hemodynamic effects of increasing hemoglobin concentration in children with a right to left ventricular shunt and relative anemia. J Am Coll Cardiol. 1985;5:357-362.
20. Carson J.L., Noveck H., Berlin J.A., Gould S.A. Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. 2002;42:812-818.
21. Carson J.L., Duff A., Poses R.M., et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055-1060.
22. Lackritz E.M., Campbell C.C., Ruebush T.K., et al. Effect of blood transfusion on survival among children in a Kenyan hospital. Lancet. 1992;340:524-528.
23. Lackritz E.M., Hightower A.W., Zucker J.R., et al. Longitudinal evaluation of severely anemic children in Kenya: The effect of transfusion on mortality and hematologic recovery. AIDS. 1997;11:1487-1494.
24. English M., Ahmed M., Ngando C. Blood transfusion for severe anaemia in children in a Kenyan hospital. Lancet. 2002;359:494-495.
25. Holzer B.R., Egger M., Teuscher R. Childhood anemia in Africa: To transfuse or not transfuse? Acta Trop. 1993;55:47-51.
26. Lacroix J., Hébert P.C., Hutchison J.H., et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609-1619.
27. Karam O., Tucci M., Ducruet T., et al. Red blood cell transfusion thresholds in pediatric septic patients. Pediatr Crit Care Med. 2010. epub
28. Rouette J., Trottier H., Ducruet T. Red blood cell transfusion threshold in post-surgical pediatric intensive care patients. a randomized clinical trial. Ann Surg., 2010;251:421-427.
29. Experts Working Group. Guidelines for red blood cell and plasma transfusions for adults and children. Can Med Assoc J. 1997;156(Suppl 11):S1-24.
30. Gibson B.E., Todd A., Roberts I., et al. Transfusion guidelines for neonates and older children. Br J Haematol. 2004;124:433-453.
31. Armano R., Gauvin F., Ducruet T. Determinants of red blood cell transfusions in a pediatric critical care unit: A prospective descriptive epidemiological study. Crit Care Med. 2005;33:2637-2644.
32. Tucci M., Lacroix J., editors. Goal-directed blood transfusion therapies. Des Plaines, IL: Society of Critical Care Medicine, 2006.
33. Waters J.H. Perioperative blood management. A physician’s handbook. Bethesda, MD: AABB; 2006.
34. Orlov D., O’Farrell R., McCluskey S.A., et al. The clinical utility of an index of global oxygenation for guiding red blood cell transfusion in cardiac surgery. Transfusion. 2009;49:682-688.
35. Zygun D.A., Nortje J., Hutchison P.J. The effect of red blood cell transfusion on cerebral oxygenation and metabolism after severe traumatic brain injury. Crit Care Med. 2009;37:1074-1078.
36. Walsh T.S., McArdle F., McLellan S.A., et al. Does the storage time of transfused red blood cells influence regional or global indexes of tissue oxygenation in anemic critically ill patients? Crit Care Med. 2004;32:364-371.
37. Palmieri T.L., Lee T., O’Mara M.S., Greenhalgh D.G. Effects of restrictive blood transfusion policy on outcomes in children with burn injury. J Burn Care Res. 2007;28:65-70.
38. Goodman A.M., Pollack M.M., Patel K.M., Luban N.L.C. Pediatric red blood cell transfusions increase resource use. J Pediatr. 2003;142:123-127.
39. Meliones J.N., Nichols D.G., Wetzel R.C., Greeley W.J. Perioperative management of patients with congenital heart disease: a multidisciplinary approach. In: Nichols D.G., Cameron D.E., Greeley W.J., editors. Critical heart disease in infants and children. St Louis: Mosby; 1995:553-579.
40. Paridon S.M. Consequence of chronic hypoxemia and pulmonary vascular disease. In: Garson A.J.Jr., Bricker J.T., Fisher D.J., Neish S.R., editors. The science and practice of pediatric cardiology. Baltimore, MD: Williams & Wilkins; 1998:2261-2272.
41. Cholette J., Rubenstein J., Powers K. Cyanotic children undergoing open heart surgery do not appear to benefit from higher hemoglobin levels: Results of a restrictive v liberal RBC transfusion strategy. Crit Care Med. 2009;37:A434.
42. Kawaguchi A., Bergsland J., Subramanian S. Total bloodless open heart surgery in the pediatric age group. Circulation. 1984;70:1-30.
43. Willems A., Harrington K., Lacroix J., et al. Comparison of two red-cell transfusion strategies after pediatric cardiac surgery. Crit Care Med. 2010;38:649-656.
44. Society of Thoracic Surgeons Blood Conservation Guideline Task Force, et al. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007;83:S27-S86.
45. Riddington C., Wang W., editors. Blood transfusion for preventing stroke in people with sickle cell disease (Cochrane review). The Cochrane library. Oxford: Update Software, 2002.
46. Roseff S.D., editor. Pediatric transfusion. Bethesda, MD: AABB, 2006.
47. Riddington C., Williamson L., editors. Preoperative blood transfusions for sickle cell disease (Cochrane review). The Cochrane library. Oxford: Update Software, 2002.
48. Goodnough L.T., Shander A., Spence R. Bloodless medicine: clinical care without allogeneic blood transfusion. Transfusion. 2003;43:668-676.
49. Henry D.A., Carless P.A., Moxey A.J., et al, editors. Pre-operative autologous donation for minimising perioperative allogeneic blood transfusion (Cochrane review). The Cochrane library. Oxford: Update Software, 2002.
50. Weldon B.C. Blood conservation in pediatric anesthesia. Anesthesiol Clin North Am. 2005;23:347-361.
51. Henry D.A., Moxey A.J., Carless P.A., et al, editors. Desmopressin for minimising perioperative allogeneic blood transfusion (Cochrane review). The Cochrane library. Oxford: Update Software, 2002.
52. Mathew P., Winter S.S., Frost J.D. Novel applications of recombinant factor VIIa for the management of pediatric coagulopathic diseases. J Pediatr Hematol Oncol. 2003;25:499-502.
53. Wong E.C. Acute normovolemic hemodilution: a critical evaluation of its safety and utility in pediatric patients. Transfus Alternatives Transfus Med. 2004;6:10-21.
54. Carless P.A., Henry D.A., Moxey A.J.Cell salvage for minimising perioperative allogeneic blood transfusion Cochrane Database Syst 2003:Rev 4;CD001888
55. Fowler R.A., Rizoli S.B., Levin P.D., Smith T. Blood conservation for critically ill patients. Crit Care Clin. 2004;20:313-324.
56. Rickard C.M., Couchman B.A., Schmidt S.J. A discard volume of twice the deadspace ensures clinically accurate arterial blood gases and electrolytes and prevents unnecessary blood loss. Crit Care Med. 2003;31:1654-1658.
57. Widness J.A., Madan A., Grindeanu L.A. Reduction in red blood cell transfusions among preterm infants: Results of a randomized trial with an in-line blood gas and chemistry monitor. Pediatrics. 2005;115:1299-1306.
58. Hobisch-Hagen P., Wiedermann F., Mayr A., et al. Blunted erythropoietic response to anemia in multiply traumatized patients. Crit Care Med. 2001;29:743-747.
59. van Iperen C.E., Gaillard C.A.J.M., Kraaijenhaged R.J. Response of erythropoiesis and iron metabolism to recombinant human erythropoietin in intensive care unit patients. Crit Care Med. 2000;28:2773-2778.
60. Corwin H.L., Gettinger A., Pearl R.G., et al. Efficacy of recombinant human erythropoietin in critically ill patients. JAMA. 2002;288:2827-2835.
61. Arif B., Ferhan K. Recombinant human erythropoietin therapy in low-birthweight preterm infants: a prospective controlled study. Pediatr Int. 2005;47:67-71.
62. Maier R.F., Obladen M., Müller-Hansen I., et al. Early treatment with erythropoietin ß ameliorates anemia and reduces transfusion requirements in infants with birth weight below 1000 g. J Pediatr. 2002;141:8-15.
63. Ohls R.K., Ehrenkranz R.A., Wright L.L., et al. Effects of early erythropoietin therapy on the transfusion requirements of preterm infants below 1250 grams birth weight: a multicenter, randomized, controlled trial. Pediatrics. 2001;108:934-942.
64. Bierer R., Roohi M., Peceny C., Ohls R.K. Erythropoietin increases reticulocyte counts and maintains hematocrit in neonates requiring surgery. J Pediatr Surg. 2009;44:1540-1545.
65. Liet J.M., Paranon S., Baraton L. Is a preventive treatment by erythropoietin relevant to reduce red blood cell transfusion in PICU? Pediatr Crit Care Med., 2006;7:541-544.
66. Jacobs B.R., Lyons K., Brilli R.J. Erythropoietin therapy in children with bronchiolitis and anemia. Pediatr Crit Care Med. 2003;4:44-48.
67. Lacroix J., Toledano B. Erythropoietin for critically ill children. Pediatr Crit Care Med. 2003;4:123-124.
68. Lacroix J. Transfusion in the pediatric intensive care unit. Transfus Altern Transfus Med. 2007;9:S12.
69. Hess J.R. Red cell storage. J Prot. 2010;73:368-373.
70. Roseff S.D., Luban N.L., Manno C.S. Guidelines for assessing appropriateness of pediatric transfusion. Transfusion. 2002;42:1398-1413.
71. Baumgartner J.M., Silliman C.C., Moore E.E. Stored red blood cell transfusion induces regulatory T cells. J Am Coll Surg. 2009;208:110-119.
72. Zubair A.C. Clinical impact of blood storage lesions. Am J Hematol. 2009. epub
73. Silva M. Standards for blood banks and transfusion services. Bethesda, MD: AABB; 2004.
74. Laupacis A., Brown J., Costello B., et al. Prevention of posttransfusion CMV in the era of universal WBC reduction: a consensus statement. Transfusion. 2001;41(4):560-569.
75. Innerhofer P., Klingler A., Klimmer C. Risk for postoperative infection after transfusion of white blood cell-filtered allogeneic or autologous blood components in orthopedic patients undergoing primary arthroplasty. Transfusion. 2005;45:103-110.
76. Goodnough L.T., Shander A., Brecher M.E. Transfusion medicine: looking to the future. Lancet. 2003;361:161-169.
77. Silverman T.A., Weiskopf R.B. Hemoglobin-based oxygen carriers: Current status and future directions. Transfusion. 2009;49:2495-2515.
78. Elizalde J.I., Clemente J., Marin J.L., et al. Early changes in hemoglobin and hematocrit levels after packed red cell transfusion in patients with acute anemia. Transfusion. 1997;37:573-576.
79. Zimrin A.B., Hess J.R. Current issues relating to the transfusion of stored blood cells. Vox Sanguinis. 2009;96:93-103.
80. Heddle N.M. Pathophysiology of febrile nonhemolytic transfusion reactions. Curr Opin Hematol. 1999;6(6):420-426.
81. Smith K.J., Sierra E.R., Nelson E.J. Histamine, IL-1ß and IL-8 increase in packed RBCs stored for 42 days but not in RBCs leukodepleted pre-storage. Transfusion. 1993;33:53S.
82. Parshuram C., Doyle J., Lau W., Shemie S.D. Transfusion-associated graft versus host disease. Pediatr Crit Care Med. 2002;3:57-62.
83. Schroeder M.L. Transfusion-associated graft-versus-host disease. Br J Haematol. 2002;117(2):275-287.
84. Lelubre C., Piagnerelli M., Vincent J.L. Association between duration of storage of transfused red blood cells and morbidity and mortality in adult patients: myth or reality? Transfusion. 2009;49:1384-1394.
85. Karam O., Tucci M., Bateman S.T., et al. Association between length of storage of red blood cell units and outcome of critically ill children. Crit Care. 2010;14:R57.
86. Gauvin F., Spinella P.S., Lacroix J., et al. Association between length of storage of transfused red blood cells and multiple organ dysfunction syndrome in pediatric intensive care. Transfusion. 2010;50:1902-1913.
87. Mou S.S., Giroir B.P., Molitor-Kirsch E.A., et al. Fresh whole blood versus reconstituted blood for pump priming in heart surgery in infants. N Engl J Med. 2004;351:1635-1644.
88. Wong E.C., Schreiber S., Criss V.R., et al. Feasibility of red blood cell transfusion through small bore central venous catheters used in neonates. Pediatr Crit Care Med. 2004;5:69-74.
89. Stanworth SJ, Tinmouth A: Plasma Transfusion and the use of albumin. In Simon TL, Snyder EK, Solheim BG, et al, editors: Rossi’s principles of transfusion medicine, Philadelphia, 2009, Lippincott Williams & Wilkins.
90. Stanworth S.J. The evidence-based use of FFP and cryoprecipitate for abnormalities of coagulation tests and clinical coagulopathy. Hematology Am Soc Hematol Educ Program. 2007:179-186.
91. Liumbruno G., Bennardello F., Lattanzio A. Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) Work Group. Recommendations for the transfusion of plasma and platelets. Blood Transfus. 2009;7:132-150.
92. Abdel-Wahab O.I., Healy B., Dzik W.H. Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion. 2006;46:1279-1285.
93. Holland L.L., Brooks J.P. Toward rational fresh frozen plasma transfusion: The effect of plasma transfusion on coagulation test results. Am J Clin Pathol. 2006;126:133-139.
94. Spinella P.C., Perkins J.G., Grathwohl K.W., et al. Fresh whole blood transfusions in coalition military, foreign national, and enemy combatant patients during Operation Iraqi Freedom at a U.S. combat support hospital. World J Surg. 2008;32:2-6.
95. Gajic O., Dzik W.H., Toy P. Fresh frozen plasma and platelet transfusion for nonbleeding patients in the intensive care unit: benefit or harm? Crit Care Med. 2006;34(Suppl 5):S170-S173.
96. Krishnan J., Morrison W., Simone S., Ackerman A. Implications of thrombocytopenia and platelet course on pediatric intensive care unit outcomes. Pediatr Crit Care Med. 2008;9:502-505.
97. Drews R.E. Critical issues in hematology: anemia, thrombocytopenia, coagulopathy, and blood product transfusions in critically ill patients. Clin Chest Med. 2003;24:607-622.
98. Verma A.K., Levine M., Shalansky S.J. Frequency of heparin-induced thrombocytopenia in critical care patients. Pharmacotherapy. 2003;23:745-753.
99. Hardy J.F., De Moerloose P., Samama M. Massive transfusion and coagulopathy: pathophysiology and implications for clinical management. Can J Anaesth. 2004;51:293-310.
100. Gauvin F., Toledano B., Champagne J., Lacroix J. Reactive hemophagocytic syndrome presenting as a component of multiple organ system failure. Crit Care Med. 2000;28:3341-3345.
101. London M.J., Franks M., Verrier E.D. The safety and efficacy of ten percent pentastarch as a cardiopulmonary bypass priming solution. A randomized clinical trial. J Thorac Cardiovasc Surg. 1992;104:284-296.
102. Slitchter S.J. Relationship between platelet count and bleeding risk in thrombocytopenic patients. Transf Med Rev. 2004;18:153-167.
103. Stroncek D.F., Rebulla P. Transfusion medicine 2. Platelet transfusions. Lancet. 2007;370:427-438.
104. Strauss R., Wehler M., Mehler K. Thrombocytopenia in patients in the medical intensive care unit: bleeding prevalence, transfusion requirements, and outcome. Crit Care Med. 2002;30:1765-1771.
105. Murray D.J., Olson J., Strauss R., Tinker J. Coagulation changes during packed red cell replacement of major blood loss. Anesthesiology. 1988;69:839-845.
106. Thompson C.B., Jakubowski J.A., Quinn P.G. Platelet size and age determine platelet function independently. Blood. 1984;63:1372-1375.
107. Gerday E., Baer V.L., Lambert D.K., et al. Testing platelet mass versus platelet count to guide platelet transfusions in the neonatal intensive care unit. Transfusion. 2009;49:2034-2039.
108. Josephson C.D., Su L.L., Christensen R.D., et al. Platelet transfusion practices among neonatalogists in the United States and Canada: results of a survey. Pediatrics. 2009;123:278-285.
109. Blajchman M.A., Slichter S.J., Heddle N.M., Murphy M.F. New strategies for the optimal use of platelet transfusions. Hematology Am Soc Hematol Educ Program. 2008:198-204.
110. Warkentin T.E., Kelton J.G. Temporal aspects of heparin-induced thrombocytopenia. N Engl J Med. 2001;344:1286-1292.
111. Gauvin F., Lacroix J., Robillard P. Acute transfusion reaction in pediatric intensive care unit. Transfusion. 2006;46:1899-1908.
112. Stainsby D., Jones H., Wells A.W. Adverse outcomes of blood donation in children: Analysis of UK reports to the serious hazards of transfusion scheme 1996-2005. Br J Haematol. 2008;141:73-79.
113. Curtis B.R., McFarland J.G. Mechanisms of transfusion-related acute lung injury (TRALI): Anti-leukocyte antibodies. Crit Care Med. 2006;34(Suppl 5):S118-S123.
114. Silliman C.C. The two-event model of transfusion-related acute lung injury. Crit Care Med. 2006;34(5 Suppl):S124-S131.
115. Kopko P., Paglieroni T.G., Popovsky M.A. TRALI: correlation of antigen-antibody and monocyte activation in donor-recipient pairs. Transfusion. 2003;43:177-184.
116. Silliman C.C., Boshkov L.K., Mehdizadehkashi Z., et al. Transfusion-related acute lung injury: Epidemiology and a prospective analysis of etiologic factors. Blood. 2003 Jan 15;101(2):454-462.
117. Kleinman S., Caulfield T., Chan P., et al. Toward an understanding of transfusion-related acute lung injury: Statement of a consensus panel. Transfusion. 2004;44:1774-1789.
118. Marik P.E., Corwin H.L. Acute lung injury following blood transfusion: Expanding the definition. Crit Care Med. 2008;36:3080-3084.
119. Church G.D., Matthay M.A., Liu K. Blood product transfusions and clinical outcomes in pediatric patients with acute lung injury. Pediatr Crit Care Med. 2009;10:297-302.
120. Gajic O., Gropper M.A., Hubmayr R.D. Pulmonary edema after transfusion: How to differentiate transfusion-associated circulatory overload from transfusion-related acute lung injury. Crit Care Med. 2006;34(Suppl 5):S109-S113.
121. Webert K.E., Blajchman M.A. Transfusion-related acute lung injury. Transfus Med Rev. 2003;17:252-262.
122. Moore S.B. Transfusion-related acute lung injury (TRALI): clinical presentation, treatment, and prognosis. Crit Care Med. 2006;34(Suppl 5):S114-S117.
123. Kleinman S., Chan P., Robillard P. Risks associated with transfusion of cellular blood components in Canada. Transfus Med Rev. 2003;17(2):120-162.
124. Popovsky M.A. Circulatory overload. In: Popovsky M.A., editor. Transfusion reactions. ed 2. Bethesda, MD: AABB Press; 2001:255-260.
125. Gauvin F., Toledano B., Hume H.A., Lacroix J. Hypotensive reactions associated with platelet transfusion through leucocyte reduction filters. J Intensive Care Med. 2000;14:329-332.
126. Hume H.A., Adam A. Hypotensive transfusion reactions. In: MA P., editor. Transfusion reactions. ed 2. Bethesda, MD: AABB Press; 2001:213-234.
127. Heddle N., Kelton J.C. Febrile nonhemolytic transfusion reactions. In: Popovsky M.A., editor. Transfusion reactions. ed 2. Bethesda, MD: AABB Press; 2001:45-82.
128. Sanders R.P., Maddirala S.D., Geiger T.L., et al. Premedication with acetaminophen or diphenhydramine for transfusion with leucoreduced blood products in children. Br J Haematol. 2005;130:781-787.
129. Wang S.E., Lara P.N., Lee-Ow A., et al. Acetaminophen and diphenhydramine as premedication for platelet transfusions: a prospective randomized double-blind placebo-controlled trial. Am J Hematol. 2002;70:191-194.
130. Fergusson D., Hebert P.C., Lee S.K., et al. Clinical outcomes following institution of universal leukoreduction of blood transfusions for premature infants. JAMA. 2003;289:1950-1956.
131. Dzik W.H., Anderson J.K., O’Neill E.M. A prospective, randomized clinical trial of universal WBC reduction. Transfusion. 2002;42(9):1114-1122.
132. Davenport R.D. Hemolytic transfusion reactions. In: MA P., editor. Transfusion reactions. ed 2. Bethesda, MD: AABB Press; 2001:1-44.
133. Linden J.V., Wagner K., Voytovich A.E., Sheehan J. Transfusion errors in New York State: An analysis of 10 years’ experience. Transfusion. 2000;40:1207-1213.
134. Vamvakas E., Pineda A.A. Allergic and anaphylactic reactions. In: Popovsky M.A., editor. Transfusion reactions. ed 2. Bethesda, MD: AABB Press; 2001:83-128.
135. Jacobs M.R., Palavecino E., Yomtovian R. Don’t bug me: the problem of bacterial contamination of blood components–challenges and solutions. Transfusion. 2001;41:1331-1334.
136. Webb I.J., Anderson K.C. Transfusion-associated graft-vs-host disease. In: Popovsky M.A., editor. Transfusion reactions. ed 2. Bethesda, MD: AABB Press; 2001:171-186.
137. Juji T., Takahashi K., Shibata Y., et al. Post-transfusion graft-versus-host disease in immunocompetent patients after cardiac surgery in Japan. N Engl J Med. 1989;321:56.
138. Callum J.L., Pinkerton P.H. Bloody easy: Blood transfusions, Blood alternatives and transfusion reactions, A guide to transfusion medicine. Toronto: Savattuq Inc; 2003.
139. Pineda A.A., Vamvakas E.C., Gorden L.D. Trends in the incidence of delayed hemolytic and delayed serologic transfusion reactions. Transfusion. 1999;39:1097-1103.
140. McFarland J.G. Posttransfusion purpura. In: Popovsky M.A., editor. Transfusion reactions. ed 2. Bethesda, MD: AABB Press; 2001:187-212.
141. Harvey M.P., Greenfield T.P., Sugrue M.E., Rosenfeld D. Massive blood transfusion in a tertiary referral hospital. Clinical outcomes and haemostatic complications. Med J Aust. 1995;163(7):356-359.
142. Uhl L., Kruskall M. Complications of massive transfusion. In: Popovsky M.A., editor. Transfusion reactions. ed 2. Bethesda, MD: AABB Press; 2001:339-357.
143. Hébert P.C., Wells G., Blajchman M.A., et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409-417.
144. Taylor R.W., Manganaro L., O’Brien J. Impact of allogenic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit Care Med. 2002;30:2249-2254.
145. Despond O., Proulx F., Carcillo J.A., Lacroix J. Pediatric sepsis and multiple organ dysfunction syndrome. Curr Opin Pediatr. 2001;13:247-253.
146. van de Watering L.M.G., Hermans J., Houbiers J.G.A., et al. Beneficial effects of leukocyte depletion of transfused blood on postoperative complications in patients undergoing cardiac surgery. Circulation. 1998;97:562-568.
147. Karam O., Tucci M., Toledano B.J., et al. Length of storage and in vitro immunomodulation induced by prestorage leukoreduced red cell concentrates. Transfusion. 2009;49:2326-2335.
148. Schneider S.O., Rensing H., Gräber S., et al. Impact of platelets and fresh frozen plasma in contrast to red cell concentrate on unstimulated and stimulated cytokine release in an in vitro model of transfusion. Scand J Immunol. 2009;70:101-105.
149. Watson G.A., Sperry J.L., Rosengart M.R., et al. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009;67:221-227.
150. Sarani B., Dunkman W.J., Dean L. Transfusion of fresh frozen plasma in critically ill surgical patients is associated with an increased risk of infection. Crit Care Med. 2008;36:1114-1118.
151. Taylor C., Cohen H., Mold D., et al The 2008 Annual SHOT Report 2009
152. MacDonald N., Scott J.W., McCombie N. Transfusion risk of infection in Canada: Update 2006. Can J Infect Dis Med Microbiol. 2006;10:149-153. 2006
153. Kamram M., Rachoin J.S., Schort C.A. Risk of cardiac arrhythmias and conduction abnormalities in patients with severe sepsis and septic shock receiving packed red blood cell transfusions. Crit Care Med. 2008;36:A155.
154. Elder A.F. Transfusion reactions. In: Hillyer C.D., Strauss R.G., Luban N.L.C., editors. Handbook of pediatric transfusion medicine. San Diego: Elsevier Academic Press, 2004.
155. Parshuram C.S., Cox P.N. Neonatal hyperkalemic-hypocalcemic cardiac arrest associated with initiation of blood-primed continuous venovenous hemofiltration. Pediat Crit Care Med. 2002;3:67-69.
156. Tang C., Ristagno G., Sun S., Weil M.H. Increased oxygen releasing capacity together with progressive acidosis in human red blood cells during storage. Crit Care Med. 2008;36:A25.
157. Singer R., Giulivi A., Bodie-Collins M., et al. Transfusion-related malaria in Canada. Can Med Assoc J. 2001;164:377-379.
158. Kain K.C., Bu Jassoum S., Fong W., Hannach B. Transfusion-transmitted babesiosis in Ontario: First reported case in Canada. Can Med Assoc J. 2001;164:1721-1723.
159. Rolain J.M., Foucault C., Guieu R. Bartonella quintana in human erythrocytes. Lancet. 2002;360:226-228.
160. Wroe S.J., Pal S., Siddique D., et al. Clinical presentation and pre-mortem diagnosis of variant Creutzfeldt-Jakob disease associated with blood transfusion: a case report. Lancet. 2006;368:2061-2067.