CHAPTER 91 TRANSFUSION: MANAGEMENT OF BLOOD AND BLOOD PRODUCTS IN TRAUMA
Approximately 15% of all blood transfusions in the United States are used in the care of patients that have sustained traumatic injury. Blood transfusion in trauma is life-saving for those patients in hemorrhagic shock who are unresponsive to crystalloid fluid resuscitation. Importantly, concomitant attempts at prompt cessation of hemorrhage are also necessary. Blood transfusion in trauma has also been identified as an independent predictor of multiple-organ failure (MOF), systemic inflammatory response syndrome (SIRS), increased postinjury infection, and increased mortality in multiple studies. The cumulative risks of blood transfusion have been related to the number of units of packed red blood cells (PRBCs) transfused, increased storage time of transfused blood, and possibly donor leukocytes. Lack of efficacy of red blood cell transfusion in critically ill patients has also been documented.1 Therefore, once hemorrhage control has been established in acute trauma we should attempt to minimize the use of blood transfusion for the treatment of asymptomatic anemia in trauma patients.
A number of potential mechanisms that may mediate adverse affects associated with blood transfusion in trauma have been proposed, including increased systemic inflammatory response, immunomodulation, microcirculatory dysfunction due to altered RBC deformability, increased nitric oxide binding by free hemoglobin and vasoconstriction, and others. These data have led some to conclude that blood transfusion in the injured patient should be minimized whenever possible.2
INCIDENCE: WHO NEEDS BLOOD TRANSFUSION IN TRAUMA?
Trauma patients in hemorrhagic shock have an absolute indication for PRBC transfusion if they are unresponsive to isotonic crystalloid fluid resuscitation, have ongoing significant hemorrhage, and manifest physiologic signs of persistent shock (hypotension, tachycardia, oliguria, lactic acidosis, abnormal base deficit)—indicating that oxygen consumption is dependent on hemoglobin concentration (critical oxygen delivery).3 In these patients, the prompt transfusion of PRBCs in conjunction with prompt hemorrhage control can be life-saving.
Patients with hemorrhagic shock, identified by a metabolic acidosis and increasing base deficit, have been documented to require increased blood and plasma transfusion (Table 1). A single-institution study in calendar year 2000 documented that 8% (479 of 5645) of acute trauma patients received PRBCs, using 5219 units. The majority (62%) of transfusions were administered in the first 24 hours of care. Only 3% of patients (n = 147) received more than 10 units of PRBCs, and these patients also received plasma and platelet transfusions to treat actual or anticipated dilutional coagulopathy. Mortality rates in trauma patients who require blood transfusion is high, ranging from 27% to 39%.4
A recent study identified independent risk factors for blood transfusion in trauma (n = 1103), including increased age, admission from scene, trauma mechanisms of motor vehicle crash or fall from height, admission systolic blood pressure <120 mm Hg, free fluid on abdominal ultrasound, and unstable pelvis on clinical examination (Table 2). The probability of RBC transfusion in the emergency department was significantly increased if these risk factors were present, and increased substantially with multiple risk factors. An Emergency Room Transfusion Score (ETS) was calculated from these rapidly assessable parameters, ranging from 1 point to 9.5 points maximum. The probability of blood transfusion exponentially increased with the sum of points in the ETS (i.e., from 0.7% at 1 point to 5% at 3 points and 97% at the maximum of 9.5 points).5
Table 2 Risk Factors Associated with Blood Transfusion in Emergency Room After Severe Trauma
| Variable | Score | Odds Ratio (95% CI) |
|---|---|---|
| Age (Years) | ||
| 0–20 | 0 | 2.0 (0.9–4.3) |
| 20–60 | 0.5 | 5.6 (2.3–13.4) |
| >60 | 1.5 | |
| Admission | ||
| From scene | 1.0 | 2.4 (1.3–4.5) |
| From other hospital | 0 | |
| Trauma Mechanism | ||
| Traffic | 1 | 3.2 (1.7–6.0) |
| Fall from height >3 m | 1 | 2.4 (1.1–5.2) |
| Blood Pressure (mm Hg) | ||
| 0–90 | 2.5 | 12.2 (6.4–23.4) |
| 90–120 | 1.5 | 4.1 (2.3–13.4) |
| >120 | 0 | |
| Abdominal Ultrasound | ||
| Free fluid | 2 | 8.4 (4.3–16.2) |
| No fluid | 0 | |
| Pelvis on Clinical Examination | ||
| Unstable | 1.5 | |
| Stable | 0 | 4.7 (2.1–10.3) |
| Total risk factor points | 9.5 | |
Adapted from Como JJ, Dutton RP, Scalea TM, Edelman BB, Hess JR: Blood transfusion rates in the care of acute trauma. Transfusion 44(6):809–813, 2004.
For severe hemorrhagic shock, type O blood should be transfused.6 Rh-negative blood should be used in women of childbearing age if possible. A prompt transition to the use of type-specific (ABO, Rh-matched) blood should be accomplished as quickly as possible, and use is continued until fully cross-matched units of blood are available. Once a trauma patient has been administered more than one blood volume and the initial antibody screen is negative, there is no point attempting compatibility testing except for ABO matching.
Once hemorrhage control has been established and the patient has completed resuscitation from hemorrhagic shock, all efforts to restrict RBC transfusion in trauma are advisable. Anemia is common in critically injured trauma patients and persists throughout the duration of critical illness, as documented in a post hoc analysis of a subset of trauma patients (n = 576) from a prospective multicenter observational cohort study in the United States.7 While in the intensive care unit (ICU), 319 (55.4%) trauma patients received a total of 1858 units of blood (or 5.8 ± 5.5 units of blood each) on average. The majority (87.1%, n = 278) of all ICU trauma patients requiring transfusions received them within the first 4 days, accounting for almost half of all ICU transfusions. However, 5%–10% of patients continued to receive blood transfusions. Importantly, this study documented that a large number of blood transfusions were administered when the hemoglobin concentration was greater than 10 g/dl.
National guidelines regarding blood transfusion differ, such that the American Society of Anesthesiology8 recommends maintaining hemoglobin (Hb) greater than 6 gm/dl—whereas the National Institutes of Health9 (NIH) recommends maintaining Hb greater than 7 g/dl for patients who are critically ill. A Cochrane Database Systematic Review titled Transfusion Thresholds and Other Strategies for Guiding Allogeneic Red Blood Cell Transfusion concluded that the limited published evidence (10 trials, n = 1780 patients) supports the use of restrictive transfusion triggers (blood transfusion only if Hb <7 g/dl) in patients who are free of cardiac disease.10
An analysis from the prospective multicenter randomized controlled trial (Transfusion Requirements in Critical Care, TRICC) compared the use of restrictive (transfuse if Hb <7 g/dl) and liberal (transfuse if Hb <10 g/dl) transfusion strategies in resuscitated critically ill trauma patients (n = 203). The average hemoglobin concentrations (8.3 ± 0.62 g/dl vs. 10.4 ± 1.2 g/dl; p < 0.0001) and the RBC units transfused per patient (2.3 ± 4.4 vs. 5.4 ± 4.3; p < 0.0001) were significantly lower in the restrictive group than in the liberal group. No differences in mortality, multiple-organ dysfunction, or ICU or hospital length of stay were identified—suggesting that a restrictive RBC transfusion strategy appears to be safe for critically ill multiple trauma patients.11
RISKS OF BLOOD TRANSFUSION
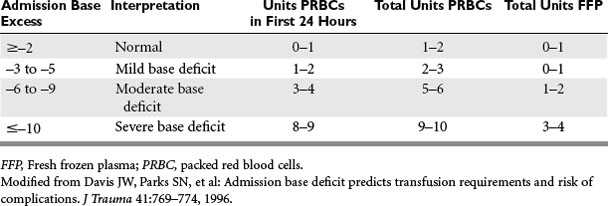
The blood supply in the United States has never been as safe as it is now. During the past several decades, there have been dramatic progressive reductions in the risk of transfusion-transmitted clinically significant blood-borne infections.12 Figure 1 summarizes the significant decline in human immunodeficiency virus (HIV), hepatitis B (HBV), and hepatitis C (HCV) risks of transmission through blood transfusion.
Although there has been a 10,000-fold reduction in the risk to patients from transfusion-transmitted infectious diseases in recent decades, there has been little progress in reducing the risk of noninfectious hazards of transfusion. A recent analysis of 366 spontaneously reported deaths and major complications of transfusion in the United Kingdom and Ireland identified that the majority (53%) of these adverse events were related to the incorrect blood component being transfused (i.e., a clerical or human-related error).13 As a result, patients today are harmed from noninfectious serious hazards of transfusion at a rate that exceeds infectious hazards by 100-fold to 1000-fold (Table 3).
| Type of Risk | Incidence |
|---|---|
| Noninfectious Risks | |
| Depressed erythropoiesis | Universal |
| Volume overload, pulmonary edema | 10%–40% |
| Febrile reaction | 1/10–1/100 |
| Urticarial reaction | 1/33–1/100 |
| Transfusion-related acute lung injury (TRALI) | 1/1120–1/5000 |
| Delayed hemolytic transfusion reaction | 1/2500 |
| Hemolytic transfusion reactions | 1/38,000–1/70,000 |
| Fatal hemolytic transfusion reactions | 1/600,000 |
| Anaphylactic shock | 1/500,000 |
| Immunosuppression | Unknown |
| Graft-versus-host disease | 1/400–1/10,000 |
| Alloimmunization (RBCs) | 1/100 |
| Alloimmunization (platelets) | 1/10 |
| ABO-Rh mismatch | |
| Occurrence | 1/6,000–1/20,000 |
| Mortality | 1/100,000–1/600,000 |
| Infectious Risks | |
| Cytomegalovirus conversion | 7% |
| Epstein-Barr virus | 0.5% |
| Bacterial contamination (PRBCs + platelets) | 1/2000 |
| Hepatitis B transmission | 1/220,000 |
| PRBC-related bacterial sepsis | 1/500,000–1/786,000 |
| Hepatitis A transmission | 1/1,000,000 |
| West Nile Virus transmission | 1/1,400,000 |
| Hepatitis C transmission | 1/1,600,000 |
| HIV transmission | 1/1,800,000 |
Adapted from Klein HG: Allogenic transfusion risks in the surgical patient. Am J Surg 170;6A:21S–26S, 1995; Silliman CC, Moore EE, Johnson JL, et al: Transfusion in the injured patient: proceed with caution. Shock 21(4):291–299, 2004; Busch MP, Kleinman SH, Nemo GJ: Current and emerging infectious risks of blood transfusions. JAMA 289(8):959–962, 2003; and Spiess BD: Risk of transfusion: outcome focus. Transfusion 44:4S–14S, 2004.
Emerging risks of blood transfusion include West Nile virus (WNV) transmission via blood transfusion. Between August of 2002 and January of 2003, there were 23 confirmed cases of transfusionassociated WNV from 14 donors. Experimental WNV nucleic acid amplification test assays were implemented for blood donor testing in July of 2003. Reports through September 2003 indicated that more than 600 infected units of blood were identified from 2.5 million donations during a period of 4137 known WNV infections reported to the Centers for Disease Control and Prevention (CDC).14 Although nucleic acid amplification testing of blood donations prevented hundreds of cases of WNV infection, it failed to detect units with a low level of viremia—some of which were antibody negative and infectious. These data support the use of targeted nucleic acid amplification testing of individual donations in high-prevalence WNV regions, a strategy that was implemented successfully in 2004.15
Transfusion-Related Acute Lung Injury
Transfusion-related acute lung injury (TRALI) is a life-threatening complication of blood transfusion. TRALI is now the leading cause of transfusion-related mortality, even though it is probably still underdiagnosed and underreported. The National Heart, Lung and Blood Institute of the NIH convened a working group on TRALI, and a common definition has been established—defined as new acute lung injury occurring during or within 6 hours after a blood transfusion.16 TRALI, like the acute respiratory distress syndrome (ARDS), may be a two-event phenomenon—with both recipient predisposition and factors in the stored blood units playing major roles.17 The overall prevalence has been reported as 1 in 1120 cellular components transfused.
A recent prospective cohort study documented a significant independent association between the amount of transfused blood and the development of ARDS and hospital mortality. The association between the amount of transfused blood and the development of ARDS remained significant in a multivariable logistic regression model accounting for differences in severity of illness, type of trauma, race/ethnicity, gender, and base deficit.18 A larger study in 5260 blunt trauma patients documented that delayed blood transfusion (defined as no blood transfusion received within the initial 48 hours after admission) was independently associated with ventilator-associated pneumonia, ARDS, and death in trauma regardless of injury severity.19 Similarly, an additional study that examined clinical predictors of ARDS identified that PRBC transfusion was an independent risk factor for increased development of and increased mortality in ARDS.20 All of these studies mandate a judicious transfusion policy after trauma resuscitation, and emphasize the need for safe and effective blood substitutes and transfusion alternatives.
MASSIVE TRANSFUSION
Massive blood transfusion is most commonly defined as complete replacement of a patient’s blood volume within a 24-hour period or more than 10 units of PRBCs in 24 hours. Newer definitions include an ongoing blood loss of more than 150 ml/min, or the replacement of 50% of the circulating blood volume in 3 hours or less.21 These newer definitions have the benefit of allowing early recognition of major blood loss and of the need for effective intervention to prevent hemorrhagic shock and other complications of massive hemorrhage and transfusion.
Massive transfusion therapy for the treatment of hemorrhagic shock requires a coordinated and detailed approach with the fundamental components listed in Table 4. In the absence of a predefined massive transfusion protocol, access to the appropriate blood products (and adequate volume of these products) may be significantly delayed. Without prompt replacement of these blood products, the resultant coagulopathy may worsen and bleeding will continue. In fact, the implementation of an organized “Massive Transfusion Policy” to address exsanguinating hemorrhage in the trauma population has proven of benefit in patient outcomes and in reducing blood product utilization. Studies have demonstrated an increase in survival (16%–45%) in patients with exsanguinating hemorrhage following the implementation of such a protocol.22,23 Guidelines for the treatment of acute massive blood loss are listed in Table 5.
Table 4 Management of Massive Transfusion for Hemorrhagic Shock
Table 5 Acute Massive Blood Loss: Template for Guidelines
| Goal | Procedure | Comments |
|---|---|---|
| Treat underlying cause if possible. |
Adapted from Stainsby D, Maclennan S, Hamilton PJ: Management of massive blood loss: a template guideline. Br J Anaesth 85:487–491, 2000; and McClelland DBL: Clinical use of blood products. In McClelland DBL, editor: The Handbook of Transfusion Medicine, 3rd ed. London, The Stationery Office, 2001, p. 79.
Blood Component Therapy: Fresh Frozen Plasma, Platelets, and Cryoprecipitate
Coagulopathy and thrombocytopenia are a common occurrence during major trauma resuscitation, and hemorrhage remains a major cause of traumatic deaths. Current coagulation factor replacement practices vary, and may be inadequate. A recent pharmacokinetic model was used to simulate the dilutional component of coagulopathy during hemorrhage and compared various fresh frozen plasma (FFP) transfusion strategies for the prevention or correction, or both, of dilutional coagulopathy. This study documented that once excessive deficiency of factors has developed and bleeding is unabated 1–1.5 units of FFP must be given for every unit of PRBC transfused. If FFP transfusion should start before plasma factor concentration drops below 50% of normal, an FFP:PRBC transfusion ratio of 1:1 would prevent further dilution. They concluded that during resuscitation of a patient who has undergone major trauma the equivalent of whole-blood transfusion is required to correct or prevent dilutional coagulopathy.24
Ultimately, additional blood product administration for the treatment of dilutional and consumptive coagulopathy and thrombocytopenia in trauma must be guided by blood coagulation testing, with regular monitoring of hemoglobin, platelet count, prothrombin time (PT), partial thromboplastin time (PTT), and fibrinogen levels (see Table 5). Component replacement therapy recommendations differ in many institutions. Some advocate 1:1:1 unit replacements of PRBCs, FFP, and platelets. Others recommend platelet concentrations (1 pack/10 kg) if platelet count falls below 50,000, recognizing that each platelet concentrate also provides about 50 ml of fresh plasma. FFP (12 ml/kg) is administered if the INR, PT, or PTT are greater than 1.5 times control levels. Cryoprecipitate (1–1.5 packs/10 kg) is administered if fibrinogen concentrations are lower than 0.8 g/l. Some suggested massive transfusion protocols include:
MANAGEMENT OF COMPLICATIONS RELATED TO BLOOD TRANSFUSION
Blood Transfusion and Postinjury Multiple-Organ Failure
Blood transfusion was first identified as an independent risk factor for MOF in a 3-year single-institution prospective cohort study (n = 394) aimed at finding a predictive model for postinjury MOF.25 Trauma patients (n = 394) with Injury Severity Score (ISS) of greater than 15 and survival over 24 hours were examined. The following variables were identified as early independent predictors of MOF: age greater than 55 years, ISS 25 or higher, and more than six units of RBCs in the first 12 hours after admission. In addition, base deficit greater than 8 mEq/l (0–12 hours) and lactate greater than 2.5 mmol/l (12–24 hours) were independent predictors of MOF in the subgroup of patients that had these measurements obtained. Whether blood transfusion was simply a surrogate measure of severity of hemorrhagic shock in this study was not fully explored.
A subsequent prospective study by this group confirmed that blood transfusion was an independent risk factor of postinjury MOF, controlling for other indices of shock—including base deficit and lactate.26 This study had a similar experimental design, with 513 trauma patients with ISS greater than 15 admitted to the ICU who survived >48 hours. A dose–response relationship between early blood transfusion and postinjury MOF was identified and blood transfusion was confirmed as an independent risk factor in 13 of the 15 multiple logistic regression models tested. The odds ratios were high, especially in the early MOF models.
Most recently, this group identified that the incidence, severity, and attendant mortality of postinjury MOF has decreased over the last 12 years despite an increased MOF risk. This is related to improvements in trauma and critical care and to the decreased use of blood transfusion during resuscitation.27
Blood Transfusion and SIRS/Mortality
Blood transfusion (in the first 24 hours) in trauma was associated with an increased incidence of systemic inflammatory response syndrome (SIRS, defined as SIRS score ≥2) in 7602 trauma patients studied at a single institution.28 Blood transfusion within the first 24 hours was administered to 954 patients, comprising 10% of the study cohort. Blood transfusion and increased total volume of blood transfusion was a significant independent predictor of SIRS, ICU admission, and mortality in trauma patients by multinomial logistic regression analysis after stratification for ISS, Glasgow Coma Scale (GCS), and age. Trauma patients who received blood transfusion had a two- to nearly six-fold increase in SIRS (p < 0.0001) and more than a four-fold increase in ICU admission (odds ratio [OR] 4.62) and mortality (OR 4.23, p < 0.0001), as well as significantly longer ICU and hospital length of stay compared to nontransfused trauma patients.
A recent prospective study confirmed that trauma patients are heavily transfused with allogeneic blood throughout the course of their hospital stay and transfusions are administered at relatively high pretransfusion hemoglobin levels (mean of 9 g/dl). One hundred and four patients (87%) received a total of 324 transfusions, 20 (6%) of which were given in the emergency room, 186 (57%) in the SICU, 22 (7%) post-SICU, and 96 (30%) in the operating room. The mean volume of blood per patient transfused was 3144 (± 2622 ml). Transfusion of more than four units of blood was an independent risk factor for SIRS. Strategies for limiting blood transfusions should be investigated in this population.29
BLOOD TRANSFUSION AND MORTALITY
A follow-up study assessed the effect of blood transfusion within the first 24 hours postinjury on outcome in trauma in a larger sample size (n = 15,534, 3-year study, 1998–2000). The study controlled for all potential confounding shock variables (including base deficit, serum lactate, and shock index [heart rate/systolic blood pressure]) on admission, as well as stratification by age, gender, race/ethnicity, GCS, and ISS.30 Blood transfusion was a strong independent predictor of mortality (OR 2.83, 95% confidence interval [CI] 1.82–4.40, p < 0.001), ICU admission (OR 3.27, 95% CI 2.69–3.99, p < 0.001), ICU length of stay (LOS) (p < 0.001), and hospital LOS (coefficient 4.37, 95% CI 2.79–5.94, p < 0.001) when stratified by indices of shock (base deficit, serum lactate, shock index, and anemia). Patients who underwent blood transfusion were almost three times more likely to die. Blood transfusion early after injury (within the first 24 hours) was therefore confirmed as an independent predictor of mortality, ICU admission, ICU LOS, and hospital LOS in trauma after controlling for severity of shock by admission base deficit, lactate, shock index, and anemia.
We also examined blood transfusion and outcome in trauma, dependent on whether patients were transfused less than or more than 24 hours postinjury (Table 6).31 This retrospective study confirmed our trauma registry data with blood bank data, and delineated the association of blood transfusion and mortality—which was higher (OR 4.13 vs. OR 3.10) when patients were transfused early (<24 hours) after injury.
A retrospective 4-year single-institution review of all adults with blunt hepatic and/or splenic injuries admitted to a Level I trauma recently examined blood transfusion as an independent risk factor for outcome.32 Of 316 patients presenting with blunt hepatic and/or splenic injuries, 143 (45%) received blood transfusion within the first 24 hours. Of the total, 230 patients (72.8%) were selected for nonoperative management, of whom 75 (33%) required transfusion in the first 24 hours. Transfusion was an independent predictor of mortality in all patients (OR 4.75, 95% CI 1.37–16.4, p = 0.014) and in those managed nonoperatively (OR 8.45, 95% CI 1.95–36.53, p = 0.0043) after controlling for indices of shock and injury severity. The risk of death increased with each unit of PRBCs transfused (OR per unit 1.16, 95% CI 1.10–1.24, p < 0.0001). Blood transfusion was also an independent predictor of increased hospital length of stay (coefficient 5.45, 95% CI 1.64–9.25, p = 0.005). Transfusionassociated mortality risk was highest in the subset of patients managed nonoperatively. The authors suggested that prospective examination of transfusion practices in treatment algorithms of blunt hepatic and splenic injuries is warranted.
Another single-institution study examined whether elderly patients are disproportionately affected by blood transfusion in trauma.33 To determine the possible interaction among age, PRBC transfusion volume, and mortality after injury, a 6-year retrospective review (January 1995 through December 2000) of adult patients who received blood transfusion within the first 24 hours after injury was completed. Of the 1312 patients who received PRBCs in the first 24 hours postinjury, 1028 (78%) were aged 55 years and younger and 284 (22%) were over 55—and overall mortality was 21.2%. Age, ISS, GCS, and PRBC transfusion volume emerged as independent predictors of mortality. Mean PRBC transfusion volume for elderly survivors (4.6 units) was significantly less than that of younger survivors (6.7 units). No patient aged over 75 years with a PRBC transfusion volume greater than 12 units survived. The authors concluded that age and PRBC transfusion volume act independently, yet synergistically, to increase mortality following injury.
Most recently, a 5-year single-institution study confirmed that low hemoglobin, abnormal prothrombin and partial thromboplastin time, and physiologic signs of shock (low systolic blood pressure and elevated base deficit) were independent predictors of mortality in trauma.34 Currently, the only treatment available for low hemoglobin and signs of hemorrhagic shock in a trauma patient is the transfusion of stored RBCs. Blood transfusions are also associated with increased mortality in two large prospective multicenter studies quantifying the incidence of anemia and the use of RBC transfusions in critically ill patients.35,36
BLOOD TRANSFUSION AND INFECTION
Immunosuppression is a consequence of allogeneic blood transfusion in humans and is associated with an increased risk in cancer recurrence rates after potentially curative surgery, as well as with an increase in the frequency of postoperative bacterial infections. A recent metaanalysis has been reported demonstrating the relationship of allogeneic blood transfusion to postoperative bacterial infection.37 Twenty peer-reviewed articles published from 1986 to 2000 were included in a metaanalysis. Criteria for inclusion included a clearly defined control group (nontransfused) compared with a treated (transfused) group and statistical analysis of accumulated data that included stepwise multivariate logistic regression analysis. In addition, a subgroup of publications that included only the traumatically injured patient was included in a separate metaanalysis.
A recent single-institution study documented that blood transfusions (within the first 28 hours after admission) correlate with infections in trauma patients in a dose-dependent manner.38 All adult patients (n = 1593) admitted to the trauma service of a Level I trauma center from November 1996 to December 1999 were studied. Of these, 12.6% developed at least one infection. The overall transfusion rate was 19.4%. The infection rate in patients who received at least one transfusion was significantly higher (p < 0.0001), at 33.0 versus 7.6% in patients receiving no blood transfusion. Transfusions per patient ranged from 0 to 46 units. There was a clear exponential correlation in patients receiving between 0 and 15 transfusions (R2 = 0.757). Multivariate logistic regression, which was used to identify risk factors for the development of infection, confirmed that transfusion of PRBCs was an independent risk factor for infection (OR 1.084, 95% CI 1.028–1.142, p = 0.0028). This study documented a clear dosedependent correlation between PRBC transfusion and the development of infection in trauma patients. Similarly, studies in critically ill patients have documented increased rates of nosocomial infection in transfused patients compared to nontransfused patients after stratification of severity of illness and age.39,40
POTENTIAL MECHANISMS FOR TRANSFUSION-ASSOCIATED ADVERSE OUTCOME
A recent review of blood transfusion in the critically ill concluded the following: (1) RBC transfusion does not improve tissue oxygen consumption consistently in critically ill patients, either globally or at the level of the microcirculation; (2) RBC transfusion is not associated with improvements in clinical outcome in the critically ill and may result in worse outcomes in some patients; (3) specific factors that identify patients who will improve from RBC transfusion are difficult to identify; and (4) lack of efficacy of RBC transfusion is likely related to storage time, increased endothelial adherence of stored RBCs, nitric oxide binding by free hemoglobin in stored blood, donor leukocytes, host inflammatory response, and reduced RBC deformability.1
Decreased Red Blood Cell Deformability
Erythrocyte aggregation parameters and deformability and shape descriptors were analyzed in blood stored for 35 days.41 RBC deformability was reduced up to 5% compared with that of fresh samples. Hovav et al.42 reported that blood storage induced changes in RBCs associated with a continuous increase in their aggregability (Figure 2). Another recent study demonstrated that human RBC deformability decreased by 34% after 4 weeks of storage.43 During storage, erythrocytes underwent a time-dependent echinocytic shape transformation in another investigation.44 This transformation increased the suspension viscosity at high and low shear rates. These investigators also confirmed that prestorage leukocyte depletion decreased these effects.
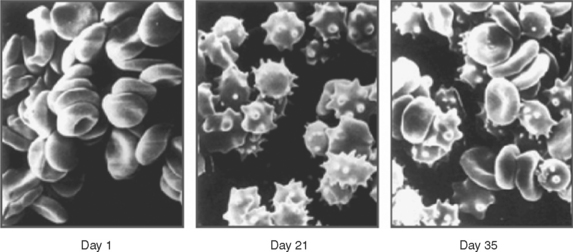
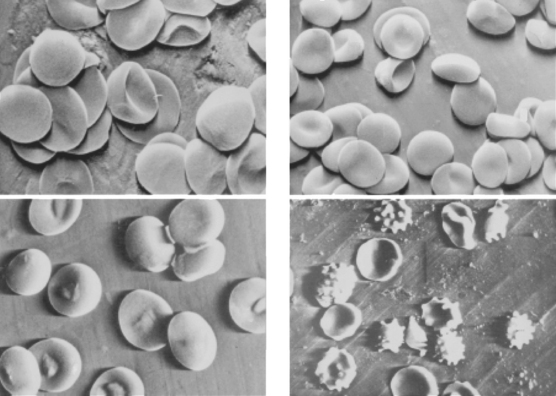
Figure 2 Changes in red blood cell shape after traumatic injury.
(From Hovav T, Yedgar S, Manny N, Barshstein G: Alteration of red cell aggregability and shape during blood storage. Transfusion 39(3):277–281, 1999.)
Traumatic injury is also accompanied by a decrease in RBC deformability. RBC shape was examined by scanning electron microscopy in 43 patients with multisystem trauma.45 Blood samples were taken at admission and every 24 hours afterward for 4–10 days. A significant decrease in the percentage of discoid erythrocytes, compared with the volunteers, was observed in both groups of patients at admission (p < 0.01). The percentage of irreversibly changed RBC (spherostomatocytes, spherocytes) was lower in survivors (12.9 ± 2.0% vs. 20.3 ± 9.4%, p < 0.01). This study confirmed that RBC shape alterations appear within the first hours after trauma and persist for at least 7–10 days (Figure 3), and these changes are more severe in patients with secondary septic complications.
Hemoglobin-Based Oxygen Carriers
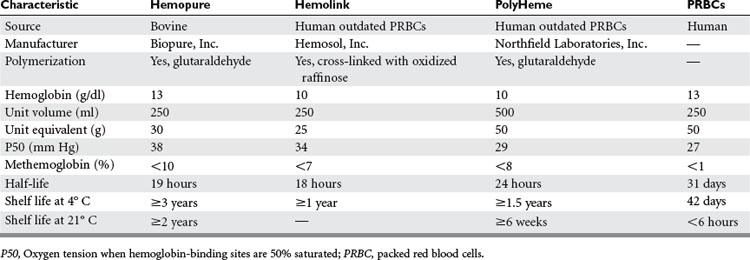
The potential role for hemoglobin-based oxygen carriers (HBOCs) in trauma care is significant. Most authorities believe that the greatest need for HBOCs is in patients with unanticipated acute blood loss, and trauma is the most likely scenario.46 The advantage of HBOCs is that they do not require blood typing, testing, or cross-matching; can be readily available; do not require refrigeration; and have long shelf lives. Some disadvantages to the HBOCs include their short half-life in the circulation; the possibility of hemoglobin binding to nitric oxide, resulting in vasoconstriction (less likely with the current products due to polymerization); inaccuracy of oxygen saturation monitoring because of methemoglobin; and potential interference with laboratory tests that are based on colorimetric changes from the dissolved plasma hemoglobin. The newer HBOCs currently in clinical investigation and their characteristics compared to stored PRBCs are listed in Table 7.
Table 7 Characteristics of Hemoglobin-Based Oxygen Carriers Used in Phase III Clinical Trials Versus Stored Red Blood Cells
Clinical trials and preclinical animal studies have documented that HBOCs attenuate the systemic inflammatory response associated with transfusion of stored RBCs, and decrease neutrophil priming, endothelial activation, and systemic release of interleukin 6 (Figure 4).47 A multicenter prehospital trial is currently ongoing in which severely injured patients with major blood loss (systolic blood pressure <90 mm Hg) are randomized to initial field resuscitation with crystalloid versus a human polymerized HBOC (PolyHeme). During the hospital phase, the control group is further resuscitated with stored PRBCs—whereas the study group receives HBOC (up to 6 units) in the first 12 hours. The primary study endpoint is 30-day mortality, with secondary endpoints including reduction in allogeneic RBC transfusion, hemoglobin concentrations <5 g/dl, uncrossmatched RBC use, and MOF. A bovine HBOC (Hemopure) is also undergoing additional clinical investigation.
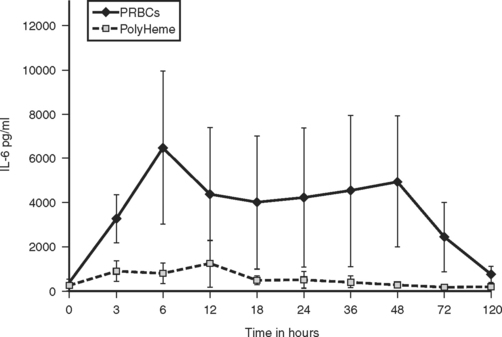
(From Johnson J, et al: Alteration of the postinjury hyperinflammatory response by means of resuscitation with a red cell substitute. J Trauma 54:133–140, 2003.)
CONCLUSIONS
Despite evolving evidence that transfusion risks outweigh benefits in some patients, the critically injured continue to receive large quantities of blood. Transfusion of the injured patient with stored PRBCs requires careful vigilance during the acute resuscitative and recovery phases postinjury. At present, blood transfusion is the only option for treatment of severe hemorrhagic shock. A more conservative approach to blood transfusion should be utilized in the trauma patient with stable asymptomatic anemia. Development of institutional protocols for transfusion of blood (Figure 5) can assist in appropriate utilization of this scarce resource. In an effort to minimize adverse events, immunosuppression, and hyperinflammation, all attempts to minimize the use of blood transfusion in trauma patients is warranted. The future of HBOCs in the treatment of hemorrhagic shock holds great promise and may ultimately lead to better outcomes for injured patients.
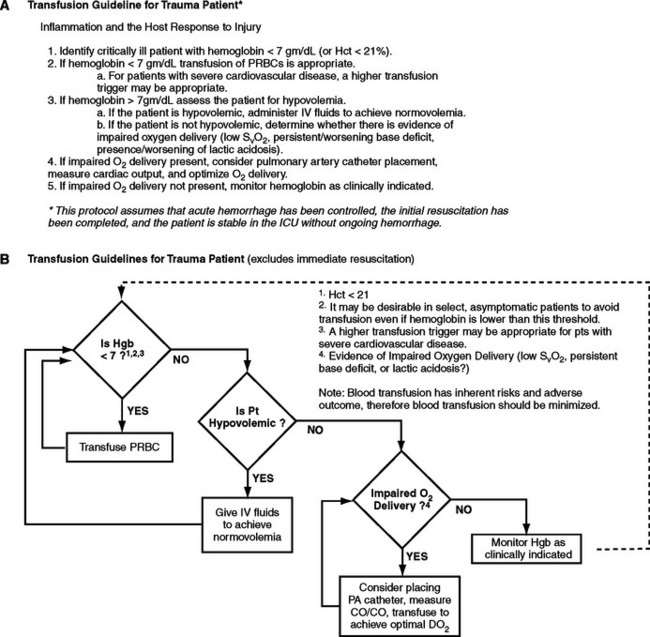
Figure 5 Trauma transfusion guideline. Summary of Protocol for Bedside Use.
(From West MA, et al: Inflammation and the host response to injury, a large-scale collaborative project: Patient-oriented research core-standard operating procedures for clinical care. IV. Guidelines for transfusion in the trauma patient. J Trauma 61(2):436–439, 2006.
1 Napolitano LM, Corwin HL. Efficacy of red blood cell transfusion in the critically ill. Crit Care Clin. 2004;20(2):255-268.
2 Silliman CC, Moore EE, Johnson JL, Gonzalez RJ, Biffl WL. Transfusion of the injured patient: proceed with caution. Shock. 2004;21(4):291-299.
3 Napolitano LM. Resuscitation endpoints in trauma. Transfus Alternatives Transfus Med. 2005;6(4):6-14.
4 Como JJ, Dutton RP, Scalea TM, Edelman BB, Hess JR. Blood transfusion rates in the care of acute trauma. Transfusion. 2004;44(6):809-813.
5 Ruchholtz S, Pehle B, Lewan U, et al. The emergency room transfusion score (ETS): prediction of blood transfusion requirements in initial resuscitation after severe trauma. Transfus Med. 2006;16(1):49-56.
6 Dutton RP, Shih D, Edelman BB, et al. Safety of uncrossmatched type-O red cells for resuscitation from hemorrhagic shock. J Trauma. 2005;59(6):1445-1449.
7 Shapiro MJ, Gettinger A, Corwin HL, Napolitano LM, et al. Anemia and blood transfusion in trauma patients admitted to the intensive care unit. J Trauma. 2003;55:269-274.
8 Practice guidelines for blood component therapy: a report by the American Society of Anaesthesiologists Task Force on Blood Component Therapy. Anesthesiology. 1996;84:732-747.
9 National Institutes of Health Consensus Conference. Perioperative red blood cell transfusion. JAMA. 1988:2700-2703.
10 Hill SR, Carless PA, Henry DA, Carson JL, Hebert PC, McClelland DBL, Henderson KM. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2000;1:CD002042.
11 McIntyre L, Hebert PC, Wells G. Canadian Critical Care Trials Group: is a restrictive transfusion strategy safe for resuscitated and critically ill trauma patients? J Trauma. 2004;57:563-568.
12 Busch MP, Kleinman SH, Nemo GJ. Current and emerging infectious risks of blood transfusion. JAMA. 2003;289(8):959-962.
13 Williamson LM, Lowe S, Love EM, et al. Serious hazards of transfusion (SHOT) initiative: analysis of the first two annual reports. BMJ. 1999;319(7201):16-19.
14 Goodnough LT. Risks of blood transfusion. Crit Care Med. 2003;31:S678-S686. (Suppl)
15 Busch MP, Caglioti S, Robertson EF, et al. Screening the blood supply for West Nile virus RNA by nucleic acid amplification testing. N Engl J Med. 2005;4353(5):460-467.
16 Toy P, Popovsky MA, Abraham E. National Heart, Lung and Blood Institute Working Group on TRALI: Transfusion-related acute lung injury: definition and review. Crit Care Med. 2005;33(4):721-726.
17 Silliman CC, Boshkov LK, Mehdizadehkashi Z, et al. Transfusion-related acute lung injury: epidemiology and a prospective analysis of etiologic factors. Blood. 2003;101(2):454-462.
18 Silverboard H, Aisiku I, Martin GS, Adams M, Rozycki G, Moss M. The role of acute blood transfusion in the development of acute respiratory distress syndrome in patients with severe trauma. J Trauma. 2005;59(3):717-723.
19 Croce MA, Tolley EA, Claridge JA, Fabian TC. Transfusions result in pulmonary morbidity and death after a moderate degree of injury. J Trauma. 2005;59(1):19-23. discussion 23–24
20 Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33(6):1191-1198.
21 National Blood Users Group: A guideline for the use of blood and blood components in the management of massive haemorrhage. November 2002. http://www.ibts.ie/docs/120-MassiveHaemorrhageGuideline.pdf
22 Cinat ME, Wallace WC, Nastanski F, et al. Improved survival following massive transfusion in patients who have undergone trauma. Arch Surg. 1999;134:964-970.
23 Vaslef SN, Knudson NW, Neligan PJ, et al. Massive transfusion exceeding 50 units of blood products in trauma patients. J Trauma. 2002;53:291-296.
24 Ho AM, Dion PW, Cheng CA, et al. A mathematical model for fresh frozen plasma transfusion strategies during major trauma resuscitation with ongoing hemorrhage. Can J Surg. 2005;48(6):470-478.
25 Sauaia A, Moore FA, Moore EE, Haenel JB, Read RA, Lezotte DC. Early predictors of postinjury multiple organ failure. Arch Surg. 1994;129(1):39-45.
26 Moore FA, Moore EE, Sauaia A. Blood transfusion. An independent risk factor for postinjury multiple organ failure. Arch Surg. 1997;132(6):620-624. discussion 624–625
27 Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. A 12-year prospective study of postinjury multiple organ failure: has anything changed? Arch Surg. 2005;140(5):432-438. discussion 438–440
28 Dunne JR, Malone DL, Tracy JK, Napolitano LM. Allogenic blood transfusion in the first 24 hours after trauma is associated with increased systemic inflammatory response syndrome (SIRS) and death. Surg Infect (Larchmt). 2004;5(4):395-404.
29 Beale E, Zhu J, Chan L, et al. Blood transfusion in critically injured patients: a prospective study. Injury. 2006;37:455-465.
30 Malone DL, Dunne J, Tracy JK, Putnam AT, Scalea TM, Napolitano LM. Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. J Trauma. 2003;54(5):898-905. discussion 905–907
31 Malone D, Edelman B, Hess J, Tracy JK, Scalea T, Napolitano L. Age of blood transfusion in trauma: does it alter outcome? Crit Care Med. 2003;30:A21. (Suppl)72
32 Robinson WP3rd, Ahn J, Stiffler A, Rutherford EJ, Hurd H, Zarzaur BL, Baker CC, Meyer AA, Rich PB. Blood transfusion is an independent predictor of increased mortality in nonoperatively managed blunt hepatic and splenic injuries. J Trauma. 2005;58(3):437-444. discussion 444–445
33 Mostafa G, Gunter OL, Norton HJ, McElhiney BM, Bailey DF, Jacobs DG. Age, blood transfusion, and survival after trauma. Am Surg. 2004;70(4):357-363.
34 MacLeod J, Lynn M, McKenney MG, Jeroukhimov I, Cohn SM. Predictors of mortality in trauma patients. Am Surg. 2004;70(9):805-810.
35 Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499-1507.
36 Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, et al. Anemia and blood transfusion in the critically ill: current clinical practice in the United States. The CRIT study. Crit Care Med. 2004;32:39-52.
37 Hill GE, Frawley WH, Griffith KE, Forestner JE, Minei JP. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma. 2003;54(5):908-914.
38 Claridge JA, Sawyer RG, Schulman AM, McLemore EC, Young JS. Blood transfusions correlate with infections in trauma patients in a dose-dependent manner. Am Surg. 2002;68(7):566-572.
39 Taylor RW, Manganaro LA, O’Brien J, Trottier SJ, Parkar N, Veremakis C. Impact of allogeneic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit Care Med. 2002;30:2249-2254.
40 Shorr AF, Duh MS, Kelly KM, Kollef MH. CRIT Study Group: Red blood cell transfusion and ventilator-associated pneumonia: a potential link? Crit Care Med. 2004;32(3):666-674.
41 Nagaprasad V, Singh M. Sequential analysis of the influence of blood storage on aggregation, deformability and shape parameters of erythrocyte. Clin Hemorheol Microcirc. 1998;18(4):273-284.
42 Hovav T, Yedgar S, Manny N, Barshstein G. Alteration of red cell aggregability and shape during blood storage. Transfusion. 1999;39(3):277-281.
43 d’Almeida MS, Jagger J, Duggan M, White M, Ellis C, Chin-Yee IH. A comparison of biochemical and functional alterations of rat and human erythrocytes stored in CPDA-1 for 29 days: implications for animal models of transfusion. Transfus Med. 2000;10(4):291-303.
44 Solberger T, Walter R, Brand B. Influence of prestorage leucocyte depletion and storage time on rheologic properties of erythrocyte concentrates. Vox Sang. 2002;82(4):191-197.
45 Berezina TL, Zaets SB, Machiedo GW. Alterations of red blood cell shape in patients with severe trauma. J Trauma. 2004;57(1):82-87.
46 Moore EE, Johnson JL, Cheng AM, Masuno T, Banerjee A. Insights from studies of blood substitutes in trauma. Shock. 2005;24(3):197-205.
47 Moore EE. Blood substitutes: the future is now. J Am Coll Surg. 2003;196(1):1-17.