Chapter 36 TransFix Anterior Cruciate Ligament Femoral Fixation
Background
More than 100,000 anterior cruciate ligament (ACL) reconstructions are estimated to be performed annually in the United States.1,2 There has been a tremendous amount of research on both graft selection and fixation methods.3–5 The increased use of soft tissue grafts and the concern regarding soft tissue interference screw fixation (e.g., graft pullout, slippage, damage) have led to the development and use of femoral cross-pin fixation in ACL reconstruction. One successful technique for cross-pin fixation is the TransFix ACL reconstruction technique (Arthrex, Naples, FL).
Biomechanical and Clinical Results
The Arthrex TransFix implant is made of titanium, and the Bio-TransFix implant is made of poly-L-lactic acid (PLLA), as shown in Fig. 36-1. In vivo, the Bio-TransFix implant hydrolyzes into lactic acid, which is then metabolized into CO2 and H2O. A proprietary degradation study of the Bio-TransFix demonstrated that it does not lose shear strength through 52 weeks.
Fabbriciani et al used the TransFix system in conjunction with fresh, ovine, doubled Achilles tendons and ovine femurs.5 Cyclical loading comparisons of bioabsorbable and metal RCI screws (Smith & Nephew, Andover, MA), the LINX HT (Mitek, Norwood, MA), and the TransFix implant showed significantly lower mean values of graft elongation for the TransFix construct (1.5 ± 0.1 mm) over 1000 cycles. The maximum load to failure (LTF) for the TransFix was 890N ± 175N, which, unlike the other devices in the study, is comparable to that of the intact ovine ACL (725N ± 77N).
Becker et al showed that the stiffness of the TransFix construct (184 N/mm) approximates the stiffness of the human ACL (242 N/mm, as reported by Woo et al6) and provides significantly greater ultimate strength than interference screw fixation.7 This study compared three fixation methods using a porcine femur model: (1) TransFix fixation of a quadruple tendon, (2) 8- × 20-mm biodegradable interference screw fixation of a quadruple tendon, and (3) 8- × 20- mm titanium screw fixation of a patellar tendon–bone graft using a porcine femur model. Interference screw fixation of the patellar tendon and quadruple tendon resisted only 59% and 37%, respectively, of the pullout strength of the TransFix (1303N ± 282N). The TransFix had significantly less construct displacement during cyclical loading than the interference screw/quadruple graft construct.
Ahmad et al demonstrated that interference screw fixation and the Rigidfix cross-pin technique were inferior to the Bio-TransFix and the Endobutton for graft slippage during cyclical loading and ultimate LTF.8 After 1000 cycles, the graft displacement for the Bio-TransFix was 1.13 mm compared with greater than 5 mm for the interference screw and Rigidfix. This study also showed significantly greater LTF of the Bio-TransFix (746N ± 119N) as compared with the interference screw technique (539N ± 114N).
As these and other studies have demonstrated, the use of the TransFix system offers considerable advantages compared with other femoral fixation systems in terms of yield load, stiffness, and deformation and elongation under cyclical loading. These results offer stable fixation of the graft during the postoperative period, before graft healing has occurred. The inherent rigidity of the TransFix limits graft-tunnel motion during physiological loading. Intratunnel motion has been associated with tunnel widening.9 Fauno and Kaalund reported a significant reduction in tunnel widening in the femur when TransFix was used compared with Endobutton fixation at 1-year follow-up for a prospective randomized study.10 Unlike interference screw techniques, in which the graft is squeezed and possibly damaged during screw insertion, graft strength is maximized with the TransFix technique.
In 1998, Wolf11 reported his initial results with the TransFix fixation. Eighty-eight percent of patients at follow-up described their postoperative knees to be normal or near normal. The 27 patients had a mean KT-1000 side-to-side laxity difference of 1.5 mm at follow-up. (Ahmad reports that greater than 5 mm may be considered clinical failure.8)
Recently, Harilainen et al12 showed no significant difference in IKDC scores at 2-year follow-up for TransFix cross-pin fixation versus metal screw fixation. In this controlled prospective randomized study, 85% of the TransFix group and 73% of the screw patients were in the IKDC A or B categories.
Surgical Technique
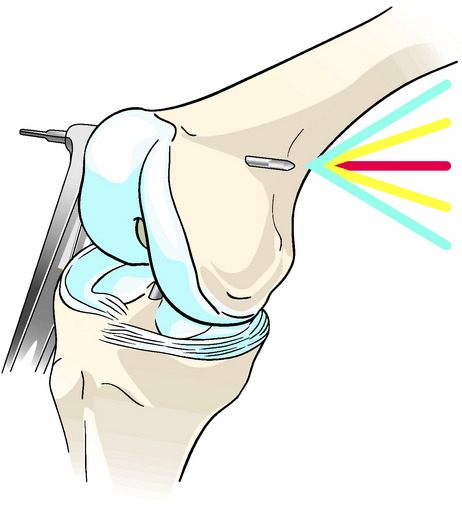
The TransFix technique requires that a 3-mm drill pin be passed from lateral to medial. Although no neurovascular complications have been reported, theoretically the medial (and lateral) neurovascular structures are at risk. The author’s lab has shown that a defined “safe zone” exists in which a distal femoral cross-pin can be reliably placed without damaging the local neurovascular structures.13 In this anatomical cadaveric study, the absolute neurovascular safe zone during cross-pin guidewire placement is from +20 degrees (0 degrees equals “parallel to the floor” line) and –40 degrees (lowering the guide more posteriorly) (Fig. 36-2).
Tip 1: Make sure the graft runs easily through the selected diameter.
Tip 2: The knee flexion angle must be maintained until the TransFix is implanted.
If orifice fixation is desired, this can be achieved with the addition of a cancellous bone block15 or 20-mm femoral retroscrew (Arthrex) placed distal to the TransFix. Ishibashi et al demonstrated that proximal fixation resulted in reduced anteroposterior translation compared with more distal fixation.16 Anatomical fixation close to the joint line results in increased knee stability and graft isometry. Fixation of the graft in the tunnel by an interference screw or bone block also may mitigate synovial fluid infiltration into the tunnel.
1 Brown CH, Carson EW. Revision anterior cruciate ligament surgery. Clin Sports Med. 1999;18:109-171.
2 Orthopedic soft tissue repair. Norwalk, CT: Windhover Information, 2005.
3 Brand J, Weiler A, Caborn D, et al. Current concepts: graft fixation in cruciate ligament reconstruction. Am J Sports Med. 2000;28:761-774.
4 Kousa P, Jarvinen TL, Vihavainen M, et al. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part I: femoral site. Am J Sports Med. 2003;31:174-181.
5 Kousa P, Jarvinen TL, Vihavainen M, et al. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction: part II: tibial site. Am J Sports Med. 2003;31:182-188.
6 Woo SL, Hollis JM, Adams DF, et al. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med. 1991;19:217-225.
7 Becker R, Voight D, Starke C, et al. Biomechanical properties of quadruple tendon and patellar tendon femoral fixation techniques. Knee Surg Sports Traumatol Arthrosc. 2001;9:337-342.
8 Ahmad CS, Gardner TR, Groh M, et al. Mechanical properties of soft tissue femoral fixation devices for anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:635-640.
9 L’Insalata JC, Klatt B, Fu FH, et al. Tunnel expansion following anterior cruciate ligament reconstruction: a comparison of hamstring and patellar tendon autografts. Knee Surg Sports Traumatol Arthrosc. 1997;5:234-238.
10 Fauno P, Kaalund S. Tunnel widening after hamstring anterior cruciate ligament reconstruction is influenced by the type of graft fixation used: a prospective randomized study. Arthroscopy. 2005;21:1337-1341.
11 Wolf EM. Semitendinosus and gracilis anterior cruciate ligament reconstruction using the TransFix technique. Tech Orthop. 1998;13:329-336.
12 Harilainen MD, Sandelin J, Jansson K. Cross-pin fixation versus metal interference screw in ACL with hamstring tendons: results of a controlled prospective randomized study with 2-year follow-up. Arthroscopy. 2005;21:25-33.
13 McKeon BP, Gordon M, Deconciliis G, et al. The “safe zone” for femoral cross-pin fixation. An anatomical study. Am J Knee Surg; In Press
14 McKeon BP, Heming JD, Langeland R, et al. The Krackow stitch: a biomechanical evaluation of changing the number of locking loops versus the number of sutures. Arthroscopy. 2006;22:33-37.
15 Hantes ME, Dailiana Z, Zachos VC, et al. Anterior cruciate ligament reconstruction using the Bio-TransFix femoral fixation device and anteromedial portal technique. Knee Surg Sports Traumatol Arthrosc. 2006;14:497-501.
16 Ishibashi Y, Rudy T, Livesay G, et al. The effect of anterior cruciate ligament graft fixation site at the tibia on knee stability: evaluation using a robotic testing system. Arthroscopy. 1997;13:177-182.