Transesophageal echocardiography
Overview
After development of an esophageal stethoscope, the first transesophageal echocardiographic (TEE) equipment provided simple M-mode images to measure left ventricular (LV) dimensions. First used for intraoperative monitoring of LV function and intracardiac air more than 30 years ago,1 TEE is now widely considered the standard of care for a number of cardiac surgical procedures.2,3 In parallel with transthoracic echocardiography (TTE), development of phased-array transducers; spectral and color Doppler; biplane, multiplane, and three-dimensional (3D) imaging; and progressive miniaturization of equipment has increased the quality and range of information available from the technique. For 20 years it has been recognized that TEE can provide additional information to other hemodynamic monitors (including the pulmonary artery catheter), which changes patient management and may improve outcomes.4 Currently, in the United States, the majority of anesthesiologists trained in TEE believe the technique to be superior to other hemodynamic monitors.5 Guidelines for performance of and training in TEE have been published by a number of national and international bodies (see Chapter 27). Although in general, TTE should be the first imaging mode used, its usefulness in the intensive care unit (ICU) may be limited by the patient (immobility, body habitus, pulmonary pathology), the pathology (i.e., prosthetic valve endocarditis), or ICU interventions (intermittent positive-pressure ventilation [IPPV], dressings/drains). In these instances, TEE may be required. Despite not being considered essential for basic critical care echocardiography, TEE is increasingly regarded as an essential skill for those who wish to perform more advanced ICU echocardiography,6,7 with experienced practitioners using the two techniques in a complementary and potentially interchangeable manner.
The TEE probe
This comprises a miniaturized ultrasound transducer incorporated into a custom-made transesophageal probe. The device uses an array of piezoelectric crystals and phased-array technology to steer the ultrasound beam through 180 degrees. The frequency range used varies (generally) between 5 to 10 MHz, and all modalities available in TTE are potentially available for TEE, including the ability to acquire real-time high-quality 3D images (Figure 29-1). Progressive technologic development has allowed availability of pediatric and neonatal TEE probes, and a disposable miniaturized probe has been devised for use as a continuous hemodynamic monitor in the ICU.
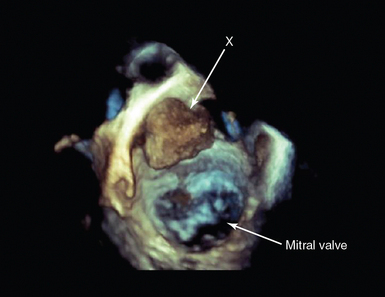
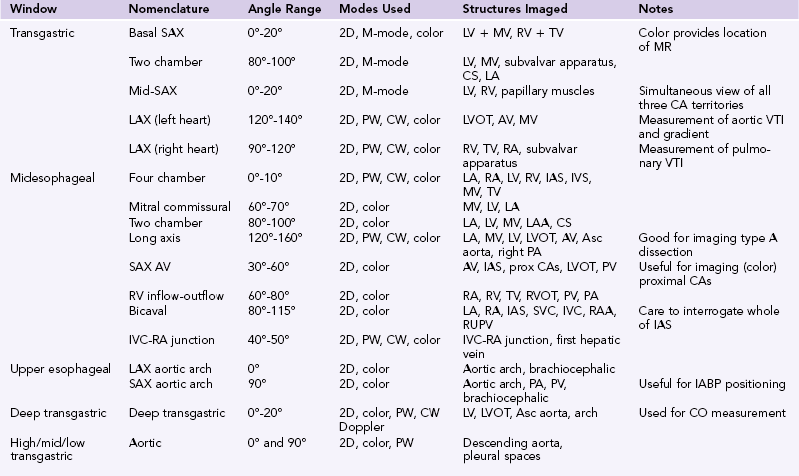
Figure 29-1 Three-dimensional (3D) transesophageal echocardiography (TEE). 3D-TEE in a patient presenting with acute cerebrovascular accident in multiple carotid artery territories. The image is a full-volume rendition, manipulated to be looking down on the mitral valve from the roof of the left atrium. There is a mass (X) attached to the atrial wall. At surgery, this was shown to be a left atrial myxoma.
Preparation for TEE in the critically ill
Process review
This should include infection control (knowledge of local and national guidelines, probe sterilization, cleaning, and tracking), and care of the probe (including procedures for checking integrity before each use). Nasogastric tubes are often in situ, and to ensure interruption of feed is minimized, gastric contents should be aspirated before the study, and postprobe removal protocols should be in place to ensure no displacement has occurred, and feeding is recommenced only and as soon as judged safe.3,7 The process of consent varies between countries, and although invasive procedures are usually undertaken only after consent is obtained, exceptions are generally allowed in the case of life-threatening emergencies and/or when the patient is unable to give informed consent. In some countries, informing the patient’s documented next of kin before a nonemergency invasive procedure is normal practice. Where research is undertaken, ethical approval must be obtained and consent obtained according to the agreed research protocol and local guidelines. The study should be recorded and reported according to local, national, and international guidelines and recommendations.
Patient review
This should include the indication for TEE and the patient history in terms of the relative and absolute contraindications to TEE (Table 29-1) and to its risk-benefit to the patient. The most significant serious complication from TEE in the outpatient population is esophageal perforation, with the usually quoted rate of 1:10,000 in studies; however, this is variable and may increase with an increasingly aged patient population.8 TEE in the intensive care is generally regarded as safe; however, because the critically ill patient is frequently coagulopathic, knowledge of the coagulation status of the patient is vital and, where indicated, possible correction undertaken. When the patient is fully anticoagulated or coagulopathic, probe insertion with laryngoscopic guidance is recommended. This is important if the patient is receiving extracorporeal support; in this circumstance, oropharyngeal bleeding can be life threatening. Critically ill patients undergoing TEE will be intubated and ventilated, and consistent with guidelines in many ICUs, there should be an independent ICU practitioner managing the airway, ventilation, and hemodynamic status of the patient during the study.
TABLE 29-1
Contraindications to Transesophageal Echocardiography3,7,8
| Relative | Absolute |
| History of esophageal surgery | Esophageal obstruction |
| Esophageal varices or diverticulum | Esophageal stricture |
| Gastric/esophageal bleeding | Poor airway control |
| Oropharyngeal pathology (i.e., pharyngeal pouch) | |
| Severe coagulopathy | |
| Cervical spine injury |
Image acquisition: The standard views
Guidelines for performance of a comprehensive TEE study have been published, with recommendations for a minimum dataset in a number of circumstances.3,7 The practitioner will then focus on the specific question that requires answering. In existing guidelines and recommendations, these predominantly relate to the perioperative cardiac surgical setting; however, it is not the mode of echo (TTE vs. TEE) but, rather, the information required that drives the study. Indeed, in undertaking repeated focused studies and/or using TEE as a hemodynamic monitor, only limited views may be required.
The standard order of image acquisition depends upon a number of factors, including the urgency of the study and time frame for acquisition, the type (comprehensive vs. focused), and individual practitioner preference. Ideally, in the critically ill patient, the amount of probe manipulation should be minimized, in particular in the elderly and/or coagulopathic patient. Movement of the probe is described in terms of its position with respect to the patient: withdraw/advance, rotate left/right, anteflex/retroflex, and flex right/left. Additional movement of the ultrasound beam relates to electronic rotation (from 0-180 degrees). As with TTE, as there is anatomic variation between patients, although numbers are given regarding the degree of electronic rotation, these are for guidance only. The standard views are described in Table 29-1, together with the main structures seen (Table 29-2).
Of note, the deep transgastric view is not achievable in all patients. Again, care should be taken to avoid excessive probe manipulation. Imaging of the pulmonary veins can be achieved by standard views (as described in Table 29-1) and additionally at 0 degrees, interrogating the right and left sides of the left atrium (both superiorly and inferiorly) in turn.
Indications for transesophageal echocardiography in the critically ill
The indications for echocardiography in the ICU have been discussed in Chapters 26 and 27, including the choice of TTE versus TEE. Images of diagnostic quality are unobtainable in up to 30% of the general ICU patient population, rising to 85% in those with severe, acute respiratory failure referred for extracorporeal respiratory support (Figure 29-2).9 Thus, when image quality precludes obtaining adequate information from the transthoracic route, TEE may be indicated. In addition, there are specific indications for TEE as opposed to TTE (Table 29-3). Guidelines for the appropriateness of undertaking TEE have been published; however, these will inevitably change with the ongoing development of critical care technologies, for example, extracorporeal support.10,11
TABLE 29-3
Specific Indications for Transesophageal Echocardiography
| Indication | Notes |
| Intracardiac thrombus/mass | Most commonly, LAA thrombus in patient with AF May see LV thrombus related to akinetic segment (NB apex difficult to visualize with TEE) Potential for paradoxic embolization with PFO/ASD |
| Endocarditis | Prosthetic valve endocarditis frequently paraprosthetic leak only Infection may be associated with indwelling lines/wires All patients, expert echocardiographic review required |
| Extracorporeal support | Useful for insertion and positioning of IABP ECMO and VAD: Highly specialized echocardiography |
| Aortic disease | Not all the aorta can be imaged, alternative imaging may be required Aortic dissection: Origin of coronary arteries (true vs. false lumen) |
| Valvular disease | Mitral MR may be dynamic, stress +/− volume loading may be required Assess mechanism of MR Critical MS: Suitability for valvuloplasty Indicated to assess potential prosthesis malfunction |
| Aortic Critical AS: Suitability for valvuloplasty Indicated to assess potential prosthesis malfunction |
|
| Cardiac monitoring | Multiple potential uses; however, operator dependent and intermittent measure of cardiac function |
| Unexplained hypoxia | Intracardiac shunting not uncommon in ventilated patients Potential to visualize intracardiac/pulmonary artery thrombus |
| Tamponade | Low sensitivity of TTE postcardiac surgery: May be required to exclude small and/or localized collection |
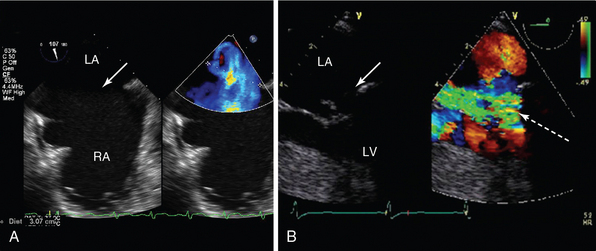
Figure 29-2 The role of transesophageal echocardiography (TEE) in patients referred with severe acute respiratory failure. TEE studies in two patients with the diagnosis of severe acute respiratory failure referred with refractory hypoxemia for ECMO. In both cases, there were no transthoracic echocardiographic windows. A, Bicaval view showing a large secundum ASD) (arrow). Color flow Doppler showed biphasic flow (current frame demonstrating left-right flow in blue). B, Midesophageal view focusing on the mitral valve in systole, showing a flail posterior mitral valve leaflet (arrow) with ruptured chordae and a corresponding jet of severe mitral regurgitation (broken arrow). Information from these two studies guided management. In patient A, ECMO was not instituted; the patient was managed in the standard manner, and after recovery from respiratory disease, the patient underwent subsequent formal hemodynamic evaluation with successful closure of the ASD. In patient B, the diagnosis was revised to pulmonary edema secondary to severe structural mitral valve disease. An intraaortic balloon pump was inserted, and the patient underwent successful mitral valve repair. ASD, Atrial septal defect; ECMO, extracorporeal membrane oxygenation; LA, left atrium; LV, left ventricle; RA, right atrium.
Evaluation for intracardiac thrombus
Intracardiac thrombus is most commonly found in the left atrial appendage (LAA) in patients with atrial arrhythmias. Guidelines have been published regarding the use of TEE for the evaluation of the LAA in atrial fibrillation before cardioversion.12 Critical care patients may be relatively prothrombotic, and therefore the indication for TEE in the critically ill, to exclude the presence of thrombus, may lie outside the guidelines (Figure 29-3). More research is required in this area, and when the patient is hemodynamically stable and no contraindications to TEE exist, there should be a low threshold for performing a study.
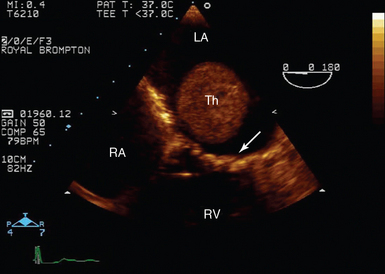
Figure 29-3 Transoesophageal echocardiography of left atrial thrombus. Midesophageal four-chamber view in a patient in atrial fibrillation with mitral stenosis (mitral valve, arrow). There is a large thrombus (Th) visible in mid-LA despite full, therapeutic anticoagulation. LA, Left atrium; RA, right atrium; RV, right ventricle.
Patients with myocardial infarction and extensive areas of akinesia are at risk of developing ventricular thrombus. Care must be taken when using TEE to evaluate the apex of the LV because foreshortening may occur in the midesophageal views. A modified long-axis transgastric long-axis view can be useful in these circumstances.13 Where an associated apical ventricular septal defect (VSD) is suspected, rotation of the probe between the LV and right ventricle (RV) in this modified view can be used to demonstrate the apical portion of the interventricular septum.
In patients receiving extracorporeal respiratory and/or cardiac support, there is a significant risk of developing intracardiac thrombus despite full anticoagulation. This may be either associated with a cannula, and/or with a nonopening valve, or an akinetic area of the LV.14 Here TEE is pivotal to making the diagnosis and planning a treatment strategy (Figure 29-4).
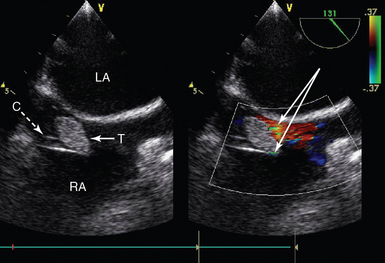
Figure 29-4 Intracardiac thrombus in a patient receiving extracorporeal support. Midesophageal bicaval view showing a cannula in the RA (inlet cannula for VA ECMO) and a large associated thrombus (solid arrow) at the mouth of the cannula. C, Cannula; LA, left atrium; RA, right atrium; T, thrombus; VA ECMO, venoarterial extracorporeal membrane oxygenation.
Infective endocarditis
Guidelines for the use of TEE in the diagnosis of endocarditis have been published,15 including prosthetic valve endocarditis.15 When endocarditis is suspected, there should be a low threshold for undertaking TEE, and it is indicated in all critical care patients with suspected prosthetic valve endocarditis (Figure 29-5). Examination should not be limited to the valves and associated structures but should include lines, cannulas, pacing wires, and any intracardiac patch or device. In prosthetic valve endocarditis, large vegetations are rarely seen, the more usual echocardiographic findings being paraprosthetic regurgitation and/or abscess or fistula formation (Figure 29-6). In intensive care, the diagnosis of severe acute respiratory failure with associated bilateral pulmonary infiltrates in a patient with previous left-sided valve repair/replacement should prompt close interrogation of the relevant valve, including TEE because significant paraprosthetic regurgitation must be actively excluded (see Figure 29-2B).
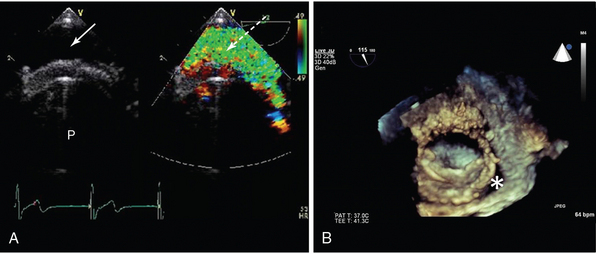
Figure 29-5 Two-dimensional (2D) and three-dimensional (3D) TOE in assessment of valve prosthesis malfunction. A, Conventional 2D imaging shows patient with a bileaflet mechanical valve (P), an echo-free space between the inferoposterior annulus, and the valve sewing ring (arrow). Corresponding color Doppler shows a broad jet of paraprosthetic regurgitation (broken arrow). The patient had the clinical features of cardiogenic shock and pulmonary edema in the context of a history suggestive of endocarditis. Note the valve anatomy is difficult to discern, and there are no obvious vegetations. B, 3D transesophageal echocardiogram (TEE) of a single tilting disc mitral valve seen from the atrial aspect. An echo-free space is seen (*) that corresponds to a jet of mitral regurgitation. The clinical features were of severe anemia resulting from hemolysis. The patient subsequently underwent TEE-guided catheter-based intervention to close the paraprosthetic leak.
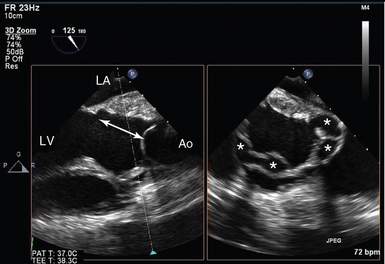
Figure 29-6 Complicated infective endocarditis. Biplane imaging in a patient with previous homograft aortic root replacement, representing clinical features of infective endocarditis. On the left, a conventional left ventricular outflow tract (LVOT) view is shown, with discontinuity between the base of the accessory mitral valve leaflet (AMVL) and the aortic leaflets (double arrow). On the right, the orthogonal view of the LVOT is seen, with multiple abscess cavities (*). Ao, Ascending aorta; LA, left atrium; LV, left ventricle.
Extracorporeal support
The use of echocardiography in extracorporeal support is described in detail in Chapter 28. Regular echocardiography in this patient population is highly recommended; however, repeated TEE studies should be avoided (because of the risk of hemorrhage), and TTE should generally be the first modality used.14 TEE does have specific roles in confirming the underlying diagnosis and/or estimation of left atrial pressure (patients referred for venovenous–extracorporeal membrane oxygenation [VV-ECMO]), assisting in cannula placement (either real time or postprocedurally), and detecting associated complications, including cannula displacement, worsening valvular regurgitation, tamponade, thrombus formation (cannulas and/or valves, see Figure 29-4), and inadvertent overload or excessive offloading of one or both ventricles.
Aortic disease
In the diagnosis of aortic dissection, TEE is comparable to computed tomography (CT) and aortography and superior to TTE (Video 29-1![]() ). It has the additional advantages of providing a shorter time to diagnosis and intervention, demonstration of the entry site (and coronary ostia), and characterization of the mechanism and severity of any aortic regurgitation (AR), in which holodiastolic reverse flow greater than 15 cm/sec indicates severe regurgitation (Figure 29-7). It is, however, inferior in its ability to image the descending aorta and aortic arch because of interference from the left main bronchus. In aortic trauma, TEE has been shown to have a high specificity (91%-100%) but variable sensitivity (57%-100%) compared with aortography in demonstration of aortic interruption and hematoma.16
). It has the additional advantages of providing a shorter time to diagnosis and intervention, demonstration of the entry site (and coronary ostia), and characterization of the mechanism and severity of any aortic regurgitation (AR), in which holodiastolic reverse flow greater than 15 cm/sec indicates severe regurgitation (Figure 29-7). It is, however, inferior in its ability to image the descending aorta and aortic arch because of interference from the left main bronchus. In aortic trauma, TEE has been shown to have a high specificity (91%-100%) but variable sensitivity (57%-100%) compared with aortography in demonstration of aortic interruption and hematoma.16
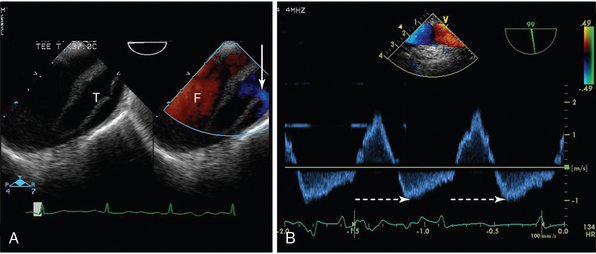
Figure 29-7 Transesophageal echocardiogram (TEE) of the aortic arch in a patient with aortic dissection. A, On the two-dimensional image to the left, the true lumen (T) is smaller than the false lumen (F), and a connection between the two (with flow from true to false lumen) is shown with an arrow. B, In the image to the right, pulsed wave Doppler of aortic flow is shown, with holodiastolic flow reversal (broken arrow).
Transesophageal echocardiography as a cardiac monitor
There are a number of publications that support TEE as a hemodynamic monitor17 (Video 29-2![]() ). Cardiac output (CO) measured using TEE has been shown to provide quantitative assessment of CO that correlates well with continuous thermodilution methods.18 However, when compared directly, it tends to underestimate CO in both ventilated and nonventilated patients, thereby precluding their use interchangeably. The use of echocardiography to measure CO can be useful, however, in patients where placement of a pulmonary artery (PA) catheter is relatively or absolutely contraindicated, or where there are multiple cardiac factors that potentially limit CO and advanced interrogation of the heart is required.
). Cardiac output (CO) measured using TEE has been shown to provide quantitative assessment of CO that correlates well with continuous thermodilution methods.18 However, when compared directly, it tends to underestimate CO in both ventilated and nonventilated patients, thereby precluding their use interchangeably. The use of echocardiography to measure CO can be useful, however, in patients where placement of a pulmonary artery (PA) catheter is relatively or absolutely contraindicated, or where there are multiple cardiac factors that potentially limit CO and advanced interrogation of the heart is required.
Left atrial pressure may be estimated using a combination of TEE-based techniques (combining E/E´, color Doppler propagation and pulmonary venous flow, in conjunction with ejection fraction19). Although gray zones still remain, and, as with TTE, it is not possible to measure the pressure within any cardiac chamber, extreme values may prove helpful. When a high left atrial pressure is suspected but not found on TEE in a patient who fails to wean from mechanical ventilation because of respiratory distress, stress echocardiography should be considered. The nature of stressor used will depend upon the suspected etiology of the underlying pathology (dobutamine in suspected ischemia, volume and pressor loading in shock). TEE has been proposed as a potential monitor of volume responsiveness, by analysis of the changes in stroke volume with respiration and/or inferior vena cava (IVC) diameter in response to respiration in positive-pressure ventilated patients. However, a number of technical and patient-related limitations exist.20 Indeed, TEE has inherent limitations when compared with the features of an ideal monitor, requiring repeated studies and skill for performance and interpretation and providing only intermittent information. Further, many of the published studies had significant relevant exclusions to the critical care patient population. To minimize patient risk and avoid the requirement for repeated intermittent studies, a miniaturized TEE device has been devised for continuous monitoring.15 However, the greatest strength of echocardiography over all other monitors is that it provides additional insight into the underlying diagnosis and can thus be used to determine potential interventions to optimize cardiac output (Figure 29-8).
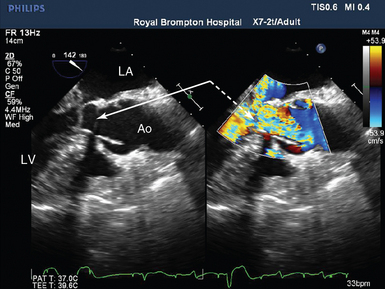
Figure 29-8 Severe SAM with outflow tract limitation in a patient with falling cardiac output in response to increasing inotropic support. Midesophageal LVOT view in systole showing the anterior mitral valve leaflet is in the LVOT (solid arrow) and is associated with a high-velocity aliasing jet (broken arrow). Conventional CO monitoring had revealed a falling cardiac index in response to increasing inotropic support. The TEE findings resulted in cessation of inotropic therapy, volume, and pressor loading, with resolution of the LVOTO, and a rise in cardiac index. Ao, Aorta; CO, cardiac output; LA, left atrium; LV, left ventricle; LVOT, left ventricular outflow tract; LVOTO, left ventricular outflow tract obstruction; SAM; systolic anterior motion of the mitral valve; TEE, transesophageal echocardiogram.
Valvular disease
There are numerous publications regarding the use of echocardiography in the evaluation of valvular heart disease.21 Valvular heart disease relating to or causing ICU admission is likely to be severe and affect the left-sided valves. When significant valve disease is suspected but TTE is nondiagnostic, TEE has a role in confirming or refuting the diagnosis, in evaluating possible endocarditis (and local complications), and in assessing of prosthesis malfunction (i.e., thrombosis or paraprosthetic regurgitation or valve degeneration). When critical aortic stenosis (AS) or mitral valve stenosis (MS) is present, TEE is additionally indicated to define suitability for valvuloplasty and exclusion of intracardiac thrombus.21 When there is severe regurgitation, TEE will aid the surgeon in planning the intraoperative strategy. Mitral regurgitation may be dynamic and reduce significantly with positive-pressure ventilation and sedation or anesthesia (Figure 29-9). Here stress echocardiography should be considered with TEE when TTE images are inadequate.21
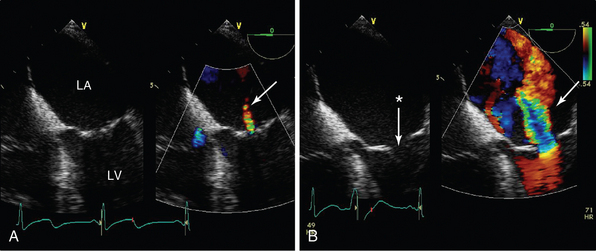
Figure 29-9 The effects of sedation and ventilation on mitral regurgitation. Midesophageal four-chamber view focused on the mitral valve in a patient with dynamic secondary mitral regurgitation undergoing stress echocardiography. In A, the patient is sedated and ventilated, and only a small jet of mitral regurgitation can be seen (arrow) and the anterior and posterior leaflets are coapting (albeit with minimal coaptation length). Because dynamic mitral regurgitation (MR) was suspected, the patient underwent volume and pressor loading (B), the mitral valve leaflets no longer coapt (*), and a broad jet of severe mitral regurgitation is seen (arrow).
Unexplained hypoxia
TTE is recommended to assist in the diagnosis of pulmonary embolism when the patient is too hemodynamically unstable to transfer to the CT scanner.22 When TEE is undertaken in critically ill patients with disproportionate/unexplained hypoxemia, on occasion, thrombus may be seen transiting the right heart, in the main pulmonary artery, or in the proximal right or left pulmonary arteries (Video 29-3![]() ); however, TEE is not the investigation of choice in patients with suspected pulmonary embolism.
); however, TEE is not the investigation of choice in patients with suspected pulmonary embolism.
TEE can be used to demonstrate presence and mechanism of intracardiac shunting. The anatomy of the interatrial septum and effects of ventilation on size and magnitude also may suggest intrapulmonary shunting, in which the timing of arrival of bubbles (and the site of their arrival, that is, from pulmonary veins) may suggest the diagnosis. In all patients with SARF, the clinician must consider the presence of an interatrial shunt because this may significantly impact management (see Figure 29-2A).
Pericardial disease
Usually evaluated in the outpatient setting with physiologic parameters of venous return, the most common pericardial disease encountered in the ICU is tamponade. When a patient has a pericardial fluid collection sufficient to cause hemodynamic instability, it is generally large, readily detected using TTE, and TEE is not indicated. An important exception is postcardiac surgery. Here the nature of the patient’s underlying cardiac disease and size and location of collections make detection difficult, with up to 50% of hemodynamically important collections missed using TTE. Although primarily a clinical diagnosis, where uncertainty exists that a patient has tamponade,TEE can be undertaken (Video 29-4![]() ). Here collections causing may be small, and/or localized (Figure 29-10), and with no respiratory variation in cardiac filling/emptying.23
). Here collections causing may be small, and/or localized (Figure 29-10), and with no respiratory variation in cardiac filling/emptying.23
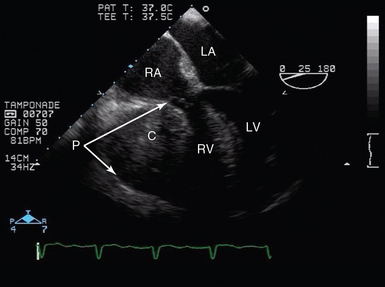
Figure 29-10 Tamponade after cardiac surgery. TEE view (midesophagus, four-chamber view) in a patient with a localized pericardial collection resulting in profound hemodynamic compromise. Here, although measured filling pressures were normal, there was a significant hematoma within the pericardial space, compressing the RV and atrioventricular groove, resulting in tamponade. C, Collection; LA, left atrium; LV, left ventricle; P, pericardium; RA, right atrium; RV, right ventricle, TEE, transesophageal echocardiogram.
Coronary artery disease
TEE can be used to visualize the first 1 to 2 cm of the coronary arteries (Figure 29-11) and has been specifically evaluated in patients after mitral valve surgery. Its use in demonstrating significant coronary artery disruption has been validated in the cardiac perioperative setting but not in the general ICU population. However, in a ventilated patient with borderline CO or an abnormal echocardiogram, abnormal coronary Doppler flow patterns and velocities seen in association with a corresponding regional wall motion abnormality suggest significant coronary artery disease (Video 29-5![]() ).
).
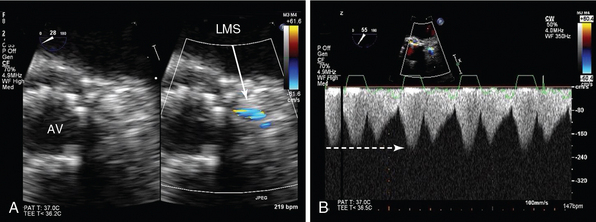
Figure 29-11 Imaging of proximal coronary arteries. Two-dimensional TEE (midesophageal view) focusing on the LMS coronary artery. A, On the left is a short-axis view of a transcatheter aortic valve implant (AV), with corresponding color Doppler of the LMS (arrow). There is high-velocity aliasing at the ostium, which appears narrowed. B, CW Doppler of the LMS shows high velocity (broken arrow) that never returns to zero (continuous flow). Coronary angiography confirmed LMS compression because of displacement of calcium during the transcatheter procedure. AV, Aortic valve; CW, continuous wave; LMS, left mainstem coronary artery; TEE, transesophageal echocardiogram.
Pearls and highlights
• The indications for TEE in the ICU include suboptimal TTE image quality and specific indications for TEE.
• The experienced practitioner will be trained in both TEE and TTE and able to apply each appropriately to the clinical question being asked.
• In the critically ill patient, TEE must never be performed with an unprotected airway.
• An independent and appropriately experienced practitioner should manage the airway and hemodynamic status of the patient during the study.
• In every patient, the risk:benefit ratio of undertaking TEE must be considered before performing a study.
• Evolving techniques in the ICU are likely to result in an expansion of indications for TEE in this patient population.
References
1. Hisanga, K, Hisanga, A, Nagata, K, et al. A new transesophageal real time two-dimensional echocardiographic system using a flexible tube and its clinical application. Proc Jpn Soc Ultrasonic Med. 1972; 32:43–44.
2. Matsumoto, M, Oka, Y, Strom, J, et al. Application of transesophageal echocardiography to continuous intraoperative monitoring of left ventricular performance. Am J Cardiol. 1980; 46:95–105.
3. Shanewise, JS, Cheung, AT, Aronson, S, et al, ASE/SCA guidelines for performing a comprehensive intraoperative multiplane transesophageal echocardiography examination: recommendations of the American Society of Echocardiography Council for Intraoperative Echocardiography and the Society of Cardiovascular Anesthesiologists Task Force for Certification in Perioperative Transesophageal Echocardiography. J Am Soc Echocardiog. 1999; 12:884–900.
4. Poelaert, JI, Trouerbach, J, De Buyzere, M, et al. Evaluation of transesophageal echocardiography as a diagnostic and therapeutic aid in a critical care setting. Chest. 1995; 107:774–779.
5. Jacka, MJ, Cohen, MM, To, T, et al. The use of and preferences for the transesophageal echocardiogram and pulmonary artery catheter among cardiovascular anesthesiologists. Anesth Analg. 2002; 94:1065–1071.
6. , Expert Round Table on Ultrasound in ICU: International expert statement on training standards for critical care ultrasonography. Intensive Care Me. 2011; 37:1077–1083.
7. Price, S, Via, G, Sloth, E, et al, Echocardiography practice, training and accreditation in the intensive care: document for the World Interactive Network Focused on Critical Ultrasound (WINFOCUS). Cardiovasc Ultrasoun. 2008; 6:49.
8. Hilberath, JN, Oakes, DA, Shernan, SK, et al. Safety of transesophageal echocardiography. J Am Soc Echocardiogr. 2010; 23:1115–1127.
9. Nguyen, TT, Dhond, M, Sabapathy, R, et al. Contrast microbubbles improve diagnostic yield in ICU patients with poor echocardiographic windows. Chest. 2001; 120:1287–1292.
10. Douglas, PS, Khandheria, B, Stainback, RF, et al. ACCF/ASE/ACEP/ASNC/SCAI/SCCT/SCMR 2007. Appropriateness Criteria for Transthoracic and Transesophageal Echocardiography. A Report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American Society of Echocardiography, American College of Emergency Physicians, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance Endorsed by the American College of Chest Physicians and the Society of Critical Care Medicine. J Am Coll Cardiol. 2007; 50:187–204.
11. Mansour, IN, Lang, RM, Furlong, KT, et al. Evaluation of the application of the ACCF/ASE appropriateness criteria for transesophageal echocardiography in an academic medical center. J Am Soc Echocardiogr. 2009; 22:517–522.
12. European Heart Rhythm Association, European Association for Cardio-Thoracic Surgery, Camm, AJ, Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart . 2010; 31:2369–2429.
13. Voci, P, Bilotta, F, Agati, L, Apical transgastric echocardiography: new imaging projections. Eur Heart . 1993; 14:1669–1674.
14. Platts, DG, Sedgwick, JF, Burstow, DJ, et al. The role of echocardiography in the management of patients supported by extracorporeal membrane oxygenation. J Am Soc Echocardiogr. 2012; 25:131–141.
15. Horstkotte, D, Follath, F, Gutschik, E, et al. Guidelines on prevention, diagnosis and treatment of infective endocarditis executive summary; the task force on infective endocarditis of the European Society of Cardiology. Eur Heart J. 2004; 25:267–276.
16. Patel, NH, Hahn, D, Comess, KA, Blunt chest trauma victims: role of intravascular ultrasound and transoesophageal echocardiography in cases of abnormal thoracic aortogram. J Traum. 2003; 55:330–337.
17. De Backer, D, Cholley, BP, Slama, M. Hemodynamic monitoring using echocardiography in the critically ill. New York: Springer; 2011.
18. Costachescu, T, Denault, A, Guimond, JG, et al, The hemodynamically unstable patient in the intensive care unit: hemodynamic vs. transesophageal echocardiographic monitoring. Crit Care Me. 2002; 30:1214–1223.
19. Vignon, P, AitHssain, A, François, B, et al, Echocardiographic assessment of pulmonary artery occlusion pressure in ventilated patients: a transesophageal study. Crit Car. 2008; 12:R18.
20. Teboul, JL, Monnet, X. Prediction of volume responsiveness in critically ill patients with spontaneous breathing activity. Curr Opin Crit Care. 2008; 14:334–339.
21. Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC), European Association for Cardio-Thoracic Surgery (EACTS), Vahanian, A, et al, Guidelines on the management of valvular heart disease. Eur Heart . 2012; 33:2451–2496. [(version 2012.].
22. Torbicki, A, Perrier, A, Konstantinides, S, Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart . 2008; 29:2276–2315.
23. Price, S, Prout, J, Jaggar, SI, et al, ‘Tamponade’ following cardiac surgery: terminology and echocardiography may both mislead. Eur J Cardiothorac Sur. 2004; 26:1156–1160.