Chapter 23 Transcranial Surgery for Pituitary Macroadenomas
Transcranial approaches are used in approximately 1% to 4% of pituitary tumors that require surgical management.1,2 The most common indications for transcranial surgery for pituitary tumors are large extrasellar tumor extension without sellar enlargement, marked frontal, middle fossa, or clival extension of tumors (particularly dumbbell shaped), very fibrous tumors not amenable to trans-sphenoidal resection (meningiomas), a persistent visual field deficit after incomplete decompression via the trans-sphenoidal approach, a coexistent aneurysm, loss of oculomotor function, ectatic midline carotid arteries, or sphenoid sinusitis.1–3 Treatment selection depends on the tumor characteristics and its associated findings. A thorough understanding of the advantages and disadvantages of each transcranial approach is critical when choosing the most appropriate surgical option.
History
In 1893, Caton and Paul recorded the first attempted, but unsuccessful, pituitary tumor resection using a two-stage lateral subtemporal decompression in a patient with acromegaly.4 This approach was suggested by Sir Victor Horsley, who from 1904 to 1906, used a subfrontal approach and a lateral middle fossa approach to operate on 10 patients with pituitary tumors.5 In comparison to the 50% to 80% mortality rate of his colleagues, Horsley reported a mortality rate of 20% using this approach. In 1904, Kilani showed an extensive bifrontal intradural approach using cadavers.6 In 1905, Krause demonstrated that it was possible to reach the sella turcica via a frontal transcranial approach in a living patient.7 In 1912, McArthur described an extradural approach via resection of the supraorbital ridge and orbital plate.8 These approaches formed the basic foundation upon which subsequent neurosurgeons improved and modified surgical treatment of these pathologies.8,9
Harvey Cushing played a central role in standardizing the preference for transcranial surgery over the trans-sphenoidal approach. Cushing’s initial experience with transcranial approaches to sellar neoplasms was very discouraging and as such, he adopted the trans-sphenoidal approach that was undergoing marked improvement at the time.10 Cushing had a mortality rate of 5.6% in his series of 231 patients from 1910 to 1925 who had undergone surgical resection via the trans-sphenoidal approach.11 The morbidity and mortality associated with the trans-sphenoidal approach at the time were primarily attributed to infection, most often associated with postoperative cerebrospinal fluid (CSF) leak, postoperative edema, and hemorrhage.9 These complications coupled with Cushing’s extreme interest in intracranial surgery prompted the development of a novel transcranial approach to sellar-based neoplasms. Cushing’s increased experience with transcranial procedures, and subsequently the reduced mortality he experienced utilizing these approaches, led to a preferential treatment of sellar tumors using an intracranial approach.9 The transfrontal craniotomy, performed via a direct midline approach, allowed Cushing to attain a more extensive resection, better visualization and decompression of the optic nerve and chiasm, lower recurrence rates, and better recovery of vision.9,12 The transfrontal approach also avoided the potentially fatal complication of infection seen with the trans-sphenoidal approach. For these reasons, Cushing discarded the trans-sphenoidal operation, and with his prominence in American neurosurgery, he helped to usher in a strong preference for the transfrontal approach.11,13,14
From the 1930s to the 1950s, transcranial approaches to the pituitary dominated neurosurgical practice and teaching. In the late 1950s and 1960s, the trans-sphenoidal procedure received renewed interest as many technological and surgical advances improved this surgical approach including: (1) development of a lighted speculum retractor by Dott in 1956,15 (2) enhanced surgical accuracy with the intraoperative radiofluoroscopy to define nasal passage anatomy and control the position of the instruments by Guiot,16 (3) development of antibiotics in the 1950s to reduce the surgical mortality associated with this approach,17 and (4) introduction of corticosteroids allowing safer surgeries to be performed on the pituitary gland.12 Some of these advances were utilized by Hardy in 1971 in his landmark paper that demonstrated the trans-sphenoidal operation on over 300 patients undergoing hypophysectomy.18 In his paper, he described the technical aspects of the trans-sphenoidal hypophysectomy and also reported a morbidity and mortality rate that was less than with transcranial approaches. Specifically, the risk of CSF leak was markedly reduced when the arachnoid was left intact. Since then, trans-sphenoidal surgery has dominated neurosurgical treatment of sellar-based lesions.
Specific Indications
While the trans-sphenoidal approach is standard for resection of sellar/parasellar region tumors, there are specific indications for transcranial approaches. The usual reason for using a transcranial approach is doubt about the diagnosis.19 For instance, if the lesion is not a pituitary adenoma, but instead perhaps a meningioma or craniopharyngioma, a craniotomy is advised because these lesions are more safely removed transcranially.19 For tumors with a wide extension on the cranial base, craniotomy remains a superior approach. Craniotomy is more effective in a variety of other surgical cases as well because it increases access to tumors, facilitates preservation of the surrounding neurovascular structures, improves visualization at the dural edge, and allows earlier identification of the cranial nerves and the feeding vessels.20
Failed Trans-Sphenoidal Surgery
A major indication for a transcranial procedure is a failed trans-sphenoidal surgery. Failure can occur for multiple different reasons. Any intrinsic tumor characteristic that does not allow it to fall into the sella compromises the efficacy of trans-sphenoidal surgery. For instance, if a pituitary adenoma is too fibrous, a trans-sphenoidal approach will fail. In those circumstances, it is advisable to take no more than a biopsy specimen and obliterate the sphenoid sinus. The latter action may seem unnecessary with a large tough tumor between the nasal cavity and the CSF. The consistency of the tumor, not the size, limits the effectiveness of trans-sphenoidal surgery.19
Failure of the suprasellar extension to descend is another reason for failed trans-sphenoidal surgery. The usual cause is that a component of the tumor is extending superiorly, laterally, or anteriorly. If the suprasellar extension has pushed vertically, it usually falls into the tumor cavity created by the trans-sphenoidal surgeon. If an extension of tumor hooks around the optic nerve or carotid artery, this extension prevents descent of the tumor, and the tumor extension above the carotid artery or chiasm is inaccessible.19 The anterior extension is particularly troublesome for the trans-sphenoidal surgeon because it is at the wrong angle for the line of approach.19 In addition, recurrent pituitary adenomas may not fall down satisfactorily because of adhesions between the tumor and the surrounding brain. In these circumstances, the combined transcranial/trans-sphenoidal approach may be the best method to completely remove the tumor. Thus, it may be justified if the tumor has recurred despite previous irradiation. Radical surgery is also indicated for hormone-secreting adenomas such as those that occur in patients with acromegaly (or Cushing syndrome), in which hormonal cure depends on total removal of the tumor.19 A trans-sphenoidal procedure is also insufficient when the surgery fails to remove adequate amounts of tumor, particularly enough to decompress the optic chiasm.19,21 The failure often manifests after incomplete decompression as a persistent visual field deficit or persistent loss of vision.
Para/Extrasellar Extension
In cases with large extrasellar tumor extension without sellar enlargement, the pituitary gland may be vulnerable to a trans-sphenoidal approach. Usually in this case, the suprasellar component either encases optic nerves or intracranial arteries or it spreads over the surface of the planum sphenoidale.21
Also, pituitary adenomas can invade one or both cavernous sinuses. While the trans-sphenoidal approach is limited to the compartment of the cavernous sinus medial to the C4 segment of the internal carotid artery, this approach can be used in patients with non-secreting adenomas in which tumor debulking and neurovascular decompression is sufficient.21 Transcranial approaches are warranted, however, if the adenoma is secretory, or if the parasellar extension ventures beyond the cavernous sinus into the middle fossa.21 Transcranial approaches are also indicated when restoration of oculomotor function is the goal.
Dumbbell-shaped pituitary adenomas that have a ‘narrow’ waist created by a thick diaphragm sellae and a small pituitary stalk opening warrant a transcranial approach over a trans-sphenoidal. One can predict the failure of trans-sphenoidal surgery for a dumbbell shaped lesion based on tumor compression into the sella by intraoperative lumbar intrathecal injection of sterile saline and endoscopic navigation of the opening in the diaphragm sellae.21
Other
Co-existent aneurysms, ectatic carotid arteries, and severe sinus infection are indications for transcranial surgery. Aneurysms are found concomitantly in approximately 1.1% of all pituitary adenoma cases.21,22 If an aneurysm adjacent to a pituitary adenoma (i.e. an aneurysm of the anterior cerebral artery) is detected preoperatively, both lesions can be potentially treated in the same operation. Co-treatment of these lesions is more applicable when an aneurysm will be affected by manipulation of the regional anatomy.21 Alternative treatment strategies include a staged-procedure, surveillance of either lesion or nonsurgical treatments such as endovascular coiling for the aneurysm and radiotherapy for the pituitary adenoma.21 In terms of ectatic vessels, the tumor’s relationship to the intercavernous carotid arteries should be taken into account when deciding on an appropriate approach. There is commonly a 1 to 3 mm separation between the medial margin of the internal cerebral arteries and the lateral surface of the pituitary gland.23 Ectatic carotid arteries can veer into the midline trajectory preventing a trans-sphenoidal approach.9 Also, if a sinus infection is severe and surgical delay will pose a threat of acute neurological deterioration, a transcranial approach is warranted.20,21
Diagnosis and Workup
The preoperative work-up for patients with signs or symptoms of a sellar neoplasm should include formal testing of the patient’s visual fields and pituitary function. A T1-weighted magnetic resonance imaging (MRI) scan with and without gadolinium in the sagittal and coronal planes should also be performed to allow proper visualization of the optic nerves, optic chiasm, carotid arteries, cavernous sinuses and surrounding soft tissue.19,24 A T2-weighted scan may be useful in determining the fibrotic nature of a tumor in this region to aid in surgical planning. Although no reliable predictor exists, evidence suggests that pituitary adenomas with a homogeneously isointense, as opposed to hypertense, signal on T2-weighted MRI are predicted to be firm and fibrotic, although this is not routinely used radiologic criteria.20,21,25
The position of the optic chiasm can be predicted on the sagittal MRI scan by finding the anterior communicating artery.19 While the length of the intracranial optic nerves varies from patient to patient, tumor extension influences the distance of the optic nerves to the tuberculum sella. If the suprasellar extension is thrust between the optic nerves, it pushes the chiasm upward and backward, allowing good surgical access. On occasion the suprasellar extension pushes the chiasm upward and forward, severely limiting surgical access.19 This is called a “prefixed chiasm.”
Craniotomy for tumors in this region carries the same general risks as any operation (i.e., deep vein thrombosis, bleeding, infection, anesthetic risks), however there are specific risks associated such as damage to the optic nerves (resulting in impaired vision or visual fields), damage to the internal carotid artery or anterior cerebral artery, and damage to the pituitary stalk (resulting in hypopituitarism and diabetes insipidus).19 The transcranial approach almost inevitably causes hypopituitarism because the normal pituitary tissue is pushed superiorly under the diaphragm sella, and this tissue is specifically coagulated and cut by the surgeon en route to the tumor.19 The potential for harm to the vascular structures in this area necessitates cross-matching of 2 pints of blood. Anticonvulsants should be started preoperatively because epilepsy is possible after transcranial surgery. Cerebrospinal fluid rhinorrhea or anosmia can occasionally occur, especially if excessive retraction of the frontal lobe is indicated.19
Pterional
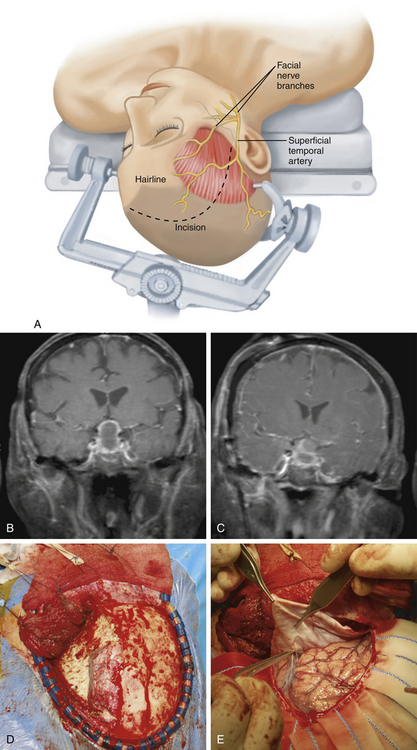
The frontosphenotemporal or pterional craniotomy is the most commonly used transcranial approach to pituitary tumors (Fig. 23-1). It was popularized by Yaşargil as an approach to intracranial aneurysms and is now the most widely used transcranial approach in neurosurgery.26–28 It provides a direct path to the sella turcica and allows removal of large pituitary tumors with minimal brain retraction. It should also be the transcranial approach of choice for tumors when a prefixed chiasm is present as the tumor can be resected safely underneath the optic chiasm.27
Technique
After reflecting the skin flap and fat pad, the temporalis muscle is elevated. An incision is made in the temporalis fascia 1.5 cm posterior to the frontozygomatic process and continued posteriorly along the linea temporalis. A 1- to 2-cm cuff is left attached to the linea temporalis for reattachment of the temporalis flap during closure.29 Using subperiosteal dissection, the temporalis myofascial flap is elevated off of the skull.30 Once the myofascial flap is completely reflected anteriorly and inferiorly, it is held in place using fish hooks.
The frontosphenotemporal craniotomy may be created using varying numbers of burr holes and either a standard router with the footplate attachment or with the Gigli saw. We prefer to make our craniotomy using two burr holes: (1) in the squamous portion of the temporal bone just superior to the root of zygoma and (2) at the MacCarty keyhole. The ideal location for the MacCarty keyhole burr hole is to create it on the frontosphenoid suture approximately 5 to 6 mm posterior to the junction of the frontozygomatic, the sphenozygomatic, and the frontosphenoid sutures.31 A Penfield #3 dissector is used to dissect the dura from the inner table.A standard router with footplate on a pneumatic drill is utilized to turn the craniotomy. The supraorbital foramen serves as the medial border of the craniotomy. Once the bone flap it ready to be elevated, it is important to free the dura that remains attached to the inner table with a Penfield #3 dissector.
After the bone flap is removed, extradural bony removal continues prior to opening the dura. The frontal and temporal dura are dissected off of the ridge of the sphenoid bone with a Penfield #1 dissector. The bony sphenoid ridge is removed using rongeurs. The sharp edges of bone may be smoothed out with a pneumatic drill with a #2 or #3 diamond bit. It is important to constantly irrigate the drill to prevent thermal injury to the dura and underlying optic nerve. The optic canal is skeletonized and the anterior clinoid process completely removed. The underlying clinoidal segment of the internal carotid artery is then visualized. When approaching tumors with wide parasellar extension or that encroach the cavernous sinus, uncovering the foramen rotundum and foramen ovale gives additional needed exposure to minimize temporal retraction.26
Orbitozygomatic Craniotomy
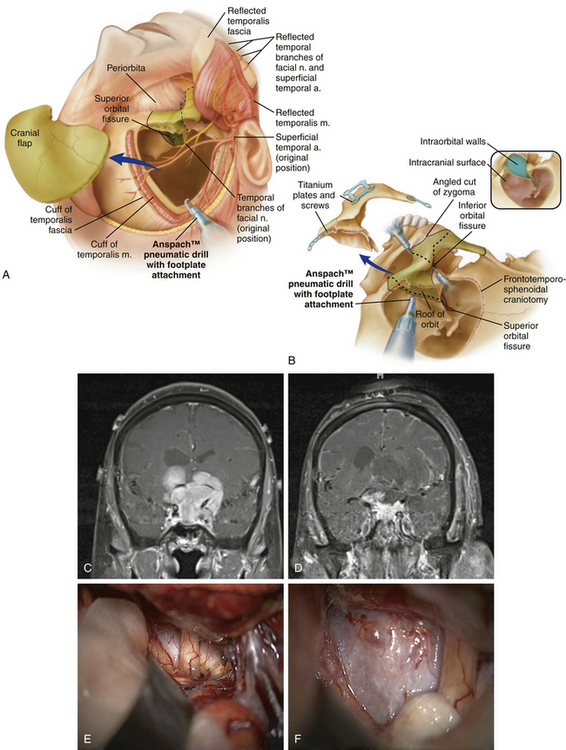
The orbitozygomatic craniotomy is a second option in the treatment of sellar masses that have significant extension (Fig. 23-2). It arose from the supraorbital craniotomy first described by Jane et al. and evolved into an approach incorporating the orbitozygomatic and pterional cranial segments as described by Pellerin et al. and Hakuba and colleagues.32–34 This approach provides a greater degree of exposure with reduced brain retraction compared to the pterional approach. It is especially useful in approaching lesions with considerable superior extension, those that extend laterally into the cavernous sinus, and those with significant parasellar and interpeduncular involvement.26 However, given that it is less commonly used than the pterional approach, familiarity with the technical nuances of the orbitozygomatic approach is critical to minimize the increased risks associated with removal of the orbital rim.
Technique
Planning, positioning, and initial flap creation are done according to the previous description for the pterional craniotomy. The temporalis dissection differs due to the need for inferior reflection of the temporalis muscle flap in order to gain additional bony exposure to perform the orbitozytomatic osteotomies. The incision in the temporalis muscle is made starting 2 cm posterior to the frontozygomatic process and carried posterior 1 cm below the superior temporal line. The incision is angled inferiorly and terminates at the root of the zygoma. The myofascial temporalis flap is then dissected using a fan periosteal elevator. The temporalis flap is then reflected inferiorly underneath the zygomatic process until the inferior orbital fissure is visualized. It is critical to have adequate exposure of the inferior orbital fissure since two of the osteotomies pass through it. The masseter can be detached from the inferior portion of the zygomatic process. Alternatively, the masseter can be left attached and simply reflected inferiorly after the osteotomies are made in order to reduce the risk of postoperative trismus.35
The first step is to create a frontosphenotemporal craniotomy as described above. The second step is to create the orbitozygomatic osteotomies to release the superior orbital roof, lateral orbital wall, and zygoma. These have been described using varying number of cuts and instruments (e.g., oscillating saw). We accomplish this by making five osteotomies and prefer to use a long router bit with footplate for all of the cuts. The footplate provides a protective barrier that protects the soft tissues of the orbit. The first cut separates zygomatic arch from the root of zygoma. Placement of a dog-bone plate on the zygomatic arch that incorporates the location of the first osteotomy prior to making such cut will facilitate the reconstruction. The second cut separates the frontozygomatic process from the body of the zygoma. To accomplish this, a small retractor is placed gently on the periorbita, exposing the inferior orbital fissure. The footplate is then placed in the anterolateral portion of the inferior orbital fissure to initiate the cut. The third cut separates the temporal process of the zygoma from the zygomatic body. The footplate is placed on the inferior portion of the temporal process of the zygoma and directed obliquely to meet the second cut. The fourth osteotomy cuts through the superior orbital rim and orbital roof. Retractors are again used to protect the orbital contents and the frontal dura. The footplate is placed inside the orbit and directed immediately lateral to the superior orbital notch, over the supraorbital rim, and guided toward the superior orbital fissure. At the posterior orbital rim, the craniotome is directed laterally until reaching the junction of the superior and lateral orbital walls. It is then guided down the lateral wall of the orbit. The fifth osteotomy connects the fourth cut with the posterolateral portion of the inferior orbital fissure. The footplate is placed in the inferior orbital fissure in the infratemporal fossa and directed superiorly to complete second portion of the orbitozygomatic craniotomy.
Bifrontal and Extended Bifrontal
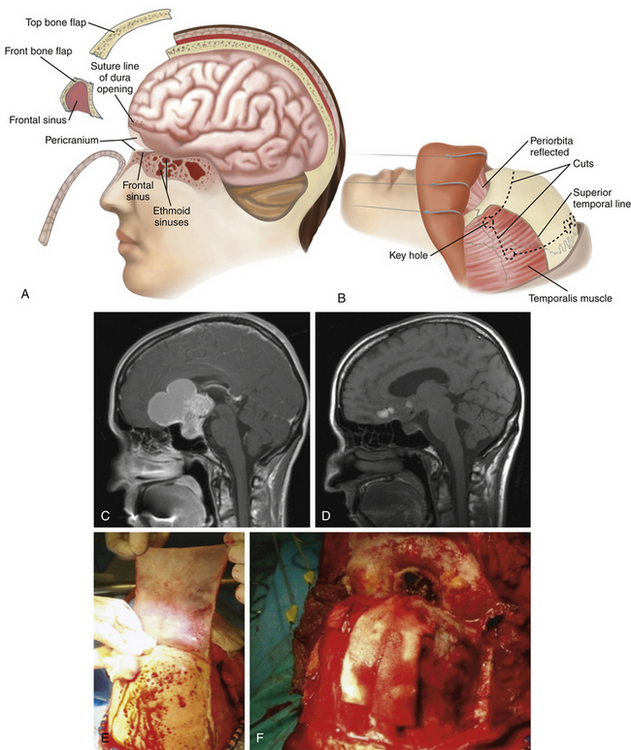
The bifrontal and extended bifrontal are third-line transcranial options to approach pituitary tumors. The original concept of a bifrontal craniotomy was described by Frazier in 1913 and subsequently applied and developed to correction of craniofacial abnormalities by Tessier and to approaching skull base tumors by Derome.36–38 These approaches are useful for tumors with significant superior and two-sided lateral extension and for those tumors that involve the medial orbits, clivus, upper air sinuses, and third ventricle. Removal of the orbital bar in the extended bifrontal craniotomy provides wide exposure of the anterior skull base and allows extensive inferior-superior viewing with minimal brain retraction.
Technique
The bifrontal and bifrontal-extended approaches are options that can be used when a suprasellar tumor has significant superior, inferior, and/or lateral extension (Fig. 23-3). In the bifrontal extended approach the orbital bar is removed, which increases the inferior-to-superior mobility and allows generous bilateral exposure to the cavernous sinuses. Specifically, the orbital contents can be mobilized to widen the path of exposure. With the additional exposure afforded by removal of the orbital rim, the amount of retraction on the frontal lobes is minimized. However, these approaches are considered as secondary alternatives to the more commonly used pterional and orbitozygomatic approaches because the amount of exposure attained is rarely needed for resection of pituitary macroadenomas.
The superior sagittal sinus is suture ligated next to the crista galli and the falx is cut until its deep edge. Further dissection beginning with the olfactory tracts is performed under the microscope. The olfactory nerves should be covered during dissection to prevent dessication. They are then dissected from the pia-arachnoid surface of the frontal lobes. If additional removal of bone from the skull base is required, the olfactory nerves cannot be preserved. Sacrifice of the olfactory nerves allows separation of the dura from the cribriform plate and the crista galli. During dissection, a single silicon-covered brain retractor is utilized to minimize unnecessary, prolonged retraction.
Supraorbital (Keyhole)
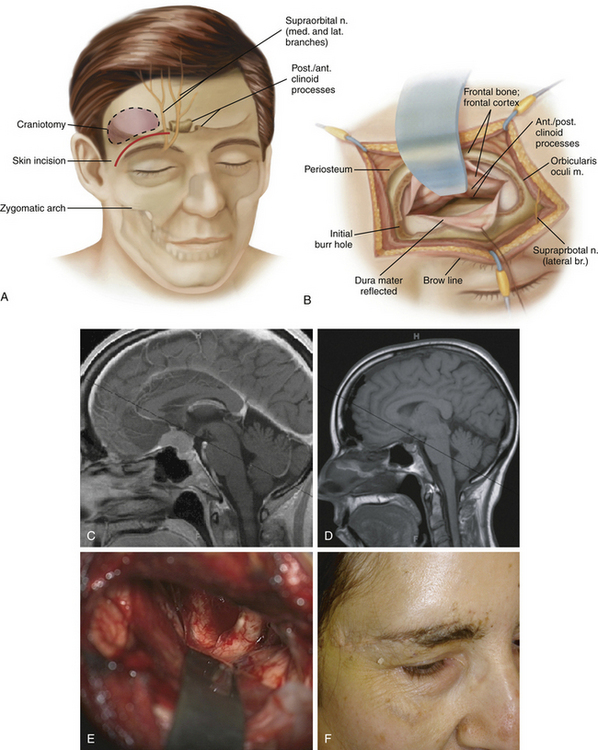
The supraorbital (keyhole) craniotomy has been previously described to approach anterior and middle fossa lesions, and specifically sellar lesions39–43 (Fig. 23-4). The advantages of this approach are that it requires a smaller incision, a single burr hole, carries a lower risk of injury to neurovascular structures that supply the temporalis muscle, minimizes the need for excessive brain retraction, and results in excellent cosmetic outcomes.40,41 Pituitary macroadenomas that are confined to the sellar or region or those with minimal parasellar extension can be approached using the supraorbital (keyhole) approach. A supraorbital approach is not appropriate for pituitary tumors with extreme parasellar extension as the exposure is too limited for these tumors.40,44 The presence of a large frontal sinus on preoperative imaging should discourage the use of this approach as well.40 Overall, the selection of this approach will depend on the individual tumors and the surgeon’s comfort level given that it is not the standard craniotomy for pituitary macroadenomas.40,41 See Chapter 35 for the technical description of the supraorbital (keyhole) craniotomy.
Complications
Frontal Lobe Cyst Formation
Cystic enlargement of an area of frontal lobe damage, sufficient to act as a space-occupying lesion, may occur. Adams described one such case and speculated on the causal mechanism.19,45
Damage to Internal Carotid, Anterior Cerebral, or Anterior Communicating Arteries
The previous author of this chapter described the unpleasant occasion of cutting an internal carotid artery.19 The patient was elderly, had undergone two previous craniotomies and radiotherapy for the pituitary tumor, and the artery was embedded in scar tissue. Although the artery was not visible, it should have been traced proximally from the middle cerebral artery. This tracing can be extremely difficult. On another occasion, he described damaging both anterior cerebral arteries. The tumor was firmly adherent to these vessels, and in hindsight the author stated he should have stopped the operation, leaving tumor behind around the vessels.
Salt-Wasting Syndrome
Salt-wasting syndrome is rare. The syndrome is also alarming, and usually occurs 1 to 2 weeks after the operation.4–7 Surgeons have described their patients developing a headache, rapidly lapsing into a coma, and on admission presenting with a low sodium value. The mechanism is unknown, but salt-wasting syndrome is assumed to be caused by inappropriate antidiuretic hormone secretion. It is difficult to measure antidiuretic hormone, and so not enough is known about this rare condition. It is advisable to place a central venous line to determine if the problem is primarily low sodium (low venous pressure) or water overload (high venous pressure). Patients should be treated empirically with rapid infusions of fluids and salt, then restricted fluids to increase the serum sodium. Until more is known about this mysterious condition, it can be treated only empirically. Kelly and colleagues46 recommend using urea for salt-wasting syndrome, pointing out that urea enhances sodium reabsorption at the kidney.
Postoperative Visual Deterioration
Olson and co-workers47 have described acute deterioration after trans-sphenoidal surgery resulting from herniation of a chiasm into the pituitary fossa. This condition is amazingly rare, considering how, after trans-sphenoidal surgery, the diaphragma rapidly descends to the floor of the pituitary fossa on many occasions without visual impairment. The question can be asked if herniation per se (apart from the case reported by Olson and co-workers) can cause visual deterioration.
Occasionally, a unilateral loss of vision occurs about 8 days after surgery. Morello and Frera48 describe a patient, and the author agrees with their conclusion that ischemic damage is the most likely explanation. Insidious visual deterioration is almost always caused by recurrent tumor, which usually reproduces the original visual field deficit (i.e., a bitemporal hemianopia). Another possibility is radiation damage to the optic nerves or chiasm after radiotherapy. This visual deterioration usually occurs 9 to 18 months after radiotherapy and is often sudden and unilateral. The mechanism is vascular damage and hence similar to stroke. Some improvement may occur. Guy and colleagues49 suggest gadolinium-enhanced MRI scanning to confirm the diagnosis of radiation damage.
Couldwell W.T. Transsphenoidal and transcranial surgery for pituitary adenomas. J Neurooncol. 2004;69:237-256.
Couldwell W.T., Simard M.F., Weiss M.H., Norton J.A. Pituitary and adrenal. In: Schwartz S.I., Shires G.T., Spencer F.C. Principles of Surgery. 7th ed. New York: McGraw-Hill; 1999:1613-1658.
Czirjak S., Szeifert G.T. Surgical experience with frontolateral keyhole craniotomy through a superciliary skin incision. Neurosurgery. 2001;48:145-149. discussion 9-50
Day J.D. Surgical approaches to suprasellar and parasellar tumors. Neurosurg Clin North Am. 2003;14:109-122.
Derome P. Transbasal approach to tumors invading the skull base. In: Schmidek H., Sweet W. Operative Neurosurgical Techniques Indications, Methods, and Results. 4th ed. Philadelphia: WB Saunders Company; 1993:427-441.
Frazier C. An approach to the hypophysis through the anterior cranial fossa. Ann Surg. 1913;7:145-150.
Hakuba A., Liu S., Nishimura S. The orbitozygomatic infratemporal approach: a new surgical technique. Surgical Neurol. 1986;26:271-276.
Hayashi N., Hirashima Y., Kurimoto M., et al. One-piece pedunculated frontotemporal orbitozygomatic craniotomy by creation of a subperiosteal tunnel beneath the temporal muscle: technical note. Neurosurgery. 2002;51:1520-1523. discussion 1523-1524
Henderson W.R. The pituitary adenomata. A follow-up study of the surgical results in 338 cases (Dr. Harvey Cushing’s series). Br J Surg. 1939;26:811-921.
Jallo G.I., Bognar L. Eyebrow surgery: the supraciliary craniotomy: technical note. Neurosurgery. 2006;59:ONSE157-ONSE158. discussion ONSE-8
Jallo G.I., Suk I., Bognar L. A superciliary approach for anterior cranial fossa lesions in children. Technical note. J Neurosurg. 2005;103:88-93.
Jane J.A., Park T.S., Pobereskin L.H., Winn H.R., Butler A.B. The supraorbital approach: technical note. Neurosurgery. 1982;11:537-542.
Kadri P.A., Al-Mefty O. The anatomical basis for surgical preservation of temporal muscle. J Neurosurg. 2004;100:517-522.
Kelly D.F., Laws E.R.Jr., Fossett D. Delayed hyponatremia after transsphenoidal surgery for pituitary adenoma: report of nine cases. J Neurosurg. 1995;83:363-367.
Liu J.K., Weiss M.H., Couldwell W.T. Surgical approaches to pituitary tumors. Neurosurg Clin North Am. 2003;14:93-107.
Musleh W., Sonabend A.M., Lesniak M.S. Role of craniotomy in the management of pituitary adenomas and sellar/parasellar tumors. Expert Rev Anticancer Ther. 2006;6(suppl 9):S79-S83.
Pellerin P., Lesoin F., Dhellemmes P., et al. Usefulness of the orbitofrontomalar approach associated with bone reconstruction for frontotemporosphenoid meningiomas. Neurosurgery. 1984;15:715-718.
Raza S.M., Thai Q.A., Pradilla G., Tamargo R.J. Frontozygomatic titanium cranioplasty in frontosphenotemporal (“pterional”) craniotomy. Neurosurgery. 2008;62:262-264. discussion 4-5
Rhoton A.L.Jr., Hardy D.G., Chambers S.M. Microsurgical anatomy and dissection of the sphenoid bone, cavernous sinus and sellar region. Surg Neurol. 1979;12:63-104.
Sanchez-Vazquez M.A., Barrera-Calatayud P., Mejia-Villela M., et al. Transciliary subfrontal craniotomy for anterior skull base lesions. Technical note. J Neurosurg. 1999;91:892-896.
Shimizu S., Tanriover N., Rhoton A.L.Jr., et al. MacCarty keyhole and inferior orbital fissure in orbitozygomatic craniotomy. Neurosurgery. 2005;57:152-159. discussion 159
Tessier P., Guiot G., Derome P. Orbital hypertelorism. II. Definite treatment of orbital hypertelorism (OR.H.) by craniofacial or by extracranial osteotomies. Scand J Plast Reconstr Surg. 1973;7:39-58.
van Lindert E., Perneczky A., Fries G., Pierangeli E. The supraorbital keyhole approach to supratentorial aneurysms: concept and technique. Surg Neurol. 1998;49:481-489. discussion 489-490
Vishteh A., Marciano F., David C., et al. The pterional approach. Oper Tech Neurosurg. 1998;1:39-49.
Youssef A.S., Agazzi S., van Loveren H.R. Transcranial surgery for pituitary adenomas. Neurosurgery. 2005;57:168-175. discussion 168-175
1. Wilson C.B. A decade of pituitary microsurgery. The Herbert Olivecrona lecture. J Neurosurg. 1984;61:814-833.
2. Wilson C.B. Endocrine-inactive pituitary adenomas. Clin Neurosurg. 1992;38:10-31.
3. Wilson C.B. Role of surgery in the management of pituitary tumors. Neurosurg Clin North Am. 1990;1:139-159.
4. Caton R., Paul F.T. Notes of a case of acromegaly treated by operation. BMJ. 1893;2:1421-1423.
5. Horsley V. On the technique of operations on the central nervous system. BMJ. 1906;2:411-423.
6. Kilani O.G.T. Some remarks on tumors of the chiasm, with a proposal how to reach the same by operation. Ann Surg. 1904;40:35-43.
7. Hirnchirurgie Krause F. (freilegung do hypophyse). Deutchse Klin. 1905;8:953-1024.
8. McArthur L.L. An aseptic surgical access to the pituitary body and its neighborhood. JAMA. 1912;58:2009-2011.
9. Couldwell W.T. Transsphenoidal and transcranial surgery for pituitary adenomas. J Neurooncol. 2004;69:237-256.
10. Cushing H. The Weir Mitchell Lecture. Surgical experiences with pituitary disorders. JAMA. 1914;63:1515-1525.
11. Henderson W.R. The pituitary adenomata. A follow-up study of the surgical results in 338 cases (Dr. Harvey Cushing’s series). Br J Surg. 1939;26:811-921.
12. Welbourn R.B. The evolution of transsphenoidal pituitary microsurgery. Surgery. 1986;100:1185-1190.
13. Couldwell W.T., Simard M.F., Weiss M.H., Norton J.A. Pituitary and adrenal. In: Schwartz S.I., Shires G.T., Spencer F.C. Principles of Surgery. 7th ed. New York: McGraw-Hill; 1999:1613-1658.
14. Rosegay H. Cushing’s legacy to transsphenoidal surgery. J Neurosurg. 1981;54:448-454.
15. Horwitz N.H. Library: historical perspective. Norman M. Dott (1897-1973). Neurosurgery. 1999;45:944-948.
16. Hardy J., Wigser S.M. Trans-sphenoidal surgery of pituitary fossa tumors with televised radiofluoroscopic control. J Neurosurg. 1965;23:612-619.
17. Zervas N.T. Reflections on the surgery of the pituitary. Clin Neurosurg. 1980;27:124-132.
18. Hardy J. Transsphenoidal hypophysectomy. J Neurosurg. 1971;34:582-594.
19. Adams C.B. Transcranial surgery for pituitary macroadenomas. In: Schmidek H.H., Roberts D.W. Schmidek and Sweet’s Operative Neurosurgical Techniques. Indications, Methods, and Results.. 5th edition. Philadelphia: WB Saunders; 2005:348-354.
20. Musleh W., Sonabend A.M., Lesniak M.S.. Role of craniotomy in the management of pituitary adenomas and sellar/parasellar tumors. Expert Rev Anticancer Ther, :2006;6 suppl 9:S79-S83
21. Youssef A.S., Agazzi S., van Loveren H.R. Transcranial surgery for pituitary adenomas. Neurosurgery. 2005;57:168-175. discussion 175
22. Pia H.W., Obrador S., Martin J.G. Association of brain tumours and arterial intracranial aneurysms. Acta Neurochir (Wien). 1972;27:189-204.
23. Rhoton A.L.Jr, Hardy D.G., Chambers S.M. Microsurgical anatomy and dissection of the sphenoid bone, cavernous sinus and sellar region. Surg Neurol. 1979;12:63-104.
24. Karnaze M.G., Sartor K., Winthrop J.D., Gado M.H., Hodges F.J.3rd. Suprasellar lesions: evaluation with MR imaging. Radiology. 1986;161:77-82.
25. Snow R.B., Johnson C.E., Morgello S., et al. Is magnetic resonance imaging useful in guiding the operative approach to large pituitary tumors? Neurosurgery. 1990;26:801-803.
26. Day J.D. Surgical approaches to suprasellar and parasellar tumors. Neurosurg Clin North Am. 2003;14:109-122.
27. Liu J.K., Weiss M.H., Couldwell W.T. Surgical approaches to pituitary tumors. Neurosurg Clin North Am. 2003;14:93-107.
28. Vishteh A., Marciano F., David C., et al. The pterional approach. Oper Tech Neurosurg. 1998;1:39-49.
29. Raza S.M., Thai Q.A., Pradilla G., Tamargo R.J. Frontozygomatic titanium cranioplasty in frontosphenotemporal (“pterional”) craniotomy. Neurosurgery. 2008;62:262-264. discussion 264-265
30. Kadri P.A., Al-Mefty O. The anatomical basis for surgical preservation of temporal muscle. J Neurosurg. 2004;100:517-522.
31. Shimizu S., Tanriover N., Rhoton A.L.Jr, et al. MacCarty keyhole and inferior orbital fissure in orbitozygomatic craniotomy. Neurosurgery. 2005;57:152-159. discussion 159
32. Hakuba A., Liu S., Nishimura S. The orbitozygomatic infratemporal approach: a new surgical technique. Surg Neurol. 1986;26:271-276.
33. Jane J.A., Park T.S., Pobereskin L.H., et al. The supraorbital approach: technical note. Neurosurgery. 1982;11:537-542.
34. Pellerin P., Lesoin F., Dhellemmes P., et al. Usefulness of the orbitofrontomalar approach associated with bone reconstruction for frontotemporosphenoid meningiomas. Neurosurgery. 1984;15:715-718.
35. Hayashi N., Hirashima Y., Kurimoto M., et al. One-piece pedunculated frontotemporal orbitozygomatic craniotomy by creation of a subperiosteal tunnel beneath the temporal muscle: technical note. Neurosurgery. 2002;51:1520-1523. discussion 1523-1524
36. Derome P. Transbasal approach to tumors invading the skull base. In: Schmidek H., Sweet W. Operative Neurosurgical Techniques Indications, Methods, and Results. 3rd ed. Philadelphia: WB Saunders; 1995:427-441.
37. Frazier C. An approach to the hypophysis through the anterior cranial fossa. Ann Surg. 1913;57:145-150.
38. Tessier P., Guiot G., Derome P. Orbital hypertelorism. II. Definite treatment of orbital hypertelorism (OR.H.) by craniofacial or by extracranial osteotomies. Scand J Plast Reconstr Surg. 1973;7:39-58.
39. Frazier C. An approach to the hypophysis through the anterior cranial fossa. Ann Surg. 1913;7:145-150.
40. Jallo G.I., Bognar L. Eyebrow surgery: the supraciliary craniotomy: technical note. Neurosurgery. 2006;59:ONSE157-ONSE158. discussion ONSE-158
41. Jallo G.I., Suk I., Bognar L. A superciliary approach for anterior cranial fossa lesions in children. Technical note. J Neurosurg. 2005;103:88-93.
42. Sanchez-Vazquez M.A., Barrera-Calatayud P., Mejia-Villela M., et al. Transciliary subfrontal craniotomy for anterior skull base lesions. Technical note. J Neurosurg. 1999;91:892-896.
43. van Lindert E., Perneczky A., Fries G., Pierangeli E. The supraorbital keyhole approach to supratentorial aneurysms: concept and technique. Surg Neurol. 1998;49:481-489. discussion 9-90
44. Czirjak S., Szeifert G.T. Surgical experience with frontolateral keyhole craniotomy through a superciliary skin incision. Neurosurgery. 2001;48:145-149. discussion 9-50
45. Adams C.B.T. A Neurosurgeon’s Notebook. Oxford: Blackwell Scientific; 1998.
46. Kelly D.F., Laws E.R.Jr, Fossett D. Delayed hyponatremia after transsphenoidal surgery for pituitary adenoma: report of nine cases. J Neurosurg. 1995;83:363-367.
47. Olson D.R., Guiot G., Derome P. The symptomatic empty sella: prevention and correction via the transsphenoidal approach. J Neurosurg. 1972;37:533-537.
48. Morello G., Frera C. Visual damage after removal of hypophyseal adenomas: possible importance of vascular disturbances of the optic nerve and chiasm. Acta Neurochir (Wien). 1966;15:1-10.
49. Guy J., Mancuso A., Beck R., et al. Radiation-induced optic neuropathy: a magnetic resonance imaging study. J Neurosurg. 1991;74:426-432.