CHAPTER 347 Transcranial Doppler Ultrasonography and Neurosonology
Background and Principles of Ultrasonography
History of Doppler Ultrasound
The use of Doppler ultrasound to measure blood flow was initially reported by Satomora in 1959.1 Satomora and Kaneko were originally interested in measuring cerebral blood flow; however, they concluded that the skull was an insurmountable barrier to the passage of ultrasound and focused on the extracranial carotid arteries in their initial investigations. After refinements in the equipment and advances in the technology, Doppler ultrasound was introduced as a clinical tool to examine blood flow velocity in the extracranial and peripheral arteries. Refinements in signal processing and the introduction of B-mode imaging, as well as duplex imaging (simultaneous display of B-mode tissue imaging and vascular flow velocity measurements), followed by the development of color flow ultrasound, have continued to improve its diagnostic capabilities for extracranial and intracranial vascular pathology. It has recently been demonstrated that sonothrombolysis (the use of ultrasound to help dissolve blood clots) can be used as a treatment of stroke and potential treatment of intracerebral hemorrhage. These observations have extended the applications of Doppler ultrasound from diagnostics to therapeutics in the brain.
Intraoperative Ultrasonography
The size of the craniotomy or laminectomy determines the size of the transducer used. Subcortical lesions can be insonated at a frequency of 7 to 10 MHz, which provides a high-resolution image. Deeper lesions require a lower frequency transducer (perhaps 3 MHz) because attenuation is less with lower frequency sound. For spinal lesions, intraoperative ultrasonography is helpful in determining the appropriate degree of exposure and in localizing the tumor. It can also evaluate the surgical bed for complete resection.2,3 Several investigators have described the benefits of using intraoperative ultrasonography.4,5 In a study of 186 patients, Rubin and Dohrmann found intraoperative ultrasonography to be more useful for small, subcortical lesions.6 The literature describes a number of novel uses of intraoperative ultrasonography ranging from the more traditional localization of subcortical lesions to the localization of contusions in trauma7 and monitoring of ventricular catheter placement.8 Another recent application of intraoperative ultrasound in cranial neurosurgery has been to correct for the effect of brain shift after craniotomy on stereotactic localization of lesions.9
Duplex Ultrasonography
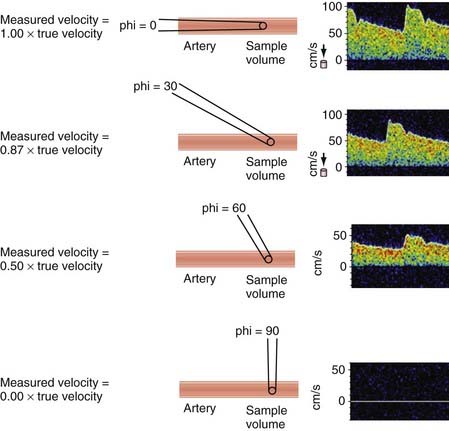
These vascular diagnostic modalities were later integrated with B-mode imaging to produce duplex ultrasonography.10 Modern duplex scanning uses one transducer (5 to 7 MHz) to produce simultaneous B-mode images and pulsed Doppler waveform analysis. The screen of the display module provides real-time anatomic B-mode data and a graphic representation of the Fourier-transformed pulsed spectral analysis. An additional advantage of this diagnostic tool is that it has the ability to correct for variable angles of insonation, which if not kept between 55 and 65 degrees, could significantly alter the recorded frequencies (Fig. 347-1).
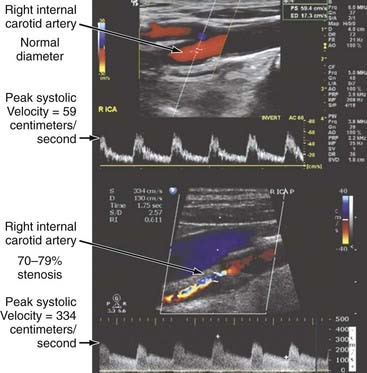
One of the major limitations of duplex scanning is that just a small region of the artery can be studied at any one time. This technology is also only a two-dimensional representation of a three-dimensional dynamic process. Color flow imaging, which combines real-time, B-mode, gray-scale imaging with color encoding of multigated Doppler flow information, begins to address these issues by sampling the mean Doppler frequency shift at various depths over the entire scan area. The color assigned to the frequency data depends on the magnitude and direction of flow, with hue (red versus blue) depicting the direction of flow, the hue’s saturation denoting the magnitude of the frequency shift, and the brightness (luminance) demonstrating the variance in mean flow (i.e., the turbulence), which is superimposed on a gray-scale B-mode image depicting the surrounding anatomy. Color Doppler allows more rapid identification of the component vessels than duplex alone does, and it also provides rapid identification of laminar flow patterns for placement of a single pulsed Doppler gate for acquisition of spectral waveform data. Today, the combined use of B-mode two-dimensional imaging and the real-time, color-enhanced spectral analysis of pulsed wave Doppler have enabled experienced operators to recognize carotid stenosis with a sensitivity approaching 100% (Fig. 347-2).
Transcranial Doppler
Aaslid and colleagues first reported the ability to record blood flow velocity in the intracranial arteries with Doppler ultrasound in 1982 and introduced TCD ultrasonography.11 TCD ultrasonography used an optimized 2-MHz frequency with a pulsed Doppler range-gated design. The lower 2-MHz frequency allowed penetration through the cranium in the thin portions of the bone. The introduction of TCD ultrasound permitted examination of the intracranial vasculature, which has improved the diagnosis of intracranial disease. The technique of TCD ultrasound was initially used in the Department of Neurosurgery in Bern, Switzerland, for the diagnosis of vasospasm after subarachnoid hemorrhage.12 Many subsequent uses have been described for this technology and are reviewed in this chapter.
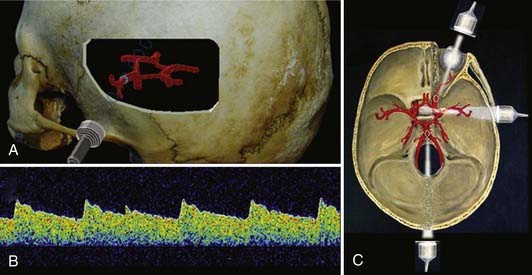
A complete TCD examination normally includes examination through three transcranial windows: the transtemporal, transorbital, and transoccipital windows.13 Through these three windows, most of the basal intracranial arteries can be examined (Fig. 347-3). The transtemporal window is used to examine the middle cerebral artery, (MCA), anterior cerebral artery, intracranial internal carotid artery (ICA), and proximal posterior cerebral artery. The transorbital window is normally used to examine the ophthalmic artery and the intracavernous and supraclinoid ICA. The transoccipital window is used for examination of the posterior circulation, specifically the two vertebral arteries and the basilar artery. The origin of the posterior inferior cerebellar arteries can be examined in many patients.
The original technique for examination was a handheld method in which manual manipulation of the probe with recordings of sample volumes at preselected depths was used to examine specific sites in the intracranial vasculature. Normally, as the distal intracranial arteries become vertically oriented in the sylvian and intrahemispheric fissures, they are considered beyond the scope of the normal TCD examination.14 The distal intracranial arteries can occasionally be examined in unusual circumstances, however, such as after craniotomy, when bone is removed and examination windows are created.
The Doppler signal reflected from intracranial arteries is used to measure ultrasound frequency shifts, which are converted into blood flow velocity in centimeters per second. To determine the actual blood flow in milliliters per minute, the total average velocity, vessel diameter, and angle of insonation need to be known precisely,15 and these parameters are not usually measured during routine TCD examinations. The angle of insonation (angle between the ultrasound beam and the vessel being recorded from) is important to consider when measuring TCD velocity. The true velocity and observed velocity are equal when the angle of insonation is zero, as often happens when examining the MCA trunk. As the angle of insonation increases, there is a reduction in the observed frequency as a function of the cosine of the angle (see Fig. 347-1). For example, if the angle between the ultrasound beam and the flow reflector is 30 degrees, 97% of the true velocity can be observed by the recording device. As the angle of insonation increases, the proportion of the true velocity observed decreases. If the angle of insonation is 60 degrees, 50% of the true velocity is observed by the recording equipment. TCD examination techniques incorporate this strategy to reduce the insonation angle to as low as possible when recording from different intracranial arteries. The introduction of color flow ultrasound adds the ability to determine the angle of insonation to some extent during the examination. Figure 347-3 illustrates a typical velocity recording from the MCA.
Advances in computerized recording devices and the miniaturization of computerized digital processing have allowed easier recording of Doppler signals for analyses. This development has permitted continuous recording of spectral outline and full spectral signals, which enables more detailed analysis of physiologic events.16 Digital recording with the capability of displaying continuous data over time has allowed calculation of changes in velocity secondary to alterations in blood flow evoked by physiologic stimuli, as well as analysis of cerebrovascular control mechanisms, such as CO2 autoregulation and changes in flow caused by cortical activation. Two-channel TCD ultrasound was introduced to resolve physiologic questions regarding control of the cerebral circulation and to document simultaneous changes in venous and arterial flow.15 Subsequently, multichannel TCD has been used to register signals simultaneously from both cerebral hemispheres recorded from both MCAs.17 Multichannel TCD has allowed detection of emboli by recording from multiple sample volumes simultaneously.18 Reflected power recordings have enabled calculation of relative flow volume, which can be used for scientific purposes to calculate changes in cerebral blood flow.15 Power Doppler can be performed transcranially to provide further detail in imaging intracranial vascular structures, such as aneurysms and arteriovenous malformations. Miniaturization has allowed TCD units to become more portable, and in the future, battery-operated devices may serve as a neurovascular stethoscope to permit rapid determination of vessel patency and flow characteristics.
Applications of Duplex Scanning in Cerebrovascular Disease
The most common neurosurgical use of extracranial vascular duplex ultrasound is for the management of stroke and cerebrovascular disease by noninvasive examination of the carotid and vertebral arteries. Certain characteristic waveforms have been recognized to correlate well with the degree of vascular obstruction.19,20 Gentle spectral broadening, usually in the downward slope of the systolic peak and in early diastole, is known to appear in mild stenosis (<50% reduction in diameter or <75% reduction in area). With moderate (50% to 70%) and sometimes severe (80% to 99%) stenosis, increases in waveform frequencies are seen, and the spectral broadening increases to such a degree that the window beneath the spectral waveform is filled (see Fig. 347-2). In severe stenosis (>90%), blood flow is limited despite compensatory increases in velocity, thereby resulting in a decrease in signal amplitude. If the ICA is completely occluded, no flow is visualized within the lumen, and the common carotid waveform begins to resemble the external carotid artery waveform. If the common carotid artery is occluded, the ICA may fill from the external carotid artery, and reversal of flow may be evident in the external carotid artery throughout the cardiac cycle.
Besides waveform characteristics, the following additional parameters are used to determine the severity of stenosis: (1) degree of stenosis by B-mode imaging, (2) peak systolic frequency (velocity), (3) peak diastolic frequency, (4) end-diastolic frequency, (5) ratio of systolic frequency between the internal and common carotid arteries, and (6) ratio of diastolic frequency between the internal and common carotid arteries. The peak systolic frequency, end-diastolic frequency, and systolic frequency ratio are considered the most important and the most predictive of stenosis.19,21 Together, the aforementioned parameters raise the sensitivity and specificity of duplex sonography for the evaluation of carotid stenosis to 99% and 84%, respectively. Correlation between the degree of stenosis determined by duplex and that determined with angiography is excellent.22 Carotid duplex ultrasonography is capable of detecting critical stenosis with nearly 100% sensitivity and specificity. By comparison, arteriography, which is the “gold standard” against which all noninvasive tests are compared, provides little physiologic information regarding flow and nearly no insight on the nature of the plaque (especially “low-grade” ulceration). When compared with surgical findings at endarterectomy, arteriography is also less sensitive than duplex ultrasonography in detecting anatomic stenosis (91% versus 99%).23
When the two modalities were compared in blinded fashion with direct anatomic examination of the plaque after removal, it was noted that duplex scanning not only was as sensitive as angiography in detecting greater than 50% luminal narrowing but also was associated with an overall 96% sensitivity and 100% specificity, as opposed to 92% sensitivity and 100% specificity for angiography.23 Duplex scanning was significantly more accurate than angiography in the detection of smaller plaque ulcerations and significantly more accurate in predicting vessel wall irregularities. Duplex sonography (via its B-mode imaging) was also able to denote plaque morphology, as well as the presence of intraplaque hemorrhage.
Applications of Transcranial Doppler in Stroke and Cerebrovascular Disease
Intracranial Stenosis
Atherosclerotic disease of the intracranial vessels has received much less attention than similar lesions of the extracranial cerebral vessels.24 Although intracranial arterial stenosis secondary to atherosclerosis is less common than extracranial stenosis in Western populations, intracranial atherosclerotic lesions can be a significant cause of stroke and transient ischemic attacks. Bogousslavsky and coworkers analyzed the natural history of patients with MCA stenosis or occlusion entered into the Extracranial-Intracranial Bypass Study Group.25 During a follow-up period of 42 months, 11.7% of the patients per year experienced recurrent cerebrovascular events (transient ischemic attack or stroke).
Several early studies reviewed the accuracy of TCD for the diagnosis of intracranial stenosis secondary to atherosclerosis.26–28 Spencer and Whisler compared carotid siphon stenosis assessed by angiography and TCD.27 In a group of 33 carotid siphons visualized angiographically, 11 showed stenosis ranging from 30% to 75%. Comparison of TCD with angiography revealed a sensitivity of 73% and specificity of 95%. Ley-Pozo and Ringelstein compared intra-arterial digital subtraction angiography with TCD in detecting occlusive disease of the carotid siphon and MCA.28 Sixteen of 17 cases of carotid siphon stenosis were identified correctly with TCD. Rorick and colleagues compared TCD with angiography for the diagnosis of intracranial stenosis.29 These investigators found that the presence of coexisting extracranial stenosis can be a confounding variable affecting the accuracy of TCD in detecting intracranial lesions. It has been useful to examine the intracranial arteries with TCD in patients with cerebrovascular symptoms who are not found to have significant pathology in the extracranial arteries.
Intracranial Hemodynamics
Maintenance of adequate cerebral blood flow depends on sufficient cerebral perfusion pressure through the inflow vessels along with adequate blood pressure, intact anatomy of the extracranial and intracranial vasculature, and compensatory mechanisms when the vasculature or blood pressure becomes compromised. The normally configured circle of Willis functions as a manifold to normalize perfusion pressure to the distal cerebral vessels if one or more extracranial vessels becomes occluded or hemodynamically compromised secondary to stenosis. The circle of Willis normally allows nearly complete compensation in the event of carotid occlusion.30
Compensation for carotid occlusion usually occurs via (1) crossover through the anterior communicating artery and reversed flow in the proximal anterior cerebral artery (A1) ipsilateral to the occlusion, (2) forward flow in the posterior communicating artery ipsilateral to the occlusion, or (3) reversed flow in the ipsilateral ophthalmic artery.31 Major differences in the functional capacity of the circle of Willis are found in the general population, however, and some patients cannot recruit sufficient flow to maintain adequate cerebral perfusion pressure.
Testing of VMR with TCD can provide valuable physiologic information regarding prognosis in patients with certain vascular lesions. Kleiser and Widder reported the results of a natural history study of patients with unilateral carotid occlusion after VMR testing.30 In a group of 86 patients with unilateral carotid occlusion, 11 had exhausted their VMR and had an increased ipsilateral stroke rate (17% per year for 3 years as compared with an ipsilateral stroke rate of 3% per year for the entire group, which is comparable to previously published series). Similar findings of increased stroke risk in patients with carotid occlusion and impaired vascular reserve, measured with other methods, including xenon-enhanced computed tomography (CT) with acetazolamide challenge and positron emission tomography (PET), have been reported.32,33 Patients with impaired vascular reserve and recent symptoms distal to ICA occlusion are the subject of an ongoing randomized clinical trial comparing extracranial-to-intracranial bypass surgery with medical therapy for prevention of stroke (Carotid Occlusion Surgery Study [COSS]).
The prognosis of patients with carotid stenosis may be influenced by VMR. Gur and associates reported that patients with asymptomatic carotid stenosis and impaired VMR had a worse prognosis than did those with intact VMR.34 With the use of VMR testing it appears possible to identify patients with poor hemodynamic reserve and carotid stenosis or occlusion who have a high risk for stroke and may benefit from medical or surgical therapy to improve cerebral perfusion.
Cerebral Autoregulation
Cerebral autoregulation is the ability of the brain to maintain constant cerebral blood flow despite changes in cerebral perfusion pressure. The methodology for determining cerebral autoregulation in the past was cumbersome and invasive and required radioisotopes for cerebral blood flow measurement and vasoactive medication to change the blood pressure. TCD can be used to determine autoregulation noninvasively in the MCA perfusion territories.35,36 Preliminary clinical testing in patients with cerebrovascular occlusive disease indicates that cerebral autoregulation is absent in patients with severely impaired CO2 reactivity.36,37 Noninvasive testing of cerebral autoregulation with TCD may prove useful in the complete hemodynamic evaluation of patients with cerebrovascular occlusive disease and may also be helpful in managing patients after head injury.
Positional Vertebral Artery Obstruction
Cerebrovascular insufficiency can sometimes occur in the posterior circulation secondary to positional obstruction of one or both vertebral arteries in the setting of impaired collateral pathways from the anterior circulation. Several reports have found TCD monitoring of the posterior cerebral arteries bilaterally during various head positions to be useful in detecting transient hemodynamic insufficiency in this condition.38,39 The most common cause of positional vertebral artery obstruction is cervical spondylosis, which can be treated surgically by anterolateral removal.39 The essential diagnostic findings on TCD are a transient drop in posterior cerebral artery velocity signals with head turning and rebound hyperemia on return to a neutral position. Examination with TCD is helpful in differentiating true positional ischemia from positional vertigo and can identify patients who require angiography to define the location and nature of the obstruction.
Intracranial Emboli
Interest has developed in the ability of TCD to directly detect intracranial microemboli in the basal cerebral vessels and their relationship to stroke and ischemic symptoms in patients with occlusive vascular disease. Doppler ultrasound was previously used clinically to detect intravascular air microemboli audibly during cardiac surgery40 and during neurosurgical procedures in the sitting position.41 The ability of TCD to detect intracranial microemboli was first recognized by monitoring MCA velocity during carotid endarterectomy (CEA) and cardiac surgery.42,43
Clinical correlation of detection of intracranial and extracranial microemboli has been described.44 Intracranial microemboli have been detected in patients with atrial fibrillation, prosthetic heart valves,45 carotid stenosis,46,47 fibromuscular dysplasia, arterial dissection,48,49 and intracranial stenosis, as well as during invasive procedures such as angiography,50 angioplasty,51 and vascular and heart surgery52 and after aneurysm treatment.53 Monitoring for intracranial air microemboli after venous injection has been helpful in identifying patients with cardiac and pulmonary defects causing right-to-left circulation shunts.54 Most microemboli do not cause overt symptoms; however, multiple microemboli have been associated with impaired neuropsychological function after cardiac surgery.55 The clinical utility of intracranial monitoring of emboli is still being established, but much has been learned with TCD about microembolism to the brain. Monitoring of emboli with TCD has played a useful role in identifying the site of active embolization in the arterial system in patients with transient ischemic attack or recent stroke, distinguishing embolic versus hemodynamic causes of stroke and transient ischemic attack, identifying high-risk stages of neurovascular and surgical procedures, and detecting patients with vascular lesions at higher risk for ischemic events.
Transcranial Doppler applications in Neurosurgery and Neuro–Critical Care
One of the most common applications of TCD in the care of neurosurgical patients is for detection of cerebral vasospasm after subarachnoid hemorrhage. TCD can be used to monitor the onset, time course, and resolution of vasospasm, and it can be combined with other blood flow measurement techniques to offer useful information to clinicians managing patients with subarachnoid hemorrhage.56
Although the physiology of cerebral vasospasm is complex and not completely understood, prompt diagnosis and institution of treatment of delayed ischemic neurological deficits (DINDs) secondary to vasospasm can be lifesaving and may prevent permanent ischemic brain damage after subarachnoid hemorrhage. It is useful to consider the physiology of large-vessel spasm, which can produce transient or permanent DINDs. It is generally accepted that the risk for vasospasm is closely related to the degree of subarachnoid hemorrhage that occurs at the time of aneurysm rupture or is present after trauma, which can be assessed by the amount of blood clot in the basal cisterns on CT performed within several days of the bleeding episode.57,58 Most patients have some degree of vessel narrowing after subarachnoid hemorrhage, and angiographically identified vasospasm can develop in 70% of patients at some time during their hospital course; however, in only approximately 30% of patients does symptomatic vasospasm characterized by DINDs develop.59 Factors in addition to the degree of vessel narrowing may determine patients in whom DINDs may develop as a result of vasospasm, but the degree and extent of basal vessel narrowing appear to play a major role in determining in which patients delayed ischemia will eventually develop. In addition, the blood pressure response to medical treatment, hematocrit, and viscosity, which may affect oxygen delivery capacity, function of cerebral autoregulation, collateral circulation, intracranial pressure (ICP), and the degree of overall brain dysfunction, as well as other factors, may influence the development of symptoms.60 It is useful to think about large-vessel spasm as progressing through stages that can affect the degree of narrowing in the basal cerebral vessels and the resulting cerebral blood flow.
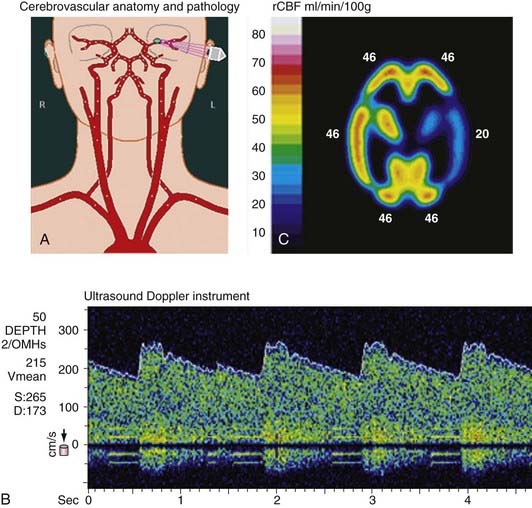
In the early stages of vasospasm, proximal vessel narrowing occurs, and velocity values in the basal vessels increase on TCD examination often before any change is visible angiographically. The next stage is characterized by further vessel narrowing with increased flow velocity through the narrow portion of the vessel but maintenance of blood flow (stage I). During the following stage, as the vessel narrows further, the degree of stenosis may be flow limiting; however, cerebral autoregulation can compensate by lowering distal vascular resistance to maintain adequate cerebral blood flow (stage II). In some patients after subarachnoid hemorrhage, especially those with a poor initial grade, the autoregulatory mechanism may not be functioning at full capacity, which may predispose patients to earlier critical reductions in cerebral blood flow. As further vessel narrowing occurs and the autoregulatory mechanism becomes exhausted, cerebral blood flow decreases in the given vascular distribution, and reduced cerebral blood flow may be detected on blood flow imaging studies such as xenon-CT, single-photon emission computed tomography (SPECT), and PET or with other methods for measuring cerebral blood flow.61,62 As cerebral blood flow is reduced, increased oxygen extraction occurs, and cerebral metabolism and neuronal function are maintained (stage III). As further vessel narrowing occurs, blood flow drops to ischemic thresholds, synaptic transmission no longer functions, and neurological deficits ensue (stage IV). As blood flow drops to lower levels or the time of ischemia is prolonged, permanent neuronal damage leading to infarction takes place.63,64 Figure 347-4 illustrates an example of velocity recordings and effect on cerebral blood flow in a patient with vasospasm after subarachnoid hemorrhage.
Effect of Vessel Narrowing from Subarachnoid Hemorrhage on Blood Flow Velocity
Normal values for cerebral blood flow velocity have been published by multiple investigators. Under normal conditions, the average flow velocity in the MCA is 62 cm/sec. Velocities greater than 120 cm/sec correlate with vasospasm, as evidenced by vessel narrowing on angiography. Flow velocities greater than 200 cm/sec often correlate with severe vasospasm seen angiographically and are associated with clinical episodes of ischemia and infarction.12,58,65,66
Early studies performed by Aaslid and Seiler and their colleagues in Bern, Switzerland, established the correlation between elevated velocities and cerebral infarction from vasospasm.58,67,68 Seiler and colleagues observed a group of 39 patients with subarachnoid hemorrhage monitored by TCD ultrasonography. In no patient did a cerebral infarct develop if blood flow velocities had not exceeded 140 cm/sec. Blood flow velocities greater than 200 cm/sec were associated with ischemic episodes and infarctions, but some patients remained asymptomatic.58,67,68 These observations are in keeping with previous angiographic studies of vasospasm indicating that there is not an absolute concordance between the degree of vessel narrowing and the development of delayed ischemia. Most patients in whom major episodes of delayed ischemia develop have significant vessel narrowing; however, other patients progress to severe vessel narrowing but preserve cerebral blood flow and DINDs do not develop. Occasionally, diffuse distal spasm unaccompanied by proximal vessel spasm may develop in patients with subarachnoid clots located in the distal cerebral fissures.14 Frequently, careful examination of the CT scan for the presence of distally located blood clots can help predict patients at risk for distal vessel vasospasm. Cerebral blood flow measurements and angiography are required to confirm the diagnosis.
Subsequent studies have correlated the degree of angiographic vasospasm with TCD velocities and found the best correlations in the MCA and distal ICA.69,70 Vasospasm in the distal anterior cerebral artery is not normally accessible with TCD, and the proximal anterior cerebral arteries can sometimes be problematic because of congenital atresia and other factors that lead to poor correlation between vessel diameter and increased velocity. Multiple physiologic factors have been shown to affect blood flow velocity in patients with vasospasm, including ICP, hematocrit, increased blood pressure from induced hypertension, hyperdynamic therapy, and hyperemia, which can result from aggressive hemodynamic support.71,72 Aaslid and colleagues initially described a mathematical ratio of flow velocities,67 which was analyzed further by Lindegaard and associates,70 between the MCA and the extracranial ICA (VMCA/VICA) to correct for flow changes induced by vasospasm. Because velocity can change from alterations in either flow or vessel diameter, the extracranial-to-intracranial ratio can be helpful in distinguishing hyperemia from elevated velocities caused by spasm. If vasospasm progresses to such a severe degree that cerebral blood flow is significantly reduced, intracranial velocities may begin to decrease as the vessels become severely attenuated and thus lead to a decrease in blood flow velocity in the extracranial carotid artery as well, which decreases intracranial velocity but results in a persistently elevated VMCA/VICA ratio.
Predictive Value of Transcranial Doppler Ultrasound in Vasospasm
The time course of vasospasm can vary according to the severity of the subarachnoid hemorrhage and the extent of the spasm. The peak incidence of vasospasm occurs in a delayed fashion after subarachnoid hemorrhage. Weir and coworkers carefully analyzed angiograms from 293 patients with aneurysms.73 These investigators found that angiographically detected vasospasm initially occurred 3 days after subarachnoid hemorrhage and appeared to be maximal between days 6 and 8 and was much reduced by day 12. Subsequent studies using TCD confirmed the delayed onset and time course of vasospasm.67,74,75 Multiple investigators have observed that patients who have early and more severe increases in flow velocity are at higher risk for the development of DINDs. The use of TCD has addressed several controversies in the past regarding the timing of vasospasm. Romner and associates were unable to find significant vasospasm within 12 hours after subarachnoid hemorrhage in a group of patients harboring aneurysms.76 It was previously thought that an early and late phase of vasospasm existed, thus leading to management decisions based on these preconceptions. These authors also showed that progression of vasospasm was more likely to occur in patients operated on 49 to 96 hours after aneurysmal rupture than in patients operated on within 48 hours of rupture, thereby supporting the notion that early aneurysm surgery may be less likely to aggravate vasospasm than surgery during the time when vasospasm begins to occur to a significant extent.77
Grosset and colleagues showed that rapid increases in velocity are predictive of patients in whom DINDs will develop.78 These investigators repeatedly examined 121 consecutive patients after acute subarachnoid hemorrhage frequently for 14 days. The average highest velocity attained was greater in the 47 patients in whom DINDs developed (186 cm/sec) than in the 74 patients in whom DINDs did not develop (149 cm/sec). There was no significant difference, however, between symptomatic and asymptomatic patients in the velocities recorded before onset of the neurological deficits, but the rate of increase in TCD velocities was greater in patients in whom DINDs subsequently developed. In these patients, there was a maximum increase in velocity of 65 ± 5 cm/sec per 24-hour period versus increases of 47 ± 3 cm/sec per 24-hour period in patients in whom DINDs did not develop. An increase greater than 50 cm/sec per 24-hour period may help predict patients in whom DINDs will eventually develop; information gathered from sequential recording may be useful in managing these patients.
Several treatments are available for cerebral vasospasm after subarachnoid hemorrhage, and it is useful to consider the effects of these treatments on vessel diameter and TCD values. Triple-H therapy (hypertension, hypervolemia, and hemodilution) may have unpredictable effects on basal vessel velocity recordings with TCD. Reports have indicated that in some cases increases in velocity after this treatment can be due to increases in blood flow and not further reduction in basal vessel diameter.79,80 The state of cerebral autoregulation may play a role in determining the changes in velocity after induction of triple-H therapy.80 These reports underscore the need for combined assessment with cerebral blood flow measurements and the use of intracranial-to-extracranial ratios to correct for blood flow changes when using TCD to monitor patients undergoing triple-H therapy for vasospasm.
Calcium channel blockers have been shown by several prospective randomized trials to be useful for preventing vasospasm and reducing morbidity and mortality after subarachnoid hemorrhage.81 It has been shown that cerebroselective calcium channel blockers cause reductions in subarachnoid hemorrhage–induced elevated intracranial blood flow velocities measured with TCD.82
Endovascular therapy has been useful in reversing vessel narrowing from vasospasm and improving cerebral blood flow. TCD ultrasonography and cerebral blood flow studies have shown improvement in blood flow and a reduction in elevated blood flow velocities secondary to spasm after treatment.82,83 Interventional treatment with papaverine has been found to decrease cerebral blood flow velocities and improve cerebral blood flow, although the effect appears to be more transient.
Arteriovenous Malformations
Hemodynamic changes in arteriovenous malformations have been well characterized by blood flow studies, direct pressure measurements, and TCD ultrasonography.84 Classic arteriovenous malformations show high velocity in feeding arteries corresponding to elevated flow volume and have a low pulsatility index on TCD, which indicates decreased vascular resistance. It has been shown by color flow Doppler that arteriovenous malformations can be imaged transcranially.85 Characteristic features on color flow imaging and the hemodynamic changes on TCD can lead to the diagnosis of arteriovenous malformation in patients examined for neurological symptoms such as headaches, seizures, or other focal findings that may lead to routine examination. For decision making regarding treatment planning, other imaging modalities such as CT, magnetic resonance imaging (MRI), and angiography are essential.
Detection of Intracranial Aneurysms
Standard TCD has not generally been useful as a screening test to detect cerebral aneurysms; however, with the introduction of color flow, two-dimensional transcranial color-coded sonography (TCCS) has shown increased sensitivity for the detection of aneurysms.86 The addition of intravascular contrast agents has been useful to improve the signal-to-noise ratio for examination. Klotzsch and colleagues reported the use of TCCS in 30 patients with known intracranial aneurysms.87 Twenty-nine angiographically proven aneurysms with diameters ranging from 3 to 16 mm and a mean of 7.2 ± 3.6 mm were detected by TCCS. In another study, Baumgartner and associates evaluated 29 patients with 30 radiographically proven cerebral aneurysms.86 Twenty-three of 27 (85%) nonthrombosed aneurysms with diameter 6 to 25 mm were identified.
Monitoring during Carotid Endarterectomy
Halsey published results from a collaborative study of 1495 CEAs in an attempt to define the relationship between intraoperative measurements of VMCA and the need for shunt placement.88 Patients were categorized according to their risk for ischemia (cross-clamp hypoperfusion) based on the reduction in VMCA values during carotid artery clamping. The study indicated that patients at high risk for ischemia (85% reduction in VMCA) who did not undergo shunting were at high risk for perioperative stroke. In more than 75% of patients with a low risk for ischemia (VMCA fell by <60%), there was a better outcome in those in whom shunting was not performed. In the group at intermediate risk for ischemia (VMCA fell 60% to 85%), there was no difference in outcome between patients with shunting and those without. Based on these data, if VMCA stabilizes to at least 40% of the preclamp value, it is unlikely the patient would benefit from a shunt. Comparison studies have been conducted between TCD and other methods of identifying patients at risk for cross-clamp ischemia who may benefit from an intraluminal shunt. There appears to be excellent correlation between TCD and electroencephalographic (EEG) criteria for ischemia.89,90
Cerebral emboli are a major cause of perioperative stroke from CEA.91,92 Microemboli detected by TCD are associated with stroke and ischemic events during and after CEA. Intra-arterial microemboli can be recognized easily with TCD monitoring by the characteristic chirping, clicking, or whistling sounds on the audio output and spectral display. The TCD features of particulate and gas emboli have been characterized and software has been developed for automatic detection and counting of emboli.93,94 The prevalence and timing of perioperative cerebral emboli associated with CEA must be considered when using TCD monitoring. Some cerebral microemboli are detected during most CEAs (97% in one report95); however, most microemboli do not result in stroke. Most emboli are detected on restoration of ICA perfusion, but these are predominately air emboli, which are believed to be less harmful than particulate emboli and not associated with ischemic events. Emboli noted during dissection of the carotid artery96 or frequent emboli detected immediately postoperatively are associated with postoperative stroke and ischemic events.92,97–99 Dextran or other antiplatelet drugs can be effective in reducing postoperative microemboli.100,101 Information gained from TCD monitoring may allow the surgeon to alter surgical technique or institute treatment postoperatively to reduce ischemic events.
Intraoperative or postoperative occlusion may be due to technical problems at the site of endarterectomy, such as thrombosis or an intimal flap, and may be affected by coagulability factors. Findings on TCD that suggest impending or completed carotid occlusion include frequent and increasing microemboli and a decrease in VMCA to postclamp values.92 Neurological deterioration in the recovery room should prompt immediate TCD evaluation for impending or completed postoperative occlusion, and if ipsilateral flow is poor, rapid re-exploration may be indicated to prevent stroke.
Postoperative hyperperfusion syndrome occurs in about 1% of patients after CEA, and when cerebral hemorrhage occurs, the prognosis is poor.91,102 The syndrome is thought to occur when abnormally high blood flow develops in vascular beds that have been habituated to low perfusion pressure and are suddenly exposed to normal arterial pressure after restoration of normal perfusion pressure during CEA.103,104 Hyperperfusion can be associated with defective cerebral autoregulation at normal blood pressure105 and can be diagnosed by TCD before clinical signs develop. Elevations in ipsilateral VMCA ranging from 30% to 230% are found in 10% to 20% of patients after CEA, but only in some of these patients do headaches or more serious sequelae develop.102 Early diagnosis with TCD allows strict control of blood pressure to restore flow velocities to the normal range and avoid serious complications.
Posttraumatic Vasospasm
Cerebral vasospasm can occur after head injury and is associated with traumatic subarachnoid hemorrhage. Posttraumatic vasospasm has been well documented and has been shown to be responsible for clinical deterioration and infarction in some patients with closed head injury.56,106–108 TCD is useful in monitoring head-injured patients for this complication. Initial investigations by Weber and colleagues reported a 40% incidence of vasospasm, by TCD criteria, in a group of severely head-injured patients.109 Martin and coworkers reported a 27% incidence of vasospasm with the use of TCD in a group of head-injured patients.107 Detection of vasospasm in head-injured patients with TCD is made more difficult by the concurrent existence of cerebral hyperemia after head injury, which may result in false-positive values for vasospasm if the criteria for vasospasm in patients with subarachnoid hemorrhage are used. Blood flow velocity recordings should be obtained from the most distal portion of the extracranial ICA in the neck to allow calculation of VMCA/VICA ratios. The VMCA/VICA ratio should be used to correct for hyperemia when using TCD to detect posttraumatic vasospasm. Measurement of cerebral blood flow with quantitative techniques is helpful in identifying patients at risk for cerebral ischemia.106
Vascular Dissection
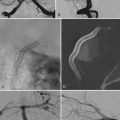
Carotid artery dissection extracranially and at the skull base can be a complication after head injury that can sometimes produce devastating delayed cerebral infarction. One of the diagnostic clues to extracranial dissection can be the presence of intracranial arterial emboli. We determined the incidence of intracranial emboli in the MCA on the side of ICA dissection in 10 patients with ICA dissection secondary to trauma and in 7 with spontaneous ICA dissection.48 The diagnosis was confirmed by carotid angiography and studied by TCD from the time of diagnosis through initiation of therapy.
Transcranial Doppler Findings with Increased Intracranial Pressure
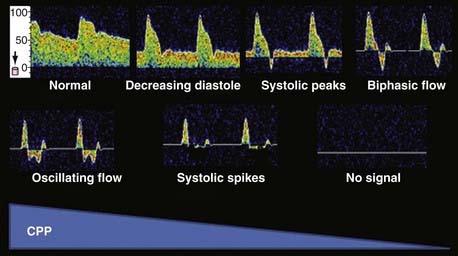
Changes in VMCA recordings have been described with increasing ICP. With early rises in ICP, the pulsatility index increases with a progressive reduction in diastolic velocity and initially no change in mean velocity. With further increases in ICP, there is a simultaneous reduction in mean velocity and a further increase in the pulsatility index.110 It is likely that these changes occur as a result of progressive increases in resistance of the cerebrovascular bed. At high ICP values that produce arrest of the cerebral circulation, the VMCA waveform progresses through a predictable sequence. This sequence begins with a progressive reduction in diastolic velocity initially to zero and then commencement of an alternating flow pattern with a reversed diastolic component (Fig. 347-5). Further obstruction to flow causes progression to alternating flow, which is antegrade in systole and retrograde in diastole with equal components. Further progression leads to small systolic peaks and then an absence of the waveform entirely. These characteristic TCD changes have correlated with previously described angiographic findings of nonfilling of the cerebral arterial tree in patients undergoing cerebral angiography.110 It has been documented by cerebral angiography and radionuclide isotope scanning that an alternating flow pattern on TCD corresponds to arrest of the cerebral circulation (Fig. 347-5).110,111
Changes in the pulsatility index and mean velocity alone are not specific for increased ICP. A high resistance in the cerebral vascular bed that produces increased pulsatility and decreased velocity can be seen with intense vasoconstriction. Intense vasoconstriction can occur as a result of vigorous hyperventilation, low cerebral metabolism, and cerebral vasoconstricting drugs such as barbiturates. For these reasons, mean velocity and the pulsatility index, when taken in isolation, cannot predict ICP reliably. The pulsatility index can be affected markedly by cardiac factors.112 Progressive increases in the pulsatility index can indicate a progressive rise in ICP in patients who are observed carefully and known to not have concomitant cardiac changes and alterations in CO2 or other factors that produce vasoconstriction.
Cerebrovascular tone pressure, as well as the critical closing pressure of the cerebral circulation, can be determined by analysis of the VMCA waveform and the arterial pressure waveform. It may be possible in the future to predict ICP more reliably from these parameters with TCD and arterial blood pressure recordings.113,114
Transcranial Doppler as a Confirmatory Test for Brain Death
TCD sonography is useful as a confirmatory test in the diagnosis of brain death. Arrest of the intracranial circulation can be detected reliably with TCD, which is often but not always present when brain death occurs. The true diagnosis of brain death is a clinical diagnosis, and blood flow studies such as cerebral angiography, radioisotope scanning, and TCD are confirmatory tests. According to the President’s Commission on guidelines for determination of brain death in the United States, confirmatory tests can be used to shorten the observation period in patients who fulfill the clinical criteria for brain death before organ retrieval or discontinuation of mechanical ventilation can proceed.111
Many studies have examined the role of TCD in the determination of brain death by comparing clinical findings and the results of blood flow studies with sonographic waveforms in patients progressing to brain death.111,115–118 Most of the studies comparing TCD with clinical criteria for brain death have indicated a sensitivity and specificity of TCD approaching 100%.
Several caveats apply to TCD and any other cerebral blood flow methods as confirmatory tests for brain death. False-negative and false-positive results can occur in rare circumstances. False-positive TCD recordings have been reported in several situations. Cerebral circulatory arrest can be transient and reverse after a period of minutes in patients with subarachnoid hemorrhage who have a marked increase in ICP that results in temporary arrest of the cerebral circulation.119,120 For this reason, when using TCD to confirm arrest of the cerebral circulation, it should be repeated or be documented that cerebral circulatory arrest is present for a sufficient period (i.e., 30 minutes under normothermic conditions) to be incompatible with survival. Another possible cause of false-positive TCD findings can be found in patients who have sustained cerebral circulatory arrest but have residual circulation to the brainstem, which may sustain respiratory effort or minimal cranial nerve function for a short time. False-positive TCD tracings showing alternating flow velocity patterns have been seen in unusual situations in which abnormally low diastolic pressure is present, such as in patients with intra-aortic balloon pumps.
False-negative TCD results (in which TCD indicates preserved flow and patients are clinically brain-dead) have been reported in patients with clinical brain death. When massive destruction of the brainstem occurs as a result of destructive lesions or posterior fossa lesions, brainstem function may be absent, and the patient fulfills the clinical criteria for brain death, but with preserved supratentorial blood flow. EEG activity may persist, and TCD waveforms can indicate preserved blood flow.121 These examples of false-positive and false-negative studies are not an indication of failure of the TCD technique itself, but they emphasize why confirmatory tests do not always concur 100% with the clinical diagnosis of brain death. Despite these shortcomings, TCD can be especially useful in patients in whom the clinical diagnosis of brain death is made difficult because of extensive trauma and cranial nerve damage and in patients after trauma who have been given paralytic agents, which makes clinical evaluation difficult. TCD examination may be helpful in avoiding unnecessary surgery in this situation.
Neurosonology as a Treatment Modality
Sonothrombolysis
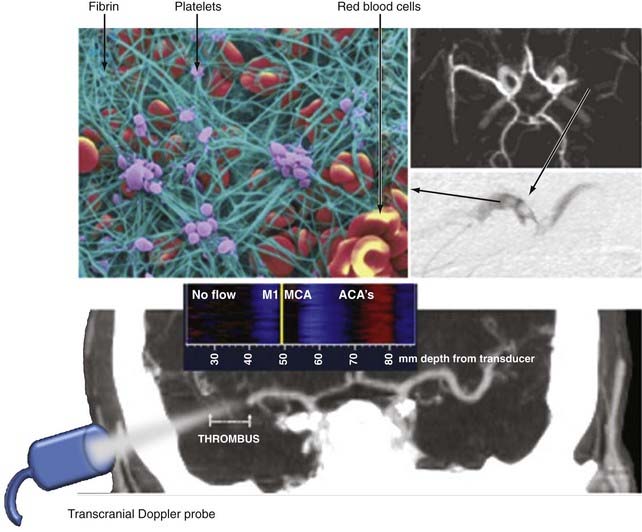
The majority of investigations of the utility of neurosonology have been directed toward diagnostic applications of this technology. Experimental and clinical work in ultrasound has revealed that ultrasound has potential therapeutic uses, including treatments used in dentistry and for musculoskeletal disorders, hemostasis, treatment of fibroid tumors, lipolysis, and other applications. One property of ultrasound with potential applications in the nervous system that has recently been discovered and characterized is the ability to accelerate the dissolution of blood clots, a process referred to as sonothrombolysis. It has also been demonstrated that ultrasound can potentate the effect of tissue plasminogen activator (t-PA) on the dissolution of blood clots. Intravascular catheters with small ultrasound-emitting probes have been used in combination with t-PA for the dissolution of intravenous and intra-arterial blood clots in patients with peripheral vascular disease. More recently, intracranial intra-arterial thrombectomy via endovascular sonothrombolysis has been evaluated in clinical trials. Independent of this research, it was serendipitously discovered as part of a clinical routine in which t-PA–treated stroke patients are monitored with TCD for MCA recanalization that some patients can recanalize earlier than expected and experience dramatic clinical recovery.122 Subsequently, the feasibility and safety of sonothrombolysis of MCA clots in stroke patients at the bedside with TCD have been established.122,123 These results have been confirmed by other groups worldwide that monitor t-PA infusion with diagnostic ultrasound.124,125
Because intravenous t-PA by itself works by induction of mostly partial recanalization of large thrombi,122,123,126 early augmentation of fibrinolysis to improve arterial recanalization is desirable. Fibrin strands are cross-linked and held by activated platelets to form a net-like structure that captures red blood cells (Fig. 347-6). To capture red blood cells, the net should have openings of less than 6 µm (note that the average size of erythrocytes is 6 to 8 µm). Thrombus size and structure will further create an area of high resistance to the antegrade movement of red blood cells, and once the embolus reaches the lumen of a vessel comparable to its size, it acts like a plug precluding flow if arterial pressure is insufficient to further distend the vessel. Delivery of intravenous t-PA to the thrombus is probably dependent on residual blood flow around the thrombus and plasma flow through the thrombus. Mechanical agitation with ultrasound can expose layers of thrombi to circulating t-PA and facilitate streaming of plasma through the thrombus, thus bringing more t-PA to binding sites. Ultrasound is a pressure wave that can travel through tissues and deliver this mechanical energy to stagnant flow areas and clot interfaces. Numerous in vitro experiments have confirmed the ability of ultrasound to enhance fibrinolysis through disaggregation of non–cross-linked fibrin strands and thereby further thin them and promote plasma flow and delivery of lytic drug to the clot.127–129 Kilohertz frequencies are believed to induce more mechanical stretching of fibrin, whereas megahertz ultrasound promotes fibrinolysis more through enzymatic mechanisms.130,131
A multicenter collaborative group was formed to confirm the effect of TCD in accelerating t-PA–induced thrombolysis in stroke patients in a clinical trial. In the Combined Lysis of Thrombus in Brain Ischemia Using Transcranial Ultrasound and Systemic TPA (CLOTBUST) trial,123 73% of patients achieved any degree of recanalization (46% complete, 27% partial) with t-PA plus TCD versus 50% (17% complete, 33% partial) with t-PA alone within 2 hours of treatment (P < .001). The rate of sustained complete recanalization at 2 hours was 38% versus 13%, respectively (P = .03). The symptomatic intracerebral hemorrhage rate was 3.8% in both groups (not significant).123 This trial was the first properly powered clinical trial that confirmed the effect of ultrasound-enhanced thrombolysis in human subjects and demonstrated a positive biologic effect of diagnostic low-power ultrasound.
A recent meta-analysis of six randomized and three nonrandomized clinical studies of sonothrombolysis suggested that any diagnostic ultrasound monitoring can at least double the chance of early complete arterial recanalization at no increase in the risk for symptomatic intracerebral hemorrhage.132
Because the application of frequencies below diagnostic range (i.e., kilohertz) in humans resulted in increased symptomatic bleeding rates133 whereas 2 MHz showed a strong signal of efficacy and safety,123 mechanisms by which kilohertz and megahertz frequencies interact with the clot–residual flow interface, endothelium, and brain tissues are currently under investigation. Catheter-based ultrasound delivery to arterial thrombi and intraventricular clots is the subject of ongoing clinical trials.
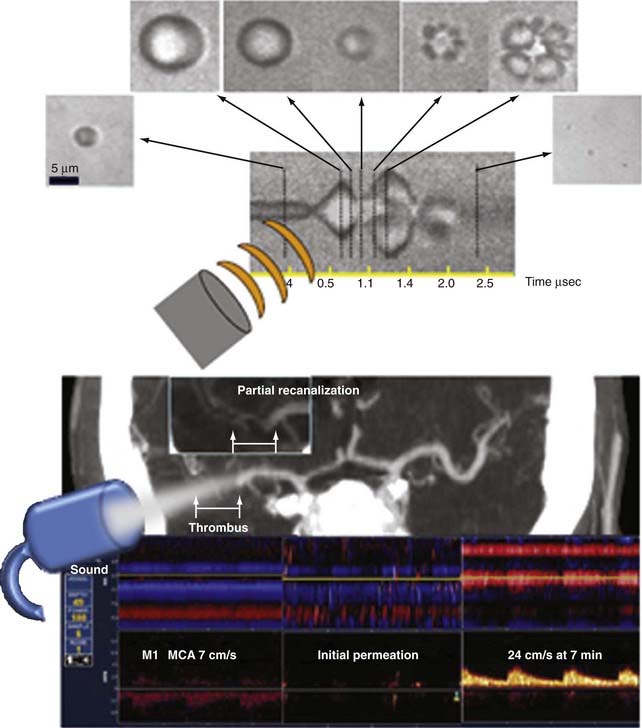
Molina and colleagues pioneered the use of gaseous microspheres in combination with CLOTBUST monitoring methods and reported safety and recanalization rates with the first-generation air-filled microspheres (Levovist), as well as with the newer diagnostic microspheres.134 These microspheres were originally designed as ultrasound contrast agents to improve the quality of ultrasound images. After intravenous injection, the microspheres circulate in the bloodstream and cross lung capillaries into the arterial circulation. When contacted by an ultrasound beam, the microspheres undergo expansion in size followed by transient oscillation or complete breakup (Fig. 347-7). The likelihood of oscillation or cavitation with microsphere destruction may depend not only on the resonant frequency but also on the power of the ultrasound delivered to the target tissues.
Observations of in vitro experiments with a temporal bone/MCA flow model have indicated that even low-power TCD with conventional TCD equipment can destroy some of the intra-arterial microspheres and thereby lead to a mechanical effect. When microspheres oscillate or burst, they expand like microscopic balloons,135 move in three dimensions, and transmit mechanical energy momentum from passing ultrasound waves to surrounding fluid and structures. If an ultrasound beam intercepts microspheres at the clot–residual flow interface, fibrinolysis is facilitated with or even without t-PA.
Therapeutic use of the newest generation of microspheres, such as perflutren-lipid microspheres, which are more stable in solution (they tend to “bubble up” less than their predecessors) and are therefore suitable for continuous infusion, has been further explored. This in turn enables continuous replenishment of microspheres at the clot–residual flow interface during the entire t-PA infusion. A multicenter feasibility study showed that perflutren-lipid microspheres reached intracranial thrombi in all patients, and in 75% they immediately permeated through or around thrombi and reached areas with no detectable flow (Fig. 347-7).136 The first dose of these microspheres was administered safely during t-PA infusion and did not cause any symptomatic intracerebral hemorrhage. Perflutren-lipid microspheres, TCD, and t-PA lysed 50% of proximal MCA occlusions.136 An additional multicenter microsphere dose escalation study called TUCSON (Transcranial Ultrasound in Clinical SONothrombolysis, NCT00504842) was recently completed and the results are pending. Transcranial ultrasound delivery in an operator-independent and dose-controlled manner is now being tested in a separate clinical trial.
Aaslid R, Huber P, Nornes H. Evaluation of cerebrovascular spasm with transcranial Doppler ultrasound. J Neurosurg. 1984;60:37.
Aaslid R, Markwalder T-M, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769.
Aaslid R, Newell DW, Stooss R, et al. Assessment of cerebral autoregulation dynamics from simultaneous arterial and venous transcranial Doppler recordings in humans. Stroke. 1991;22:1148.
Alexandrov AV, Demchuk AM, Felberg RA, et al. High rate of complete recanalization and dramatic clinical recovery during tPA infusion when continuously monitored with 2-MHz transcranial doppler monitoring. Stroke. 2000;31:610.
Alexandrov AV, Mikulik R, Ribo M, et al. A pilot randomized clinical safety study of sonothrombolysis augmentation with ultrasound-activated perflutren-lipid microspheres for acute ischemic stroke. Stroke. 2008;39:1464.
Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170.
Dawson DL, Zierler RE, Strandness DEJr, et al. The role of duplex scanning and arteriography before carotid endarterectomy: a prospective study. J Vasc Surg. 1993;18:673.
Dohrmann GJ, Rubin JM. Dynamic intraoperative imaging and instrumentation of brain and spinal cord using ultrasound. Neurol Clin. 1985;3:425.
Ducrocq X, Hassler W, Moritake K, et al. Consensus opinion on diagnosis of cerebral circulatory arrest using Doppler-sonography: Task Force Group on cerebral death of the Neurosonology Research Group of the World Federation of Neurology. J Neurol Sci. 1998;159:145.
Grosset DG, Straiton J, du Trevou M, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage by rapidly increasing transcranial Doppler velocity and cerebral blood flow changes. Stroke. 1992;23:674.
Grubb RLJ, Derdeyn CP, Fritsch SM, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA. 1998;280:1055.
Gur AY, Bova I, Bornstein NM. Is impaired cerebral vasomotor reactivity a predictive factor of stroke in asymptomatic patients? Stroke. 1996;27:2188.
Kaneko Z. First steps in the development of the Doppler flowmeter. Ultrasound Med Biol. 1986;12:1877.
Kleiser B, Widder B. Course of carotid artery occlusions with impaired cerebrovascular reactivity. Stroke. 1992;23:171.
Lee JH, Martin NA, Alsina G, et al. Hemodynamically significant cerebral vasospasm and outcome after head injury: a prospective study. J Neurosurg. 1997;87:221.
Letteboer MM, Willems PW, Viergever MA, et al. Brain shift estimation in image-guided neurosurgery using 3-D ultrasound. IEEE Trans Biomed Eng. 2005;52:268.
Levi CR, O’Malley HM, Fell G, et al. Transcranial Doppler detected cerebral microembolism following carotid endarterectomy. High microembolic signal loads predict postoperative cerebral ischaemia. Brain. 1997;120:621.
Lindegaard KF, Bakke SJ, Aaslid R, et al. Doppler diagnosis of intracranial artery occlusive disorders. J Neurol Neurosurg Psychiatry. 1986;49:510.
Lindegaard KF, Nornes H, Bakke SJ, et al. Cerebral vasospasm after subarachnoid haemorrhage investigated by means of transcranial Doppler ultrasound. Acta Neurochir Suppl. 1988;42:81.
Markus H. Transcranial Doppler detection of circulating cerebral emboli. A review. Stroke. 1993;24:1246.
Newell DW, Aaslid R, Lam A, et al. Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke. 1994;25:793.
Newell DW, Aaslid R, Stooss R, et al. Evaluation of hemodynamic responses in head injury patients with transcranial Doppler monitoring. Acta Neurochir (Wein). 1997;139:804.
Spencer MP. Transcranial Doppler monitoring and causes of stroke from carotid endarterectomy. Stroke. 1997;28:685.
Srinivasan J, Newell DW, Sturzenegger M, et al. Transcranial Doppler in the evaluation of internal carotid artery dissection. Stroke. 1996;27:1226.
Sturzenegger M, Newell DW, Douville C, et al. Dynamic transcranial Doppler assessment of positional vertebrobasilar ischemia [see comments]. Stroke. 1994;25:1776.
1 Kaneko Z. First steps in the development of the Doppler flowmeter. Ultrasound Med Biol. 1986;12:1877.
2 Montalvo BM, Quencer RM. Intraoperative sonography in spinal surgery: current state of the art. Neuroradiology. 1986;28:551.
3 Rubin JM, Dohrmann GJ. The spine and spinal cord during neurosurgical operations: real-time ultrasonography. Radiology. 1985;155:197.
4 Dohrmann GJ, Rubin JM. Use of ultrasound in neurosurgical operations: a preliminary report. Surg Neurol. 1981;16:362.
5 Dohrmann GJ, Rubin JM. Dynamic intraoperative imaging and instrumentation of brain and spinal cord using ultrasound. Neurol Clin. 1985;3:425.
6 Rubin JM, Dohrmann GJ. Efficacy of intraoperative US for evaluating intracranial masses. Radiology. 1985;157:509.
7 Andrews BT, Bederson JB, Pitts LH. Use of intraoperative ultrasonography to improve the diagnostic accuracy of exploratory burr holes in patients with traumatic tentorial herniation. Neurosurgery. 1989;24:345.
8 Rubin JM, Dohrmann GJ. Use of ultrasonically guided probes and catheters in neurosurgery. Surg Neurol. 1982;18:143.
9 Letteboer MM, Willems PW, Viergever MA, et al. Brain shift estimation in image-guided neurosurgery using 3-D ultrasound. IEEE Trans Biomed Eng. 2005;52:268.
10 Blackshear WMJr, Phillips DJ, Thiele BL, et al. Detection of carotid occlusive disease by ultrasonic imaging and pulsed Doppler spectrum analysis. Surgery. 1979;86:698.
11 Aaslid R, Markwalder T-M, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769.
12 Aaslid R, Huber P, Nornes H. Evaluation of cerebrovascular spasm with transcranial Doppler ultrasound. J Neurosurg. 1984;60:37.
13 Fujioka KA, Douville CM. Anatomy and freehand examination techniques. In: Newell DW, Aaslid R, editors. Transcranial Doppler. New York: Raven Press; 1996:9-31.
14 Newell DW, Grady MS, Eskridge JM, et al. Distribution of angiographic vasospasm after subarachnoid hemorrhage: implications for diagnosis by transcranial Doppler ultrasonography. Neurosurgery. 1990;27:574.
15 Aaslid R, Newell DW, Stooss R, et al. Assessment of cerebral autoregulation dynamics from simultaneous arterial and venous transcranial Doppler recordings in humans. Stroke. 1991;22:1148.
16 Newell DW, Aaslid R, Stooss R, et al. Evaluation of hemodynamic responses in head injury patients with transcranial Doppler monitoring. Acta Neurochir (Wein). 1997;139:804.
17 Newell DW, Aaslid R, Stooss R, et al. The relationship of blood flow velocity fluctuations to intracranial pressure B waves. J Neurosurg. 1992;76:415.
18 Nabavi DG, Georgiadis D, Mumme T, et al. Detection of microembolic signals in patients with middle cerebral artery stenosis by means of a bigate probe. A pilot study. Stroke. 1996;27:1347.
19 Chan A, Beach KW, Langlois Y, et al. Evaluation of extracranial carotid artery disease by ultrasonic duplex scanning: a clinical perspective. Aust N Z J Surg. 1982;52:562.
20 Breslau PJ, Fell G, Phillips DJ, et al. Evaluation of carotid bifurcation disease: the role of common carotid artery velocity patterns. Arch Surg. 1982;117:58.
21 Knox RA, Breslau PJ, Strandness DEJr. A simple parameter for accurate detection of severe carotid disease. Br J Surg. 1982;69:230.
22 Dawson DL, Zierler RE, Strandness DEJr, et al. The role of duplex scanning and arteriography before carotid endarterectomy: a prospective study. J Vasc Surg. 1993;18:673.
23 Rubin JR, Bondi JA, Rhodes RS. Duplex scanning versus conventional arteriography for the evaluation of carotid artery plaque morphology. Surgery. 1987;102:749.
24 Toole JF. Middle cerebral artery stenosis—a neglected problem? Surg Neurol. 1987;27:44.
25 Bogousslavsky J, Barnett HJ, Fox AJ, et al. Atherosclerotic disease of the middle cerebral artery. Stroke. 1986;17:1112.
26 Lindegaard KF, Bakke SJ, Aaslid R, et al. Doppler diagnosis of intracranial artery occlusive disorders. J Neurol Neurosurg Psychiatry. 1986;49:510.
27 Spencer MP, Whisler D. Transorbital Doppler diagnosis of intracranial arterial stenosis. Stroke. 1986;17:916.
28 Ley-Pozo J, Ringelstein EB. Noninvasive detection of occlusive disease of the carotid siphon and middle cerebral artery. Ann Neurol. 1990;28:640.
29 Rorick MB, Nichols FT, Adams RJ. Transcranial Doppler correlation with angiography in detection of intracranial stenosis. Stroke. 1994;25:1931.
30 Kleiser B, Widder B. Course of carotid artery occlusions with impaired cerebrovascular reactivity. Stroke. 1992;23:171.
31 Wilterdink JL, Feldmann E, Furie KL, et al. Transcranial Doppler ultrasound battery reliably identifies severe internal carotid artery stenosis. Stroke. 1997;28:133.
32 Yonas H, Smith HA, Durham SR, et al. Increased stroke risk predicted by compromised cerebral blood flow reactivity. J Neurosurg. 1993;79:483.
33 Grubb RLJ, Derdeyn CP, Fritsch SM, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA. 1998;280:1055.
34 Gur AY, Bova I, Bornstein NM. Is impaired cerebral vasomotor reactivity a predictive factor of stroke in asymptomatic patients? Stroke. 1996;27:2188.
35 Newell DW, Aaslid R, Lam A, et al. Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke. 1994;25:793.
36 Newell DW, Aaslid R, Douville CM, et al. Comparison of autoregulation and CO2 reactivity in occlusive disease. Stroke. 1994;25:748.
37 White RP, Markus HS. Impaired dynamic cerebral autoregulation in carotid artery stenosis. Stroke. 1997;28:1340.
38 Fujioka KA, Ernsberger AM, Nicholls SC, et al. Transcranial Doppler assessment of mechanical compression of the vertebral arteries. J Vasc Surg. 1991;15:254.
39 Sturzenegger M, Newell DW, Douville C, et al. Dynamic transcranial Doppler assessment of positional vertebrobasilar ischemia. Stroke. 1994;25:1776.
40 Spencer MP, Lawrence GH, Thomas GI, et al. The use of ultrasonics in the determination of arterial aeroembolism during open-heart surgery. Ann Thorac Surg. 1969;8:489.
41 Maroon JC, Albin MS. Air embolism diagnosed by Doppler ultrasound. Anesth Analg. 1974;53:399.
42 Spencer MP, Thomas GI, Nicholls SC, et al. Detection of middle cerebral artery emboli during carotid endarterectomy using transcranial Doppler ultrasonography. Stroke. 1990;21:415.
43 Padayachee TS, Parsons S, Theobold R, et al. The detection of microemboli in the middle cerebral artery during cardiopulmonary bypass: a transcranial Doppler ultrasound investigation using membrane and bubble oxygenators. Ann Thorac Surg. 1987;44:298.
44 Babikian VL, Hyde C, Pochay V, et al. Clinical correlates of high-intensity transient signals detected on transcranial Doppler sonography in patients with cerebrovascular disease. Stroke. 1994;25:1570.
45 Georgiadis D, Baumgartner RW, Karatschai R, et al. Further evidence of gaseous embolic material in patients with artificial heart valves. J Thorac Cardiovasc Surg. 1998;115:808.
46 Siebler M, Kleinschmidt A, Sitzer M, et al. Cerebral microembolism in symptomatic and asymptomatic high-grade internal carotid artery stenosis. Neurology. 1994;44:615.
47 Siebler M, Sitzer M, Rose G, et al. Silent cerebral embolism caused by neurologically symptomatic high-grade carotid stenosis. Event rates before and after carotid endarterectomy. Brain. 1993;116:1005.
48 Srinivasan J, Newell DW, Sturzenegger M, et al. Transcranial Doppler in the evaluation of internal carotid artery dissection. Stroke. 1996;27:1226.
49 Koennecke HC, Trocio SHJr, Mast H, et al. Microemboli on transcranial Doppler in patients with spontaneous carotid artery dissection. J Neuroimaging. 1997;7:217.
50 Dagirmanjian A, Davis DA, Rothfus WE, et al. Silent cerebral microemboli occurring during carotid angiography: frequency as determined with Doppler sonography. AJR Am J Roentgenol. 1993;161:1037.
51 Markus HS, Clifton A, Buckenham T, et al. Carotid angioplasty. Detection of embolic signals during and after the procedure. Stroke. 1994;25:2403.
52 Markus H. Transcranial Doppler detection of circulating cerebral emboli. A review. Stroke. 1993;24:1246.
53 Giller CA, Giller AM, Landreneau F. Detection of emboli after surgery for intracerebral aneurysms. Neurosurgery. 1998;42:490.
54 Di Tullio M, Sacco RL, Venketasubramanian N, et al. Comparison of diagnostic techniques for the detection of a patent foramen ovale in stroke patients. Stroke. 1993;24:1020.
55 Stump DA, Rogers AT, Hammon JW, et al. Cerebral emboli and cognitive outcome after cardiac surgery. J Cardiothorac Vasc Anesth. 1996;10:113.
56 Newell DW, Winn HR. Transcranial Doppler in cerebral vasospasm. Neurosurg Clin N Am. 1990;1:319.
57 Kistler JP, Crowell RM, Davis KR, et al. The relation of cerebral vasospasm to the extent and location of subarachnoid blood visualized by CT scan: a prospective study. Neurology. 1983;33:424.
58 Seiler RW, Grolimund P, Aaslid R, et al. Cerebral vasospasm evaluated by transcranial ultrasound correlated with clinical grade and CT-visualized subarachnoid hemorrhage. J Neurosurg. 1986;64:594.
59 Heros RC, Zervas NT, Varsos V. Cerebral vasospasm after subarachnoid hemorrhage: an update. Ann Neurol. 1983;14:599.
60 Lodi CA, Ursino M. Hemodynamic effect of cerebral vasospasm in humans: a modeling study. Ann Biomed Eng. 1999;27:257.
61 Sekhar LN, Wechsler LR, Yonas H, et al. Value of transcranial Doppler examination in the diagnosis of cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery. 1988;22:813.
62 Lewis DH, Newell DW, Winn HR. Delayed ischemia due to cerebral vasospasm occult to transcranial Doppler. An important role for cerebral perfusion SPECT. Clin Nucl Med. 1997;22:238.
63 Powers WJ, Grubb RLJr, Raichle ME. Physiological responses to focal cerebral ischemia in humans. Ann Neurol. 1984;16:546.
64 Powers WJ, Grubb RLJr, Baker RP, et al. Regional cerebral blood flow and metabolism in reversible ischemia due to vasospasm. Determination by positron emission tomography. J Neurosurg. 1985;62:539.
65 Vora YY, Suarez-Almazor M, Steinke DE, et al. Role of transcranial Doppler monitoring in the diagnosis of cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery. 1999;44:1237.
66 Seiler R, Grolimund P, Huber P. Transcranial Doppler sonography. An alternative to angiography in the evaluation of vasospasm after subarachnoid hemorrhage. Acta Radiol Suppl. 1986;369:99.
67 Aaslid R, Huber P, Nornes H. Evaluation of cerebrovascular spasm with transcranial Doppler ultrasound. J Neurosurg. 1984;60:37.
68 Seiler R, Grolimund P, Huber P. Transcranial Doppler sonography. An alternative to angiography in the evaluation of vasospasm after subarachnoid hemorrhage. Acta Radiol Suppl. 1986;369:99.
69 Sloan MA, Haley ECJr, Kassell NF, et al. Sensitivity and specificity of transcranial Doppler ultrasonography in the diagnosis of vasospasm following subarachnoid hemorrhage. Neurology. 1989;39:1514.
70 Lindegaard KF, Nornes H, Bakke SJ, et al. Cerebral vasospasm after subarachnoid haemorrhage investigated by means of transcranial Doppler ultrasound. Acta Neurochir Suppl. 1988;42:81.
71 Klingelhofer J, Dander D, Holzgraefe M, et al. Cerebral vasospasm evaluated by transcranial Doppler ultrasonography at different intracranial pressures. J Neurosurg. 1991;75:752.
72 Lindegaard KF. The role of transcranial Doppler in the management of patients with subarachnoid haemorrhage—a review. Acta Neurochir Suppl. 1999;72:59.
73 Weir B, Grace M, Hansen J, et al. Time course of vasospasm in man. J Neurosurg. 1978;48:173.
74 Seiler RW, Grolimund P, Aaslid R, et al. Cerebral vasospasm evaluated by transcranial ultrasound correlated with clinical grade and CT-visualized subarachnoid hemorrhage. J Neurosurg. 1986;64:594.
75 Harders AG, Gilsbach JM. Time course of blood velocity changes related to vasospasm in the circle of Willis measured by transcranial Doppler ultrasound. J Neurosurg. 1987;66:718.
76 Romner B, Ljunggren B, Brandt L, et al. Transcranial Doppler sonography within 12 hours after subarachnoid hemorrhage. J Neurosurg. 1989;70:732.
77 Romner B, Ljunggren B, Brandt L, et al. Correlation of transcranial Doppler sonography findings with timing of aneurysm surgery. J Neurosurg. 1990;73:72.
78 Grosset DG, Straiton J, du Trevou M, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage by rapidly increasing transcranial Doppler velocity and cerebral blood flow changes. Stroke. 1992;23:674.
79 Clyde BL, Resnick DK, Yonas H, et al. The relationship of blood velocity as measured by transcranial Doppler ultrasonography to cerebral blood flow as determined by stable xenon computed tomographic studies after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1996;38:896.
80 Manno EM, Gress DR, Schwamm LH, et al. Effects of induced hypertension on transcranial Doppler ultrasound velocities in patients after subarachnoid hemorrhage. Stroke. 1998;29:422.
81 Barker FG, Ogilvy CS. Efficacy of prophylactic nimodipine for delayed ischemic deficit after subarachnoid hemorrhage: a metaanalysis. J Neurosurg. 1996;84:405.
82 Harders A, Gilsbach J. Haemodynamic effectiveness of nimodipine on spastic brain vessels after subarachnoid haemorrhage evaluated by the transcranial Doppler method. A review of clinical studies. Acta Neurochir Suppl. 1988;45:21.
83 Lewis DH, Eskridge JM, Newell DW, et al. Brain SPECT and the effect of cerebral angioplasty in delayed ischemia due to vasospasm. J Nucl Med. 1992;33:1789.
84 Harders A, Bien S, Eggert HR, et al. Haemodynamic changes in arteriovenous malformations induced by superselective embolization: transcranial Doppler evaluation. Neurol Res. 1988;10:239.
85 Klotzch C, Henkes H, Nahser HC, et al. Transcranial color-coded duplex sonography in cerebral arteriovenous malformations. Stroke. 1995;26:2298.
86 Baumgartner RW, Mattle HP, Kothbauer K, et al. Transcranial color-coded duplex sonography in cerebral aneurysms. Stroke. 1994;25:2429.
87 Klotzsch C, Nahser HC, Fischer B, et al. Visualisation of intracranial aneurysms by transcranial duplex sonography. Neuroradiology. 1996;38:555.
88 Halsey JHJr. Risks and benefits of shunting in carotid endarterectomy. The International Transcranial Doppler Collaborators. Stroke. 1992;23:1583.
89 Fiori L, Parenti G, Marconi F. Combined transcranial Doppler and electrophysiologic monitoring for carotid endarterectomy. J Neurosurg Anesthesiol. 1997;9:11.
90 Jansen C, Vriens EM, Eikelboom BC, et al. Carotid endarterectomy with transcranial Doppler and electroencephalographic monitoring. A prospective study in 130 operations. Stroke. 1993;24:665.
91 Riles TS, Imparato AM, Jacobowitz GR, et al. The cause of perioperative stroke after carotid endarterectomy. J Vasc Surg. 1994;19:206.
92 Spencer MP. Transcranial Doppler monitoring and causes of stroke from carotid endarterectomy. Stroke. 1997;28:685.
93 Smith JL, Evans DH, Bell PR, et al. Time domain analysis of embolic signals can be used in place of high-resolution Wigner analysis when classifying gaseous and particulate emboli. Ultrasound Med Biol. 1998;24:989.
94 Droste DW, Hagedorn G, Notzold A, et al. Bigated transcranial Doppler for the detection of clinically silent circulating emboli in normal persons and patients with prosthetic cardiac valves. Stroke. 1997;28:588.
95 Smith JL, Evans DH, Gaunt ME, et al. Experience with transcranial Doppler monitoring reduces the incidence of particulate embolization during carotid endarterectomy. Br J Surg. 1998;85:56.
96 Jansen C, Ramos LM, van Heesewijk JP, et al. Impact of microembolism and hemodynamic changes in the brain during carotid endarterectomy. Stroke. 1994;25:992.
97 Gaunt M, Naylor AR, Lennard N, et al. Transcranial Doppler detected cerebral microembolism following carotid endarterectomy [letter]. Brain. 1998;121:389.
98 Levi CR, O’Malley HM, Fell G, et al. Transcranial Doppler detected cerebral microembolism following carotid endarterectomy. High microembolic signal loads predict postoperative cerebral ischaemia. Brain. 1997;120:621.
99 Cantelmo NL, Babikian VL, Samaraweera RN, et al. Cerebral microembolism and ischemic changes associated with carotid endarterectomy. J Vasc Surg. 1998;27:1024.
100 Hayes P, Lennard N, Smith J, et al. Vascular surgical society of Great Britain and Ireland: transcranial Doppler-directed dextran therapy in the prevention of postoperative carotid thrombosis. Br J Surg. 1999;86:692.
101 Lennard N, Smith J, Dumville J, et al. Prevention of postoperative thrombotic stroke after carotid endarterectomy: the role of transcranial Doppler ultrasound. J Vasc Surg. 1997;26:579.
102 Jansen C, Sprengers AM, Moll FL, et al. Prediction of intracerebral haemorrhage after carotid endarterectomy by clinical criteria and intraoperative transcranial Doppler monitoring. Eur J Vasc Surg. 1994;8:303.
103 Schroeder T, Sillesen H, Boesen J, et al. Intracerebral haemorrhage after carotid endarterectomy. Eur J Vasc Surg. 1987;1:51.
104 Schroeder T, Sillesen H, Sorensen O, et al. Cerebral hyperperfusion following carotid endarterectomy. J Neurosurg. 1987;66:824.
105 Jorgensen LG, Schroeder TV. Defective cerebrovascular autoregulation after carotid endarterectomy. Eur J Vasc Surg. 1993;7:370.
106 Martin NA, Patwardhan RV, Alexander MJ, et al. Characterization of cerebral hemodynamic phases following severe head trauma: hypoperfusion, hyperemia, and vasospasm. J Neurosurg. 1997;87:9.
107 Martin NA, Doberstein C, Zane C, et al. Posttraumatic cerebral arterial spasm: transcranial Doppler ultrasound, cerebral blood flow, and angiographic findings. J Neurosurg. 1992;77:575.
108 Lee JH, Martin NA, Alsina G, et al. Hemodynamically significant cerebral vasospasm and outcome after head injury: a prospective study. J Neurosurg. 1997;87:221.
109 Weber M, Grolimund P, Seiler RW. Evaluation of posttraumatic cerebral blood flow velocities by transcranial Doppler ultrasonography. Neurosurgery. 1990;27:106.
110 Hassler W, Steinmetz H, Gawlowski J. Transcranial Doppler ultrasonography in raised intracranial pressure and in intracranial circulatory arrest. J Neurosurg. 1988;68:745.
111 Newell DW, Grady MS, Sirotta P, et al. Evaluation of brain death using transcranial Doppler. Neurosurgery. 1989;24:509.
112 Newell DW. Transcranial Doppler ultrasonography. Neurosurg Clin N Am. 1994;5:619.
113 Aaslid R. Cerebral hemodynamics. In: Newell DW, Aaslid R, editors. Transcranial Doppler. New York: Raven Press; 1992:49-55.
114 Aaslid R, Newell DW. Pressure flow relationships in the cerebral circulation. J Cardiovasc Tech. 1990;9:90.
115 Bode H, Sauer M, Pringsheim W. Diagnosis of brain death by transcranial Doppler sonography. Arch Dis Child. 1988;63:1474.
116 Ducrocq X, Hassler W, Moritake K, et al. Consensus opinion on diagnosis of cerebral circulatory arrest using Doppler-sonography: Task Force Group on Cerebral Death of the Neurosonology Research Group of the World Federation of Neurology. J Neurol Sci. 1998;159:145.
117 Petty GW, Mohr JP, Pedley TA, et al. The role of transcranial Doppler in confirming brain death: sensitivity, specificity, and suggestions for performance and interpretation. Neurology. 1990;40:300.
118 Zurynski Y, Dorsch N, Pearson I, et al. Transcranial Doppler ultrasound in brain death: experience in 140 patients. Neurol Res. 1991;13:248.
119 Eng CC, Lam AM, Byrd S, et al. The diagnosis and management of a perianesthetic cerebral aneurysmal rupture aided with transcranial Doppler ultrasonography. Anesthesiology. 1993;78:191.
120 Grote E, Hassler W. The critical first minutes after subarachnoid hemorrhage. Neurosurgery. 1988;22:654.
121 Ropper AH, Kehne SM, Wechsler L. Transcranial Doppler in brain death. Neurology. 1987;37:1733.
122 Alexandrov AV, Demchuk AM, Felberg RA, et al. High rate of complete recanalization and dramatic clinical recovery during tPA infusion when continuously monitored with 2-MHz transcranial doppler monitoring. Stroke. 2000;31:610.
123 Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170.
124 Molina CA, Montaner J, Abilleira S, et al. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke. 2001;32:1079.
125 Eggers J, Koch B, Meyer K, et al. Effect of ultrasound on thrombolysis of middle cerebral artery occlusion. Ann Neurol. 2003;53:797.
126 Alexandrov AV, Burgin WS, Demchuk AM, et al. Speed of intracranial clot lysis with intravenous tissue plasminogen activator therapy: sonographic classification and short-term improvement. Circulation. 2001;103:2897.
127 Francis CW. Ultrasound-enhanced thrombolysis. Echocardiography. 2001;18:239.
128 Kimura M, Iijima S, Kobayashi K, et al. Evaluation of the thrombolytic effect of tissue-type plasminogen activator with ultrasound irradiation: in vitro experiment involving assay of the fibrin degradation products from the clot. Biol Pharm Bull. 1994;17:126.
129 Lauer CG, Burge R, Tang DB, et al. Effect of ultrasound on tissue-type plasminogen activator-induced thrombolysis. Circulation. 1992;86:1257.
130 Blinc A, Francis CW, Trudnowski JL, Carstensen EL. Characterization of ultrasound-potentiated fibrinolysis in vitro. Blood. 1993;81:2636.
131 Suchkova V, Siddiqi FN, Carstensen EL, et al. Enhancement of fibrinolysis with 40-kHz ultrasound. Circulation. 1998;98:1030.
132 Tsivgoulis G, Molina CA, Eggers J, et al. Safety and efficacy of ultrasound-enhanced thrombolysis: a meta-analysis of randomized and non-randomized studies [abstract]. Stroke. 2008;39:593.
133 Daffertshofer M, Gass A, Ringleb P, et al. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator: results of a phase II clinical trial. Stroke. 2005;36:1441.
134 Molina CA, Ribo M, Rubiera M, et al. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke. 2006;37:425.
135 Sharma VK, Tsivgoulis G, Lao AY, et al. Quantification of microspheres appearance in brain vessels: implications for residual flow velocity measurements, dose calculations, and potential drug delivery. Stroke. 2008;39:1476.
136 Alexandrov AV, Mikulik R, Ribo M, et al. A pilot randomized clinical safety study of sonothrombolysis augmentation with ultrasound-activated perflutren-lipid microspheres for acute ischemic stroke. Stroke. 2008;39:1464.