Transcranial doppler in the diagnosis of cerebral circulatory arrest
(CONSULTANT LEVEL EXAMINATION)
Overview
The role of transcranial color coded Doppler (TCCD) in neurocritical monitoring has been illustrated in previous chapters. We will here focus on its use as a confirmatory test in the diagnosis of brain death (BD). The concept of death in Western civilization has been linked to the cessation of breathing and heart beat, irrespective of cultural and religious variability. Advances in medical technology after the Second World War and the development of critical care integrating the use of mechanical ventilators, as well as the advent of successful transplantation of vital organs, presented new ethical, legal, and medical dilemmas.1
In 1968, the Ad Hoc Committee of the Harvard Medical School to Examine the Definition of BD concluded that BD is a strictly clinical diagnosis, defined as the irreversible cessation of all hemispheric, cerebellum, and brainstem neurologic functions.2,3 Analyzing BD is beyond the scope of this chapter; however, readers are referred to the White Paper published by the U.S. President’s Council on Bioethics in 2009, which illustrates controversies in the diagnosis of BD, while introducing the term “total brain failure” and defining the irreversible cessation of whole-brain function.4,5 Controversies in the diagnosis of BD and the use of the traditional cardiopulmonary standard in the organ procurement practice, known as “controlled donation after cardiac death,” have led to the development of confirmatory tests in BD diagnostic protocols.4,6 These tests are recommended whenever specific elements of the clinical examination may be unreliable and are rarely implemented by law in certain countries.6 Patients with severe brain injury are usually treated with barbiturates; moreover, the presence of various metabolic, thermoregulatory, respiratory, and other disturbances may prevent determination of BD by clinical criteria.7 Confirmatory tests are used in children and neonates because clinical diagnosis of BD can be challenging in these cases.8,9 Confirmatory tests are divided into those diagnosing cerebral circulatory arrest (CCA) (e.g., angiography) and those that demonstrate loss of bioelectrical activity (e.g., electroencephalography).10,11
Transcranial doppler in the diagnosis of cerebral circulatory arrest
CCA is an element of the destructive pathophysiologic process leading toward BD. Of note, brain neurons are irreversibly damaged after several minutes of CCA, and global brain destruction can be evident within 30 minutes.12 However, BD may occur regardless of CCA and vice versa because the specific pathophysiology can be different among patients progressing toward BD.10–14 The common pattern is underlined by an increase in intracranial pressure (ICP) that will eventually lead to brain “tamponade” because ICP rises above mean arterial pressure (MAP), resulting thus in CCA. Another pattern is characterized by ICP increments that may not exceed MAP, although there is pathology affecting the brain on a cellular level, resulting in edema and tissue necrosis.10,11 Early testing for CCA may lead to false-negative findings, and thus testing for neuronal function and viability may be advocated; however, in cases where brain damage becomes irreversible, CCA could finally emerge.14 Detecting CCA requires careful timing of the initial and follow-up examinations in patients with severe brain injury progressing toward BD. Several ancillary tests to detect CCA were developed, such as cerebral angiography, intravenous digital subtraction angiography, intravenous radionuclide angiography, single-photon emission computed tomography, echoencephalography, measurement of arm-to-retina circulation time, ophthalmic artery pressure, rheoencephalography, xenon-enhanced computed tomography (CT), magnetic resonance imaging angiography, CT angiography and perfusion, and TCD.11 Invasive angiography remains the gold standard examination in detecting CCA; however, recent developments in CT angiography (e.g., multirow CT, allowing reconstructions of intracranial vessels) is rapidly shifting current practice. This practice change is occurring even though more studies are required to evaluate its efficacy to diagnose CCA. Lack of portability and use of iodinated contrast remain major disadvantages of invasive and CT angiography.11,14,15 Angiographic patterns indicative of BD are (1) absent filling of intracranial arteries at the skull entry (at the foramen magnum in the posterior circulation and at the petrosal portion of the carotid artery in the anterior circulation) and (2) minimal arterial opacification with absent parenchymal and venous phases.10–14
TCCD was used in the diagnosis of CCA after studies that proved its high agreement with invasive angiography.11,13–15 The sensitivity and specificity of TCCD for BD confirmation, when compared with angiography, are 88% and 100%, respectively.11,15 TCCD remains largely operator dependent, and its use is limited by various technical issues (i.e., absence of an acoustic window in up to 20% of cases), but it is a noninvasive, portable, dye-contrast agents free, and relatively cheap examination.11,15 The American Academy of Neurology Therapeutics and Technology Assessment Subcommittee concluded that TCCD is highly specific and sensitive in detecting CCA, whereas the Neurosonology Research Task Force Group of the World Federation of Neurology emphasized that extracranial and intracranial TCCD can detect CCA and thus may be considered optional in BD diagnostic protocols.15,16 Recommended parameters for TCCD examinations were detailed in preceding chapters. In brief, patients are placed in dorsal decubitus position, while aiming to maintain systolic blood pressure above 90 mm Hg, heart rate above 60 beats/min, and oxygen saturation by pulse oximetry (Spo2) greater than 95%. Technical parameters are use of 1.5- to 5-MHz phased-array transducer, color and velocity scales adjusted for low amplitude, sample volume of 6 to 10 mm (Doppler mode), maximal gain, and small sample size (color Doppler mode), thus avoiding angle adjustments on Doppler mode.
CCA-specific signals depicted by TCCD are oscillating flow, systolic spikes, or no demonstrable flow in a case with previously documented flow on TCCD (Figure 4-1). As ICP is gradually increasing in brain-injured patients, TCCD diastolic and mean flow velocities are accordingly decreasing, resulting in increments of the Doppler-derived pulsatility index. Hence laminar flow of small-caliber intracranial vessels is disrupted, and this can be documented by TCCD and color M-mode analysis (Figure 4-2).14 As brain injury deteriorates and autoregulation fails, cerebral flow is further affected, and end-diastolic TCCD velocities tend to become zero, while ICP has reached diastolic blood pressure; however, forward flow may continue in systole. When ICP equals or exceeds the systolic blood pressure forward, and reverse flow are nearly identical, a pattern known as oscillating or biphasic flow may be evident (see Figures 4-1 and 4-2). Oscillating flow may persist in patients with severe traumatic brain injury who underwent salvage decompressive craniectomy.17 Oscillating flow may be depicted on color mode as a transitory “flickering” of cerebral circulation in both intracranial and extracranial arteries ![]() (Videos 4-1 and 4-2).13 Progression of these phenomena can generate systolic spikes or absent flow on TCCD. Inability to detect flow signals because of an absent acoustic window may be solved by using transorbital and other approaches, but these techniques have not yet gained wide acceptance.18 TCCD findings should always be evaluated along with clinical and laboratory findings, and repeating the examination within at least 30 minutes is essential.13–20 To confirm diagnosis, extracranial bilateral scanning of the common carotid, internal carotid, and vertebral arteries should be performed.15,16
(Videos 4-1 and 4-2).13 Progression of these phenomena can generate systolic spikes or absent flow on TCCD. Inability to detect flow signals because of an absent acoustic window may be solved by using transorbital and other approaches, but these techniques have not yet gained wide acceptance.18 TCCD findings should always be evaluated along with clinical and laboratory findings, and repeating the examination within at least 30 minutes is essential.13–20 To confirm diagnosis, extracranial bilateral scanning of the common carotid, internal carotid, and vertebral arteries should be performed.15,16
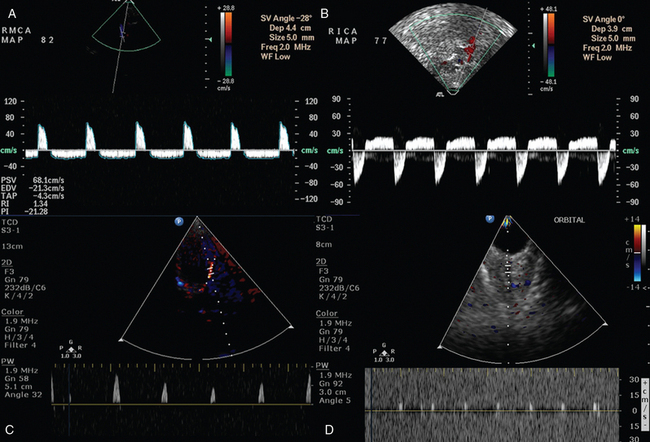
Figure 4-1 A and B, Oscillating flow depicted in the right middle cerebral (RMCA) and internal carotid arteries (RICA), respectively, in a patient with severe traumatic brain injury who was thereafter diagnosed as brain dead. C and D, Systolic spikes depicted in the left middle cerebral and ophthalmic arteries, respectively, in a brain-dead subject.
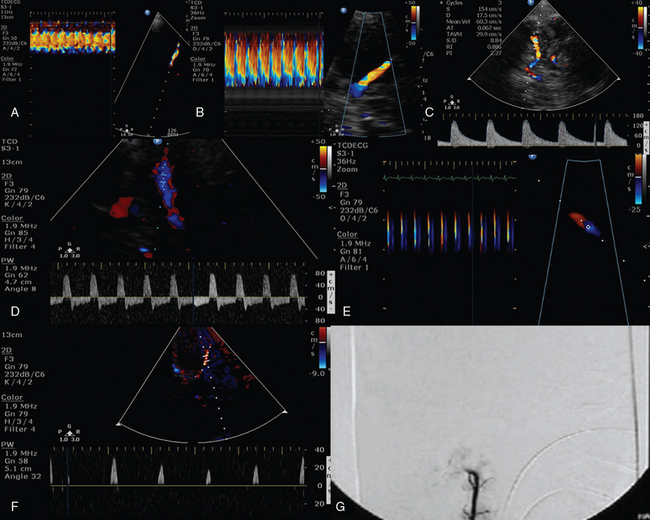
Figure 4-2 A, Color M-mode depicting normal laminar flow in the left middle cerebral artery (MCA). Flow anomalies in the left MCA (color M-mode), which are confirmed by increments of peak systolic velocity (B), while diastolic and mean velocities (Doppler mode) are gradually decreasing, resulting thus in increased pulsatility index (PI) values greater than 2 (C) in a brain-injured patient with increased intracranial pressure. Oscillating flow depicted by Doppler (D) and color M-mode analysis (E) in a brain-injured patient with decompressive craniectomy. The above signals persisted for approximately 48 hours; thereafter, systolic spikes (F) and absent intracranial flow were evident (G).
Suggested guidelines for the use of TCCD in detecting CCA are (1) CCA-specific TCCD signals are present in both intracranial and extracranial arteries when recorded bilaterally on two examinations within a 30-minute interval; (2) CCA-specific signals are systolic spikes or oscillating flow present in any arterial segment of the circle of Willis and depiction of the same findings in extracranial arterial segments, such as the common carotid, internal carotid, and vertebral arteries; and (3) lack of a signal during transcranial insonation of the basal cerebral arteriesis not a reliable finding, because this can be observed due to signal transmission issues—but the disappearance of intracranial flow signals in conjunction with typical extracranial signals can be accepted as proof of CCA; and (4) ventricular drains or large openings of the skull that possibly interfere with the development of the ICP are not present.15,16 However, patients with ventricular drains and/or large skull openings may in fact develop CCA because progression toward brain “tamponade” may be slow and follow-up examinations may demonstrate CCA-specific TCCD signals.13,14,17–20 Hence the designated 30-minute interval may be not enough to establish diagnosis of CCA in individual cases, and continuous TCCD monitoring for longer periods may be necessary.13,14,17–20 Also, performing TCCD measurements under controlled hemodynamic, metabolic, and respiratory conditions increases the reliability of findings.13–20 Despite its limitations, TCCD has a high level of agreement with angiography in the diagnosis of CCA, and its use may be considered optional in BD diagnostic protocols.
Pearls and highlights
• Brain death is a strictly clinical diagnosis, although controversies about its definition still exist, leading to complex ethical, legal, and medical dilemmas.
• Ancillary tests that diagnose CCA or loss of bioelectrical activity may be integrated in BD diagnostic protocols in case clinical diagnosis is complicated or not possible.
• CCA is an element of the destructive pathophysiologic process leading toward BD.
• CCA-specific TCCD findings are oscillating flow and systolic spikes or absence of flow in a case with previously demonstrable flow.
• Despite its limitations, TCCD has a high agreement with conventional angiography in the diagnosis of CCA.
References
1. Machado, C. Determination of death. Acta Anaesthesiol Scand. 2005; 49:592–593.
2. Harvard Medical School Ad Hoc Committee to Examine the Definition of Brain Death. A definition of irreversible coma. JAMA. 1968; 205:337–340.
3. Guidelines for the determination of death. Report of the medical consultants on the diagnosis of death to the President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. JAMA. 1981; 246:2184–2186.
4. The President’s Council on Bioethics. Controversies in the determination of death. Available at http://bioethics.georgetown.edu/pcbe/reports/death/index.html, December 2008. [Accessed March 11, 2013].
5. Miller, FG, Truog, RD, The incoherence of determining death by neurological criteria: a commentary on “Controversies in the determination of death”, a White Paper by the President’s Council on Bioethics. Kennedy Inst Ethics . 2009; 19:185–193.
6. Wijdicks, EF, Brain death worldwide: accepted fact but no global consensus in diagnostic criteria. Neurolog. 2002; 58:20–25.
7. Powner, DJ. Drug-associated isoelectric EEGs. A hazard in brain-death certification. JAMA. 1976; 236:1123.
8. Ashwal, S. Clinical diagnosis and confirmatory testing of brain death in children. In: Wijdicks EFM, ed. Brain death. Philadelphia: Lippincott Williams & Wilkins; 2001:91–114.
9. Mejia, RE, Pollack, MM. Variability in brain death determination practices in children. JAMA. 1995; 274:550–553.
10. Wijdicks, EF. The diagnosis of brain death. N Engl J Med. 2001; 344:1215–1221.
11. Wijdicks, EFM. Clinical diagnosis and confirmatory testing in brain death in adults. In: Wijdicks EFM, ed. Brain death. Philadelphia: Lippincott Williams & Wilkins; 2001:61–90.
12. Bernat, JL. On irreversibility as a prerequisite for brain death determination. Adv Exp Med Biol. 2004; 550:161–167.
13. Karakitsos, D, Poularas, J, Karabinis, A, et al. Considerations for the utilization of transcranial Doppler sonography in the study of progression towards cerebral circulatory arrest. Intensive Care Med. 2011; 37:368–370.
14. Poularas, J, Karakitsos, D, Kouraklis, G, et al. Comparison between transcranial color Doppler ultrasonography and angiography in the confirmation of brain death. Transplant Proc. 2006; 38:1213–1217.
15. Ducrocq, X, Hassler, W, Moritake, K, et al, Consensus opinion on diagnosis of cerebral circulatory arrest using Doppler-sonography: Task Force Group on cerebral death of the Neurosonology Research Group of the World Federation of Neurology. J Neurol Sc. 1998; 159:145–150.
16. Sloan, MA, Alexandrov, AV, Tegeler, CH, et al, Assessment: transcranial Doppler ultrasonographyreport of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurolog. 2004; 62:1468–1481.
17. Cabrer, C, Dominnguez-Roldan, JM, Manyalich, M, et al. Persistence of intracranial diastolic flow in transcranial Doppler sonography exploration of patients in brain death. Transplant Proc. 2003; 35:1642–1643.
18. Conti, A, Iacopino, DG, Spada, A, et al, Transcranial Doppler ultrasonography in the assessment of cerebral circulation arrest: improving sensitivity by trancervical and transorbital carotid insonation and serial examinations. Neurocrit Car. 2009; 10:326–335.
19. Hadani, M, Bruk, B, Ram, Z, et al. Application of transcranial Doppler ultrasonography for the diagnosis of brain death. Intensive Care Med 1999. 1999; 25:822.
20. Newell, DW, Grady, MS, Sirotta, P. Evaluation of brain death using transcranial Doppler. Neurosurgery. 1989; 24:509–513.