Training and competence in ultrasound-guided vascular access
Overview
Despite well-established evidence on the use of ultrasound for placement of any vascular access,1 a formal training program on how to perform ultrasound-guided vascular access (UGVA) and how to certify adequate competence has not been clearly defined.2,3 According to recommendations by the National Institute for Clinical Excellence, those involved in UGVA should undertake appropriate training to achieve competence.4 Defining a competent practitioner in objective terms is a difficult task. Recently, the American Society of Echocardiography declared that training in UGVA should include a minimum of 10 proctored procedures, but the learning curve needed to perform these procedures was not defined, nor was it specified who should be considered expert and supervise new users.5
Evidence-based guidelines for defining a common educational process and establishing credentialing for any type of UGVA that can be considered as a model for any kind of training course in UGVA were recently published by the World Conference on Vascular Access.6 The guidelines emphasized that the use of ultrasound reduces insertion-related complications, increases the success rate at first attempt, assists in selection of the correct vascular device according to safety, and enables preservation of veins.
Didactic education
Educational programs facilitate a reduction in delayed complications such as infection and thrombosis.7,8 The didactic contents of UGVA courses should be specifically directed at how to perform UGVA. Clinicians’ knowledge of certain subject areas and competence in device insertion affect the rate of UGVA-related complications. The following topics should be discussed in formal educational training programs:
• Ultrasound physics: characteristics of sound waves, effects of interactions with ultrasound waves, and creation of image from ultrasound beams
• Sonographic anatomy of relevant structures such as vessels, nerves, bony structures, soft tissues, muscles, lung, pleura, and basic cardiac anatomy
• Knobology and interpretation of vascular images (transverse and longitudinal views) and needle visualization (out of plane and in plane)
• Techniques for insertion and assessment (“4 P’s” technique: preprocedure scanning of relevant ultrasound anatomic structures, preparation of a sterile field and sterile ultrasound probe for vascular access, puncture of the vein or artery, placement of catheters)
• Sonographic detection of the tip of the catheter (contrast-enhanced echocardiography9)
• Evaluation and management of complications: accidental carotid or posterior venous wall puncture, hematoma, pneumothorax, and abnormal position of the tip of the catheter
• UGVA in neonates and pediatric patients and ultrasound-guided cannulation of peripheral veins and arteries
All didactic content should be included in a training manual so that trainees can read it before beginning the didactic portion of the training. A minimum of 6 to 8 hours of didactics has been suggested. Theoretic competence should then be evaluated with a multiple-choice test that has at least 100 questions. The trainee must pass the test with at least 70% correct answers before proceeding to hands-on training.6
Simulation training
Training laboratories should offer a variety of ultrasound machines to facilitate understanding of their basic functions such as depth, gain, and freeze (knobology). Training should start with examination of normal vascular anatomy in healthy volunteers and with hands-on simulation (models). Several inanimate models of vascular access are available. The best simulation model should not only include vessels but also mimic the normal body anatomy in terms of muscles, soft tissues, and bones. Inanimate animal models such as turkey breasts may be useful to demonstrate these anatomic findings. In the future, virtual simulators could be useful for measuring the correct hand-eye coordination of the trainee. Although it is not possible to include visualization of abnormal vascular anatomy in inanimate models, a collection of videos should be incorporated into the didactic material to show all significant abnormal findings during preliminary evaluation of the vessels before cannulation (e.g., intraluminal thrombosis, significant collapse of the internal jugular vein during inspiration). Trainees should complete 6 hours of hands-on training on normal human volunteers for detection of normal ultrasound anatomy and 4 hours of hands-on training on inanimate models.2,6
Learning curves and training time
Application of the learning-curve concept to UGVA placement results in a 50% reduction in major complications during the learning process. There is still no evidence to establish the ideal number of supervised procedures for trainees to reach the learning curve.2,3,5 The main goal of supervision is to guide the trainee in avoiding major complications while performing the procedure so that the required minimal skill competence with the lowest rate of major and minor complications can be achieved. The suggested number of tutored procedures to achieve minimal learning curves ranges from 10 to 25 UGVA placements, but this number could be different in trainees already experienced in placement of a central venous line, as well as if minor complications are to be considered.10 Research should be conducted to establish a suggested number of procedures for reaching the learning curve benchmark or for calculating a personal learning curve according to the time needed to successfully perform UGVA.
Accreditation and level of performance of ultrasound-guided vascular access
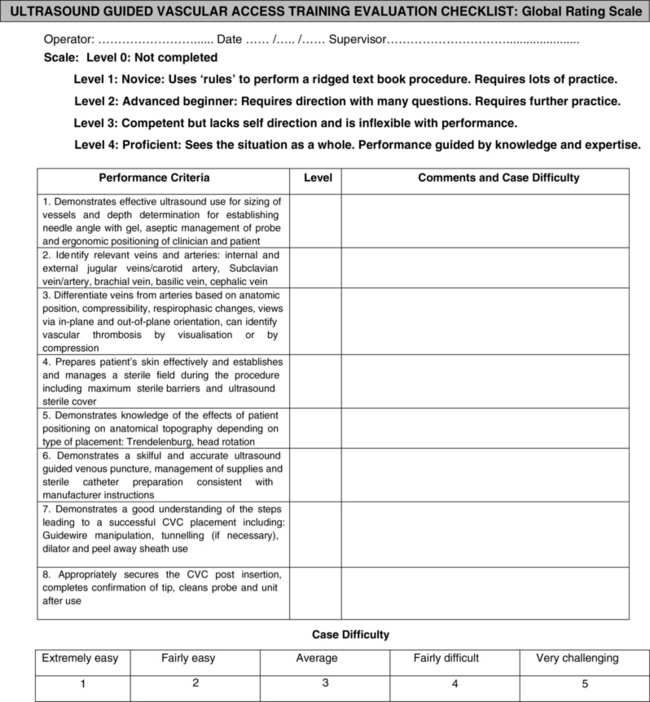
After appropriate tutored training, the trainee should be examined according to an objective step-forward checklist called the Global Rating Score (Figure 60-1).11 This final audit evaluates levels of a trainee’s performance. In case of failure, the trainee should have to continue practicing until all practical goals have been achieved. Once an ultrasound technique for access at a specific site is achieved, it may be applied to other sites accordingly. A quality control registry includes the recording of all complications (including minor ones such as multiple needle punctures) in a personal logbook.
Skill learning, maintenance of skills, and self-training
It remains crucial that operators still undergo training and become proficient in the standard landmark method of insertion of a central venous catheter.12 Maintenance of skills is also crucial. It is suggested that an operator could perform at least 10 UGVA placements per year to maintain minimal proficiency in this technique. Self-training is integral to proficiency in all operator-dependent techniques. Self-training should be used in conjunction with and not instead of didactics and instruction from supervisors.13 Notably, whenever UGVA was performed by two operators, the experience of the physician controlling the needle did not influence procedural success or the complication rate, whereas both were significantly reduced if the physician manipulating the transducer was experienced.14 This explains the fact that there is a learning curve associated with sonography and that guidance based on misinformation can harm patients.
Specific requirements of training for ultrasound-guided vascular access in neonates and children
UGVA in neonates and pediatric patients is a challenging practice, mainly because of reduced diameters of vessels and a thinner layer of subcutaneous fat, as well as the need for sedation to achieve a stable position and allow a safe puncture. However, UGVA in children and neonates reduces the frequency of failures during insertion of central venous lines and time to cannulation as a result of increased first-time success.15,16 Specific technical requirements for safe performance of UGVA in neonates and children are small (2 cm), high-frequency (10 to 15 MHz), broadband transducers; short, steel microneedles (21 or 22 gauge); soft, floppy, straight-tipped nitinol guidewires; soft peel-away introducers; and short polyurethane catheters (8 and up to 12 cm in length). The operator should place the head of the child in a stabilized position by putting pillows under the head if it is turned to the opposite side of the cannulation. Operators should sit down while performing preliminary scans of all vessels. Once the vein to cannulate is determined, operators should have their hands stabilized by placing their elbows down on the bed of the patient. In the case of very small vessels (2 to 3 mm) it could be useful to perform the maneuver with two operators. The first operator punctures the vein and the second simultaneously places the guidewire to perform real-time, ultrasound-guided venous cannulation. UGVA in children requires excellent hand-eye coordination. It was suggested that previous UGVA experience in adults with at least 15 tutored procedures is necessary to become proficient in performing the procedure in pediatric patients.17
Training costs of ultrasound-guided vascular access
UGVA educational programs should be mandatory in every setting in which venous and arterial cannulation is routinely performed and should be included in the responsibilities and job descriptions of doctors and nurses. UGVA training expenses include the cost of ultrasound machines (including maintenance), didactic courses, hands-on training, final audit, and revalidation. These costs were calculated to be higher than £8 per cannulation. However, this calculation should be upgraded because cost is estimated to be around 1000 Euros per operator, with a break-even point of totally successful procedures being reached after the completion of 200 ultrasound-guided cannulations.18 After this point a real increase in benefits and zeroing of costs related to complications are achieved.
Pearls and highlights
• UGVA training includes a didactic manual plus tutoring by certified supervisors. Didactics should include knobology, sonographic anatomy and its variations, ultrasound techniques, detection and treatment of complications, and care and maintenance of vascular devices.
• Hands-on training on inanimate phantoms should be used to evaluate the visual-manual competence of trainees before any practice on patients.
• Initially, practice on patients needs to be supervised. A final audit after 25 procedures should be done to evaluate a trainee’s proficiency and competence. Training for performing UGVA in neonates and children is more prolonged.
References
1. Lamperti, M, Bodenham, A, Pittiruti, M, et al. International evidence-based recommendations on ultrasound guided vascular access. Intensive Care Med. 2012; 38:1105–1117.
2. American College of Emergency Physicians. ACEP policy statement: emergency ultrasound guidelines, ACEP, 2008:1–38.
3. Royal College of Radiologists RCR Board of the Faculty of Clinical Radiology Available at http://www.rcr.ac.uk/docs/radiology/pdf/ultrasound.pdf, Accessed March 9, 2012. Ultrasound training recommendations for medical and surgical specialties, RCR, 2004:1–56.
4. National Institute for Health and Clinical Excellence (NICE). Technology appraisal No 49: guidance on the use of ultrasound locating devices for placing central venous catheters, 2002, 1–24. Available at http://www.nice.org.uk/nicemedia/live/11474/32461/32461.pdf [Accessed March 9, 2012].
5. Troianos, C, Hartman, G, Glas, K, et al, Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiog. 2011; 24:91–318.
6. Moureau N, Lamperti M, Kelly LJ, et al: An evidence-based consensus on the insertion of central venous access devices: definition of minimal requirements for training, Br J Anaesth 110: 347-356
7. Warren, DK, Zack, JE, Mayfield, JL, et al. The effect of an education program on the incidence of central venous catheter–associated bloodstream infection in a medical ICU. Chest. 2004; 126:1612–1618.
8. Grove, J, Pevec, W. Venous thrombosis related to peripherally inserted central catheters. J Vasc Interv Radiol. 2000; 11:837–840.
9. Vezzani, A, Brusasco, C, Palermo, S, et al, Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Me. 2010; 38:533–538.
10. Lamperti, M, Subert, M, Cortellazzi, P, et al. Is a neutral head position safer than 45-degree neck rotation during ultrasound-guided internal jugular vein cannulation? Results of a randomized controlled clinical trial. Anesth Analg. 2012; 114:777–784.
11. Evans, LV, Morse, JL, Hamann, CJ, et al. The development of an independent rater system to assess residents’ competence in invasive procedures. Acad Med. 2009; 84:1135–1143.
12. Calvert, N, Hind, D, McWilliams, RG, The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation. Health Technol Asses. 2003; 7:1–84.
13. Feller-Kopman, D. Real-time sonography with central venous access. The role of self-training. In response. Chest. 2007; 132:2061–2062.
14. Mey, U, Glasmacher, A, Hahn, C, et al. Evaluation of an ultrasound-guided technique for central venous access via the internal jugular vein in 493 patients. Support Care Cancer. 2003; 11:148–155.
15. Verghese, ST, McGill, WA, Patel, RI, et al. Comparison of three techniques for internal jugular vein cannulation in infants. Paediatr Anaesth. 2000; 10:505–511.
16. Lamperti, M, Caldiroli, D, Cortellazzi, P, et al. Safety and efficacy of ultrasound assistance during internal jugular vein cannulation in neurosurgical infants. Intensive Care Med. 2008; 34:2100–2105.
17. Breschan, C, Platzer, M, Jost, R, et al. Consecutive, prospective case series of a new method for ultrasound-guided supraclavicular approach to the brachiocephalic vein in children. Br J Anaesth. 2011; 106:732–737.
18. Calvert, N, Hind, D, McWilliams, R, Ultrasound for central venous cannulation: economic evaluation of cost-effectiveness. Anaesthesi. 2004; 59:1116–1120.