Tracheostomy
History
Most medications, devices, and surgical techniques employed in today’s intensive care unit (ICU) have resulted from twentieth century medical advances. However, one of the most common surgical procedures, tracheostomy, has been described for nearly 6000 years. The terms tracheostomy and tracheotomy are derived from the Greek words tracheia arteria, translated “rough artery,” and refer to the trachea being the vital conduit of air.1 Tracheostomy means permanent opening (stoma, Greek for “mouth”), to be distinguished from the temporary nature of a tracheotomy (tome, “to cut”). Today the terms are used interchangeably for any artificial airway created in the trachea, with tracheostomy used more commonly.
McClelland divides the history of the tracheostomy into five periods beginning with “The Period of Legend” (2000 BCE to 1546 CE).2 The first written reference to tracheostomy is in the sacred book of Hindu medicine, the Rig Veda, dated between 2000 BCE and 1000 BCE, and describes “the bountiful one, who without a ligature, can cause the windpipe to reunite when the cervical cartilages are cut across.”3,4 The earliest depictions of a tracheostomy being performed date back to about 3600 BCE and show two Egyptian kings undergoing a tracheostomy.5,6 Homer referred to tracheostomy as a way of relieving choking persons by cutting open the trachea, and Alexander the Great is described to have used the point of his sword to open the trachea of one of his soldiers who was choking while eating.2 The next successful tracheostomy was performed in the second century CE by Antyllus as documented 400 years later by Paul of Aegina, who wrote that the use of tracheostomy was encouraged in cases of upper airway obstruction and provided a technical description of the operation.7 Mention of tracheostomy can be found in the Roman and Arabic literature, although during the Dark Ages of medicine and science the technique of tracheostomy (and virtually all other surgeries) was forgotten for nearly 1000 years.6,8
The second period, “The Period of Fear” (1536 to 1833),2 starts with the Renaissance, when European physicians experimented with tracheostomy for trauma, aspirated foreign bodies, drowning, and Ludwig’s angina. Fabricius of Aquapendente tells us that the timidity at that time was due partly to “lack of knowledge of anatomy, partly to fear of a loss of reputation, should the patient die after the operation.” In his day, tracheostomy was known as the “scandal of surgery.” About the same time, in 1546, the first definite account of a successful tracheostomy was recorded by Antonius Brasavola, who told of “opening the windpipe and saving the life of a patient near death from angina and an abscess which was obstructing the canalis pulmonis.”1,8 Over the next 2 centuries, opposition to tracheostomy diminished because of the interest in anatomy and autopsy, as evidenced by the drawings and writings of Leonardo da Vinci, Julius Casserius, and others.6,9–11
The clinical importance of tracheostomy became evident during sporadic diphtheria epidemics, and multiple publications of successful tracheostomies can be found in many European countries after 1620.8 In 1730, Scottish surgeon George Martine treated upper airway obstruction resulting from diphtheria with tracheostomy. He also recommended the use of an inner cannula for ease of care and recognized that tracheal wounds heal spontaneously without the need for surgical repair.8,12 In America, fear of tracheostomy was still quite prevalent, as evidenced in the well-known controversy surrounding the death of George Washington in 1799, when bloodletting won over relieving an epiglottitis-related upper airway obstruction with a tracheostomy.6,13 Until 1825, during these first several thousand years in the history of tracheostomy, only 28 tracheostomies are verifiable.
After Napoleon Bonaparte’s nephew died of diphtheria in 1807, a grand prize was offered for new insights into this disease and its treatment. This heralded the “Period of Drama,” which lasted from 1833 to 1931. Based on subsequent research, especially by Bretonneau and his pupil Trousseau, tracheostomy became a relatively established procedure, particularly for croup and diphtheria. Before Bretonneau, tracheostomy had been known under many different names, including bronchotomy, laryngotomy, pharyngotomy, sectio epiglottis, scisio cannae, and incisio cannae pulmonis. In 1718 Heister had introduced the term tracheotomy and recommended that all other terms be discarded, but it was not until Bretonneau used this term in a paper describing a successful operation in 1825 for diphtheria that it gained widespread acceptance.8,14 Trousseau described a series of 200 French children, most dying of diphtheria, in whom he reduced mortality rate from nearly 100% to 75% with tracheostomy.15 American surgeon Thomas Shastid published an account of performing tracheostomies on children in the 1890s in a small Illinois town during a diphtheria epidemic.16,17
In the mid-1800s, Snow and Trendelenburg advocated tracheostomy for administration of inhaled anesthetics,3,18 but endotracheal intubation, performed by MacEwan in 187819 and O’Dwyer in 1880,20 and popularized by Bartholomay and Dufor in 1907 and Kelly in 1912, soon replaced tracheostomy as the route for delivering general anesthesia.3,21 This put the performance of tracheostomy squarely in the hands of surgeons experienced in upper airway problems, most notably Chevalier Jackson, whose name became virtually synonymous with tracheostomy. In his hands, the rate of mortality attributable to tracheostomy decreased from 25% to less than 5%.22
The fourth period, the “Period of Enthusiasm” (1932 to 1965), was heralded by a revolutionary shift in the indications for the procedure and major advances in the technique. During this period the main sentiment was “if you think of tracheostomy, do it,” without consideration of possible adverse outcomes. Up to then the main indication for tracheostomy was upper airway obstruction, mostly caused by severe croup and diphtheria, which practically disappeared as a result of the development of antibiotics and immunization. In the 1950s, with the poliomyelitis epidemic in Europe and North America, the need for positive-pressure ventilation (PPV) and tracheobronchial suctioning created new indications for tracheostomy.23 At present, these are still the major indications for tracheostomy in the ICU, and they caused the number of tracheostomies performed at Massachusetts General Hospital to increase from fewer than 10 in 1947 to more than 150 in 1959. Before 1958, not a single tracheostomy was performed there for the sole purpose of providing PPV.24
The exponential increase in tracheostomy in the 1950s and 1960s revived older controversies. In 1921, Chevalier Jackson discussed proper technique and complications.25 He condemned the use of high tracheostomy, believing it caused laryngeal stenosis. For 50 years, this was the prevailing wisdom of neck surgery and airway maintenance. Jackson drew his conclusions, however, primarily from unsterile emergency tracheostomies performed by inexpert practitioners.25 Laryngeal stenosis probably resulted from factors other than the site of incision, especially when performed with preexistent laryngeal disease.26,27 Jackson’s reputation made surgeons reluctant to perform high tracheostomies until the advent of cardiac surgery made them necessary to avoid contaminating median sternotomy incisions. In 1976, two cardiac surgeons, Brantigan and Grow, published the first large series of high tracheostomies and found few complications.28 Acceptance of high tracheostomy permitted reintroduction of PT; a technique described 20 years earlier.
As previously described, when ICUs were developed, tracheostomy became one of the most frequently performed procedures in critically ill patients.29 This created a need for a safe, cost-effective bedside procedure that would eliminate the necessity for transport of the patient from the ICU to the operating room, with its attendant risks, which are discussed subsequently.
In 1955, Shelden and associates30 described the first PT, using a slotted needle to guide a cutting trocar into the trachea. This technique was abandoned after fatalities resulted from trocar lacerations of vital structures adjacent to the airway.31,32 In 1969 Toye and Weinstein33 described a modified Seldinger technique in which a splitting needle was inserted into the trachea, and through this a guidewire was placed. A single lead dilator was passed over the guidewire, the needle split away, and the tracheostomy tube placed. This technique had a 1% incidence of perioperative death and a 6% incidence of paratracheal insertion, which ultimately caused it also to be abandoned.34
In 1985, Ciaglia and colleagues,35 drawing from experience with cricothyroidotomy, described a true Seldinger technique for quick and easy bedside tracheostomy: percutaneous dilational tracheostomy (PDT). In this technique, multiple curved dilators of gradually increasing size are placed over a guidewire, creating an opening for a tracheostomy tube.
Subsequently, several different versions of PT have been described. In 1988, Hazard and associates36 had few complications using three straight dilators instead of Ciaglia’s seven curved dilators. In 1989, Schachner and colleagues37 reported a dilating forceps technique (Rapitrac), which is no longer available for use because of its high incidence of complications. In 1990, Griggs and coworkers38 described passing a blunt-tipped modified Kelly forceps over a guidewire to allow dilation of an aperture adequate to place a tracheostomy tube (guidewire dilator forceps [GWDF] technique). This technique is similar to the Rapitrac, but with a lower incidence of complications. In 1999, Ciaglia developed a soft-tipped, tapered dilator (Blue Rhino, Cook Critical Care, Inc., Bloomington, IN) that was used to create a stoma via a single-step dilation.39 This approach replaced the multiple dilations necessary in Ciaglia’s original kit, theoretically reducing complications and the time necessary to perform PDT. Fantoni’s translaryngeal tracheotomy technique was introduced in 1993 and modified in 1996,40 in which a guidewire is directed in retrograde fashion from the trachea to the mouth over which a cuffed cone-cannula is placed. The cannula is drawn through the neck, and a cuffed tube is placed in the trachea over the cannula as it is removed. The PercuTwist, developed in 2002, uses a dilator with a threaded screw to allow the insertion of a 9-mm tracheostomy tube.41 The latest modification of the Ciaglia technique of PDT was developed in 2005 and uses balloon dilatation to create the stoma (Blue Dolphin, Cook Critical Care, Inc., Bloomington, IN).42,43
The Artificial Airway
In critical care medicine, regardless of the patient’s diagnosis, there are four indications for placement of an artificial airway, which can be either an endotracheal or tracheostomy tube: (1) relieving airway obstruction, (2) providing mechanical ventilation (MV), (3) preventing aspiration in the unprotected airway, and (4) facilitating tracheobronchial toilet.44,45
Although tracheostomy was considered the emergency airway of choice in the past, now endotracheal intubation is preferred for initial airway management. This is because more practitioners are familiar with endotracheal intubation (which requires less specialized equipment and training), and studies indicate that, in the emergency setting, endotracheal intubation has fewer life-threatening complications46–48 such as bleeding and pneumothorax, which are extremely rare. Independent of esophageal placement, mortality rate from endotracheal intubation is 0.05%, but ranges from 1% to 2% for emergency tracheostomy.29 Tracheostomy should be considered an elective or semielective procedure when the airway is already secured.
Occasionally, endotracheal intubation under direct laryngoscopic visualization is not the initial airway management of choice because of massive facial trauma, tracheal obstruction, or anomalous anatomy.49,50 In such cases, endotracheal intubation sometimes can be facilitated through fiberoptic bronchoscopy.51 When intubation is impossible even with bronchoscopy, or when bronchoscopy is unavailable, the preferred emergency airway procedure is cricothyroidotomy.52,53 The primary role of tracheostomy is long-term airway care for patients who initially were treated with ETs or cricothyroidotomies, although reports of PT performed in an emergency setting have been published.54–57
Timing of Tracheostomy
The question as to when to replace an ET with a tracheostomy tube is a subject of much discussion, remaining highly controversial independent of technique. Beatrous44 stated in 1968 that, “Timing is an aspect of tracheostomy that deserves much more emphasis than it is apparently receiving. Delay defeats the purpose of the operation.”
The debate as to when to replace an ET with a tracheostomy tube centers on the advantages and disadvantages of the tracheostomy tube, as listed in Table 14.1.58–126 Unfortunately there are very few definite studies confirming or refuting these advantages.
Table 14.1
Advantages and Disadvantages of Tracheostomy
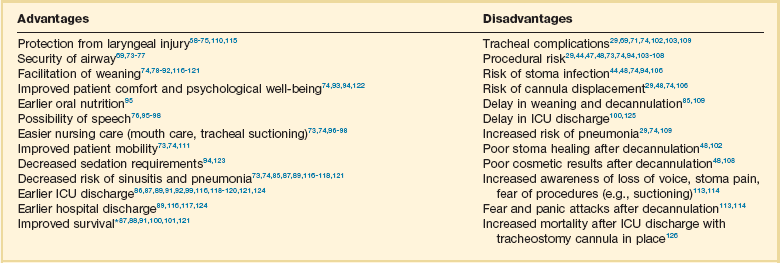
*Improved survival after tracheostomy has not been demonstrated in all studies (see text).
The decision as to when to perform a tracheostomy is more often based on personal preference of the treating physician than on data. For patients with an airway obstruction that cannot be relieved, tracheostomy is the treatment of choice because it is the only airway that can safely remain long term.127,128 In most other situations the decision is not as straightforward as ultimately only 5% to 11% of patients on MV require placement of a tracheostomy tube. Nevertheless, those patients account for 26% of all ventilator days and 14% of hospital days.101,125,129
Although the need for tracheostomy sometimes can be predicted early in the ICU course (e.g., neurologic ICU patients with infratentorial lesions who have brainstem dysfunction130 or a low Glasgow Coma Scale score90,99), in most situations this is not the case. Finding early clinical predictors that would identify patients who require a tracheostomy is most problematic in patients receiving MV.131–134 Many studies from the early and mid-1990s attempted to establish criteria to satisfy the recommendations of the 1989 Consensus Conference on Artificial Airways in Patients Receiving Mechanical Ventilation, which stated that “the decision to convert to tracheotomy should be made as early as possible . . . to minimize the duration of translaryngeal intubation. Once the decision is made, the procedure should be done without undue delay.”135
Unfortunately, good studies to determine optimal timing of tracheostomy are difficult to perform. Existing studies tend to be either retrospective reviews, which often include a large number of patients but are prone to bias, or randomized, controlled trials that are generally underpowered owing to the large number of patients required to detect significant outcome benefits.129 Multiple publications address the question of timing of tracheostomy in a variety of patient populations.85–92,115–120,124,136–138 Most are retrospective reviews of ICU databases in which timing of tracheostomy is retrospectively correlated with multiple outcomes. In these studies, the definition of “early” ranges from 3 to 21 days, and “late” ranges from 7 to 28 days, making it hard to draw any firm conclusions. It seems clear from these studies, however, that the procedural and short-term complications of tracheostomy are low, and that in general the outcome of patients with tracheostomy is at least no worse than that of patients managed with prolonged translaryngeal endotracheal intubation.
Some studies show a decrease in mortality rate in patients with tracheostomy.100,101,120,125 However, one has to question if this difference is partly explained by the fact that patients with anticipated high mortality risk are not offered a tracheostomy. A frequent finding is that placement of a tracheostomy reduces time on MV,86,87,89–92,101,116,118,119,121 but even this is not consistent. Other studies have shown no change85,115,138 or even markedly prolonged times100 on MV. This may reflect either a less aggressive approach to weaning after a potentially permanent airway has been placed or a selection bias reflecting higher early mortality rate in the patients not receiving a tracheostomy.
Randomized controlled trials of early tracheostomy versus late tracheostomy or prolonged translaryngeal intubation are rare. In 2004, Rumbak and associates87 showed that early tracheostomy in critically ill medical patients decreased time on MV, ICU length of stay, and ventilator-associated pneumonia. Additionally, early tracheostomy decreased mortality rate by 50% despite well-matched baseline characteristics. They also evaluated the patients for the most feared complication of tracheostomy, tracheal stenosis, during the hospital course and at 10 weeks. No significant differences in the incidence or severity of stenosis were seen, although there was a trend favoring early tracheostomy.87
These findings conflict with the findings of several other randomized controlled trials. A trial by Sugerman and coworkers72 showed no differences in any of the measured outcomes (ICU length of stay, death, and pneumonia). This study was significantly limited by incomplete data collection and physician bias. Another randomized controlled trial in trauma patients by Barquist and associates was prematurely terminated after the first interim analysis with only 60 patients enrolled due to lack of differences in the primary outcomes; ICU length of stay, ventilator-associated pneumonia, and ventilator days.136
A well-designed trial in France looked at multiple end points including death, pneumonia, duration of MV, complications, sedation requirements, and subjective patient comfort. There were no differences in any of the parameters, except in late laryngeal complications and patient discomfort. All patients who had an endotracheal as well as a tracheostomy tube during the course of their illness favored tracheostomy. Unfortunately, only 10% to 20% of eligible patients were actually enrolled in the trial and the study was grossly underpowered to detect any significant differences.115 Finally, a study assessing the impact of early tracheostomy (6-8 days) on the risk of developing ventilator-associated pneumonia showed a statistically insignificant trend (p = 0.07) toward reduction in the incidence of pneumonia.120 Another large trial showed no benefit of early tracheostomy except reduced use of sedatives.123 Interpreting length of ICU and hospitalization data from these trials requires knowledge of post-tracheostomy disposition, which may vary depending on the country in which the study was performed. Specifically, the availability of long-term ventilator facilities might not be apparent.
An older trial in the 1970s by Stauffer and colleagues,69 which is frequently quoted as an argument for prolonged translaryngeal intubation, found significantly more complications that were judged more severe in patients who underwent tracheostomy compared to patients with translaryngeal intubation. Most notably, the incidence of tracheal stenosis after tracheostomy was significantly higher (65% versus 19%). Nonetheless, they also found that patients treated with prolonged translaryngeal intubation followed by tracheostomy had significantly more laryngeal injury and an increased frequency of tracheal stenosis compared with patients who underwent tracheostomy after a short period of translaryngeal intubation.69
Conclusions from all these studies indicate that a tracheostomy should be offered only to patients anticipated to survive who require secure, long-term airway access. The timing of tracheostomy tube placement should be individualized and depends on three factors: (1) underlying disease, (2) indication for artificial airway, and (3) expected ultimate outcome. An article published in 2012 found that the hospital mortality rate of patients undergoing PDT was 30% with 14-day and 6-month mortality rates of 11% and 40%, respectively.122 Predictors of poor short-term outcome were found to be older age, diagnosis of malignancy, cardiogenic shock, and presence of a ventricular assist device. A realistic discussion of prognosis therefore should be part of the informed consent prior to placement of a tracheostomy.
Despite several negative trials that were generally underpowered and with the emergence of bedside PDT, a recommendation for earlier tracheostomy still seems appropriate as the early complication rate in experienced hands is low and is likely outweighed by the probable benefits, especially patient comfort. Figures 14.1 through 14.3 provide suggested algorithms regarding the timing of tracheostomy placement based on whether the patient’s pathologic condition is primarily upper airway obstruction, neuromuscular, or pulmonary.
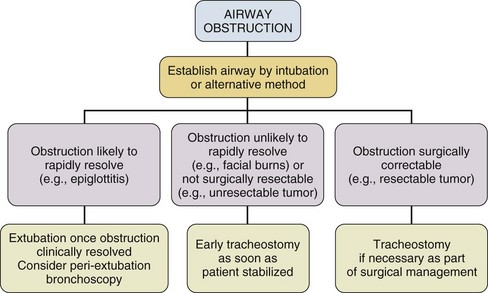
Figure 14.1 Timing of tracheostomy in airway obstruction.
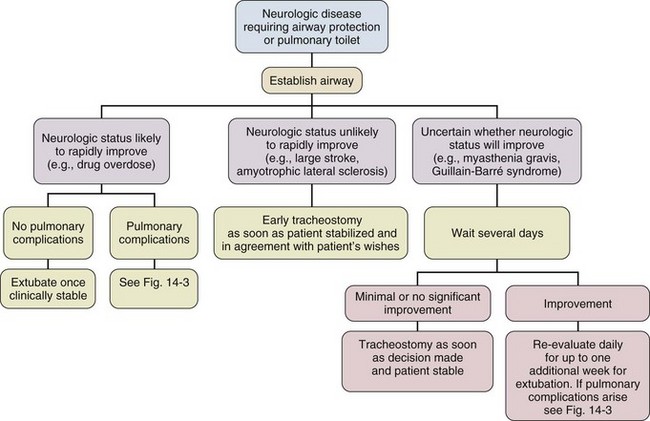
Figure 14.2 Timing of tracheostomy in neurologic disease.
Upper Airway Obstruction
In patients who have upper airway obstruction, the timing of tracheostomy depends on the likelihood of rapid resolution of the obstruction and whether surgical intervention is required (see Fig. 14.1).
Neuromuscular Conditions
Many ICU patients intubated because of stupor, coma, or neuromuscular weakness (see Fig. 14.2) are susceptible to aspiration, as they may have laryngeal dysfunction and may not be able to generate an effective cough.139 In most patients, the duration of the pathologic condition is predictable, either short (e.g., drug overdose) or long (e.g., large stroke, amyotrophic lateral sclerosis).140–145 In some cases of postoperative weakness, cerebrovascular accident, and peripheral neuromuscular weakness (e.g., Guillain-Barré syndrome, myasthenia gravis), the course is uncertain.139,146–149 A second factor involved in the decision to perform tracheostomy is the coexistence of pulmonary complications, such as atelectasis, pneumonia, or aspiration (see Fig. 14.3).
Pulmonary Conditions
The spectrum of diseases for patients intubated because they require MV is so broad that firm rules regarding tracheostomy have not been established. The approach to the timing of tracheostomy in these situations is difficult because the duration of ventilatory support is frequently hard to predict. This is demonstrated in some randomized studies comparing early and late tracheostomy in which 50% to 75% of patients randomized to delayed tracheostomy did not undergo this procedure because of either death or extubation.115,120,150 Despite this and multiple studies not supporting survival benefits or faster liberation from ventilator support but with data supporting improved patient well-being, it is advisable to consider early tracheostomy once the patient has stabilized, if the patient is expected to survive and needs a long-term airway, and a tracheostomy can be safely performed.
However, a recently published paper followed 73 ICU patients who received a tracheostomy and were transferred to a floor experienced in the care of patients with tracheostomies.126 Patients who were not decannulated in the ICU had a significantly higher mortality rate compared to the patients who were decannulated prior to transfer (11% versus 26%). The only other factors associated with increased mortality rate were the presence of tenacious sputum at ICU discharge and a body mass index (BMI) greater than 30 kg/m2. “The cause of in-ward deaths was cardiopulmonary arrest in 33% of ICU-decannulated patients, versus 90% of those discharged with a cannula in place (p = 0.08), most of which occurred overnight.” The authors feel the most likely cause of death was respiratory arrest that was likely cannula related, suggesting suboptimal monitoring and care for nondecannulated patients after ICU discharge. This study, though very concerning, was a single-center, prospective, observational trial. Therefore, this finding may reflect an institutional problem rather than a problem inherent to the procedure. Nevertheless, this issue needs further investigation.
Complications of Tracheostomy
Throughout the history of tracheostomy, complications have been the most scrutinized aspect. Complications have been categorized as occurring during performance of the tracheostomy (procedural), while the tube is in place (in situ), or after decannulation.29,102,106 Older studies report a major complication rate of approximately 15% (range, 6% to 66%), with a mortality rate of approximately 1.5% (range, 0% to 5%).29,47,48,69,71,102–107,151 More recent studies show generally lower complication rates ranging from 1.4% to 27%, and essentially negligible mortality rate.152–158 A recent multicenter review showed that major procedural complications are rare and are only dependent on operator experience, but not surgical technique or location of the procedure.157
Procedural Complications
The most common serious procedural complications are pneumothorax (0.9% to 5%) and severe hemorrhage (5%).44,47,48,71,103,105,151 Pneumothorax usually results from violation of the pleural space as it ascends into the neck. Operative hemorrhage is usually venous, originating from the anterior jugular venous system, the thyroid gland isthmus, and very rarely may be arterial from an aberrant vessel. Most of the time bleeding can be controlled by local measures alone, but occasionally will require surgical intervention for hemostasis.156 Other serious but very rare complications (<1%) include tube misplacement, tracheoesophageal perforation, aspiration, thyroid laceration, recurrent laryngeal nerve damage, and cardiopulmonary arrest.29,48,151,152,156 Less serious complications are subcutaneous and mediastinal emphysema, clinically insignificant hypotension, and desaturation.152–154,157
In Situ Complications
After a tracheostomy tube is placed, it is susceptible to obstruction and displacement, which may be potentially life-threatening, especially if a problem occurs before a secure tract has been formed and is not promptly recognized.29,48,106 Tube displacement may result from poor tracheostomy tube selection, excessive patient motion, or careless reinsertion after dislodgement. Obstruction is often caused by tenacious secretions, but can also be caused by positioning of the tracheostomy tube against the wall after poor sizing of the tracheostomy. Use of a double-cannula tube with a disposable inner cannula protects against occlusion by encrusted secretions,8,159 though this may delay successful weaning because of the increased outer diameter of the tube.160
Increasing peak airway pressure, increased resistance during manual bag ventilation, or difficulty passing a suction catheter may indicate tube obstruction or displacement. Providing adequate humidity, adhering to suctioning and tracheostomy care protocols, and minimizing tube manipulation help avert this.48,106,151
Another common in situ complication is stomal infection, which occurs in 36% of all tracheostomies.69 Trivial stomal infection can be treated topically.48 Necrotizing wound infection can occur, and may extend into the mediastinum, causing life-threatening sepsis.154,161 Many patients will have bacterial colonization of the tracheobronchial tree, especially with resistant gram-negative organisms (50% to 66%) making them more susceptible to develop nosocomial pneumonia.29,103,127,162
In contrast to intraprocedural venous hemorrhage, in situ hemorrhage usually results from the tracheostomy tube tip eroding into a major artery, generally the innominate (0.2% to 4%).29,69 The resultant tracheoinnominate fistula will present as massive bleeding, sometimes heralded by smaller bleeds or a pulsating tracheostomy tube. Treatment consists of immediate tamponade and operative ligation, despite which tracheoinnominate fistula carries a 75% mortality rate.29,48,163 Other in situ complications include mucosal ulceration, tracheal erosion, tracheal dilatation, and very rarely tracheoesophageal fistula.48,69,106,164–168
Complications After Decannulation
The most important postdecannulation complication after tracheostomy is tracheal stenosis.29,69,71,74,103,155–157,169 It can develop in three distinct areas: subglottic, at the stoma site, or at the cuff site. A mild degree of tracheal narrowing after decannulation at the site of the stoma is very common, and if patients are carefully evaluated with imaging and endoscopy, tracheal narrowing can be found in 30% to 75% of patients after tracheostomy.69,158,169 Most patients with tracheal stenosis remain asymptomatic and require no treatment.74 Symptoms generally occur when the tracheal lumen diameter is reduced by either 75% or to less than 5 mm.103 Recent studies showed a risk of symptomatic stenosis in 0.6% and 10% of patients who were followed long term.157,158,169 Risk factors for the development of tracheal stenosis at the stoma site were found to be hypotension, sepsis, stoma infection, older age, male sex, use of steroids, tight-fitting cannula, prolonged cannulation, and excessive motion. In a study published in 2012 another risk factor for stenosis was obesity with an increase in risk in the obese from 0.4% to 9.9%. This was especially true if prior to the tracheostomy the patient was intubated with an ET greater than size 7.5 (relative risk 27) and if intubation prior to tracheostomy lasted longer than 7 days (relative risk 2.5).157
Stenosis at the site of the cuff is related to mucosal pressure necrosis, exposing the tracheal rings. As the airway is often chronically colonized this will cause chondritis ultimately leading to tracheomalacia or tracheal stenosis. The introduction of the high-volume, low-pressure cuffs has decreased the incidence of this complication by a factor of 10.170–174 Despite the use of low-pressure cuffs, Stauffer reported that about 20% of patients required excessive cuff pressures to achieve a seal, though the rate was similar in patients with endotracheal or tracheostomy tubes.69 As has been shown, a low-pressure cuff does not guarantee a low pressure, especially in patients with high pulmonary inflation pressures. Therefore, cuff pressures should routinely be measured and maintained at the lowest appropriate level, ideally less than 25 mm Hg. Even this may not prevent tracheal injury because lateral wall pressure varies widely, is unmeasurable, and may compromise local tracheal blood supply.166,174–176
The question whether tracheostomy tubes cause more tracheal stenosis than ETs cannot be answered at this time as most patients who undergo tracheostomy were initially treated with translaryngeal intubation of variable duration, and often require the chosen airway device for a prolonged period. These confounding variables make it impossible to state with certainty if stenosis was caused by the endotracheal or tracheostomy tube unless it is at the site of the stoma. Subglottic stenosis, which is more commonly due to translaryngeal intubation, is more difficult to repair surgically.110,177–179 Other late complications, which occur infrequently, include tracheocutaneous fistula, tracheomalacia, tracheal granulomas, vocal cord dysfunction, and cosmetic deformities.102,152,180,181
Risks of Patient Transport
A further risk for patients who are having a tracheostomy performed is related not to the procedure itself, but to transporting patients to the operating room. The incidence of harmful events in patients during transport is 33% to 68%,182,183 with Waddell reporting that “one patient a month suffered major cardiorespiratory collapse or death as a direct result of movement.”184 To address this issue and to reduce tracheostomy complications, Ciaglia developed the technique of PDT in 1985.35
Percutaneous Tracheostomy
Many descriptive series and reviews have examined short-term and long-term complications of percutaneous tracheostomy (PT)104,158,185–195 and prospective comparisons of PT with surgical tracheostomy (ST).196–203 By virtue of these studies, PT has become an integral part of the care of critically ill patients, with PDT the most common PT performed today.204,205 In many institutions PT is the procedure of choice when a tracheostomy is needed for an ICU patient.195,206
When reading and analyzing the literature related to PT, it is essential to know which of the currently available procedures of PT are being discussed: one of the PDTs (Ciaglia Blue Rhino, Blue Dolphin, or the Portex multiple straight dilator kit), Griggs’ GWDF, Fantoni’s translaryngeal tracheostomy, or Frova’s PercuTwist207 (see “History” section earlier).
It is important to realize that all PDT techniques are percutaneous tracheostomies, but not all PTs are PDTs.207 Most PTs reported in the literature have been performed using the Ciaglia dilational technique (PDT),104,152,188,194,195,208 followed by Griggs’ GWDF technique.208 There is evident confusion with respect to the different techniques of PT, as seen in an article on surgical airway management in the ICU in which the description of PDT is that “sequential dilators are used to enlarge a tract,” but the picture of the commercial kit that “has the necessary instruments” for the procedure is one of the single-dilator Blue Rhino.209 Confusion occurs when publications describe PT performed using Portex kits (Keene, NH), but fail to clarify which of the kits that Portex makes for PT was used. One, manufactured in the United States, is made for the Ciaglia PDT technique using three straight dilators. The other is made in Europe for the Griggs GWDF technique.38 Finally, confusion regarding technique of PT is especially evident in most published meta-analyses, with only two that compared studies of only one technique of PT (namely PDT) and ST,210,211 and the others comparing at least two different techniques of PT to ST.152,212–214
Each of these techniques has its own particulars with respect to technique and complication rates. When evaluating PT techniques or comparing PT with ST, the technique that is being used must be specified.207 This was not done in many studies of PT207,212–214 and significantly affected the conclusions215 of the meta-analysis of PT versus ST by Dulguerov and coworkers.152
Clear knowledge of the technique and complication rates of the different types of PT will allow an informed judgment as to which of the techniques should be chosen to be performed. Based on the fact mentioned earlier that most reports of PT are of Ciaglia’s dilational technique, predominantly using curved dilators (either single or multiple), the most popular technique of PT seems to be PDT. In most studies that compared PT with ST, PDT was used.196–203 One compared translaryngeal tracheostomy versus ST,216 and two others compared GWDF technique versus ST.217,218
Reports by van Heerden and associates,219 Nates and colleagues,220 and Fikkers and coworkers221 have compared short-term outcomes using the Griggs GWDF technique versus PDT. Van Heerden and associates219 showed no difference in complications, Nates and colleagues220 showed a significantly higher incidence of bleeding using the GWDF technique, and Fikkers and coworkers221 showed a higher rate of minor procedural and postdecannulation complications in GWDF technique. Cantais and associates222 compared GWDF technique with translaryngeal tracheostomy, finding a higher rate of serious complications in the translaryngeal tracheostomy group. Byhahn and colleagues compared PercuTwist with PDT (single dilator)223 and found more patients in whom insertion of the cannula was difficult/impossible with the PercuTwist. Montcriol and coworkers224 found PercuTwist to take significantly longer than GWDF technique.
In an attempt to reduce the chance of injury to the posterior tracheal wall, and to speed up the procedure, Ciaglia developed the gradually tapered soft Blue Rhino dilator in 1999.39 Two randomized comparisons of multiple-dilator PDT with the single-dilator Blue Rhino PDT39,225 showed a shorter procedure duration for the single-dilator PDT. Also, no intervention was required despite a statistically higher incidence of tracheal ring fracture in the single-dilator group in one study.39 In the multiple-dilator group, there were two injuries to the posterior tracheal wall and one pneumothorax.39 The latest development in PDT uses balloon dilation and is a true single-step procedure.42,43 Cianchi and associates43 found that balloon dilation took significantly longer than the single-dilator PDT and that limited intratracheal bleeding was more frequent after balloon dilation.
Technique
The technique of PDT can be learned by attending a training course or observing the procedure, followed by assisting and performing PDT under supervision until proficiency is attained, which usually occurs after performing 10 to 20 procedures.203 The operator’s initial efforts should be performed under supervision of an experienced operator using bronchoscopic guidance,153 in an already intubated patient. It is important to remember that there is a learning curve with this procedure. Complications are more likely to occur early in the practitioner’s experience with PDT.226
Initially, PDT was thought to be contraindicated in emergencies, in children, in obese patients, and in patients who had a previous tracheostomy, uncorrectable coagulopathies, or severe anatomic neck deformities,35 but its performance has been described in all of these groups except children younger than 14 years old.54,56,57,185,186,189,195,227–232 We believe PDT remains absolutely contraindicated in young children and patients with certain severe anatomic neck deformities (e.g., fused, anterior flexed neck, overlying neck mass). An emergent PDT to establish an airway should be done only by practitioners with significant experience in the procedure.
The ET is usually positioned by either palpating the ET cuff to identify the tube position,233 or using bronchoscopic guidance.104,187,234 Besides facilitating proper tube positioning, using the bronchoscope allows direct visualization of needle insertion, ensuring midline placement and intratracheal insertion of the tracheostomy tube.104 Marx and Ciaglia235 who originally recommended nonendoscopic ET positioning, later recommended use of bronchoscopy. Recently, Mallick and colleagues236 prospectively compared capnography and bronchoscopy to confirm tracheal placement, and Jackson and coworkers237 retrospectively compared PDT with and without bronchoscopy and found no difference in complications. Other authors232,233,238,239 do not routinely use bronchoscopy to perform PDT and recommend its use for training and in selected patients with difficult airways, which has been our experience.
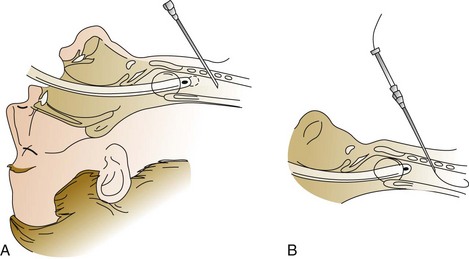
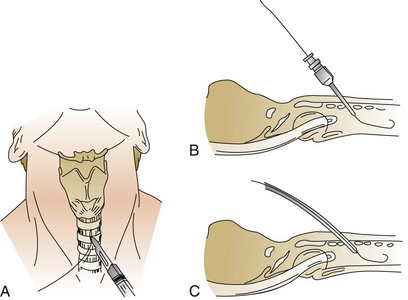
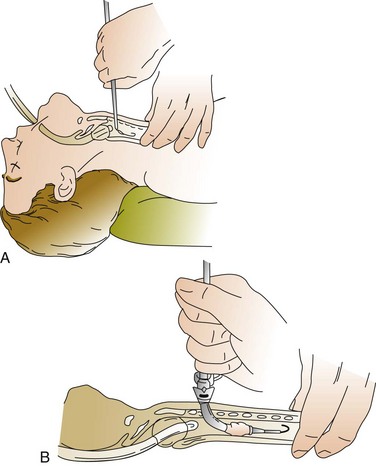
A 1-cm midline vertical or horizontal incision is made and a short punch dilator inserted to enlarge the tract from skin to trachea. A guiding catheter is placed over the guidewire, which remains in place for the duration of the procedure to add stiffness and facilitate passage of the dilator(s), which should be inserted at a right angle to the patient’s neck. Dilation of the tract may be performed with multiple curved dilators,35 three straight dilators,36,233 a single tapered dilator (Blue Rhino),39 or an expanding balloon (Blue Dolphin).42,43 The tract is dilated until an appropriately sized tracheostomy tube (that was previously loaded on a curved obturator) can be inserted (Figs. 14.4 to 14.6, see online video of the technique of PDT). The obturator, guidewire, and guiding catheter are removed; the inner cannula is inserted; and the cuff is inflated. Breath sounds, returned tidal volume, and cuff pressure must be checked before the tracheostomy is secured. A chest film should be obtained to verify tube position and the absence of barotrauma. In experienced hands, the procedure can be performed in less than 5 minutes.
Percutaneous Dilational Tracheostomy Versus Surgical Tracheostomy
As mentioned previously, it seems that the Ciaglia PDT has become the benchmark for PT. Although other techniques have been described, none has been as widely accepted,33,37,38,40,41,195,208,232 and some are no longer available.33,37 Kits currently available to perform PDT contain a single tapered dilator (Cook Blue Rhino, three straight dilators [Portex], or equipment for balloon dilation [Cook Blue Dolphin]). At this point in the history of PT, PDT is the technique that should be the focus of discussion.
Nonrandomized, observational studies of PDT show short-term and long-term complication rates (e.g., mortality, paratracheal insertion, bleeding, infection, and tracheal stenosis rates),35,36,104,153,158,185–193,195 that are comparable to or lower than those in similar studies of ST.44,47,69,71,74,102–106,108,109 The largest observational study195 of PDT followed 1000 patients and had an overall complication rate of only 1.4% (1.2% in normal risk and only 1.7% even in high-risk patients). The authors concluded that PDT “should be considered the gold standard in patients requiring elective tracheostomy in the ICU.”
However, using these articles to justify use of PDT over ST is probably not appropriate, as they are from different eras and there are differences in technique and equipment. Also, complications may have been defined or selected differently. Thus, it is important to examine the prospective trials of PDT versus ST,196–203,240,241 which presumably used the same definitions and procedures in both groups. The two latest and largest of these studies found that there was a shorter time to PDT once the decision was made to perform a tracheostomy.202,241 Additionally, Silvester and associates202 found a lower incidence of postoperative infection and a trend toward a better cosmetic sequelae in PDT, and Beltrame and colleagues241 found that PDT was faster to perform. Others showed a lower incidence of minor in situ complications of PDT.196,197,199
There have been several meta-analyses comparing PT versus ST.152,210–214 As mentioned previously, only two210,211 examined studies that used only one technique of PT, namely PDT. The others had as many as four different techniques of PT, which will likely affect the results and conclusions of the analyses.152,212–214 The largest single-technique meta-analysis of Freeman and coworkers211 showed that PDT had less procedural and in situ bleeding, a lower stomal infection rate, and lower overall postoperative complications.
Finally, a recent evidence-based analysis of PT versus ST found 13 studies of PT that reached a type II level of evidence. Of the nine that used PDT, eight were prospective studies and one was a meta-analysis. Six had complication rates that favored PDT and two favored ST. However, the authors of this analysis raised several issues: (1) that the findings of improved outcomes of PDT were based on significant reduction of minor complications, but there were no differences in major complications; (2) that the patient selection was biased in favor of PDT by excluding high-risk patients; and (3) that there was no consistent long-term follow-up.242
What can be said is that PDT has been shown to have a shorter procedure time than ST.196,197,241 It has also been shown to have less delay from the time the decision to perform the tracheostomy was made until the procedure was performed.202,215,241 Some data suggest that this may shorten ICU stay and time on the ventilator (see Table 14.1). Bedside PDT eliminates the need for transport to the operating room, reducing the morbidity and mortality rates for intrahospital transport182–184 and has been shown to be more cost-effective than ST because of decreased utilization of resources.201
References
1. Nelson, TG. Tracheotomy: A clinical and experimental study. Am Surgeon. 1957; 23:660–694.
2. McClelland, RMA. Tracheostomy: Its management and alternatives. Proc Roy Soc Med. 1972; 65:401–404.
3. Frost, EAM. Tracing the tracheostomy. Ann Otol Rhinol Laryngol. 1976; 85:618–624.
4. Wright, J. A History of Laryngology and Rhinology. Philadelphia: Lea & Febiger; 1914.
5. Pahor, AL. Ear, nose and throat in ancient Egypt. J Laryngol Otol. 1992; 106:773–779.
6. Guerrier, Y, Mounier-Kuhn, P. Histoire de Maladies de l’Oreille, du Nez et la Gorge. Paris: Les Editions Roger Dacosta; 1980.
7. Wright, J. The Nose and Throat in Medical History. St. Louis: Matthews; 1898.
8. Goodall, EW. The story of tracheostomy. Br J Child Dis. 1934; 31:167–176.
9. Stevenson, RS. A History of Otolaryngology. Edinburgh: Livingstone; 1949.
10. Garrison, FH. An Introduction to the History of Medicine. Philadelphia: Saunders; 1929.
11. Casserius, J. The larynx, organ of voice. (trans). Acta Otolaryngol Suppl. 1969; 261:1–36.
12. Guthrie, D. Early records of tracheotomy. Bull Hist Med. 1947; 15:59–64.
13. Morens, DM. Death of a president. N Engl J Med. 1999; 341:1845–1849.
14. Bretonneau, M. Sporadic tracheal diphtherite. In: Semple RH, ed. Memoirs on Diphtheria. London: The New Sydenham Society; 1859:59–71.
15. Trousseau, A, Tracheostomy in diphtheria (trans). Lectures on Clinical Medicine. Cormack, JR, eds. Lectures on Clinical Medicine; Vol. 2. The New Sydenham Society, London, 1869:594–617.
16. Shastid, TH. My Second Life. Ann Arbor: University of Michigan Press; 1944.
17. Shorter, E. Primary care. In: Porter R, ed. Cambridge Illustrated History of Medicine. Melbourne: Cambridge University Press; 1996:118–153.
18. Alberti, PW. Tracheostomy versus intubation. Ann Otol Rhinol Laryngol. 1984; 93:333–337.
19. MacEwen, W. Clinical observations on the introduction of tracheal tubes by the mouth instead of performing tracheotomy or laryngotomy. BMJ. 1880; 2:122–124.
20. O’Dwyer, J. Intubation of the larynx. N Y Med J. 1885; 4:145–157.
21. Stoller, JK. The history of intubation, tracheotomy, and airway appliances. Respir Care. 1999; 44:595–601.
22. Jackson, C. Tracheotomy. Laryngoscope. 1909; 19:285–290.
23. Hilberman, M. The evolution of the intensive care unit. Crit Care Med. 1975; 3:159–165.
24. Head, JM. Tracheostomy in the management of respiratory problems. N Engl J Med. 1961; 264:587–591.
25. Jackson, C. High tracheotomy and other errors the chief cause of laryngeal stenosis. Surg Gynecol Obstet. 1921; 32:392–398.
26. DeLaurier, GA, Hawkins, ML, Treat, RC, et al. Acute airway management. Am Surg. 1990; 56:12–15.
27. Brantigan, CO, Grow, JB, Sr. Subglottic stenosis after cricothyroidotomy. Surgery. 1982; 91:217–221.
28. Brantigan, CO, Grow, JB, Sr. Cricothyroidotomy: Elective use in respiratory problems requiring tracheotomy. J Thorac Cardiovasc Surg. 1976; 71:72–81.
29. Heffner, JE, Miller, KS, Sahn, SA. Tracheostomy in the intensive care unit: Part 1. Indications, technique, management. Part 2. Complications. Chest. 1986; 90:269–274.
30. Shelden, CH, Pudenz, RH, Freshwater, DB, et al. A new method for tracheostomy. J Neurosurg. 1955; 12:428–431.
31. Smith, VM. Perforation of trachea during tracheostomy performed with Sheldon tracheostome. JAMA. 1957; 165:2074–2076.
32. Hamilton, RD. Fatal hemorrhage during tracheostomy. JAMA. 1960; 174:530–531.
33. Toye, FJ, Weinstein, JD. A percutaneous tracheostomy device. Surgery. 1969; 65:384–389.
34. Toye, FJ, Weinstein, JD. Clinical experience with percutaneous tracheostomy and cricothyroidotomy in 100 patients. J Trauma. 1986; 6:1034–1040.
35. Ciaglia, P, Firsching, R, Syniec, C. Elective percutaneous dilatational tracheostomy. Chest. 1985; 87:715–719.
36. Hazard, PB, Garrett, HE, Adams, JW. Bedside percutaneous tracheostomy: Experience with 55 elective procedures. Ann Thorac Surg. 1988; 46:63–67.
37. Schachner, A, Ovil, Y, Sidi, J, et al. Percutaneous tracheostomy—a new method. Crit Care Med. 1989; 17:1052–1056.
38. Griggs, WM, Worthley, LIG, Gilligan, JE, et al. A simple percutaneous tracheostomy technique. Surg Gynecol Obstet. 1990; 170:543–545.
39. Byhahn, C, Wilke, HJ, Halbig, S, et al. Percutaneous tracheostomy: Ciaglia Blue Rhino versus the basic Ciaglia technique of percutaneous dilational tracheostomy. Anesth Analg. 2000; 91:882–886.
40. Fantoni, A, Ripamonte, D. A non-derivative, nonsurgical tracheostomy: The translaryngeal method. Intensive Care Med. 1997; 23:386–392.
41. Frova, G, Quintel, M. A new simple method for percutaneous tracheostomy: Controlled rotating dilation—a preliminary report. Intensive Care Med. 2002; 28:229–232.
42. Gromann, TW, Birkelbach, O, Helzer, R. Balloon dilational tracheostomy: Initial experience with the Ciaglia Blue Dolphin method. Anesth Analg. 2009; 108:1862–1866.
43. Cianchi, G, Zagli, G, Bonizzoli, M, et al. Comparison between single-step and balloon dilatational tracheostomy in intensive care unit: A single-centre, randomized controlled study. Brit J Anesthesia. 2010; 104:728–732.
44. Beatrous, WP. Tracheostomy: Its expanded indications and its present status. Laryngoscope. 1968; 78:3–55.
45. Shapiro BA, Harrison RA, Kacmarek RM, et al, eds. The artificial airway. In Clinical Application of Respiratory Care. 3rd ed. Year Book Medical Publishers, Chicago, 1985:213–241.
46. Stauffer, JL. Medical management of the airway. Clin Chest Med. 1991; 12:449–482.
47. Skaggs, JA, Cogbill, CL. Tracheostomy: Management, mortality and complications. Am Surg. 1969; 35:393–396.
48. Chew, JY, Cantrell, RW. Tracheostomy: Complications and their management. Arch Otolaryngol. 1972; 96:538–545.
49. Schwartz, DE, Wiener-Kronish, JP. Management of the difficult airway. Clin Chest Med. 1991; 12:483–495.
50. Latto, IP, Vaughn, RS. Difficulties in Tracheal Intubation, 2nd ed. London: Saunders; 1997.
51. Weiss, YG, Deutschman, CS. The role of fiberoptic bronchoscopy in airway management of the critically ill patient. Crit Care Clin. 2000; 16:445–451.
52. American College of Surgeons Committee on Trauma. Advanced Trauma Life Support Course for Physicians, Instructor’s Manual. Chicago: The College; 1985.
53. Burkey, B, Escalamado, R, Morganroth, M. The role of cricothyroidotomy in airway management. Clin Chest Med. 1991; 12:561–571.
54. Myles, PS, Venema, HR, Lindholm, DE. Trauma patient managed with the laryngeal mask airway and percutaneous tracheostomy after failed intubation. Med J Aust. 1994; 161:640.
55. Griggs’, WM, Myburgh, JA, Worthley, LIG. Urgent airway access—an indication for percutaneous tracheostomy? Anaesth Intensive Care. 1991; 19:586–587.
56. Dob, DP, McLure, HA, Soni, N. Failed intubation and emergency percutaneous tracheostomy. Anaesthesioloy. 1998; 53:69–78.
57. Ben-Nun, A, Altman, E, Best, LAE. Emergency percutaneous tracheostomy in trauma patients: An early experience. Ann Thorac Surg. 2004; 77:1045–1047.
58. Lindholm, CE. Prolonged endotracheal intubation. Acta Anaesth Scand Suppl. 1969; 33:1–131.
59. Weymuller, E, Bishop, MJ, Fink, BR, et al. Quantification of intralaryngeal pressure exerted by endotracheal tubes. Ann Otol Rhinol Laryngol. 1983; 92:444–447.
60. Steen, JA, Lindholm, CE, Brdlik, GC, et al. Tracheal tube forces on the posterior larynx. Crit Care Med. 1982; 10:186–189.
61. Supance, JS, Reilly, JS, Doyle, WJ, et al. Acquired subglottic stenosis following prolonged endotracheal intubation. Arch Otolaryngol. 1982; 108:727–731.
62. Santos, PM, Afrassiabi, A, Weymuller, EA. Risk factors associated with prolonged intubation and laryngeal injury. Otolaryngol Head Neck Surg. 1994; 111:453–459.
63. Peppard, SB, Dickens, JH. Laryngeal injury following short-term intubation. Ann Otol Rhinol Laryngol. 1983; 90:327–330.
64. Gaynor, EB, Greenberg, SB. Untoward sequelae of prolonged intubation. Laryngoscope. 1985; 95:1461–1467.
65. Donnelly, WH. Histopathology of endotracheal intubation. Arch Pathol. 1969; 88:511–520.
66. Whited, RE. A prospective study of laryngotracheal sequelae in long-term intubation. Laryngoscope. 1984; 94:367–377.
67. Bishop, MJ, Weymuller, EA, Fink, BR. Laryngeal effects of prolonged intubation. Anesth Analg. 1984; 63:335–342.
68. Benjamin, B. Laryngeal trauma from intubation: Endoscopic evaluation and classification. In: Cummings CW, Fredrickson JM, Harker LA, et al, eds. Otolaryngology Head and Neck Surgery. 3rd ed. St. Louis: Mosby; 1998:2013–2035.
69. Stauffer, JL, Olson, DE, Petty, TL. Complications and consequences of endotracheal intubation and tracheotomy. Am J Med. 1981; 70:65–76.
70. Bishop, M. Mechanisms of laryngotracheal injury following prolonged tracheal intubation. Chest. 1989; 96:185–186.
71. Marsh, HM, Gillespie, DJ, Baumgartner, AE. Timing of tracheostomy in the critically ill patient. Chest. 1989; 96:190–193.
72. Sugerman, HJ, Wolfe, L, Pasquale, MD, et al. Multicenter, randomized, prospective trial of early tracheostomy. J Trauma. 1997; 43:741–747.
73. Heffner, JE. Tracheotomy: Indications and timing. Respir Care. 1999; 44:807–815.
74. Heffner, JE. Timing of tracheotomy in ventilator-dependent patients. Clin Chest Med. 1991; 12:611–625.
75. Coppolo, DP, May, JJ. Self-extubations. Chest. 1990; 98:165–169.
76. Campbell, RS. Extubation and the consequences of reintubation. Respir Care. 1999; 44:799–803.
77. Torres, A, Gatell, JM, Aznar, E, et al. Reintubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation. Am J Respir Crit Care Med. 1995; 152:137–141.
78. Davis, K, Campbell, RS, Johannigman, JA, et al. Changes in respiratory mechanics after tracheostomy. Arch Surg. 1999; 134:59–62.
79. Heffner, JE. The role of trachestomy in weaning. Chest. 2001; 120:477S–481S.
80. Villafane, MC, Cinella, G, Lofaso, F, et al. Gradual reduction of endotracheal tube diameter during mechanical ventilation via different humidification devices. Anesthesia. 1996; 85:1341–1349.
81. Lin, MC, Huang, CC, Yang, CT, et al. Pulmonary mechanics in patients with prolonged mechanical ventilation requiring tracheostomy. Anaesth Intensive Care. 1999; 27:581–585.
82. Diehl, JL, El Atrous, S, Touchard, D, et al. Changes in the work of breathing induced by tracheostomy in ventilator-dependent patients. Am J Respir Crit Care Med. 1999; 159:383–388.
83. Wright, PE, Marini, JJ, Bernard, GR. In vitro versus in vivo comparison of endotracheal airflow resistance. Am Rev Respir Dis. 1989; 140:10–16.
84. Bersten, AD, Rutten, AJ, Vedig, AE. Additional work of breathing imposed by endotracheal tubes, breathing circuits, and intensive care ventilators. Crit Care Med. 1989; 17:671–677.
85. Boynton, JH, Hawkins, K, Eastridge, BJ, et al. Tracheostomy timing and the duration of weaning in patients with acute respiratory failure. Crit Care. 2004; 8:R261–R267.
86. Arabi, Y, Haddad, S, Shirawi, N, et al. Early tracheostomy in intensive care trauma patients improves resource utilization: A cohort study and literature review. Crit Care. 2004; 8:R347–R352.
87. Rumbak, MJ, Newton, M, Truncale, T, et al. A prospective, randomized, study comparing early percutaneous tracheotomy to prolonged translaryngeal intubation (delayed tracheotomy) in critically ill medical patients. Crit Care Med. 2004; 32:1689–1694.
88. Hsu, CL, Chen, KY, Chang, CH, et al. Timing of tracheostomy as a determinant of weaning success in critically ill patients: A retrospective study. Crit Care. 2005; 9:R46–R52.
89. Rodriguez, JL, Steinberg, SM, Luchetti, FA, et al. Early tracheostomy for primary airway management in the surgical critical care setting. Surgery. 1990; 108:655–659.
90. Bouderka, MA, Fakhir, B, Bouggad, A, et al. Early tracheostomy versus prolonged endotracheal intubation in severe head trauma. J Trauma. 2004; 57:251–254.
91. Flaatten, H, Gjerde, S, Heimdal, JH, et al. The effect of tracheostomy on outcome in intensive care unit patients. Acta Anaesthesiol Scand. 2006; 50:92–98.
92. Griffith, J, Barber, VS, Morgan, L, et al. Systematic review and meta-analysis of studies of the timing of tracheostomy in adult patients undergoing artificial ventilation. BMJ. 2005; 330:1243–1246.
93. McGeehin, WH, Scoma, R, Igidbashian, L, et al. Tracheostomy versus endotracheal intubation: The ICU nurse’s perspective. Crit Care Med. 1990; 18:S224.
94. Astrachan, DI, Kirchner, JC, Goodwin, WJ. Prolonged intubation vs. tracheotomy: Complications, practical and psychological considerations. Laryngoscope. 1988; 98:1165–1169.
95. Heffner, JE, Hess, D. Tracheostomy management in the chronically ventilated patient. Clin Chest Med. 2001; 22:55–69.
96. Kluin, KJ, Maynard, F, Bogdasarian, RS. The patient requiring mechanical ventilatory support: Use of the cuffed tracheostomy “talk” tube to establish phonation. Otolaryngol Head Neck Surg. 1984; 92:625–627.
97. Manzano, JL, Lubillo, S, Henriquez, D, et al. Verbal communication of ventilator-dependent patients. Crit Care Med. 1993; 21:512–517.
98. Bergbom-Engbert, I, Haljamae, H. Assessment of patient’s experience of discomforts during respirator therapy. Crit Care Med. 1989; 17:1068–1072.
99. Koh, WY, Lew, TWK, Chin, NM, et al. Tracheostomy in a neuro-intensive care setting: Indications and timing. Anaesth Intensive Care. 1997; 25:365–368.
100. Kollef, MH, Ahrens, TS, Shannon, W. Clinical predictors and outcomes for patients requiring tracheostomy in the intensive care unit. Crit Care Med. 1999; 27:1714–1720.
101. Freeman, BD, Borecki, IB, Coopersmith, CM, et al. Relationship between tracheostomy timing and duration of mechanical ventilation in critically ill patients. Crit Care Med. 2005; 33:2513–2520.
102. Wood, DE, Mathisen, DJ. Late complications of tracheotomy. Clin Chest Med. 1991; 12:597–609.
103. Lewis, RJ. Tracheostomies—indications, timing, and complications. Clin Chest Med. 1992; 13:137–149.
104. Kost, KM. Endoscopic percutaneous dilatational tracheotomy: A prospective evaluation of 500 consecutive cases. Laryngoscope. 2005; 115:1–30.
105. Goldstein, SI, Breda, SD, Schneider, KL. Surgical complications of bedside tracheotomy in an otolaryngology residency program. Laryngosope. 1987; 97:1407–1409.
106. Myers, EN, Carrau, MR. Early complications of tracheotomy. Clin Chest Med. 1991; 12:589–595.
107. Rosenbower, TJ, Morris, JA, Eddy, VA, et al. The long-term complications of percutaneous tracheostomy. Am Surg. 1998; 64:82–86.
108. Davis, HS, Kretchmer, HE, Bryce-Smith, R. Advantages and complications of tracheotomy. JAMA. 1953; 153:1156–1159.
109. El-Naggar, M, Sadagopan, S, Levine, H, et al. Factors influencing choice between tracheostomy and prolonged translaryngeal intubation in acute respiratory failure: A prospective study. Anesth Analg. 1976; 55:195–201.
110. Loh, KS, Irish, JC. Traumatic complications of intubation and other airway management procedures. Anesth Clin North Am. 2002; 20:953–969.
111. Nieszkowska, A, Combes, A, Luyt, CE, et al. Impact of tracheotomy on sedative administration, sedation level, and comfort of mechanically ventilated intensive care unit patients. Crit Care Med. 2005; 33:2527–2533.
112. Koitschev, A, Simon, C, Blumenstock, G, et al. Suprastomal tracheal stenosis after dilational and surgical tracheostomy in critically ill patients. Anaesthesia. 2006; 61:832–837.
113. Foster, A. More than nothing: The lived experience of tracheostomy while acutely ill. Intensive Crit Care Nurs. 2010; 26:33–43.
114. Sherlock, ZV, Wilson, JA, Exley, C. Tracheostomy in the acute setting: Patient experience and information needs. J Crit Care. 2009; 24:501–507.
115. Blot, F, Similowski, T, Trouillet, JL, et al. Early tracheostomy versus prolonged intubation in unselected severely ill ICU patients. Intensive Care Med. 2008; 34:1779–1789.
116. Moeller, MG, Slaikeu, JD, Bonelli, P, et al. Early vs late tracheostomy in the surgical intensive care unit. Am J Surg. 2005; 189:293–296.
117. Lesnik, I, Rappaport, W, Fulginiti, J, et al. The role of early tracheostomy in blunt, multiple organ trauma. Am Surg. 1992; 58:347–349.
118. Schauer, JM, Engle, LL, Maugher, DT, et al. Does acuity matter?—Optimal timing of tracheostomy stratisfied by injury severity. J Trauma. 2009; 66:220–225.
119. Arabi, YM, Alhashemi, JA, Tamin, HM, et al. The impact of time to tracheostomy on mechanical ventilation: Duration, length of stay and mortality in intensive care unit patients. J Crit Care. 2009; 24:435–440.
120. Terragni, PP, Antonelli, M, Fumagalli, R, et al. Early vs late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients. JAMA. 2010; 303:1483–1489.
121. Bickenbach, J, Fries, M, Offermanns, V, et al. Impact of early vs late tracheostomy on weaning: A retrospective analysis. Minerva Anesthesiol. 2011; 77:1176–1183.
122. Pandian, V, Gilstrap, DL, Mirski, MA, et al. Predictors of short-term mortality in patients undergoing percutaneous dilatational tracheostomy. J Crit Care. 2012; 27(4):420.
123. Harvey, E, Early versus late tracheostomy: The TracMan trial Presented at the 29th International Symposium for Intensive Care and Emergency Medicine, Brussels, Belgium. 2009.
124. Armstrong, PA, McCarthy, MC, Peoples, JB. Reduced use of resources by early tracheostomy in ventilator dependent patients with blunt trauma. Surgery. 1998; 124:763–767.
125. Frutos-Vivar, F, Esteban, A, Apezteguia, C, et al. Outcome of mechanically ventilated patients who require a tracheostomy. Crit Care Med. 2005; 33:290–298.
126. Hernandez Martinez, G, Fernandez, R, Sanchez Casado, M, et al. Tracheostomy tube in place at intensive care unit discharge is associated with increased ward mortality. Respir Care. 2009; 54:1644–1652.
127. Heffner, JE. Medical indications for tracheotomy. Chest. 1989; 96:186–190.
128. Wenig, BL, Applebaum, EL. Indications for and techniques of tracheotomy. Clin Chest Med. 1991; 12:545–553.
129. Scales, DC, Kahn, JM. Tracheostomy timing, enrollment and power in ICU clinical trials. Intensive Care Med. 2008; 34:1743–1745.
130. Qureshi, AI, Suarez, JI, Parekh, PD, et al. Prediction and timing of tracheostomy in patients with infratentorial lesions requiring mechanical ventilator support. Crit Care Med. 2000; 28:1383–1387.
131. Heffner, JE, Zamora, CA. Clinical predictors of prolonged translaryngeal intubation in patients with the adult respiratory distress syndrome. Chest. 1990; 97:447–451.
132. Johnson, SB, Kearney, PA, Barker, DE. Early criteria predictive of prolonged mechanical ventilation. J Trauma. 1992; 33:95–99.
133. Heffner, JE, Brown, LK, Barbieri, CA, et al. Prospective validation of an acute respiratory distress syndrome predictive score. Am J Crit Care Med. 1995; 152:1518–1526.
134. Seneff, MG, Zimmerman, JE, Knaus, WA, et al. Predicting the duration of mechanical ventilation. Chest. 1996; 110:469–479.
135. Plummer, AL, Gracey, DR. Consensus conference on artificial airways in patients receiving mechanical ventilation. Chest. 1989; 96:178–180.
136. Barquist, ES, Amortegui, J, Hallal, A, et al. Tracheostomy in ventilator dependent trauma patients: A prospective, randomized intention-to-treat study. J Trauma. 2006; 60:91–97.
137. Dunham, CM, Ransom, KJ. Assessment of early tracheostomy in trauma patients: A systematic review and meta-analysis. Am Surg. 2006; 72:276–281.
138. Saffle, JR, Morris, SE, Edelman, L. Early tracheostomy does not improve outcome in burn patients. J Burn Care Rehab. 2002; 23:431–438.
139. Bella, I, Chad, DA. Neuromuscular disorders and acute respiratory failure. Neurol Clin North Am. 1998; 16:391–417.
140. Steiner, T, Mendoza, G, De Georgia, M, et al. Prognosis of stroke patients requiring mechanical ventilation in a neurological critical care unit. Stroke. 1997; 28:711–715.
141. Grotta, J, Pasteur, W, Khwaja, G, et al. Elective intubation for neurologic deterioration after stroke. Neurology. 1995; 45:640–644.
142. Gujjar, AR, Deibert, E, Manno, EM, et al. Mechanical ventilation for ischemic stroke and intracerebral hemorrhage: indications, timing, and outcome. Neurology. 1998; 512:447–451.
143. Wijdicks, EF, Scott, JP. Outcome in patients with acute basilar artery occlusion requiring mechanical ventilation. Stroke. 1996; 27:1301–1303.
144. Tandan, R, Bradley, WG. Amyotrophic lateral sclerosis: Part 1. Clinical features, pathology, and ethical issues in management. Ann Neurol. 1985; 18:271–280.
145. Braun, SR. Respiratory system in amyotrophic lateral sclerosis. Neurol Clin. 1987; 5:9–31.
146. Gracey, DR, McMichan, JC, Divertie, MB, et al. Respiratory failure in Guillain-Barré syndrome. Mayo Clin Proc. 1982; 57:742–746.
147. Ropper, AH, Kehne, SM. Guillain-Barré syndrome: Management of respiratory failure. Neurology. 1985; 35:1662–1665.
148. Borel, CO, Guy, J. Ventilatory management in critical neurologic illness. Neurol Clin. 1995; 13:627–644.
149. Gracey, DR, Divertie, MB, Howard, FM. Mechanical ventilation for respiratory failure in myasthenia gravis. Mayo Clin Proc. 1983; 58:597–602.
150. Durbin, C. Tracheostomy: Why, when and how? Resp Care. 2010; 55:1056–1068.
151. Stock, MC, Woodward, CG, Shapiro, B, et al. Perioperative complications of elective tracheostomy in critically ill patients. Crit Care Med. 1986; 14:861–863.
152. Dulguerov, P, Gysin, C, Perneger, TV, et al. Percutaneous or surgical tracheostomy: A meta-analysis. Crit Care Med. 1999; 27:1617–1625.
153. Zeitouni, A, Kost, K. Tracheostomy: A retrospective review of 281 patients. J Otolaryngol. 1994; 23:61–66.
154. Goldenberg, D, Golz, A, Netzer, A, et al. Tracheostomy: Changing indications and a review of 1130 cases. J Otolaryngol. 2002; 31:211–215.
155. Fattahi, T, Vega, L, Fernandes, R, et al. Our experience with 171 open tracheostomies. J Oral Maxillofac Surg. 2012; 70(7):1699–1702.
156. Straemans, J, Schloendorff, G, Herzhoff, G, et al. Complications of midline-open tracheotomy in adults. Laryngoscope. 2010; 120:84–92.
157. Halum, SL, Ting, JY, Plowman, EK, et al. A multi-institutional analysis of tracheostomy complications. Laryngoscope. 2012; 122:38–45.
158. Law, RC, Carney, AS, Manara, AR. Long-term outcome after percutaneous dilational tracheostomy: Endoscopic and spirometry findings. Anaesthesia. 1997; 52:51–56.
159. Johnson, JT, Wagner, RL, Sigler, BA. Disposable inner cannula tracheotomy tube: A prospective trial. Otolaryngol Head Neck Surg. 1988; 99:83–84.
160. McCracken, J, Leasa, D. Trach tubes designed to maximize safety may increase risk to ventilated patients. Crit Care. 2010; 14:1008–1009.
161. Snow, N, Richardson, JD, Flint, LM. Management of necrotizing tracheostomy infections. J Thorac Cardiovasc Surg. 1986; 82:341–344.
162. Niederman, NS, Ferranti, RD, Ziegler, A, et al. Respiratory infection complicating long-term tracheostomy: The implication of persistent gram-negative tracheobronchial colonization. Chest. 1984; 85:39–44.
163. Jones, JW, Reynolds, M, Hewitt, RL, et al. Tracheoinnominate artery erosion: Successful surgical management of a devastating complication. Ann Surg. 1977; 184:194–204.
164. King, K, Mandava, B, Kamen, J. Tracheal tube cuffs and tracheal dilatation. Chest. 1975; 67:458–462.
165. Jaeger, JM, Wells, NC, Kirby, RR, et al. Mechanical ventilation of a patient with decreased lung compliance and tracheal dilatation. J Clin Anesthesiol. 1992; 4:147–152.
166. Lee, TS. Routine monitoring of intracuff pressure. Chest. 1992; 102:1309.
167. Dane, TEB, King, EG. A prospective study of complications after tracheostomy for assisted ventilation. Chest. 1975; 67:398–404.
168. Grillo, HC, Postintubation tracheoesophageal fistula. International Trends in General Thoracic Surgery. Grillo, HC, Eschapasse, H, eds. International Trends in General Thoracic Surgery; Vol. 2. Saunders, Philadelphia, 1987:61–68.
169. Norwood, S, Vallina, VL, Short, K, et al. Incidence of tracheal stenosis and other late complications after percutaneous tracheostomy. Ann Surg. 2000; 232:233–241.
170. Weber, AL, Grillo, HC. Tracheal stenosis: An analysis of 151 cases. Radiol Clin North Am. 1978; 16:291–308.
171. Grillo, HC, Cooper, JD, Geffin, B, et al. A low-pressure cuff for tracheostomy to minimize tracheal injury: A comparative clinical trial. J Thorac Cardiovasc Surg. 1971; 62:898–906.
172. Pearson, FG, Goldberg, M, daSilva, AJ. Tracheal stenosis complicating tracheostomy with cuffed tubes: Clinical experience and observation from a prospective study. Arch Surg. 1968; 97:380–392.
173. Crawley, BE, Cross, DE. Tracheal cuffs: A review and dynamic pressure study. Anaesthesia. 1975; 30:4–11.
174. Lewis, FR, Jr., Schlobohm, RM, Thomas, AN. Prevention of complications from prolonged tracheal intubation. Am J Surg. 1978; 135:452–457.
175. Guyton, D, Banner, MJ, Kirby, RR. High volume, low-pressure cuffs? Are they always low pressure? Chest. 1991; 100:1076–1081.
176. Black, AMS, Seegobin, RD. Pressures on endotracheal tube cuffs. Anaesthesia. 1981; 36:498–511.
177. Grillo, HC. Surgical treatment of post-intubation tracheal injuries. J Thorac Cardiovasc Surg. 1979; 78:860–873.
178. Grillo, HC. Acquired tracheal stenosis. In: Grillo HC, Austen WG, Wilkins EW, et al, eds. Current Therapy in Cardiothoracic Surgery. Toronto: Decker; 1989:57–61.
179. Cooper, JD, Complications of tracheostomy: Pathogenesis, treatment, and prevention. International Trends in General Thoracic Surgery. Grillo, HC, Eschapasse, H, eds. International Trends in General Thoracic Surgery; Vol. 2. Saunders, Philadelphia, 1987:21–28.
180. Friedman, Y. Indications, timing, techniques, and complications of tracheostomy in the critically ill patient. Curr Opin Crit Care. 1996; 2:47–53.
181. Dierks, EJ. Tracheostomy: Elective and emergent. Oral Maxillofac Surg Clin North Am. 2008; 20:513–523.
182. Smith, I, Fleming, S, Cernaianu, A. Mishaps during transport from the intensive care unit. Crit Care Med. 1990; 18:278–281.
183. Indeck, M, Peterson, S, Brotman, S. Risk, cost and benefit of transporting patients from the ICU for special studies. Crit Care Med. 1987; 15:350.
184. Waddell, G. Movement of critically ill patients within hospital. BMJ. 1975; 2:417–419.
185. Friedman, Y, Mayer, AD. Bedside percutaneous tracheostomy in critically ill patients. Chest. 1993; 104:532–535.
186. Toursarkissian, B, Zweng, TN, Kearney, PA. Percutaneous dilational tracheostomy: Report of 141 cases. Ann Thorac Surg. 1994; 57:862–867.
187. Winkler, WB, Karnik, R, Seelman, O, et al. Bedside percutaneous dilational tracheostomy with endoscopic guidance: Experience with 71 ICU patients. Intensive Care Med. 1994; 20:476–479.
188. Powell, DM, Price, PD, Forrest, LA. Review of percutaneous tracheostomy. Laryngoscope. 1998; 108:170–177.
189. Hill, BB, Zweng, TN, Maley, RH, et al. Percutaneous dilational tracheostomy: Report of 356 cases. J Trauma. 1996; 40:238–244.
190. Walz, MK, Peitgen, K, Thurauf, N, et al. Percutaneous dilational tracheostomy—early results and long-term outcome of 326 critically ill patients. Intensive Care Med. 1998; 24:685–690.
191. Ciaglia, P, Graniero, KD. Percutaneous dilatational tracheostomy: Results and long-term follow-up. Chest. 1992; 101:464–467.
192. Thompson, EC, Fernandez, LG, Norwood, S, et al. Percutaneous dilatational tracheostomy in a community hospital setting. South Med J. 2001; 94:208–211.
193. van Heurn, LWE, Goei, R, de Ploeg, I, et al. Late complications of percutaneous dilatational tracheostomy. Chest. 1996; 110:1572–1576.
194. Dempsey, GA, Grant, CA, Jones, TM. Percutaneous tracheostomy: A 6-year prospective evaluation of the single tapered dilator technique. Br J Anesth. 2010; 105:782–788.
195. Kornblith, LZ, Burlew, CC, Moore, EE, et al. One thousand bedside percutaneous tracheostomies in the surgical intensive care unit: Time to change the gold standard. J Am Coll Surg. 2011; 212:163–170.
196. Hazard, P, Jones, C, Benitone, J. Comparative clinical trial of standard operative tracheostomy with percutaneous tracheostomy. Crit Care Med. 1991; 19:1018–1024.
197. Friedman, Y, Fildes, J, Mizock, B, et al. Comparison of percutaneous and surgical tracheostomies. Chest. 1996; 110:480–485.
198. Crofts, SL, Alzeer, A, McGuire, GP, et al. A comparison of percutaneous and operative tracheostomies in intensive care patients. Can J Anaesth. 1995; 42:775–779.
199. Holdgaard, HO, Pederson, J, Jensen, RA, et al. Percutaneous dilatational tracheostomy versus conventional surgical tracheostomy: A clinical randomized study. Acta Anaesthesiol Scand. 1998; 42:545–550.
200. Gysin, C, Dulguerov, P, Guyot, JP, et al. Percutaneous versus surgical tracheostomy: A double-blind randomized trial. Ann Surg. 1998; 230:708–714.
201. Freeman, BD, Isabella, K, Cobb, JP, et al. A prospective, randomized study comparing percutaneous with surgical tracheostomy in critically ill patients. Crit Care Med. 2001; 29:926–930.
202. Silvester, W, Goldsmith, D, Uchino, S, et al. Percutaneous versus surgical tracheostomy: A randomized controlled study with long-term follow-up. Crit Care Med. 2006; 34:2145–2152.
203. Massick, DD, Yao, S, Powell, DM, et al. Bedside tracheostomy in the intensive care unit: A prospective randomized trial comparing open surgical tracheostomy with endoscopically guided percutaneous dilatational tracheostomy. Laryngoscope. 2001; 111:494–500.
204. Veenith, T, Ganeshamoorthy, S, Standley, T, et al. Intensive care unit tracheostomy: A snapshot of UK practice. Int Arch Med. 2008; 1:21.
205. Kluge, S, Baumann, HJ, Maier, C, et al. Tracheostomy in the intensive care unit: A nationwide survey. Anesth Analg. 2008; 107:1639–1643.
206. Cooper, RM. Use and safety of percutaneous tracheostomy in intensive care: Report of a postal survey of ICU practice. Anaesthesia. 1998; 53:1209–1227.
207. Friedman, Y. Percutaneous tracheostomy: What technique is it? Crit Care Med. 2001; 29:1289–1290.
208. Cabrini, L, Monti, G, Landoni, G, et al. Percutaneous tracheostomy, a systematic review. Acta Anesthesiol Scand. 2012; 56:270–281.
209. Pryor, JP, Reilly, PM, Shapiro, MB. Surgical airway management in the intensive care unit. Crit Care Clin. 2000; 16:473–488.
210. Cheng, E, Fee, WE. Dilational versus standard tracheostomy: A meta-analysis. Ann Otol Rhinol Laryngol. 2000; 109:803–807.
211. Freeman, BD, Isabella, K, Lin, N, et al. A meta-analysis of prospective trials comparing percutaneous and surgical tracheostomy in critically ill patients. Chest. 2000; 118:1412–1418.
212. Delaney, A, Bagshaw, SM, Nalos, M. Percutaneous dilatational tracheostomy versus surgical tracheostomy in critically ill patients: A systematic review and meta-analysis. Crit Care. 2006; 10(2):R55.
213. Higgins, KM, Punthakee, X. Meta-analysis comparison of open versus percutaneous tracheostomy. Laryngoscope. 2007; 117:447–454.
214. Oliver, ER, Gist, A, Gillespie, MB. Percutaneous versus surgical tracheostomy: An updated meta-analysis. Laryngoscope. 2007; 117:1570–1575.
215. Friedman, Y, Mizock, BA. Percutaneous versus surgical tracheostomy: Procedure of choice or choice of procedure? Crit Care Med. 1999; 27:1684–1685.
216. Antonelli, M, Michetti, V, Di Palma, A, et al. Percutaneous translaryngeal versus surgical tracheostomy: A randomized trial with 1-yr double-blind follow-up. Crit Care Med. 2005; 33:1015–1020.
217. Heikkinen, M, Aarnio, P, Hannukainen, J. Percutaneous dilational tracheostomy or conventional surgical tracheostomy. Crit Care Med. 2000; 28:1399–1402.
218. Kilic, D, Findikcioglu, MD, Akin, S, et al. When is surgical tracheostomy indicated? Surgical “U-shaped” versus percutaneous tracheostomy. Ann Thorac Cardiovasc Surg. 2011; 17:29–32.
219. van Heerden, PV, Webb, SAR, Power, BM, et al. Percutaneous dilational tracheostomy—a clinical study evaluating two systems. Anaesth Intensive Care. 1996; 24:56–59.
220. Nates, JL, Cooper, DJ, Myles, PS, et al. Percutaneous tracheostomy in critically ill patients: A prospective, randomized comparison of two techniques. Crit Care Med. 2000; 28:3734–3739.
221. Fikkers, BG, Staatsen, M, Lardenoije, SGGF, et al. Comparison of two percutaneous tracheostomy techniques, guidewire dilating forceps and Ciaglia Blue Rhino: A sequential cohort study. Crit Care. 2004; 8:R299–R305.
222. Cantais, E, Kaiser, E, Le-Goff, Y, et al. Percutaneous tracheostomy: Prospective comparison of the translaryngeal technique versus the forceps-dilational technique in 100 critically ill adults. Crit Care Med. 2002; 30:815–819.
223. Byhahn, C, Westphal, K, Meininger, D, et al. Single-dilator percutaneous trachesotomy: A comparison of PercuTwist and Ciaglia Blue Rhino technique. Intensive Care Med. 2002; 28:1262–1266.
224. Monteriol, A, Bordes, J, Asencio, Y, et al. Bedside percutaneous tracheostomy: A prospective randomized comparison of PercuTwist versus Griggs’ forceps dilational trachesotomy. Anesth Intensive Care. 2011; 39:209–216.
225. Johnson, JJ, Cheatem, ML, Sagraves, SG, et al. Percutaneous dilatational tracheostomy: A comparison of single vs. multiple dilator techniques. Crit Care Med. 2001; 29:1251–1254.
226. Feller-Kopman, D. Acute complications of artificial airways. Clin Chest Med. 2003; 24:445–455.
227. Guzman, J, Bander, J, Weinmann, MD. Percutaneous diational tracheostomy: A safe technique in patients at risk for bleeding. Am J Respir Crit Care Med. 1995; 151:A489.
228. Meyer, M, Critchlow, J, Mansharamani, N, et al. Repeat bedside percutaneous tracheostomy is a safe procedure. Crit Care Med. 2002; 30:986–988.
229. Kluge, S, Meyer, A, Kuhnelt, P, et al. Percutaneous tracheostomy is safe in patients with severe thrombocytopenia. Chest. 2004; 126:547–551.
230. Romero, CM, Cornejo, RA, Ruiz, MH, et al. Fiberoptic bronchoscopy-assistd percutaneous tracheostomy is safe in obese critically ill patients: A prospective and comparative study. J Crit Care. 2009; 24:494–500.
231. Aldawood, AS, Arabi, YM, Haddad, S. Safety of percutaneous trachestomy in obese critically ill patients: A prospective cohort study. Anaesth Intensive Care. 2008; 36:69–73.
232. Bardell, T, Drover, JW. Recent developments in percutaneous tracheostomy: Improving techniques and expanding roles. Curr Opin Crit Care. 2005; 11:326–332.
233. Friedman, Y, Franklin, C. The technique of percutaneous tracheostomy. J Crit Illness. 1993; 8:289–297.
234. Barba, CA, Angood, PB, Kauder, DR, et al. Bronchoscopic guidance makes percutaneous tracheostomy easy to teach, safe, and cost effective. Surgery. 1995; 118:879–883.
235. Marx, WH, Ciaglia, P, Graniero, KD. Some important details in the technique of percutaneous dilatational tracheostomy via the modified Seldinger technique. Chest. 1996; 110:762–766.
236. Mallick, A, Venkatanesh, D, Elliot, SC, et al. A prospective randomized controlled trial of capnography vs. bronchoscopy for Blue Rhino™ percutaneous tracheostomy. Anaesthesia. 2003; 58:864–868.
237. Jackson, LM, Davis, JW, Kaups, KL, et al. Percutaneous tracheostomy: To bronch or not to bronch—that is the question. J Trauma. 2011; 71:1553–1556.
238. Cobean, R, Beals, M, Moss, C, et al. Percutaneous dilatational trachesotomy: A safe, cost effective bedside procedure. Arch Surg. 1996; 131:265–271.
239. Ernst, A, Critchlow, J. Percutaneous tracheostomy—special considerations. Clin Chest Med. 2003; 24:409–412.
240. Porter, J, Ivatury, RA. Preferred route of tracheostomy—percutaneous versus open at the bedside: A randomized, prospective study in the surgical intensive care unit. Am Surg. 1999; 65:142–146.
241. Beltrame, F, Zussino, M, Martinez, B, et al. Percutaneous versus surgical bedside tracheostomy in the intensive care unit: A cohort study. Minerva Anesthesiol. 2008; 74:529–535.
242. Pappas, S, Maragoudakis, P, Vlastarakos, P, et al. Surgical versus percutaneous tracheostomy: An evidence-based approach. Eur Arch Otorhinolaryngol. 2011; 266:323–330.