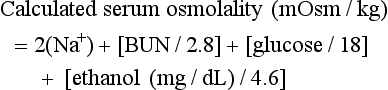Toxic Alcohols
Methanol
Principles of Disease
Methanol is absorbed rapidly from the gastrointestinal tract, and blood levels peak 30 to 60 minutes after ingestion.1 Transdermal and respiratory tract absorption also has resulted in toxicity, especially in infants. Certain occupations, including painting, glazing, varnishing, lithography, and printing, are high risk for inhalational exposure to methanol. Inhalational abuse of methanol is a recent trend that can result in toxic serum levels.2
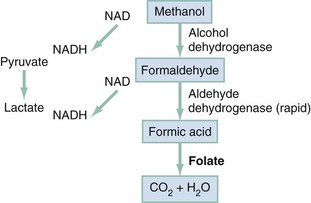
Methanol itself has little toxicity, producing less central nervous system (CNS) depression and inebriation than ethanol. Metabolites of the parent alcohol are extremely toxic, however. Although small amounts of methanol are eliminated by renal and pulmonary routes, 90% is metabolized in the liver. Methanol is oxidized by alcohol dehydrogenase (ADH) to formaldehyde, which is rapidly converted by aldehyde dehydrogenase to formic acid (Fig. 155-1). Formic acid is the primary toxicant and accounts for much of the anion gap metabolic acidosis and ocular toxicity peculiar to methanol ingestion.3 Through a folate-dependent pathway, formic acid is degraded to carbon dioxide and water.
Pathophysiology
Optic neuropathy and putaminal necrosis are the two main complications of severe methanol poisoning. Long-term morbidity takes the form of visual impairment, including blindness, and parkinsonian motor dysfunction, characterized by hypokinesis and rigidity. Formic acid has a high affinity for iron and inhibits mitochondrial cytochrome oxidase, halting cellular respiration.4 Methanol metabolism in the cytosol and mitochondria may account for a second mechanism of adenosine triphosphate depletion.5 Lactate accumulation resulting from hypotension or seizures further compounds the metabolic acidosis predominantly caused by formate. Other mechanisms of toxicity involve increased lipid peroxidation, free radical formation, and impaired protective antioxidant reactions.4,6 Severe dysfunction of subcellular metabolism from methanol also has been linked to significant disturbance in proteolytic-antiproteolytic balance.7
The primary sites of ocular injury are the retrolaminar optic nerve and retina. Selective myelin damage to the retrolaminar optic nerve has been seen at autopsy after methanol toxicity. Müller cells, the principal glial cells of retinal neurons and photoreceptors, have been proposed as the initial target in methanol-induced visual toxicity. It seems that they alone harbor the enzymes necessary to metabolize methanol to formate. Histopathologic correlates suggest that retinal cells develop intra-axonal swelling, calcium influx, mitochondrial destruction, and microtubular disruption. Ultimately, this interferes with transport of essential proteins from the retinal neuron cell body to the nerve fiber axoplasm. Oligodendrocyte involvement causes myelin degeneration and leads to visual decrements. Acidosis may accelerate this process by enhancing nonionic diffusion of formic acid into neurons and further increasing lactate production.4 This self-perpetuating cycle of acidosis, termed circulus hypoxicus,8 underscores the need for aggressive correction of pH to accomplish ion trapping of formate outside the CNS.
Methanol adversely affects other areas of the CNS, specifically the basal ganglia. Bilateral, symmetrical putaminal hypodensities, hemorrhages, or cystic lesions are characteristic, occurring in 13.5% of patients. Necrosis is described in the subcortical white matter, spinal cord anterior horn cells, and cerebellum.9 Acute signs and symptoms may be lacking or may take several days to develop despite the presence of these radiographic findings. The cellular mechanisms of injury may be similar to the mechanisms of the ophthalmologic injury, but the reason for localization of neurologic damage to the basal ganglia is unknown. Quantitative neuropathologic studies conflict as to whether concentrations of formic acid within the putamen are higher than levels in the blood or other tissues. Massive edema adjacent to the putamen shown by magnetic resonance imaging (MRI) suggests a possible localized disruption of the blood-brain barrier. Other proposed mechanisms for the vulnerability of this region include the unique pattern of arterial blood supply and venous drainage and greater metabolic activity.
Clinical Features
With individual cases of methanol poisoning, the history may be unobtainable or unreliable. The diagnosis should be considered in patients with altered mental status, visual complaints, or metabolic acidosis and in patients with occupations that put them at high risk for exposure. Because methanol is a poor substrate for ADH, a latency period exists between the time of ingestion and metabolism to the formate that causes visual or metabolic disturbance. The typical 12- to 24-hour latency may be shorter when large amounts are consumed or longer when ethanol is coingested (range, 40 minutes to 72 hours).1 Formic acid accumulation may be ongoing, with risk for significant toxicity, in patients who present early despite being initially asymptomatic. When symptoms are manifested, they are primarily neurologic, gastrointestinal, or ocular.
Although methanol is less inebriating than ethanol, early symptoms of methanol poisoning include depressed mental status, confusion, and ataxia. Nonspecific complaints of weakness, dizziness, anorexia, headache, and nausea develop; in severe cases, coma and seizures may be seen. Although vomiting and abdominal pain commonly result from mucosal irritation, the absence of gastrointestinal complaints does not rule out a serious ingestion.1 Abdominal tenderness, however, may be so severe that it suggests an acute surgical abdomen.1 This may result from pancreatitis, and elevation of serum amylase is relatively common, but increased salivary amylase isoenzyme can occur without pancreatic inflammation.
Visual disturbances are seen in 50% of patients, and their development may precede or parallel that of other symptoms.8 Patients may complain of cloudy, blurred, indistinct, or misty vision or may note yellow spots or, rarely, photophobia. The most common acute field defect is a dense central scotoma.10 Some patients compare their visual symptoms with “stepping out into a snowstorm,” a complaint unique to methanol ingestion. Patients can have a complete lack of light perception and total loss of vision. Visual acuity should be determined. On examination, optic disc hyperemia is seen at 18 to 48 hours after ingestion. Peripapillary retinal edema follows, is most striking in the nerve fiber layer along the vascular arcades, and only rarely involves the macula.5,10 Sluggishly reactive or fixed and dilated pupils indicate a poor prognosis. Pallor and cupping, indicative of optic atrophy, are late findings suggesting a poor prognosis for visual recovery. On occasion, the fundus may appear normal, even in patients with visual symptoms.
Compensatory tachypnea heralds the onset of metabolic acidosis, which often may be severe, with reported serum bicarbonate concentrations of less than 5 mEq/L and an arterial pH less than 7.0. Early tachycardia has been noted, but in general, cardiovascular abnormalities are rare.1 Hypotension and bradycardia, when present, are preterminal findings.11 Historically, death is associated with a peculiar, abrupt cessation of respiration rather than with cardiovascular collapse.1 Multiple organ failure is rare.11
Prognosis after methanol ingestion seems to correlate with the degree of acidosis, time to presentation, and initiation of treatment within 8 hours of exposure.1,12 Poor prognosis is associated with coma, seizures, or arterial pH less than 7.0.11 A large outbreak was associated with a fatality rate of 44%.13 Patients surviving the acute phase of toxicity may be left with permanent blindness or neurologic deficits, such as parkinsonism, toxic encephalopathy, polyneuropathy, cognitive dysfunction, transverse myelitis, primitive reflexes, or seizures.14
Diagnostic Strategies
The “normal” osmol gap is often cited to be less than 10 mOsm/kg when the preceding equation is used. This is an arbitrary number, and there is considerable variability in baseline osmolal gaps in patients, particularly children. An osmol gap significantly greater than 10 mOsm/kg may be a useful aid in the diagnosis of toxic alcohol ingestion. In addition to methanol, ethylene glycol, and isopropanol, other low-molecular-weight solutes, such as ethanol, acetone, propylene glycol, mannitol, glycerol, and ethyl ether, may cause elevated osmol gaps. Caution should be taken, however, in ruling out toxic alcohol ingestion with a normal osmol gap for several reasons. First, calculated serum osmolality results may vary among laboratories and must be done by the freezing point depression method. Also, delayed presentation after toxic alcohol ingestion may be associated with prior metabolism of most of the parent alcohol. Because only the parent compound is osmotically active and because the charged metabolites are electrically balanced by sodium, there may be little or no osmol gap elevation in this setting. Finally, a toxic level of either methanol or ethylene glycol may be present with a gap of only 10 mOsm/kg. If there is clinical suspicion of toxic alcohol ingestion, direct measurement of the serum toxic alcohol level is necessary, and if it is not readily available, empirical treatment is warranted.15 Rhabdomyolysis, pancreatitis, and metabolic derangements, such as hypomagnesemia, hypokalemia, and hypophosphatemia, are also described with methanol poisoning.
Computed tomography may be indicated in an intoxicated patient with altered mental status. The characteristic finding of bilateral putaminal lesions suggests methanol poisoning, but this finding also may be seen with Leigh’s syndrome, Wilson’s disease, hypoxic-ischemic insult, encephalitis, and certain metabolic disorders. Ischemic necrosis, cerebral edema, or brain hemorrhages also may be noted. Follow-up scans may have prognostic value because parkinsonian features are unlikely to develop in patients whose putaminal lesions resolve within a short time frame.16 MRI may also detect putaminal aberrations or optic neuropathy from methanol intoxication.
Ethylene Glycol
Ethylene glycol is a viscous, colorless, odorless, slightly sweet-tasting liquid. Because it lowers the freezing point of water, its primary utility is as a commercial antifreeze or coolant. Other sources include airplane deicing solutions, hydraulic brake fluids, and industrial solvents and precursors; it also is a component of certain paints, lacquers, and cosmetics. Most ethylene glycol poisonings occur with the ingestion of antifreeze. Unusual poisoning scenarios are described, including an epidemic after the contamination of water supplies and the intentional poisoning of an infant, manifested as an inherited metabolic disorder. In 2009, the American Association of Poison Control Centers reported 5977 exposures to ethylene glycol. Of those exposures, 72% were unintentional, and 11% resulted in moderate or severe effects with 19 fatalities. If it is treated early and aggressively, ethylene glycol poisoning is unlikely to result in death. Conversely, failure to treat ethylene glycol ingestion may result in multiorgan failure and death within 24 to 36 hours.17
Principles of Disease
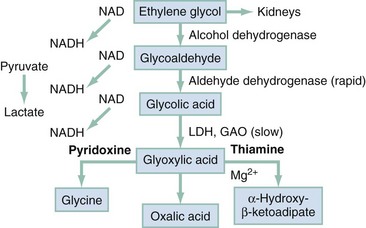
Absorption of ethylene glycol is rapid after ingestion. It distributes evenly to tissues with a volume similar to that of body water. Peak blood levels are reached within 1 to 4 hours after ingestion. In contrast to methanol and isopropanol, ethylene glycol is nonvolatile at room temperature, so absorption by inhalation is unlikely. Reported half-lives range from 3 to 8.6 hours.18 When metabolism is blocked by fomepizole or ethanol, the half-life increases to 11 to 15 hours or 17 hours, respectively.19 The toxic and lethal doses of 100% ethylene glycol have been reported as 0.2 mL/kg and 1.4 mL/kg. At the other extreme, with modern treatment, patients who have ingested 3000 mL have survived. Twenty-seven percent of ethylene glycol is excreted unchanged by the kidneys. The remainder is oxidized through hepatic ADH and other oxidative enzymes to various toxic organic aldehydes and acids (Fig. 155-2).
Pathophysiology
Unmetabolized ethylene glycol has limited toxicity, yet all metabolites are toxic. In humans, 2.3% of a dose of ethylene glycol ultimately is converted to oxalic acid, most of which is excreted in the urine. A fraction of oxalic acid combines with calcium to form calcium oxalate crystals, which precipitate in renal tubules, brain, and other tissues. Studies have shown definitively that the accumulation of calcium oxalate monohydrate crystals in kidney tissue produces renal tubular necrosis that leads to kidney failure.20 Other authors suggest that glycolate levels correlate better with disruption of CNS metabolism, development of renal failure, and mortality.21 Because the intermediate metabolite, glyoxylic acid, theoretically can be shunted toward pyridoxine-dependent or thiamine-dependent pathways to generate the nontoxic products glycine and α-hydroxy-β-ketoadipate (see Fig. 155-2), both pyridoxine and thiamine are routinely used in therapy.
On histologic examination, proximal renal tubular dilation with hydropic change and vacuolar degeneration, intratubular crystal deposition, and edema of the interstitium are seen. Relative sparing of the glomeruli is typical, although glomerular interloop space crystal deposition may occur. Glomerular function is preserved, but tubular dysfunction manifesting as protein leak is noted.22 Neuropathologic changes induced by ethylene glycol include diffuse calcium oxalate deposition with petechial hemorrhages in the retina, brain, vessel walls, and perivascular spaces, with evidence of cerebral edema and chemical meningoencephalitis. Similar changes also have been noted in the liver, spleen, pancreas, pleura, lungs, pericardium, and blood vessel walls throughout the body. Myonecrosis occurs in skeletal and myocardial muscle, and rhabdomyolysis and myocardial dilation occur. Fatty liver with focal necrosis has been noted.
Clinical Features
Stage II, the cardiopulmonary stage, occurs 12 to 24 hours after ingestion. Often, the patient exhibits mild hypertension and tachycardia. Tachypnea may reflect the underlying profound metabolic acidosis or may herald the onset of cardiogenic or noncardiogenic pulmonary edema.8 The mechanism of acute respiratory distress syndrome (ARDS) is unclear but may be related to the toxicity of glycolic and glyoxylic acids and to the deposition of calcium oxalate crystals within the lungs. Pulmonary infiltrates are described during this phase. Circulatory collapse may occur as a result of myocardial depression. Hypocalcemia, rarely associated with tetany or cardiac dysrhythmia, occurs in 30% of patients as a result of systemic chelation of calcium by oxalate. Myositis with muscle tenderness and creatine kinase (CK) elevation results from skeletal muscle inflammation and necrosis.
Stage III, the renal stage, occurs 24 to 72 hours after ingestion. In one epidemic of 36 patients, renal damage was noted in 67%. Awake patients may complain of flank pain or have costophrenic angle tenderness. Calcium oxalate monohydrate or dihydrate crystalluria is seen in only 50% of cases. Hematuria and proteinuria are common, however. As with other causes of acute tubular necrosis, oliguria is not always present, but it may develop 12 hours after ingestion and may progress to frank anuria. The outcome has not been well studied in patients who have renal failure. Prolonged hemodialysis may be necessary, although recovery of renal function has been reported. Delayed onset of ARDS has been reported to occur during this stage, but it is more typically seen in stage II. The degree of acidosis, delay in presentation, and glycolate levels correlate better than ethylene glycol levels with the development of renal failure.23
Stage IV, the delayed neurologic sequelae stage, occurs 6 to 12 days after ingestion and typically is manifested as cranial neuropathy.24 All cases have been associated with renal failure. Facial diplegia, occasionally with deafness, is most frequently encountered. Other reported findings include dysarthria, dysphagia, tongue deviation, visual deterioration, and internal ophthalmoplegia. Delayed and persistent cognitive and motor deficits, such as ataxia, chorea, coma, and late personality changes, are also reported. Total paralysis from severe axonal peripheral polyneuropathic degeneration with oxalate deposition25 and polyradiculopathy from nerve root (not distal) disease are also reported. Although some improvement in neurologic status has been noted at follow-up, most patients are left with residual neurologic deficits. Some authors have coined the term facial auditory nerve oxalosis for this delayed syndrome on the basis of heavy calcium oxalate crystal deposition along the subarachnoid portions of the seventh and eighth cranial nerves seen at autopsy. Whether calcium oxalate deposition is a cause is unclear. Direct mechanical injury from crystals, inflammatory response triggered by ethylene glycol metabolites, meningitis, and pyridoxine deficiency have been proposed as mechanisms. Because the syndrome has been reported only since 1978, it is possible that the advent of hemodialysis has allowed more patients with potentially lethal ingestions of ethylene glycol to survive to display delayed neurologic complications.
Diagnostic Strategies
Useful laboratory tests for evaluation of a patient with potential ethylene glycol toxicity include serum electrolyte values; calcium, BUN, creatinine, and serum glucose concentrations; serum osmolality; blood ethanol level; arterial blood gas analysis; ethylene glycol level; electrocardiogram; and urinalysis. Although crystalluria is considered the hallmark of ethylene glycol ingestion, its absence does not rule out the diagnosis because less than half of patients have this finding. Crystalluria may take the form of envelope-shaped calcium oxalate dihydrate crystals or needle-shaped calcium oxalate monohydrate crystals, which are occasionally mistaken for hippurate crystals.8 Other crystal shapes and composites have been noted so that in the setting of combined anion and osmolal gap elevation, the presence of any type of crystalluria warrants a search for ethylene glycol. Monohydrate crystals are thought to be more specific for ethylene glycol poisoning. Irrigating the urinary bladder with 50 to 100 mL of saline, centrifuging the irrigant, and examining the sediment for crystals may yield the diagnosis in patients who are already anuric. Other findings reflecting tubular dysfunction include decreased specific gravity, proteinuria, microscopic hematuria, pyuria, and cylindruria. Falsely elevated ethylene glycol levels may be seen in the face of elevated lactate or lactate dehydrogenase with certain enzymatic assays.
Freshly voided urine can be examined for fluorescence with Wood’s lamp. Sodium fluorescein is added to antifreeze to aid in the detection of radiator leaks. Urinary fluorescence may be seen 6 hours after ingestion of fluorescein-containing antifreeze. Gastric contents and the patient’s skin or clothing also may fluoresce under Wood’s lamp. The lack of fluorescence does not rule out ethylene glycol ingestion, however, because examiner sensitivity, specificity, and inter-rater reliability are low.26 One study found urinary fluorescence in all specimens from children evaluated for conditions unrelated to poisoning.27 Specimens should be collected in borosilicate glass test tubes or deposited directly onto gauze or filter paper because many plastic specimen containers and some glass tubes are fluorescent. The urine pH also should be checked and adjusted to 4.5 or higher before Wood’s lamp examination.
Similar to methanol, ethylene glycol often causes a profound anion gap metabolic acidosis when the metabolites glycolic acid and glyoxylic acid (and, to some extent, lactic acid) accumulate. False elevations of lactic acid levels may occur in the presence of glycolate with certain analyzers.28 Similar to methanol toxicity, an elevated osmolal gap as measured by freezing point depression can be a clue, but lethal concentrations can be associated with a normal or only slightly elevated osmolal gap. The cerebrospinal fluid may be normal or have a cloudy or bloody appearance, with increased protein or, most commonly, a polymorphonuclear pleocytosis.
Imaging studies show cerebral edema with decreased attenuation in the mediobasilar portions of the brain, typically with a return to isodensity within 1 week; this does not correspond to the clinical picture.8 MRI in one patient with delayed cranial neuropathy showed gadolinium enhancement of cranial nerve V bilaterally, possibly secondary to calcium oxalate deposition. Electroencephalographic findings have been nonspecific.
Management
Methanol and ethylene glycol are absorbed rapidly from the gastrointestinal tract; because gastric emptying has not been shown to alter clinical course or outcome and may be associated with complications, its use is restricted to possible nasogastric suction for patients who have ingested more than a swallow and arrive in the emergency department within 30 to 60 minutes of ingestion.29 Even in these cases, it is of unproven value. Forced diuresis is of no value and may cause pulmonary edema and ARDS. Early intubation may be indicated to protect the airway against aspiration and in anticipation of further deterioration in mental status.
Three treatment goals exist for patients with methanol or ethylene glycol toxicity: (1) correction of metabolic acidosis with bicarbonate; (2) ADH enzyme blockade, which inhibits the metabolism of methanol and ethylene glycol to toxic metabolites; and (3) removal of the parent alcohol and its metabolites by hemodialysis. The acidic metabolites of methanol and ethylene glycol can cause a profound bicarbonate-resistant metabolic acidosis, with several hundred milliequivalents of excess acid produced per hour. In contrast to lactic acid, these acids are not metabolized to bicarbonate, and massive amounts of bicarbonate may be necessary merely for partial correction of the acidosis.8 Early correction of metabolic acidosis may be beneficial in reversing methanol-induced visual impairment,30 most likely related to the induction of a larger fraction of dissociated formic acid, which should decrease the amount of formic acid entering the CNS. Depending on the severity of the patient’s acidosis, bicarbonate can be administered intravenously by intermittent boluses, by an initial bolus followed by an infusion, or by infusion alone. Boluses of 1 to 2 mEq/kg to attain a target serum pH of 7.45 to 7.50 followed by an infusion of 150 mEq/L of sodium bicarbonate in 5% dextrose at 1.5 to 2 times the maintenance fluid rate are suggested. With ethylene glycol, the potential for worsened hypocalcemia during the administration of large amounts of sodium bicarbonate should be considered.
To prevent further production of the toxic and acidic metabolites of methanol and ethylene glycol, metabolism of the parent compounds by the enzyme ADH must be blocked by either ethanol or fomepizole (Antizol). As experience has been gained with fomepizole, it is generally thought to be more advantageous than ethanol from a safety and convenience perspective. ADH blockade should be carried out in any adult or child with symptoms or with methanol or ethylene glycol levels greater than 20 mg/dL, even if the patient is asymptomatic. If ethanol is used to block ADH, the goal is to maintain the blood ethanol level between 100 and 150 mg/dL, which completely saturates ADH.8 The affinity of ADH for ethanol is 10 to 20 times greater than for methanol and 100 times greater than for ethylene glycol. When the metabolism of methanol and ethylene glycol is blocked by ethanol, their half-lives increase to more than 30 hours and 17 hours, respectively. Prevention of methanol and ethylene glycol metabolism by ethanol explains the delayed toxicity seen in patients who ingest ethanol in combination with either of these substances. In dosing of ethanol, it also is important to measure the patient’s initial blood ethanol level. If it is greater than 100 mg/dL, a loading dose is unnecessary, and a maintenance infusion can be started (Table 155-1). The required maintenance dose of ethanol may nearly triple during hemodialysis because ethanol also is efficiently removed. Ethanol may be given orally or intravenously. Potential side effects of intravenous administration include CNS and respiratory depression, hypotension, vomiting, hypoglycemia, and thrombophlebitis, although even in children, adverse effects are limited.31 Oral ethanol loading may be associated with gastritis. Initial close monitoring of serum ethanol and glucose levels every 1 to 2 hours is essential until a steady-state level of 100 to 150 mg/dL is achieved. Levels should be checked every 2 to 4 hours thereafter. Monitoring of ethanol therapy ideally is accomplished in the intensive care unit.
Table 155-1
Standard Range of Therapeutic Doses of Ethanol Based on Average Pharmacokinetic Values
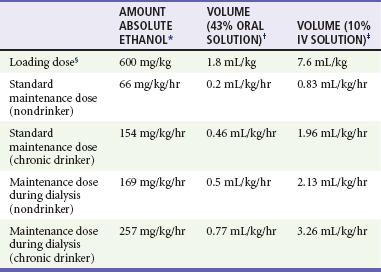
†Equivalent to 86 proof undiluted liquor (34 g/dL of ethanol).
‡Equivalent to 7.9 g/dL of ethanol.
§Assumes that initial ethanol concentration is zero; dose is independent of chronic drinking status.
Modified from Barceloux DG, Krenzelok EP, Olson K, Watson W: American Academy of Clinical Toxicology practice guidelines on the treatment of ethylene glycol poisoning. Ad Hoc Committee. J Toxicol Clin Toxicol 37:537, 1999.
Similar to ethanol, fomepizole blocks the metabolism of methanol and ethylene glycol by ADH and prevents the formation of toxic metabolites.19,32 When the metabolism of methanol and ethylene glycol is blocked by fomepizole, their half-lives increase to an average of 52 hours and 17 hours, respectively.33 Although fomepizole is a pregnancy category C drug and not approved for pediatric use, it has been used in children.31 Advantages of fomepizole over ethanol include ease of administration, predictable pharmacokinetics, improved patient safety profile, standardized and less complicated dosing regimen that does not require direct observation or frequent blood monitoring, longer duration of action, and lack of CNS depression.34 Nonetheless, simply blocking toxic alcohol metabolism does not alter the toxicity of preformed metabolites. The use of neither fomepizole nor ethanol supplants the need for hemodialysis. The main disadvantage of the use of fomepizole is its cost. The dosing regimen for adults and pediatric patients is 15 mg/kg, followed by 10 mg/kg every 12 hours for four doses. After five doses, the dose increases to 15 mg/kg every 12 hours until the ethylene glycol concentration is undetectable or less than 20 mg/dL and the patient is asymptomatic with a normal arterial pH. Dosing changes are required during hemodialysis. Side effects of fomepizole include headache, nausea, dizziness, inflammation at the site of infusion, rash, eosinophilia, and mild reversible transaminase elevation. A single case of hypotension and bradycardia related to the rapid infusion has been described.35 No prospective clinical trials have compared the relative efficacy, safety, or cost of fomepizole and ethanol in the treatment of ethylene glycol or methanol toxicity; however, a 10-year review of adverse events in patients showed fomepizole to be safer than ethanol.36
Rapid removal of the methanol or ethylene glycol through hemodialysis before it has been metabolized remains the cornerstone of therapy. In addition, hemodialysis, preferably with use of a dialysate high in bicarbonate, corrects acidosis and uremia, aids in fluid management and cardiovascular stabilization, and removes the toxic metabolites in late presenters. Hemodialysis decreases the half-life of methanol and ethylene glycol to 2.5 to 3.5 hours. Peritoneal dialysis and hemoperfusion are much less effective.36
Indications for hemodialysis in patients with methanol or ethylene glycol toxicity are controversial. In general, hemodialysis is indicated for patients who have metabolic acidosis, renal insufficiency, deterioration despite intensive care, electrolyte disturbance, or visual disturbances with methanol.8 In addition, a blood level of either substance greater than 50 mg/dL usually calls for dialysis,35,37 but in patients with normal kidneys, prolonged fomepizole treatment alone has been used without hemodialysis.38,39 For ethylene glycol, a glycolic acid level greater than 8 mmol/L, an anion gap greater than 20 mmol/L, or an initial pH less than 7.30 predicts renal failure and the need for hemodialysis.40 Methanol and ethylene glycol levels should be interpreted in the context of the clinical status and the time after ingestion. An acidotic patient with a low blood level after a delayed presentation requires more aggressive treatment than an asymptomatic patient with a high level.
In methanol poisoning, the rate of the final degradation reaction of formic acid to carbon dioxide and water depends on the cofactor folate in primates (see Fig. 155-1), so 50 mg of leucovorin (folinic acid) should be given intravenously (IV) every 4 hours to adults with methanol toxicity. Likewise, the cofactors thiamine and pyridoxine have theoretic benefit on the basis of their use in humans with primary hyperoxaluria, although no clinical trials have studied their efficacy in the treatment of ethylene glycol toxicity. Adequate cofactor levels minimize oxalic acid production by favoring the production of other, less toxic metabolites (see Fig. 155-2). The recommended adult doses are thiamine, 100 mg IV every 6 hours, and pyridoxine, 50 mg every 6 hours for 2 days. With symptomatic hypocalcemia, IV calcium should be supplemented, albeit cautiously, to avoid further precipitation of calcium oxalate crystals in tissues. Asymptomatic hypercalcemia need not be treated. Magnesium is a cofactor along with thiamine for the detoxification of glycolic acid and should be replaced in patients who are deficient.
Prevention
The bittering agent denatonium benzoate (Bitrex) has been added to consumer automotive products containing 10% or more ethylene glycol or 4% or more methanol, but the effect of this effort has not yet decreased the frequency or severity of pediatric antifreeze poisoning.41 Another opportunity for prevention is the substitution of less toxic glycols, such as propylene glycol, into commercial antifreezes.
Isopropyl Alcohol
Isopropyl alcohol (isopropanol) is a clear, colorless liquid with a slightly bitter taste. It is the second most commonly ingested alcohol after ethanol. In 2009, the American Association of Poison Control Centers reported 20,902 isopropanol exposures: 83% were unintentional, and 3% involved moderate or major effects; there was one fatality. Rubbing alcohols, which contain 70 to 91% isopropanol or ethanol, are frequent sources of exposure and may be tinted green or blue. Other sources include skin and hair products, nail polish removers, disinfectants, window and pine household cleaners, and antifreeze. Children experience toxicity most commonly from accidental ingestion, but inhalation and transdermal absorption during sponge bathing may occur. Isopropanol and its major metabolite acetone cause twice the CNS depression of ethanol, so intentional ingestion as an alcohol substitute is encountered frequently in adults. Isopropanol is not associated with the toxicity of ethylene glycol or methanol poisoning, and death after exposure is rare. The typical fatality involves a chronic, older alcoholic with mixed ethanol-isopropanol intoxication after a drinking binge.42
Principles of Disease
Absorption of isopropanol is rapid and complete; 80% of a dose is absorbed within 30 minutes of ingestion. The kidneys excrete 20% as unchanged isopropanol. A small portion is re-secreted into the stomach and saliva; the remaining 80% is metabolized in the liver to acetone by ADH (Fig. 155-3). Acetone is excreted primarily by the kidneys, with small amounts expired through the lungs.
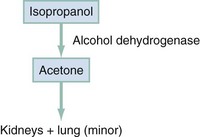
Figure 155-3 Metabolism of isopropanol.
Isopropanol blood levels peak 30 minutes to 3 hours after ingestion, and elimination follows first-order kinetics, with a half-life of 3 to 7 hours. In children, the half-life may be slightly shorter. Acetone is eliminated more slowly, with a half-life of 22 hours. The rate of elimination of isopropanol may be increased by chronic ethanol abuse and decreased by hepatic damage.42 A potentially lethal dose in adults is 150 to 240 mL (2-4 mL/kg), but adults have survived ingestions of 1 L (8-15 mL/kg).
Clinical Findings
Gastrointestinal and CNS complaints predominate clinically. Intoxication may be suggested by apparent inebriation with the odor of acetone rather than of ethanol detected on the breath. The patient may have headache or dizziness and may exhibit neuromuscular incoordination, confusion, and nystagmus. Severe ingestions may result in deep coma, which is prolonged compared with ethanol. Respiratory depression or failure may occur. Patients may have a loss of deep tendon, corneal, or protective airway reflexes and have an extensor response to plantar reflex testing. Pupillary size varies, but miosis is most common.43 Because isopropanol is a gastrointestinal irritant, the patient may complain of abdominal pain, nausea, and vomiting. Gastritis can occur with dermal and oral exposure.44 Hematemesis and upper gastrointestinal bleeding stem from gastritis but are rare.
Hypotension, although rare, signifies severe poisoning, with a mortality rate of 45%. One case series found mortality only when coma and hypotension were present.43 Sinus tachycardia is common, but other atrial and ventricular dysrhythmias are rare and generally found only with confounding hypoxia, acidosis, or shock. Hypothermia is frequent.43 Rarely, myoglobinuria, acute tubular necrosis, hepatic dysfunction, and hemolytic anemia have been reported. A distinguishing feature is that metabolic acidosis is not present with isopropanol intoxication unless it is accompanied by hypotension, gastrointestinal bleeding, or coingestants. Hyperglycemia may be noted, but hypoglycemia has not previously been described, even though it should be sought in patients who have altered mental status.
Diagnostic Strategies
Most of these patients do not need any laboratory testing unless they are severely ill. The laboratory tests primarily are used to exclude other ingestions and include serum electrolyte values, BUN and creatinine concentrations, osmolality, serum and urine ketones, and arterial blood gas analysis. Peak isopropanol levels occur soon after ingestion, whereas acetone levels peak later at 4 hours after ingestion.43 The most common laboratory abnormality is ketosis with little or no acidosis and normal blood glucose levels. The ketosis is from the metabolite acetone, which can be detected in the blood 15 minutes after ingestion and in the urine 3 hours after ingestion.43 Acetone is uncharged, so it does not elevate the anion gap. Isopropanol and acetone contribute to the increased osmol gap. In theory, an increase of 1 mg/dL in blood isopropyl alcohol concentration should result in an increase of 0.17 mOsm/kg in serum osmolality.
Differential Considerations
Patients who ingest significant amounts of isopropanol appear intoxicated or have depressed consciousness, and the differential diagnosis includes all of the causes of altered mental status in an alcoholic patient, as discussed previously. The presence of ketosis warrants a search for diabetic ketoacidosis, alcoholic ketoacidosis, salicylism, cyanide, or starvation ketosis. A distinguishing feature of isopropanol ingestion is the presence of ketosis without acidosis. An increased osmolal gap with acidosis should lead to a search for other toxic alcohols. In the setting of a high or rising creatinine concentration and normal BUN concentration, acute rhabdomyolysis also should be considered. Other substances that falsely elevate creatinine are cimetidine, nitroethane, and nitromethane, often mixed with methanol in formulating radio-controlled vehicle fuel.45 Detection of isopropanol in acetonemic patients not exposed to isopropanol (i.e., the conversion of acetone to isopropanol in vivo) is well described.
Management and Disposition
Neither gastric emptying nor activated charcoal administration is warranted. Intubation is indicated if the patient is unable to protect the airway; it may also be indicated on the basis of coingestants or if the patient’s mental status is poor and deteriorating. In contrast to methanol and ethylene glycol ingestion, ADH blockade with ethanol or fomepizole is not indicated. Hypotension should be managed with fluids and vasopressors as needed. If the patient remains hypotensive or has further vital sign deterioration despite these measures, dialysis is indicated. Some authors also recommend dialysis for isopropanol serum levels greater than 400 mg/dL. Coma itself is not an indication for dialysis but may necessitate mechanical ventilation. Peritoneal dialysis and hemodialysis have been successful, but hemodialysis is much more effective and is preferred. Because of isopropanol’s rapid absorption, patients who are hemodynamically stable without coma during the first 6 hours are at low risk for development of significant sequelae and generally do not require extracorporeal removal of isopropanol. The development of altered mental status within 2 hours is a clinical predictor of toxic blood isopropanol levels in children.46 Care is otherwise supportive and includes rewarming, evaluation for hypoglycemia, and monitoring for gastrointestinal bleeding.
References
1. Bennett, IL, Cary, FH, Mitchell, GL. Acute methyl alcohol poisoning: Review based on experiences of an outbreak of 323 cases. Medicine (Baltimore). 1953;32:431.
2. Wallace, EA, Green, AS. Methanol toxicity secondary to inhalant abuse in adult men. Clin Toxicol (Phila). 2009;47:239.
3. Martin-Amat, G, et al. Methanol poisoning: Ocular toxicity produced by formate. Toxicol Appl Pharmacol. 1978;45:201.
4. Liesivuori, J, Savolainen, H. Methanol and formic acid toxicity: Biochemical mechanisms. Pharmacol Toxicol. 1991;69:157.
5. Martinasevic, MK, et al. Folate and 10-formyltetrahydrofolate dehydrogenase in human and rat retina: Relation to methanol toxicity. Toxicol Appl Pharmacol. 1996;141:373.
6. Sandhir, R, Kaur, K. Influence of ethanol on methanol-induced oxidative stress and neurobehavioral deficits. J Biochem Mol Toxicol. 2006;20:247.
7. Skrzydlewska, E, Szmitkowski, M, Farbiszewski, R. Activity of cathepsin G, elastase, and their inhibitors in plasma during methanol intoxication. J Toxicol Environ Health. 1999;57:431.
8. Jacobsen, D, McMartin, KE. Methanol and ethylene glycol poisonings: Mechanism of toxicity, clinical course, diagnosis and treatment. Med Toxicol. 1986;1:309.
9. Jacobsen, D, McMartin, KE. Antidotes for methanol and ethylene glycol poisoning. J Toxicol Clin Toxicol. 1997;35:127.
10. Sanaei-Zadeh, H, Zamani, N, Shadnia, S. Outcomes of visual disturbances following methanol poisoning. Clin Toxicol (Phila). 2011;49:102.
11. Brent, J. Fomepizole for ethylene glycol and methanol poisoning. N Engl J Med. 2009;360:2216.
12. Hassanian-Moghaddam, H, Pajoumand, A, Dadgar, SM, Shadnia, SH. Prognostic factors in methanol poisoning. Hum Exp Toxicol. 2007;26:583.
13. Paasma, R, Hovda, KE, Tikkerberi, A, Jacobsen, D. Methanol mass poisoning in Estonia: Outbreak in 154 patients. Clin Toxicol (Phila). 2007;45:152.
14. Paasma, R, Hovda, KE, Jacobsen, D. Methanol poisoning and long term sequelae—a six years follow-up after a large methanol outbreak. BMC Clin Pharmacol. 2009;9:5.
15. Glaser, DS. Utility of the serum osmol gap in the diagnosis of methanol or ethylene glycol ingestion. Ann Emerg Med. 1996;27:343.
16. Hantson, P, Duprez, T, Mahieu, P. Neurotoxicity to the basal ganglia shown by magnetic resonance imaging (MRI) following poisoning by methanol and other substances. J Toxicol Clin Toxicol. 1997;35:151.
17. Jacobsen, D. New treatment for ethylene glycol poisoning. N Engl J Med. 1999;340:879.
18. Eder, AF, et al. Ethylene glycol poisoning: Toxicokinetic and analytical factors affecting laboratory diagnosis. Clin Chem. 1998;44:168.
19. Baud, FJ, et al. Treatment of ethylene glycol poisoning with intravenous 4-methylpyrazole. N Engl J Med. 1988;319:97.
20. McMartin, KE. Are calcium oxylate crystals involved in the mechanism of acute renal failure in ethylene glycol poisoning? Clin Toxicol (Phila). 2009;47:859.
21. Brent, J, et al. Fomepizole for the treatment of ethylene glycol poisoning. N Engl J Med. 1999;340:832.
22. Niederstadt, C, Lerche, L, Steinhoff, J. Proteinuria in ethylene glycol–induced acute renal failure. Nephron. 1996;73:316.
23. Sabeel, AI, Kurkaus, J, Lindholm, T. Intensified dialysis treatment of ethylene glycol intoxication. Scand J Urol Nephrol. 1995;29:125.
24. Reddy, NJ, Sudini, M, Lewis, LD. Delayed neurological sequelae from ethylene glycol, diethylene glycol and methanol poisonings. Clin Toxicol (Phila). 2010;48:967.
25. Tobe, TJM, Braam, GB, Meulenbelt, J, et al. Ethylene glycol poisoning mimicking Snow White. Lancet. 2002;359:444.
26. Wallace, KL, Suchard, JR, Curry, SC, et al. Diagnostic use of physicians’ detection of urine fluorescence in a simulated ingestion of sodium fluorescein–containing antifreeze. Ann Emerg Med. 2001;38:49.
27. Casavant, MJ, Shah, MN, Battels, R. Does fluorescent urine indicate antifreeze ingestion by children? Pediatrics. 2001;107:113.
28. Manini, AF, Hoffman, RS, McMartin, KE, Nelson, LS. Relationship between serum glycolate and falsely elevated lactate in severe ethylene glycol poisoning. J Anal Toxicol. 2009;33:174.
29. Caravati, EM, Erdman, AR, Christianson, G, et al. Ethylene glycol exposure: An evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila). 2005;43:327.
30. Chew, WB, et al. Alkali treatment of methyl alcohol poisoning. JAMA. 1946;130:61.
31. Brent, J. Fomepizole for the treatment of pediatric ethylene and diethylene glycol, butoxyethanol and methanol poisoning. Clin Toxicol (Phila). 2010;48:401.
32. McMartin, KE. Antidotes for alcohol and glycol toxicity: Translating mechanisms to treatment. Clin Pharmacol Ther. 2010;88:400.
33. Hovda, KE, Andersson, KS, Urdal, P, Jacobsen, D. Methanol and formate kinetics during treatment with fomepizole. Clin Toxicol (Phila). 2005;43:221.
34. Barceloux, DG, Krenzelok, EP, Olson, K, Watson, W. American Academy of Clinical Toxicology practice guidelines on the treatment of ethylene glycol poisoning. Ad Hoc Committee. J Toxicol Clin Toxicol. 1999;37:537.
35. Lepik, KJ, et al. Bradycardia and hypotension associated with fomepizole infusion during hemodialysis. Clin Toxicol (Phila). 2008;46:570.
36. Lepik, KJ, et al. Adverse drug events associated with the antidotes for methanol and ethylene glycol poisoning: A comparison of ethanol and fomepizole. Ann Emerg Med. 2009;53:439.
37. Barceloux, DG, Bond, R, Krenzelok, EP, Cooper, H, Vale, JA. American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning. J Toxicol Clin Toxicol. 2002;40:415.
38. Sivilotti, ML, Burns, MJ, McMartin, KE, Brent, J. Toxicokinetics of ethylene glycol during fomepizole therapy: Implications for management. Ann Emerg Med. 2000;36:114.
39. Mégarbane, B, et al. Treatment of acute methanol poisoning with fomepizole. Intensive Care Med. 2001;27:1370.
40. Porter, WH, Rutter, PW, Bush, B, Pappas, AA, Dunnington, JE. Ethylene glycol toxicity: The role of serum glycolic acid in hemodialysis. J Toxicol Clin Toxicol. 2001;39:607.
41. White, NC, et al. The impact of bittering agents on pediatric ingestions of antifreeze. Clin Pediatr (Phila). 2009;48:913.
42. Alexander, CB, McBay, AJ, Hudson, RP. Isopropanol and isopropanol deaths: Ten years’ experience. J Forensic Sci. 1982;27:541.
43. LaCouture, PG, et al. Acute isopropyl alcohol intoxication: Diagnosis and management. Am J Med. 1983;75:680.
44. Dyer, S, et al. Hemorrhagic gastritis from topical isopropanol exposure. Ann Pharmacother. 2002;36:1735.
45. Cook, MD, Clark, RF. Creatinine elevation associated with nitromethane exposure: A marker of potential methanol toxicity. J Emerg Med. 2007;33:249.
46. Stremski, E, Hennes, H. Accidental isopropanol ingestion in children. Pediatr Emerg Care. 2000;16:238.