Chapter 165 Total Facet Arthroplasty
All of these studies were approved by the U.S. Food and Drug Administration (FDA) as Investigational Device Exemptions (IDE) studies; however, three of the four studies are still in the preliminary recruiting phase, with minimal published outcome data. The midterm results of the Total Facet Arthroplasty System (TFAS, Archus Orthopedics, Redmond, WA) U.S. IDE trial were published more recently and were promising.1 Although the U.S. IDE trial for the Total Posterior-Element System (TOPS, Impliant, Ramat Poleg, Israel) has commenced only more recently, the potential efficacy and safety in the management of degenerative lumbar stenosis were recently underscored in a publication analyzing early clinical outcomes in 29 patients implanted with the device outside of the United States.2
Background
When surgical intervention was first advanced as a viable treatment option for lumbar degenerative disc disease by Dandy in 1929,3 relatively little was understood with regard to spinal biomechanics. Since that time, as understanding of the underlying biomechanics of the spine has evolved, so too has the application of these principles to the surgical management of various disorders of the spine. Nowhere is this application more apparent than in nonpenetrating, blunt force trauma to the spine, in which management of the resultant stereotyped injury patterns produced in response to externally applied forces has become more intuitive through the application of biomechanical principles derived from anatomic (cadaver) and computer-assisted models. The relevance of biomechanics is not limited to trauma because the application of these same principles can also contribute to restoration of anatomic balance after iatrogenic destabilization of the spine.
The early history of lumbar surgery often involved midline, transdural approaches to the disc space. As surgical technique evolved to extradural discectomy and decompression, the surrounding ligamentous and articular structures began to represent greater barriers to adequate operative visualization. The introduction of the surgical microscope to lumbar discectomy by Yaşargil4 and Caspar5 in 1977 served to lateralize and decrease the size of the working surgical field, magnifying further the intimate anatomic relationship between the disc space, lateral recess, and facet complex. As a compensatory maneuver, partial facetectomy was eventually incorporated into the surgical technique to improve both visualization and access to the compressed neural elements. The degree of facetectomy required to achieve decompression safely varies, depending on both surgeon experience and facet joint size and orientation. In a prospective, nonrandomized study, Çelik et al.6 showed that a facet angle of less than 35 degrees does not allow for a safe surgical corridor, resulting in the need for a more extensive facetectomy.
Over the past 20 years, the use of posterior instrumented fusion, primarily with pedicle screw fixation, has increased dramatically after iatrogenic destabilization of the facet joint in the context of surgical decompression for degenerative lumbar stenosis. In some cases, the facet complex represents the primary pathology, rather than a structural impediment to adequate decompression. This is typically seen in cases of severe facet arthropathy, resulting in significant lateral recess stenosis and nerve root impingement. The more recent, controversial concept of the degenerative facet joint as an independent pain generator has also contributed to the growing trend of combined aggressive facetectomy with posterior instrumented fusion as a common surgical practice.
Whatever the underlying pathology, it is generally accepted that rigid spinal fusion permanently alters both local and global spinal biomechanics. The resultant increased, compensatory range of motion (ROM) at neighboring spinal segments after instrumented fusion leads to accelerated rates of adjacent-level degeneration—25% to 40% over 5 years—often necessitating reoperation for decompression and extension of the fusion construct.7,8 In this context, the emergence of motion preservation strategies and dynamic stabilization has garnered increasing interest in the both the neurosurgical and orthopedic literature.
Although the concept of motion preservation is not a novel one, the potential restoration of near-normal lumbar kinematics via facet replacement systems is a relatively recent development. Approved by the FDA for single-level disc disease with associated mechanical back pain, the lumbar artificial disc (e.g., Charité, DePuy Spine, Raynham, MA; ProDisc, Synthes Spine, West Chester, PA) has steadily gained acceptance despite limited surgical indications (Fig. 165-1). In contrast to facet arthroplasty, lumbar disc replacement does not allow for direct posterior decompression of neural structures and can accelerate facet degeneration, potentially exacerbating facetogenic pain symptoms. For this reason, its use is contraindicated in patients with radiographic evidence of moderate to severe facet arthropathy.
Dorsal dynamic stabilization (posterior dynamic stabilization [PDS]) devices are placed via a traditional posterior approach, allowing for direct surgical decompression before implantation. Early devices, such as the Graf ligament, were initially popular, but their popularity declined because of poor mechanical wear and issues with elastomeric material properties (Fig. 165-2). Khoueir et al.9 described a useful classification scheme for PDS devices consisting of (1) interspinous spacer devices (i.e., X STOP, Medtronic, Minneapolis, MN; DIAM, Medtronic; Coflex, Paradigm Spine, New York, NY), (2) pedicle screw and rod–based devices (i.e., Dynesys, Zimmer Spine, Minneapolis, MN; Accuflex rod, Globus Medical, Audobon, PA; Isobar, Alphatec Spine, Carlsbad, CA), and (3) total facet replacement systems (i.e., TFAS, TOPS). Although more conventional pedicle screw and rod–based PDS devices were designed to reduce facet loads and preserve intersegmental kinematics, in vitro biomechanical studies have yielded mixed results. Niosi et al.10 reported significantly increased peak facet contact forces in flexion-extension and lateral bending in cadaver human spines implanted with the Dynesys system. In theory, a facet arthroplasty system more accurately mirrors the anatomic facet joint, potentially restoring the intrinsic load-sharing properties of the intact facet complex.
Facet joint surgeries to treat the synovial surfaces without replacing the joint itself are being investigated as well. These joint “resurfacing” technologies are in their infancy, holding the promise of treating a dysfunctional synovial joint through less invasive approaches (Fig. 165-3). Because of the lack of scientific data on these devices, they are not discussed further in this chapter.
Facet Biomechanics
Because the biomechanics of the facet joints is rarely discussed, a brief review of facet kinematics in the context of their contribution to the functional spine unit (FSU) is appropriate. The FSU refers to the three-joint structural arrangement of a single spinal level and consists of the intervertebral disc, the two facet joints and their investing capsule, and the associated posterior musculoligamentous supporting structures. The vertebrae articulate with one another via the two diarthrodial encapsulated facet joints at the superior and inferior aspects of the pars interarticularis. By virtue of their bilateral and posterior location, the facets come into maximal contact with one another during extension and axial rotation, contributing more to overall load sharing under these conditions. Generally, the orientation of the facet joint changes at differing locations throughout the spine, from a more coronal orientation with approximately 45 degrees of inclination from the horizontal in the cervical spine to a more sagittal orientation in the lumbar spine.11 These segment-specific differences in facet geometry and characteristics serve to impart differing patterns of movement at different spinal levels.
By virtue of their more sagittal orientation, the lumbar facet joints serve to provide greater resistance to axial rotation and significantly contribute to axial load bearing, in particular, with the spine in extension.11–14 The articular surface area also increases from L1 to S1, mirroring the greater shear loads in the lower spine relative to the upper lumbar spine.14 The application of eloquent computer-generated three-dimensional finite element analyses coupled with biomechanical data obtained from related in vitro human cadaver studies has furthered understanding of lumbosacral facet kinematics in response to iatrogenic destabilization. As anticipated, models replicating bilateral laminectomy and total facetectomy have shown marked increases in the angular ROM of the lumbar motion segment, under flexion-extension and axial rotation.12,15–18 However, the relative preservation of angular ROM in the context of lateral bending underscores the greater role of the anterior column in load bearing during lateral bending.19,20 Facet biomechanics also differ between individuals, by spinal level and laterality, making their biomechanics joint-specific.
Facetogenic Pain Syndrome
Although controversial, the concept of the facet joint as a potential pain generator has gained support in recent years. Hirsch et al.21 first raised the possibility in 1963, when they reported the production of low back pain in subjects whose facet joints were injected with hypertonic saline. Since that time, various accounts in the literature have served both to validate and to refute this theory. Some accounts report a 50% to 60% success rate with facet blocks and rhizolysis procedures,22 whereas others report a similar efficacy of pain relief in cases in which facet blocks were conducted with normal saline, suggesting a significant placebo effect.23 Central to the theory of facetogenic pain syndrome is the innervation of the facet joint, which is derived from the medial branches of the dorsal rami originating at the same level and the cephalad spinal level.24 The joint capsule itself is innervated by numerous mechanoreceptors, which can undergo extensive stretch under conditions of physiologic loading.
In their neuroanatomic and neurophysiologic analysis of the facet joint, Cavanaugh et al.25 concluded that these nerves are activated by capsular stretch and by neurogenic and non-neurogenic modulators of inflammation, including substance P, bradykinin, and phospholipase A2. This interplay of mechanical stretch and local inflammatory mediators may serve to propagate the cycle of chronic low back pain in a subset of patients with lumbago. In these patients, total facetectomy followed by facet arthroplasty may represent a viable alternative to rigid arthrodesis. Because facet arthropathy is often encountered concomitantly with advanced disc disease, replacement of the entire three-joint FSU with combination disc and facet arthroplasty may also ultimately emerge as a future surgical treatment option in patients with severe lumbar degenerative disease.
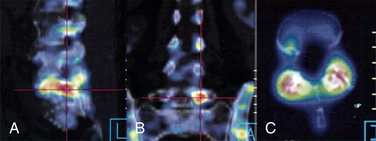
Facetogenic pain can be diagnosed with imaging studies as well. Test injections, whether intra-articular or periarticular, can be useful for diagnosing facet pain. In addition, nuclear medicine bone scans showing “hot” facets can be useful for identifying inflamed synovial joints (Fig. 165-4).26,27
Facet Arthroplasty Devices
At the present time, four TFA devices have progressed to clinical trials: TFAS, TOPS, AFRS, and Stabilimax NZ. Although all of these systems are similar in motion preservation properties and surgical technique for implantation, they are vastly different with regard to their respective design technology and build material. To date, most published clinical outcome data have come from the TFAS U.S. IDE trial,1 although limited clinical accounts regarding the relative efficacy and safety of the TOPS device in patients undergoing implantation outside of the United States can be found in the international spine literature.2
Total Facet Arthroplasty System
Implant Characteristics
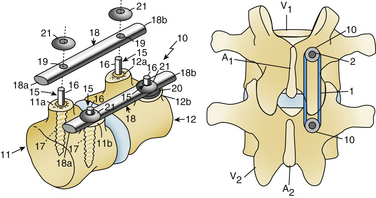
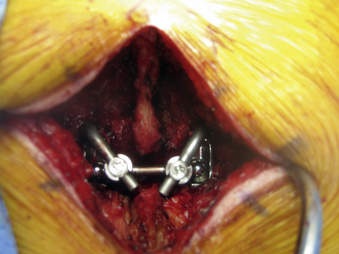
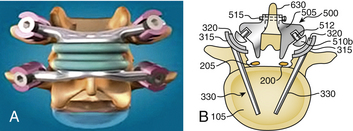
The TFAS device consists of two rostral spherical bearings attached to stems implanted into the rostral pedicles, which articulate with two proportionately sized caudal bearing surfaces, affixed to the pedicles below (Fig. 165-5). The geometry of the caudal bearing surfaces is designed to provide a gradual resistance to motion, in an attempt to recapitulate the diarthrodial facet kinematics of the intact spinal segment.15,16,18 Composed entirely of implantable grade metals, the TFAS device is placed via a standard open posterior surgical approach, following decompressive laminectomy and facetectomy. The implant-pedicle interface is augmented with polymethylmethacrylate, similar to the TOPS device, which uses hydroxyapatite to fortify the construct.2,18
Biomechanical Data
Several in vitro biomechanical studies analyzing linear and angular ROM in human cadaver lumbar spines under intact, injured (bilateral laminectomy and total facetectomy), and implanted (with TFAS) conditions, all have served to show a relative conservation of intact spinal biomechanics at implanted and adjacent levels in iatrogenically destabilized specimens.14–1618 Phillips et al.15 assessed multidirectional flexibility in nine human cadaver spines under various conditions including intact, after L3-4 laminectomy and facetectomy (injured), after L3-4 pedicle screw fixation, and after stabilization with L3-4 TFAS implantation. The injured and rigidly stabilized models had increased (injured model) and decreased (rigidly stabilized model) flexion-extension, lateral bending, and axial rotation ROM at the intervened level, whereas TFAS implantation restored intact ROM in all directions and resulted in near-normal load displacement curves.15 Additionally, the associated increase in ROM observed at adjacent spinal levels after L3-4 pedicle screw fixation was essentially restored to intact values after implantation of the TFAS device. Similar results have been published using in vitro models at L4-5 and L5-S1 and three-dimensional finite element analyses of the lumbosacral spine.14,18,28
Clinical Outcome Data
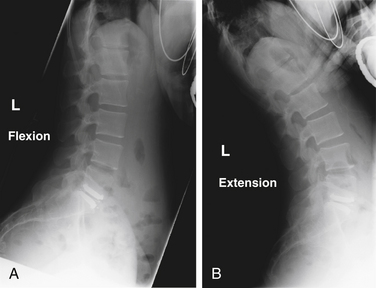
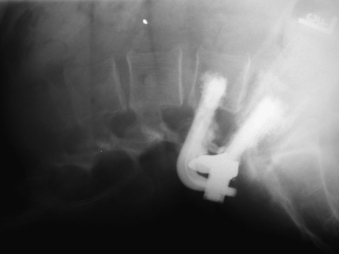
The midterm results of the TFAS U.S. IDE trial for the management of degenerative lumbar stenosis were released more recently; the longest clinical follow-up was 24 months.1 This multicenter, prospective, randomized clinical trial with a concurrent surgical (pedicle screw fixation) control was designed to compare clinical outcomes between the two surgical cohorts as a primary end point, with relative safety and radiographic evaluation of ROM as important secondary end points. Of the 104 patients enrolled in the study to date, 96 had undergone TFAS implantation, whereas only 8 patients were randomly assigned to the instrumented fusion cohort. With regard to symptomatic improvement, 84% of the TFAS patients showed significant improvement in the Zurich Claudication Questionnaire (ZCQ) symptom scores, and 81% showed significant improvement in the ZCQ function scores. Visual analogue scale scores for leg and back pain improved in 95% and 85% of TFAS patients. Radiographic analysis revealed all implanted devices to be intact and functioning, with preserved ROM at the implanted level (Fig. 165-6). No device-specific complications were encountered. Although the preliminary results of this clinical trial suggest that the comparative efficacy and relative device safety of the TFAS implant in the management of degenerative lumbar stenosis appear to be at least equal to pedicle screw fixation, the data must be interpreted with caution given the small number of patients enrolled in the control group.
Total Posterior Element System
Implant Characteristics
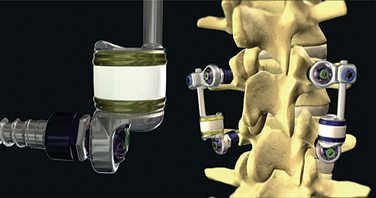
In contrast to the diarthrodial, multicomponent structure of the TFAS implant, the TOPS device is unitary in design, composed of a titanium construct with an interlocking, flexible, articulating core, surrounded by a polyurethane elastomer cover that is capable of transmitting tensile and compressive loads, in addition to shear forces (Fig. 165-7). Surgical technique involves a standard open posterior approach for complete laminectomy and facetectomy, placement of cannulated polyaxial pedicle screws under direct fluoroscopic guidance, and attachment of the unitary TOPS implant to the pedicle screw heads with standard locking set-screw caps, which are ultimately counter-torqued to their final tightness. Finally, a small amount of sterile saline is injected through a port in the bottom of the implant to serve as a lubricant.
Biomechanical Data
Compared with the TFAS implant, fewer biomechanical data exist in the literature to support the TOPS device as a viable option for PDS in the surgical management of degenerative lumbar stenosis. The largest study, an in vitro analysis of six human cadaver spines implanted with the TOPS device at L4-5 after bilateral laminectomy and total facetectomy, showed restoration of near-normal motion behavior to the implanted spinal level in left and right lateral bending and axial rotation.17 ROM in flexion-extension was 85% of that seen in an intact segment, which is higher than published results for both Dynesys and TFAS.10,18 The spines implanted with the TOPS device did not show any significant increase in mobility at the adjacent spinal levels. Intradiscal pressure monitoring revealed a significant reduction in intradiscal forces at the implanted level, while still allowing the disc to participate in near-normal load sharing.17
Clinical Outcome Data
McAfee et al.2 reported preliminary results of a prospective, multicenter, nonrandomized, clinical trial of 29 patients with degenerative lumbar stenosis implanted with the TOPS device, with longest clinical follow-up of 1 year. In this international trial, all 29 patients underwent surgery outside of the United States, with 28 of 29 having the device implanted at the L4-5 level. As a whole, the patients’ clinical status seemed to improve after surgery. Of the 11 patients with 1-year follow-up, the mean Oswestry Disability Index score decreased by 41%, the mean visual analogue scale leg pain score decreased by 86%, and the mean ZCQ score decreased by 54%.2 Radiographic analysis showed that both lumbar ROM and disc height at the implanted and adjacent levels were preserved at 1-year follow-up. Device-specific adverse events, including radiographic evidence of screw pull-out or device failure, were not observed during the brief follow-up period.
ACADIA Facet Replacement System and Stabilimax DZ
Implant Characteristics
The Stabilimax NZ implant is similarly anchored in place with pedicle screw fixation; however, motion preservation is achieved through bilateral independent concentric springs incorporated via connecting rods (Fig. 165-8). Panjabi and Timm29 tested 70 bilateral assemblies of the Stabilimax NZ implant, which exceeded static, fatigue, wear resistance, and histologic requirements, resulting in permission to initiate an IDE trial for efficacy in the surgical management of degenerative lumbar stenosis. Although the IDE trials for both the AFRS and the Stabilimax NZ systems are in their relative infancy, preliminary results presented at national spine meetings have indicated safety and efficacy comparable to the other facet replacement systems.
Butler J., Ferrara L.A., Benzel E.C. Basic biomechanically relevant anatomy. Benzel E.C., editor. Spine surgery: techniques, complication avoidance, and management. Philadelphia: Saunders. 2005;vol 2:1397-1410.
Cavanaugh J.M., Ozaktay A.C., Yamashita H.T., et al. Lumbar facet pain: biomechanics, neuroanatomy, and neurophysiology. J Biomech. 1996;29:1117-1129.
Lee K.K., Teo E.C. Effects of laminectomy and facetectomy on the stability of the lumbar motion segment. Med Eng Physics. 2004;26:183-192.
Panjabi M.M., Oxland T., Takata K., et al. Articular facets of the human spine: quantitative three-dimensional anatomy. Spine (Phila Pa 1976). 1993;18:1298-1310.
Schultz A.B., Warwick D.N., Berkson M.H., et al. Mechanical properties of human lumbar spine motion segments—responses in flexion, extension, lateral bending, and torsion. J Biomech Eng. 1979;101:46-52.
Serhan H.A., Varnavas G., Dooris A.P., et al. Biomechanics of the posterior lumbar articulating elements. Neurosurg Focus. 2007;22:1E1.
1. Sachs B., Webb S., Brown C., et al. The total facet arthroplasty system (TFAS®) in the treatment of degenerative lumbar spinal stenosis: midterm results of US IDE trial with longest follow-up of 24-months. Spine J. 2008;8:60S-61S.
2. McAfee P., Khoo L.T., Pimenta L., et al. Treatment of lumbar spinal stenosis with a total posterior arthroplasty prosthesis: implant description, surgical technique, and a prospective report on 29 patients. Neurosurg Focus. 2007;22:E13.
3. Dandy W.E. Loose cartilage from the intervertebral disc simulating tumor of the spinal cord. Arch Surg. 1989;19:660-672.
4. Yaşargil M.G. Microsurgical operations for herniated lumbar disc. Adv Neurosurg. 1977;4:81-82.
5. Caspar W. A new surgical procedure for lumbar disc herniation causing less tissue damage through a microsurgical approach. Adv Neurosurg. 1977;4:74-80.
6. Çelik S.E., Ҫelik S., Kara A., et al. Lumbar facet joint angle and its importance on joint violation in lumbar microdiscectomy. Neurosurgery. 2008;62:168-172.
7. Ghiselli G., Wang J.C., Bhatia N.N., et al. Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg [Am]. 2004;86:1497-1503.
8. Lee C.K. Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine (Phila Pa 1976). 1988;13:375-377.
9. Khoueir P., Kim K.A., Wang M.Y. Classification of posterior dynamic stabilization devices. Neurosurg Focus. 2007;22:E3.
10. Niosi C.A., Wilson D.C., Zhu Q., et al. The effect of dynamic posterior stabilization on facet joint contact forces. Spine (Phila Pa 1976). 2008;33:19-26.
11. Butler J., Ferrara L.A., Benzel E.C. Basic biomechanically relevant anatomy. Benzel E.C., editor. Spine surgery: techniques, complication avoidance, and management. Philadelphia: Saunders. 2005;vol. 2:1397-1410.
12. Lee K.K., Teo E.C. Effects of laminectomy and facetectomy on the stability of the lumbar motion segment. Med Eng Physics. 2004;26:183-192.
13. Panjabi M.M., Oxland T., Takata K., et al. Articular facets of the human spine: quantitative three-dimensional anatomy. Spine (Phila Pa 1976). 1993;18:1298-1310.
14. Serhan H.A., Varnavas G., Dooris A.P., et al. Biomechanics of the posterior lumbar articulating elements. Neurosurg Focus. 2007;22:1E1.
15. Phillips F.M., Tzermiadianos M.N., Voronov L.I., et al. Effect of total facet arthroplasty system after complete laminectomy-facetectomy on the biomechanics of implanted and adjacent segments. Spine J. 2009;9:96-102.
16. Voronov L.I., Havey R.M., Rosler D.M., et al. L5-S1 segmental kinematics after facet arthroplasty. SAS J. 2009;3:50-58.
17. Wilke H.J., Schmidt H., Werner K., et al. Biomechanical evaluation of a new total posterior-element replacement system. Spine (Phila Pa 1976). 2006;31:2790-2796.
18. Zhu Q., Larson C.R., Sjovold S.G., et al. Biomechanical evaluation of the total facet arthroplasty system. Spine (Phila Pa 1976). 2007;32:55-62.
19. Schultz A.B., Warwick D.N., Berkson M.H., et al. Mechanical properties of human lumbar spine motion segments—responses in flexion, extension, lateral bending, and torsion. J Biomech Eng. 1979;101:46-52.
20. Shirazi-Adl A. Finite-element evaluation of contact loads on facets on and L2-3 lumbar segment in complex loads. Spine (Phila Pa 1976). 1991;16:533-540.
21. Hirsch C., Ingelmark B.E., Miller M. The anatomical basis for low back pain. Acta Orthop Scand. 1963;33:1-17.
22. Helbig T., Lee C.K. The lumbar facet syndrome. Spine (Phila Pa 1976). 1988;13:61-64.
23. Lilius G., Laasonen E.M., Myllynen P., et al. Lumbar facet joint syndrome: a randomized clinical trial. J Bone Joint Surg [Br]. 1989;71:681-684.
24. Bogduk N. The laminectomy of the lumbar spine. Spine (Phila Pa 1976). 1983;8:286-293.
25. Cavanaugh J.M., Ozaktay A.C., Yamashita H.T., et al. Lumbar facet pain: biomechanics, neuroanatomy, and neurophysiology. J Biomech. 1996;29:1117-1129.
26. Kim K.A., Wang M.Y. MRI-based morphologic predictors of SPECT positive facet arthropathy in patients with axial back pain. Neurosurgery. 2006;59:147-156.
27. McDonald M., Cooper R., Wang M.Y. Use of computed tomography-single-photon emission computed tomography fusion for diagnosing painful facet arthropathy. Neurosurg Focus. 2007;22:E2.
28. Bowden A., Villarraga M. In situ biomechanics of total facet replacement using finite element analysis. Spine J. 2006;6:70S-71S.
29. Panjabi M.M., Timm J.P. Development of Stabilimax NZ from biomechanical principles. SAS J. 2007;1:2-7.