CHAPTER 65 Topectomy and Multiple Subpial Transection
Topectomy
The prevalence of epilepsy is approximately 1%. In the majority of cases, it can be effectively treated medically with antiepileptic drugs, but 30% to 40% of all new epilepsy patients will ultimately become refractory to treatment.1 Some investigators report that ongoing medication trials can be a satisfactory approach to managing refractory epilepsy.2 However, it is important to consider surgery for such patients.3
In the Montreal Neurological Institute series covering the period from 1929 to 1980, the anatomic distribution of surgical resection in 2177 patients was as follows: temporal, 56%; frontal, 18%; central, 7%; parietal, 6%; occipital, 1%; and multilobar, 11%.4
“Topectomy” generally indicates resection of focal cerebral cortex from the frontal, parietal, or occipital lobe. Outside the temporal lobe, localization of the seizure origin is much more difficult, seizure outcome is less favorable, and surgery in some brain sites is associated with higher risk for major morbidity than with temporal lobe resection.5 Although temporal lobe resection is much more common in modern epilepsy practice, topectomy is the foundation of modern epilepsy surgery.6 In 1886, Sir Victor Horsley performed what would now be considered lesionectomies with excellent results. Before the invention of electroencephalography (EEG) in 1928, epilepsy surgery was exploratory and based on clinical localization pioneered by Jackson and identification of visibly abnormal tissue. EEG removed this requirement and decreased negative explorations considerably through the 1950s and 1960s.7 Magnetic resonance imaging (MRI) now provides a priori knowledge of structural cortical abnormalities. Table 65-1 shows types of procedures for epilepsy.
A fundamental concept underlying focal cortical resection is that the epileptic focus contains electrically abnormal neurons that are the source of seizures. Hence, if this epileptogenic focus can be accurately identified and resected, the seizure will cease. Epileptogenicity is assumed to derive from physiologic events that occur within the cell body and dendrites of neurons rather than axons.8 Therefore, resection of only epileptogenic gray matter (cerebral cortex) rather than white matter should in theory be sufficient to eliminate seizures. Recent work suggests that epilepsy may arise as a result of abnormalities of a distributed cellular network.9 Therefore, intrinsic cellular abnormalities may not be required to create an epileptic condition. The most characteristic and ubiquitous pathologic changes are the presence of gliosis, loss of neurons, and dendritic abnormalities.10,11 This chapter reviews the principles and methods of surgical localization and cortical resection in patients who may be candidates for topectomy or multiple subpial transection (MST).
Patient Selection
In general, surgical treatment of epilepsy should be considered when (1) the seizures have not been controlled by adequate attempts at treatment with maximally tolerable doses of correct anticonvulsant medications; (2) the seizures interfere with psychological and intellectual development, employment, or social performance; (3) all potentially epileptogenic areas have matured, the seizure tendency (pattern and frequency) is stable, and there is no tendency toward spontaneous regression; and (4) the patient must be strongly motivated to cope with an exhaustive diagnostic regimen and a lengthy operative procedure, possibly under local anesthesia.12–14 Chronic psychosis is a contraindication to surgical treatment, but epilepsy-related acute psychosis is not. Mental retardation is not a contraindication.15,16
The length of time that patients have seizures before being referred for surgery has become shorter as referring physicians recognize that superior surgical results may be obtained when the period of uncontrolled seizures is shortened, particularly in children.17 Currently, adequate, but unsuccessful antiepileptic drug therapy for 1 to 5 years may be a sufficiently long trial period to warrant referral for consideration of epilepsy surgery.16 In exceptional cases, urgent cortical resection may be considered for the relief of status epilepticus.18,19
Table 65-2 is a representative list of neurological diagnoses that may be considered for surgery involving focal cortical resection.20 Topectomy can be considered when the seizure arises from a focal and functionally silent area of the brain, which usually means that the focus is not in the speech or motor cortex. Additionally, neuropsychological testing is performed to identify any functional deficits that may be related to the epileptic cortical region.21 Multiple widespread or bilateral foci are generally contraindications to resective surgery. A series of diagnostic studies, including EEG, are necessary to confirm the site of seizure onset. In eloquent brain regions, MST may be considered.
Presurgical Evaluation
The optimal surgery for epilepsy is resection of just enough neuronal tissue to eliminate the patient’s seizures without causing unacceptable neurological deficits. Presurgical evaluation enables the surgeon to determine the volume of cortex involved in seizure initiation and propagation and whether it can be safely resected. As stated by Rasmussen, epileptogenic lesions outside the temporal lobe are considerably more varied in extent and geographic configuration than the more common temporal lobe epileptogenic lesions.22 A variety of diagnostic tests are used for this purpose, but there is no consensus on how much information is actually needed before a particular surgical intervention can be recommended.23
The initial preoperative evaluation process includes careful examination of clinical seizure characteristics, ictal and interictal scalp EEG, neuroimaging, and neuropsychological testing. The results of this testing should provide considerable lateralizing and localizing information. Video-EEG monitoring is the “gold standard” of the evaluation. When noninvasive tests do not show the source of seizures clearly, invasive testing is considered. Localization of the seizure focus is difficult in patients with extratemporal epilepsy, and invasive EEG is almost always required.5
Noninvasive Evaluation
Symptomatic Localization
In frontal lobe epilepsy, clinical localization of the seizure origin is difficult because the ictal behavioral manifestations in patients with frontal lobe epilepsy are largely due to spread of seizures to neighboring or distant functionally connected brain regions.24 Rasmussen characterized six distinct convulsion patterns, some of which he believed indicated a frontal lobe focus:25,26
In parietal lobe seizure, most patients have attacks consisting of unilateral motor or sensory phenomena with additional features such as dizziness, cephalic sensation, contraversion, perceptual illusions, informed visual hallucinations, mental confusion, epigastric sensation, dysphasia, or automatisms.26,27
In central epilepsies, the attacks are somatomotor or somatosensory. The attacks sometimes remain localized, but in most patients a certain portion of the attacks progress to generalized convulsive seizures. Epilepsia partialis continua is particularly common.26,27
In occipital lobe seizure, visual symptoms such as transient blindness or visual hallucinations are usually characterized by flashes of lights, colored balls, and other geometric patterns. These symptoms have a strong relationship with seizure activity.26
However, some initial seizure symptoms may not reflect the region of seizure origin because many cortical regions are clinically silent. Seizures originating in these regions produce signs and symptoms only after spreading outside the epileptogenic region.27
Extracranial Electroencephalography
Scalp EEG often provides important information about the location and size of the epileptogenic area. An interictal epileptiform abnormality consisting of spikes, spike and slow wave complexes, sharp waves, and sharp and slow wave complexes repeated on several occasions may be particularly informative.28 Activation procedures such as withdrawal of medication, hyperventilation, or drug-induced sleep may enhance abnormalities or even provoke a seizure.13
In addition, long-term video-EEG monitoring is generally performed in an epilepsy monitoring unit to correlate clinical seizure activity with the accompanying electrical abnormalities with the intent of identifying the seizure origin electrographically.5 The specific ictal symptomatology can help greatly in some cases in which localization of the focus is problematic. The EEG pattern of seizure onset is most frequently rhythmic, fast, spike-like discharges, often building in amplitude.9
Neuroimaging
MRI is the imaging modality of choice in patients with intractable partial epilepsy. Computed tomography (CT) is also helpful because it demonstrates intracranial calcifications more clearly than MRI does. MRI provides visualization of focal dysplastic cortical lesions in many patients previously assigned a diagnosis of cryptogenic neocortical epilepsy.5,29 An MRI-identified lesion may prove to be the cause of the seizure disorder. These abnormalities are then correlated with the results of electrophysiologic studies to accurately identify the source of the patient’s seizures.30 MRI is also useful in the diagnosis of structural and developmental epileptogenic pathologies. The most common findings in this category are ischemic lesions or developmental abnormalities, specifically, neuronal migration disorders.31 Structural lesions include ischemic infarctions (prenatal, perinatal, or postnatal), Sturge-Weber syndrome, Rasmussen’s encephalitis, and others. Progressive unilateral cortical atrophy is demonstrated in patients with Rasmussen’s encephalitis. Unilateral megalencephaly is one characteristic example of a developmental pathology associated with seizures. The most common neuronal migration disorders are lissencephaly and diffuse pachygyria. The complete absence of gyri and sulci in lissencephaly and the development of a few thick, wide gyri separated by shallow sulci in diffuse pachygyria are clearly identifiable on MRI.31
Ictal perfusion single-photon emission computed tomography (SPECT) may demonstrate increased blood flow at the site of seizure onset.15,32–34 The tracer isotope (usually technetium 99m–labeled hexamethyl-propyleneamine-oxime [HMPAO]) is administered immediately after seizure onset, and the scan should be performed within several hours. There are some reports of localized hyperperfusion in ictal studies of patients with frontal, parietal, or occipital epilepsy.15,16 Interictal SPECT has been of only limited value; it occasionally shows local reduced blood flow.33 Timing demands and other technical issues associated with SPECT scanning present barriers to its use. There is some evidence that functional anatomic correlation is more reliable with a technique called SISCOM, an acronym for subtraction ictal single-photon tomography coregistered to MRI.35
Interictal positron emission tomography (PET) with a tracer for glucose metabolism (18F-fluorodeoxyglucose) has regularly demonstrated hypometabolism in the epileptic focus of temporal lobe epilepsy.15,36 However, PET is less reliable in identifying extratemporal, nonlesional foci.29
Neuropsychological Testing
Neuropsychological examinations contain a personality inventory and tests of memory, language function, and intelligence. These tests are useful in localization and lateralization of speech, memory, spatial, cognitive, and cerebral dominance functions. They also evaluate mental states such as mental retardation.29,37 These traditional roles of neuropsychological evaluation are now augmented by the discipline’s ability to predict cognitive and behavioral outcomes after surgery.22 Additionally, neuropsychology can have therapeutic benefit as well,38 and it can be combined with advanced imaging techniques to make highly specific predictions about cognitive function after epilepsy resection.39
Invasive Evaluation
The Wada Test
The Wada test (intracarotid amobarbital [Amytal] test) is performed to identify the dominant hemisphere for language functions and to determine the degree to which memory functions are subserved by each hemisphere.40 In the series from the Montreal Neurological Institute, in right-handers, 96% had speech lateralized to the left cerebral hemisphere and 4% to the right hemisphere. In left-handed or ambidextrous individuals 70% had speech on the left, 15% had it on the right, and 15% had some representation of speech in each hemisphere. Therefore, the Wada test is necessary in all left-handed and ambidextrous patients and in right-handed patients with some ambiguous evidence of lateralization from psychological tests, x-ray films, EEG studies, or the seizure pattern.13 Unusual language lateralization can be seen in patients who sustain left hemisphere injury in early life or have early onset of intractable epilepsy.15 Recently, work has been done to supplant the invasive Wada test with functional MRI (fMRI).41,42 Although fMRI has some cost and risk advantage,43 the diagnostic accuracy of the technique is insufficient to completely supplant the WADA test at this time.
Invasive Electroencephalography
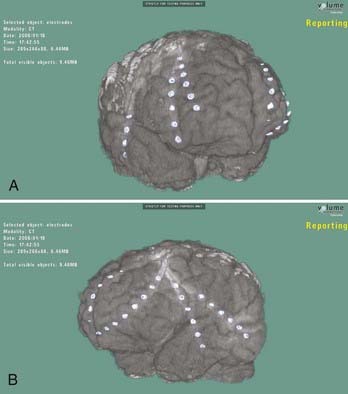
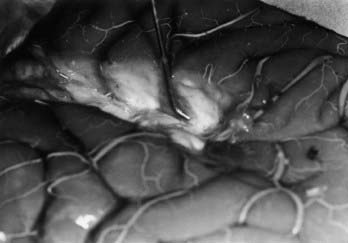
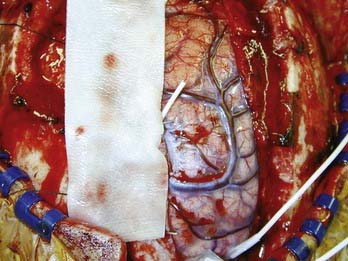
When an epileptogenic focus is not clearly lateralized, invasive EEG monitoring is necessary for cortical resection. In extratemporal epilepsy, subdural strip electrodes are used primarily to lateralize an epileptogenic focus (Fig. 65-1), and large subdural grids are used to define the limits of a focus that has already been lateralized but not sufficiently localized (Fig. 65-2). It is important to cover as much of the suspected area as possible for accurate lateralization and localization.29,44 In our center, we routinely identify electrode locations in image space by coregistering postoperative MRI and CT images.45 If the electrodes are properly indexed in the image, an electroradiographic record of the eloquent and epileptogenic regions can be obtained and used as a navigational tool during resection.
FIGURE 65-2 Cingular depth electrodes from the same patient as in Figure 65-1. The patient had intractable frontal lobe epilepsy. The goal was to determine from which side the seizures arose.
Subdural strip electrodes are placed through a bur hole and passed blindly into the subdural space. Multiple electrodes may be inserted through one bur hole to cover wide regions of the brain. In patients with bifrontal discharges, strip electrodes are placed over the medial and lateral surfaces of the posterior frontal lobe from bur holes at the coronal suture, just off the midline (see Fig. 65-1).15
Subdural grid electrodes are placed with a craniotomy (see Fig. 65-2). They are used not only for determining the site of seizure onset over the convexity of one hemisphere but also for extraoperative functional mapping (motor, sensory, speech, and so on) by stimulation of each electrode. The maximal extent of an epileptogenic focus and areas of cortical function are determined with these evaluation methods.4 With invasive EEG methods, the electrode leads are brought out through the scalp and the patient is monitored for many days. The most common complications are infection and leakage of cerebrospinal fluid, especially with a large subdural grid.42
Surgical Topectomy Procedure
General Principles from Anatomic Considerations
Anatomically, the frontal lobe Broca speech area is identified in the opercular, inferior frontal gyrus (usually the posterior 2.5 cm of this gyrus). It is difficult to identify Wernicke’s area by anatomic criteria. The parietal speech area is identified 1 to 4 cm above the sylvian fissure and 2 to 4 cm behind the postcentral sulcus. The temporal speech areas usually extend posteriorly behind the level of the postcentral sulcus and 2 to 3 cm from the adjacent convolution above, behind Heschl’s gyri. Lack of defining anatomic features for Wernicke’s area renders language mapping essential for cortical dominant hemisphere resections.13,14,46
Large frontal resections in the nondominant hemisphere can be carried out in front of the precentral gyrus. Identification of the precentral and postcentral gyri is best accomplished by stimulation under anesthesia without neuromuscular blockade.4,27 It can also be done more coarsely by identifying the somatosensory evoked potential (SSEP) phase reversal over the central sulcus.47 Resection of precentral arm or leg motor cortex is permitted only if significant contralateral paresis is already present.15,47 The lower precentral face area can be resected. The resulting contralateral facial paresis improves but may not return to normal.27
Resection of the postcentral sensory arm or leg area causes a profound proprioceptive deficit and is rarely indicated, although improvement over a period of several months is possible.27,47 Conversely, the lower 2.5 to 3 cm of the postcentral face area can be resected without significant deficit as long as the resection does not extend into the underlying white matter.27 In the nondominant hemisphere, the entire parietal cortex posterior to the postcentral gyrus can be removed without inducing a sensorimotor deficit.27,47,48 Resection in the parietal operculum may produce contralateral lower quadrantic hemianopia if resections are carried beyond the depths of the sulci into the white matter.47 In the dominant hemisphere, parietal lobe resections should be limited to the superior parietal lobule. Language functions are subserved by cortex of the inferior parietal lobule, and a disabling Gerstmann syndrome can also result from extensive parietal lobe resection.27
Large resections of occipital cortex produce a contralateral homonymous hemianopia. Therefore, if vision is intact preoperatively, the calcarine cortex and optic radiations are spared as much as possible. Because cortex essential for reading is often more widespread than that for naming, excision within 2 cm of Wernicke’s area may cause a persisting dyslexia.47
The vascular territory of each crucial artery or vein should be studied to assess the consequences of occlusion of the vessel during surgery. This approach is essential to minimize morbidity, especially with surgery on the motor and speech areas. Any ascending vein to the superior sagittal sinus draining from the central or postcentral sulci should be left intact to avoid significant morbidity.4,27
Preoperative Care and Anesthesia
It is our practice to reduce the doses of antiepileptic medications the week before surgery so that the epileptogenic cortex is as active as possible during surgery.13 Some epilepsy centers do not use this strategy, particularly in situations in which they will be carrying out awake craniotomies. When resecting noneloquent cortical areas, general anesthesia can be used.27 However, when intraoperative electrocorticography (ECoG) is required, the use of drugs that depress cortical electrical activity, such as benzodiazepines and barbiturates, should be avoided. In addition, when functional mapping of speech and sensory areas is performed, the patient should be conscious and cooperative during the procedure. In this situation, local and total intravenous anesthesia with analgesic drugs (fentanyl and droperidol or propofol) should be used.14,49 Local anesthesia alone has the disadvantages of taking more time to create a complete block and limiting the range of head positions that can be used. Furthermore, it cannot be used with uncooperative patients and young children. Constant supervision by a specially trained anesthesia team is essential.4,27
Intraoperative Electrocorticography
Sufficient brain exposure via craniotomy is essential during ECoG. ECoG is performed to further delineate the extent of the epileptogenic zone. The intention is to identify regions with primary epileptic neurons by identifying brain sites that have interictal ECoG spikes. In our experience, there is a clear relationship among the site of interictal discharges, the site of ictal onset, and the tissue that must be removed to control seizures. This ECoG hallmark is used to determine what part of the brain should be resected.4,15,50
ECoG also provides prognostic information by indicating areas with residual discharges after cortical resection. Patients with no interictal discharges on postresection recordings are more likely to be free of seizures than those with persisting discharges.4,51,51 For “standard” temporal lobectomy surgery, the value of ECoG is not as clear.52
Cortical Stimulation (Functional Mapping)
The location of the central sulcus is determined by electrical stimulation of the precentral and postcentral gyri after preliminary identification by monitoring for the SSEP phase reversal.48 The suspected site of the motor and sensory cortex is stimulated and mapped with a motor response detected by the anesthetist, measurement by electromyographic electrodes, or a report of sensory change by the patient.15,47,51 In practice, the best way to identify the postcentral gyrus is to induce sensory responses in the tongue area located at the bottom of the postcentral gyrus.27,53
The frontal, parietal, and temporal language areas in the dominant hemisphere are stimulated while the patient carries out simple verbal tasks such as naming objects shown on picture cards. A language critical area is identified if the patient is unable to speak (speech arrest) when the site is being stimulated or if the patient can speak but is unable to name objects.14,15,47,51 Although failure to produce speech arrest or anomia does not always exclude the presence of language critical sites in the stimulated cortex, intraoperative mapping is nevertheless the most reliable method currently available for indentifying these language critical sites.
Surgical Technique
A craniotomy is performed to expose the epileptic focus that will be resected. The extent of neocortical resection is based on the gross pathology and the results of ECoG and functional mapping. In general, effort is made to resect all areas with interictal discharges. Essential motor and language areas should be preserved (preferably with a 2- or 1-cm margin), regardless of involvement in the epileptic focus.15 Special attention is also given to the vascular supply of the area to be resected.47 The extent of the resection is individually tailored to each case.
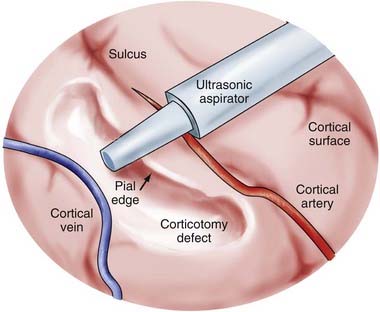
A subpial dissection technique is used for cortical resection (Fig. 65-3).9,14,15,16,47,54 This procedure was used by Horsley (1909) and has remained the technical basis of surgery for epilepsy to this day. The pial surface is coagulated and incised in a relatively avascular area. Gyral resection is then carried out in a subpial fashion. An ultrasonic aspirator at a low suction/vibration setting is extremely useful for focal resections in a subpial plane. Meticulous, slow removal of epileptogenic gray matter is carried to the bottom of the sulcus without damaging vessels within the pia that might supply other nonresected tissue. Hemostasis is achieved principally with topical agents such as Gelfoam or Surgicel and minimal use of electrocautery. With the topectomy procedure, unnecessary resection of the underlying white matter is avoided to preserve the integrity of projection, association, and commissural fibers.
Appropriate antiepileptic medication and dexamethasone are administered after cortical resection.
Surgical Outcome
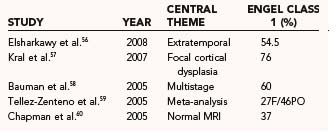
Extratemporal nonlesional resection is associated with worse seizure control rates and a higher incidence of major postoperative morbidity than is lesional or temporal lobe resection surgery. Table 65-3 presents the seizure outcome reported by Engel and colleagues in 1993.55 Extratemporal surgery results in seizure-free rates of 45% and improvement in 35%. More recent work is highlighted in Table 65-4.
| ATL (%) | ETR (%) | |
|---|---|---|
| Seizure free | 2429 (67.9) | 363 (45.1) |
| Improved | 860 (24.0) | 283 (35.2) |
| Not improved | 290 (8.1) | 159 (19.8) |
| Total | 3579 (100) | 805 (100) |
ATL, anterior temporal lobectomy; ETR, extratemporal resection.
From Engel J, Ness PCV, Rasmussen TB, et al. Outcome with respect to epileptic seizures. In: Engel J, ed. Surgical Treatment of the Epilepsies, 2nd ed. New York: Raven Press; 1993:609-621.
Multiple Subpial Transection
From the inception of epilepsy surgery, it has been clear that resective surgery for medically intractable seizure foci can be done in noneloquent areas with acceptably low rates of morbidity and a reasonable chance for seizure control in carefully selected patients. Resection of seizure foci in eloquent cortex, however, results in unacceptable deficits. The surgical procedure MST addresses this difficult problem by capitalizing on the difference between the vertical and horizontal organization of the cortical structures of the brain.61 The master organizational principle in the cerebral cortex is the functional vertical column, with its vertical orientation of incoming and outgoing fibers and blood supply.62–64 At the same time, seizures in part spread horizontally through the gray matter. MST involves disconnecting the gray matter columns that lie in eloquent cortex. This technique can inhibit synchronization and spread of the seizure focus with minimal injury to the cortex. In this section we review the history of the development of MST, patient selection, surgical indications, technique, results, pitfalls, and areas for further exploration. New horizons for the application of MST, including hippocampal transection for mesial temporal epilepsy, are also reviewed.
History
In the 1930s, two treatments emerged for patients with chronic epilepsy that brought hope for better seizure control. The anticonvulsant effects of phenytoin were discovered in 1938, and it was effective in controlling the seizures of patients with chronic epilepsy.65 At the same time, Wilder Penfield at the Montreal Neurological Institute expanded the use of surgical resection of seizure foci, primarily in the temporal lobe, for “psychomotor seizures.”66 News of Penfield’s success spread, and in 1951, Percival Bailey and Fred Gibbs at the University of Illinois Neuropsychological Institute reported their results of temporal lobectomy for psychomotor seizures guided by EEG findings.67 One class of patients who would not benefit from standard surgical therapy consisted of those with a seizure focus in eloquent cortex because excision of this seizure focus would lead to unacceptable deficits.
Several discoveries in neuroscience in the 1950s and 1960s, along with his own work, led Frank Morrell to believe that nonresective surgical therapy might be safe and effective. The first discovery, by Mountcastle, was that gray matter in the neocortex was organized in vertical functional columns with afferent and efferent connecting fiber tracts oriented perpendicular to the surface of the cortex.62,63 Although some horizontal interconnections between neurons exist, experiments by Sperry and colleagues demonstrated that if these horizontal connections are interrupted in the cat visual cortex, the function of that cortex is largely preserved.68–70 In these experiments, they placed mica plates in gray matter perpendicular to the cortical surface, thus severing the horizontal fibers in the cortex but preserving the vertical fibers. Visual testing showed preservation of function. Experiments by Morrell demonstrated the role of these horizontal fibers in the synchronization and spread of an epileptic discharge.71,72 He found that in the monkey, he could inhibit the seizure focus but preserve motor function when he transected through a penicillin-induced seizure focus in the motor cortex.61 These findings led to a long-term collaboration in the surgical treatment of seizure foci in eloquent cortex between the neurologist Morrell and the neurosurgeon Walter Whisler, who had worked at the Illinois Neuropsychological Institute with Gibbs. By 1999, Morrell and Whisler had used MST to treat more than 120 patients at Rush Epilepsy Center in Chicago, where they developed and perfected the technique. Since the report of their original series,61 many other epilepsy centers have duplicated that success and reported their findings.73–99
Patient Selection
The great majority of surgical epilepsy cases can be treated by standard resective techniques such as temporal lobectomy with amygdalohippocampectomy and extratemporal resection in noneloquent cortex. Subpial transection is reserved for patients in whom the seizure activity originates in eloquent cortex. MST has been used to treat patients with epileptogenic lesions of the speech, motor, or primary sensory cortex. Although MST has been used for Rasmussen’s encephalitis, it is of questionable benefit for progressive disease states when the underlying disease cannot be controlled.100,101
MST can be used for the treatment of carefully selected cases of
Operative Procedure
In most cases, subdural grid recording and mapping have been done, and general anesthesia with methohexital is used. Methohexital has been shown to induce epileptic activity, with some evidence that it preferentially induces epileptiform activity within the primary seizure focus.104–106 If intraoperative mapping is planned, lighter doses of methohexital are used, along with intravenous sedation and generous use of local anesthetic. Patients are positioned so that the planned operative exposure is superior in the field and the head is in three-point fixation. A standard frontotemporal craniotomy with an exposure large enough to allow ECoG is carried out. ECoG is performed with a grid, and additional strip electrodes are placed as needed. In many patients in whom MST is being considered, the seizure focus lies in both eloquent and noneloquent cortex. In these cases, surgical resection is performed in the noneloquent cortex to within 1.5 cm of eloquent cortex, as delineated by intraoperative or subdural grid stimulation mapping with standard techniques.107,108 If repeat ECoG does not show significant resolution of the interictal activity and the primary residual focus lies within eloquent cortex, MST is performed on the crown of the involved gyri at 5-mm intervals through the gray matter. Transections are made in parallel rows in the direction perpendicular to the long axis of the gyrus (Fig. 65-4). If significant resolution of the seizure focus is noted on ECoG, no further transection is done. If no significant resolution is noted and a clearly focal area is active despite transection of the crown of the gyrus in that area, transection is sometimes carried out vertically into the gray matter within the depth of the sulcus. This is illustrated in a cadaver in Figure 65-5. This transection is performed along the same plane as transection of the crown. If the primary seizure focus is truly in the MST area, these maneuvers usually stop or significantly reduce the intraictal epileptic discharges. If they do not, it is possible that the preoperative evaluation and ECoG have not shown the primary seizure focus, which may be projecting abnormal electrical activity from a distance.
Transections
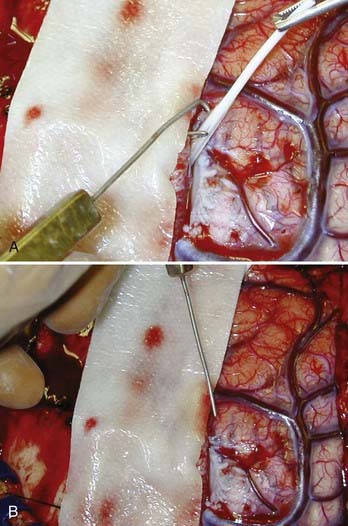
Transections are performed with a specially designed fine, malleable wire that is bent into a 4-mm blunt hook at the tip (Fig. 65-6). The hook of the subpial transector (Whisler-Morrell Subpial Transector, Redman Neurotechnologists, Lake Zurich, IL) measures 4 mm in length to match the average depth of gray matter in the neocortex.63 This hook is bent at an obtuse angle of 105 degrees relative to the main shaft of the wire. The wire is connected to a rectangular handle with the tip of the hook aligned with the flat sides of the handle. This is important because it prevents the hook from going into the cortex at any angle other than perpendicular. If the hook is advanced through the cortex at an angle off the perpendicular, extensive undercutting of the cortex will result, with corresponding deficits. The transection hook shank can be bent to any shape necessary for use in technically difficult locations. This is useful in the intrahemispheric motor and visual cortices and in the posterior temporal lobe when the sylvian fissure is opened to allow access to the depths of the fissure.
The area to be transected is carefully inspected. The gyral and microgyral patterns are noted. The course of the vascular supply and bypassing vessels is traced. After ECoG, transections are usually begun in the most dependent area because subarachnoid bleeding may occur. If a large amount of subarachnoid blood accumulates, a small opening can be made in the pia to let the blood escape. Each transection is begun by opening a hole in an avascular area of the pia. This is done at the edge of a sulcus with a 20-gauge needle. As the transector hook enters the gray matter through this opening, it is important to keep the hook vertically oriented to avoid undercutting and advancing the tip too deep and thus injuring white matter. The hook is advanced in stepwise fashion in a straight line across the crown of the gyrus in a direction perpendicular to the long axis of the gyrus. The hook is then withdrawn along the same path, with the tip of the hook visible just below the pia.109–111 As the hook is withdrawn from the pial puncture site, a small amount of blood sometimes escapes. This is easily controlled with a small piece of Gelfoam and gentle pressure. The next transection line is made parallel to and 5 mm from the first. Because the hook is 4 mm long, it can be used to estimate the distance to the next transection line. The transections are thus done along the gyrus in the area of the seizure focus. Great care must be taken to note the course of the major blood vessels, particularly around the sylvian and intrahemispheric fissures. When transection must be performed in the depths of a sulcus or fissure, the hook is inserted upside down, with the tip pointed away from the pial surface. This lessens the likelihood of damaging a vessel in the sulcus or fissure. The obtuse angle of the hook also helps lessen the likelihood of injury to vessels. In cases of perisylvian-onset epilepsy, it is often useful to open the sylvian fissure to record from the depths of the fissure and transect under direct vision. In some cases, transection at 5-mm intervals is not possible because of microgyral patterns or a large confluence of vessels that may cover the area to be transected (Fig. 65-7).
An alternative approach to transection was proposed by Wyler and colleagues in which the transection device is inserted with the point projected downward into the cortex.74 Other authors have described alterations of the technique.86,87
A recent publication by Shimizu and coauthors reported hippocampal transection in patients with temporal lobe epilepsy.97 Shimizu and associates stated that the primary indication for this procedure was normal hippocampal cases in which there is a high risk for memory loss with hippocampal resection. In their technique, the ventricle is accessed from above via a corticotomy in the superior temporal gyrus. Hippocampal ECoG is followed by transections in the hippocampus in a coronal plane to varying depths by a specially designed transection device. The amygdala is resected if ECoG shows epileptic discharges. They reported that active epileptic discharges usually disappear after transection. If further discharges are noted, they may resect the basal temporal region. In their series, they reported the results of this procedure in 21 patients with normal preoperative MRI findings. In the 17 patients who had at least 1 year of follow-up, 14 were free of seizures. Postoperative neuropsychological testing (Rey auditory verbal learning test, Benton Visual Retention Test, and Wechsler Adult Intelligence Scale–Revised) was reported to be unchanged from preoperative testing in all patients by 6 months after surgery. The confounding variables of having performed a corticotomy on the superior temporal gyrus, transgression of the temporal stem, resection of the amygdala, and in some cases, resection of basal cortex make it difficult to determine that the efficacy of the procedure is entirely due to transection of the hippocampus. Regardless, the reported stability of memory testing makes this procedure intriguing. Long-term follow-up in these patients will be required to determine the potential usefulness of this procedure.
Pathology
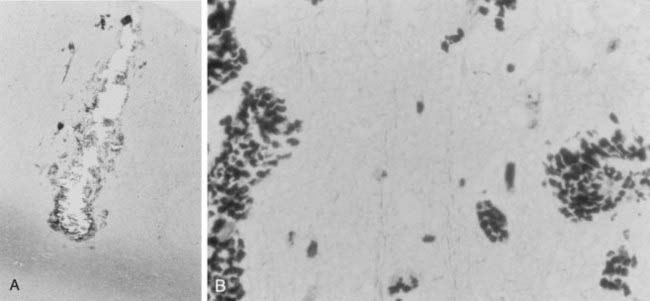
When tissue that has been treated with the MST method has been removed and analyzed, the acute pathologic changes are consistent with microscopic focal injury along the transection line (Fig. 65-8). On the transection line, microscopic hemorrhage, edema, and mild cell injury are seen. When the transection lines are done properly and perpendicular to the gyral surface, the columns of cell bodies and their vertical afferent and efferent fibers are preserved. In the study by Pierre-Louis and colleagues,112 the majority of transections were entirely in gray matter and were perpendicular to the gyral surface. Limiting transections to surface gray matter may explain the kainic acid–induced animal models of epilepsy, which show evidence that MST suppresses epileptic activity in the transected cortex but does not stop subcortical spread of epilepsy. Kaufmann and associates described more variable results with transections deviating from the perpendicular and at varying depths.113
Radiology and Functional Imaging
Hashizume and Tanaka demonstrated the effects of MST on an experimental seizure focus.114 In a kainic acid rat seizure model, they found that MST suppressed the spread of seizure activity to the ipsilateral hemisphere and reduced the frequency of seizures but did not eliminate them. They tested glucose metabolism in the transected cortex and found that it was not altered, thus suggesting that function of the cortex was preserved.
Leonhardt and coworkers reported PET findings after MST.115 In one patient who had undergone MST in the central sensorimotor cortex, they found that PET activation during a hand motor task was maintained in the transected cortex. They also found that there was more widespread activation in motor association cortex bilaterally, thus suggesting active recruitment of cortex after MST.
Complications
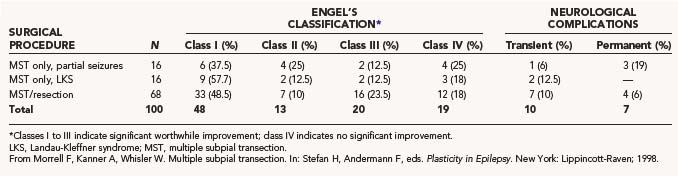
In our series of more than 100 patients and in published series by others, no deaths have been reported (Table 65-5). In the early postoperative period, most patients who undergo MST in eloquent cortex have subtle, transient deficits corresponding to the area transected. These deficits are most pronounced in the first week after surgery. As the edema and microhemorrhage resolve, most patients return to their baseline function within 2 to 4 weeks. In many patients undergoing careful, detailed examination, deficits in fine motor control or speech can be detected. Moo and coworkers reported a prospective evaluation of motor recovery after MST.116 fMRI was performed before and after transection and then repeated 6 weeks postoperatively. Findings included an initial loss of dexterity corresponding to an initial loss of focal fMRI activation. The focal fMRI activation and the dexterity had returned by 6 weeks.
In the series reported by Morrell, there was a 5% incidence of permanent, disabling complications corresponding to the area transected. In two patients, motor deficits arose after retraction and transection of the intrahemispheric leg motor cortex. Two cases of permanent postoperative dysphasia occurred after transection of speech areas. One case of hemiparesis occurred after a basal ganglia hemorrhage distant from the site of transection. Eight patients had deficits corresponding to the area transected that lasted longer than the expected 2 to 4 weeks, but they eventually resolved over a period of several months. One was related to speech, and seven were related to sensory or motor function. Seven other complications occurred that were clearly related to the resection or craniotomy. Permanent visual field loss and permanent sensory loss were clearly related to the surgical resection. A transient sixth nerve palsy was attributed to temporal lobe resection. A single case of meningitis, orchitis, and phlebitis was associated with the craniotomy procedure but resolved with appropriate treatment. The final permanent complication rate was 7% as reported by Morrell. A later publication by Smith and Byrne on 84 patients with MST, with or without additional resection, reported a complication rate of 17%.100
Complication rates similar to ours have been reported in other series (Table 65-6). A period of transient dysfunction has also been commonly noted. Higher complication rates can be expected if the interval of transection is narrowed to 4 mm or less.76 Although perpendicular transection through cortex causes little damage to the surrounding neurons, as the pathologic studies indicate, it does cause some damage.112,113 As the interval is narrowed, one would expect a higher percentage of neurons in the cortex to be injured. This would also be expected if the depths of the sulci in the cortical area were transected. This maneuver is not routinely done at our center but is performed routinely by some surgeons.
In a multicenter meta-analysis reported by Spencer and coworkers, the complication rate for MST without resection was 19% and that for MST with resection was 23% in a series of 211 patients.117 These results are similar to those reported by Smith and Byrne and reflect the nature of operating in eloquent cortex in patients with severe medically intractable epilepsy.100 Results from other series are listed in Table 65-6.
Seizure Outcome
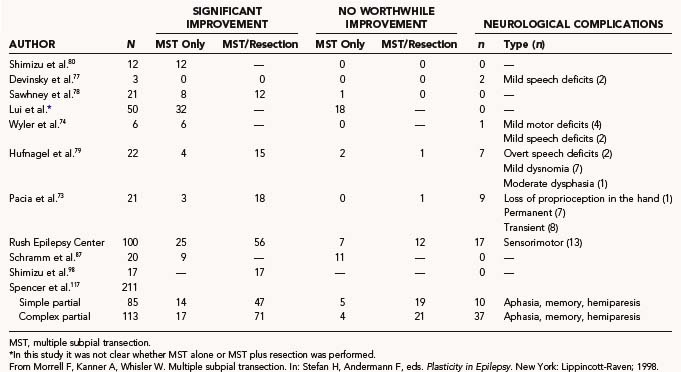
Seizure outcome after MST is analyzed in this fashion because the cortical resection done in the majority of cases introduces a confounding variable: one cannot be certain whether MST or the resection had the effect on seizure outcome or on morbidity. In these cases, one can be certain only that MST had an effect on intraoperative ECoG. The group that underwent MST as the only surgical intervention gives the clearest indication of the effect of MST on seizure outcome because there are fewer confounding variables and the pathology is more uniform in this group. In 16 patients who underwent MST alone for focal-onset seizures in eloquent cortex with at least 2 years of follow-up, 6 were made seizure free (see Table 65-5). An additional 6 patients had only rare seizures or had a 90% or greater reduction in seizures. Four patients had no worthwhile benefit. Overall, 75% of patients in this category had a worthwhile Engel’s class I to III seizure outcome. Because all of these patients would have been rejected for standard resective surgery, their outcomes should be compared with best medical therapy. Other groups have reported similar results (see Table 65-6). In Wyler and coworkers’ series of 6 patients with uniform pathology in the sensorimotor cortex, all 6 had a significant reduction in their seizures.74 There was only one permanent mild motor deficit. Schramm and colleagues reported on a series of 20 patients who underwent MST without resection.87 Forty-five percent of the patients at the 1-year follow-up had a worthwhile outcome (Engel’s class I to III). They reported that lesional epilepsy and large areas of epileptic discharge predicted a worse outcome. Mulligan and coauthors reported a 42% rate of significant improvement in seizure frequency in a series of 12 patients.86 Spencer and associates reported an international meta-analysis on 212 patients who underwent MST at six epilepsy centers.117 In cases of MST alone without resection, they reported rates of excellent outcome with regard to seizure control of 71% for generalized epilepsy, 62% for complex partial epilepsy, and 63% for simple partial seizures.
The next category of patients underwent a combination of MST and resection. This was the most common type of case in the series of Morrell and Whisler.61 It is rare for a seizure focus to lie entirely in eloquent cortex. A total of 82% of patients in this category were seizure free or had a significant reduction in their seizures (Engel’s class I to III; see Table 65-5). Although the majority of patients exhibited worthwhile improvement, the initial report by Morrell of 52% being free of seizures was not sustained later in the series.61 The meta-analysis reported by Spencer and coauthors showed similar results for patients who underwent resection plus MST, with excellent outcomes (>95% reduction) reported in 87% of patients with generalized seizures, 68% of patients with complex partial seizures, and 68% of patients with simple partial epilepsy.117 These collective data support the efficacy of MST, both as a stand-alone procedure for highly localized cases in eloquent cortex and for cases in which the epileptogenic zone overlaps eloquent cortex. The expected outcome of MST, however, is more likely to be improvement than cure. Results from other series are listed in Table 65-6.
The question of long-term efficacy has been raised. Orbach and coworkers reported an 18.5% seizure recurrence rate in a series of patients who underwent MST and had at least 5 years of follow-up.118 In a series of 20 patients who underwent pure MST, Schramm and coauthors reported that outcomes changed over time.87 Five patients improved during the long-term follow-up period, whereas 7 worsened. This phenomenon has also been described in patients who have undergone resective epilepsy surgery.
Conclusion
The initial experience with MST was reported in 1989.61 In that group of patients with intractable seizures, 11 of 20 were free of seizures after a follow-up of at least 5 years. Subsequent cases in which MST was performed without resection proved the independent efficacy of the procedure. Since then, MST has proved helpful in patients with intractable epilepsy and foci in unresectable cortex. Similar results at other centers have confirmed its efficacy and safety if done properly on well-selected patients. The exact mechanism of action of MST is being delineated with functional testing. Other centers attempting MST have added different techniques and new protocols.73,74,76 As we learn more about MST and about the nature of epileptogenic cortex, the technique will be further refined and will probably result in improved outcomes in patients with unresectable seizure foci.
Abson Kraemer DL. Diagnostic techniques in surgical management of epilepsy: strip electrodes, grids and depth electrodes. In: Schmidek HH, Roberts DW, editors. Schmidek & Sweet Operative Neurosurgical Techniques: Indications, Methods, and Results. 4th ed. Philadelphia: WB Saunders; 2000:1359-1374.
Barkovich AJ, Rowley HA, Andermann F. MR in partial epilepsy: value of high-resolution volumetric techniques. AJNR Am J Neuroradiol. 1995;16:339-343.
Bauman JA, Feoli E, Romanelli P, et al. Multistage epilepsy surgery: safety, efficacy and utility of a novel approach in pediatric extratemporal epilepsy. Neurosurgery. 2005;56:318-334.
Chapman K, Wyllie E, Najm I, et al. Seizure outcome after epilepsy surgery in patients with normal preoperative MRI. J Neurol Neurosurg Psychiatry. 2005;76:710-713.
Cohen-Gadol AA, Stoffman MR, Spencer DD. Emerging surgical and radiotherapeutic techniques for treating epilepsy. Curr Opin Neurol. 2003;16:213-219.
Devinsky O, Perrine K, Vazquez B, et al. Multiple subpial transections in the language cortex. Brain. 1994;117:255-265.
Faught E. Collective data supports efficacy of multiple subpial transection. Epilepsy Curr. 2002;2:108.
Hashizume K, Tanaka T. Multiple subpial transection in kainic acid–induced focal cortical seizure. Epilepsy Res. 1998;32:389-399.
Hufnagel A, Zenter J, Fernandez G, et al. Multiple subpial transection for control of epileptic seizures: effectiveness and safety. Epilepsia. 1997;38:678-688.
Kaufmann W, Kraus G, Uematsu S, et al. Treatment of epilepsy with multiple subpial transections: an acute histological analysis in human subjects. Epilepsia. 1996;37:342-352.
Leonhardt G, Spiekermann G, Muller S, et al. Cortical reorganization following multiple subpial transection in human brain—a study with positron emission tomography. Neurosci Lett. 2000;292:63-65.
Morrell F, Whisler WW, Bleck T. Multiple subpial transaction: a new approach to the surgical treatment of focal epilepsy. J Neurosurg. 1989;70:231-239.
Mulligan LP, Spencer DD, Spencer SS. Multiple subpial transections: the Yale experience. Epilepsia. 2001;42:226-229.
Ojemann GA. Surgical treatment of epilepsy. In: Wilkins RH, Rengachary SS, editors. Neurosurgery. 2nd ed. New York: McGraw-Hill; 1996:4173-4183.
Ojemann GA. Awake operations with mapping in epilepsy. In: Schmidek HH, Sweet WH, editors. Operative Neurosurgical Techniques. 3rd ed. Philadelphia: WB Saunders; 1995:1317-1322.
Olivier A, Awad IA. Extratemporal resections. In: Engel J, editor. Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993:489-500.
Pierre-Louis SJC, Smith MC, Morrell F, et al. Anatomical effects of multiple subpial transection. Epilepsia. 1993;34(suppl):104.
Rasmussen T. Surgery of frontal lobe epilepsy. Adv Neurol. 1975;8:197-205.
Schramm J, Aliashkevich AF, Grunwald T. Multiple subpial transections: outcome and complications in 20 patients who did not undergo resection. J Neurosurg. 2002;97:39-47.
Shimizu H, Kawai K, Sunaga S, et al. Hippocampal transection for treatment of left temporal lobe epilepsy with preservation of verbal memory. J Clin Neurosci. 2006;13:322-328.
Shimizu H, Kawai K, Sunaga S, et al. [Surgical treatment for temporal lobe epilepsy with preservation of postoperative memory function.]. Rinsho Shinkeigaku. 2004;44:868-870.
Smith MC, Byrne R. Multiple subpial transection in neocortical epilepsy: part I. Adv Neurol. 2000;84:621-634.
Spencer SS, Schramm J, Wyler A, et al. Multiple subpial transection for intractable partial epilepsy: an international meta-analysis. Epilepsia. 2002;43:141-145.
Sperry RW, Miner N, Myers RE. Visual pattern perception following subpial slicing and tantalum wire implantation in visual cortex. J Comp Physiol Psychol. 1955;48:50-58.
Tanaka T, Hashizume K, Sawamura A, et al. Basic science and epilepsy: experimental epilepsy surgery. Stereotact Funct Neurosurg. 2001;77:239-244.
Wada J, Rasmussen T. Intracranial injection of Amytal for the localization of cerebral speech dominance. J Neurosurg. 1960;17:266-282.
Whisler WW. Multiple subpial transection. In: Kaye A, Black P, editors. Operative Neurosurgery. London, United Kingdom: Churchill Livingstone, 1997.
Whisler WW. Multiple subpial transection. In: Rengachary SS, editor. Neurosurgical Operative Atlas, Vol 6. Park Ridge, IL: American Association of Neurological Surgeons; 1997:125-129.
Wyler AR, Wilkus RJ, Retard SW, et al. Multiple subpial transection for partial seizures in sensorimotor cortex. Neurosurgery. 1995;37:1122-1128.
1 Shorvon SD. The epidemiology and treatment of chronic and refractory epilepsy. Epilepsia. 1996;37(suppl 2):S1-S3.
2 Callaghan BC, Anand K, Hesdorffer D, et al. Likelihood of seizure remission in an adult population with refractory epilepsy. Ann Neurol. 2007;62:311-313.
3 Weibe S, Blume WT, Girvin JP, et al. Effectiveness and efficiency of surgery for temporal lobe epilepsy study group: a randomized controlled trial of surgery for temporal lobe epilepsy. N Engl J Med. 2001;345:311-318.
4 Olivier A, Awad IA. Extratemporal resections. In: Engel J, editor. Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993:489-500.
5 Baltuch GH. Complex partial seizures: surgical treatment. Curr Treat Options Neurol. 1999;1:353-357.
6 Connolly PJ, Cohen-Gadol AA, Spencer DD. Milestones in epilepsy surgery since 1849. In: Spencer D, ed. Operative Epilepsy (in press).
7 Feindel W. Brain stimulation combined with electroencephalography in the surgery of epilepsy: historical highlights. Electroencephalogr Clin Neurophysiol Suppl. 1998;48:1-8.
8 Wyler AR. Focal cortical resections. In: Wyler AR, Hermann BP, editors. The Surgical Management of Epilepsy. Boston: Butterworth-Heinemann; 1994:129-138.
9 Morgan RJ, Soltesz I. Nonrandom connectivity of the epileptic dentate gyrus predicts a major role for neuronal hubs in seizures. Proc Nat Acad Sci U S A. 2008;105:6179-6184.
10 Labyt E, Uva L, de Curtis M, et al. Realistic modeling of entorhinal cortex field potentials and interpretation of epileptic activity in the guinea pig isolated brain preparation. J Neurophysiol. 2006;96:363-377.
11 Auer RN, Siesjo BK. Biological differences between ischemia, hypoglycemia, and epilepsy. Ann Neurol. 1988;24:699-707.
12 Hansebout RR. Surgery of epilepsy—Current technique of cortical resection. In Schmidek HH, Roberts DW, editors: Operative Neurosurgical Techniques, Indications, Methods and Results, 5th ed, Orlando, FL: Grune & Stratton, 1988.
13 Marino R. Neurosurgical aspects of epilepsy in adults. In: Youmans JR, editor. Neurological Surgery. 3rd ed. Philadelphia: WB Saunders; 1990:4288-4326.
14 Ojemann GA. Surgical treatment of epilepsy. In: Wilkins RH, Rengachary SS, editors. Neurosurgery. 2nd ed. New York: McGraw-Hill; 1996:4173-4183.
15 Silfvenius H. Latest advances in epilepsy surgery. Acta Neurol Scand Suppl. 1995;162:11-16.
16 Engel J, Shewmon A. Who should be considered a surgical candidate? In: Engel J, editor. Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993:23-34.
17 Bourgeois M, Sainte-Rose C, Lellouch-Tubiana A, et al. Surgery of epilepsy associated with focal lesions in childhood. J Neurosurg. 1999;90:833-842.
18 Krsek P, Tichy M, Zamecnik J, et al. Life-saving epilepsy surgery for status epilepticus caused by cortical dysplasia. Epileptic Disord. 2002;4:203-208.
19 Carvalho KS, personal communication.
20 Ghatan S. Surgery for epilepsy. Pediatr Ann. 2006;35:386-392.
21 Helmstaedter C, Gleibner U, Zentner J, et al. Neuropsychological consequences of epilepsy surgery in frontal lobe epilepsy. Neuropsychologia. 1998;36:333-341.
22 Rasmussen T. Commentary: extratemporal cortical excisions and hemispherectomy. In: Engel J, editor. Surgical Treatment of the Epilepsies. New York: Raven Press; 1987:417-424.
23 Engel J. Surgery for seizures. N Engl J Med. 1996;334:647-652.
24 Quesney LF, Risinger MW, Shewmon A. Extracranial EEG evaluation. In: Engel J, editor. Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993:173-195.
25 Rasmussen T. Surgery of frontal lobe epilepsy. Adv Neurol. 1975;8:197-205.
26 Olivier A. Extratemporal cortical resections: Principles and methods. In: Lüders H, editor. Epilepsy Surgery. New York: Raven Press; 1991:559-568.
27 Williamson PD, Ness PCV, Wieser HG, et al. Surgically remediable extratemporal syndrome. In: Engel J, editor. Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993:65-76.
28 Wyler AR, Vossler DG. Surgical strategies for epilepsy. In: Grossman RG, Loftus CM, editors. Principles of Neurosurgery. 2nd ed. Philadelphia: Lippincott-Raven; 1999:737-755.
29 Barkovich AJ, Rowley HA, Andermann F. MR in partial epilepsy: value of high-resolution volumetric techniques. AJNR Am J Neuroradiol. 1995;16:339-343.
30 Kuzniecky RI, Cascino GD, Palmini A, et al. Structural neuroimaging. In: Engel J, editor. Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993:197-209.
31 Barkovich A, Chuang S, Norman D. MR of neuronal migration anomalies. AJNR Am J Neuroradiol. 1987;8:1009-1017.
32 Berkovic SF, Newton MR, Chiron C, et al. Single photon emission tomography. In: Engel J, editor. Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993:233-243.
33 Rowe CC, Berkovic SF, Sia STB, et al. Localization of epileptic foci with postictal single photon emission computed tomography. Ann Neurol. 1989;26:660-668.
34 Harvey AS, Hopkins IJ, Bowe JM, et al. Frontal lobe epilepsy: Clinical seizure characteristics and localization with ictal [99m]Tc-HMPAO SPECT. Neurology. 1993;43:1966-1980.
35 Cascino GD. Surgical treatment of epilepsy. Epilepsy Res. 2004;60:179-186.
36 Henry TR, Chugani HT, Abou-Khlil BW, et al. Positron emission tomography. In: Engel J, editor. Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993:211-232.
37 Jones-Gotman M, Smith ML, Zatorre RJ. Neuropsychological testing for localizing and lateralizing the epileptogenic region. In: Engel J, editor. Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993:245-261.
38 Arnedo M, Espinosa M, Ruiz R, et al. Neuropsychological intervention in patients with epilepsy. Rev Neurol. 2006;43(suppl):S83-S88.
39 Powell HW, Richardson MP, Symms MR, et al. Preoperative fMRI predicts memory decline following anterior temporal lobe resection. J Neurol Neurosurg Psychiatry. 2008;79:686-693.
40 Wada J, Rasmussen T. Intracranial injection of Amytal for the localization of cerebral speech dominance. J Neurosurg. 1960;17:266-282.
41 Detre JA. fMRI: Applications in epilepsy. Epilepsia. 2004;45(suppl):26-31.
42 Bargalló N. Functional magnetic resonance: new applications in epilepsy. Eur J Radiol. 2008;67:401-408.
43 Medina LS, Aguirre E, Bernal B, et al. Functional MR imaging versus Wada test for evaluation of language lateralization: cost analysis. Radiology. 2004;230:49-54.
44 Abson Kraemer DL. Diagnostic techniques in surgical management of epilepsy: strip electrodes, grids and depth electrodes. In: Schmidek HH, Roberts DW, editors. Schmidek & Sweet Operative Neurosurgical Techniques: Indications, Methods, and Results. 4th ed. Philadelphia: WB Saunders; 2000:1359-1374.
45 Connolly PJ, Danish SF, Litt B, et al. Coregistration of subdural grid electrodes for navigation during epilepsy surgery. J Neurosurg. 2008;108:A895.
46 Pilcher WH, Ojemann GA. Presurgical evaluation and epilepsy surgery. In: Apuzzo MLJ, editor. Brain Surgery: Complication Avoidance and Management. New York: Churchill Livingstone; 1993:1525-1555.
47 Cedzich C, Taniguchi M, Schafer S, et al. Somatosensory evoked potential phase reversal and direct motor cortex stimulation during surgery in and around the central region. Neurosurgery. 1996;38:962-970.
48 Corkin S, Milner B, Rasmussen T. Somatosensory thresholds: contrasting effects of postcentral gyrus and posterior parietal lobe excisions. Arch Neurol. 1970;23:41-58.
49 Ojemann GA. Awake operations with mapping in epilepsy. In: Schmidek HH, Sweet WH, editors. Operative Neurosurgical Techniques. 3rd ed. Philadelphia: WB Saunders; 1995:1317-1322.
50 Ojemann GA. Intraoperative electrocorticography and functional mapping. In: Wyler AR, Hermann BP, editors. The Surgical Management of Epilepsy. Boston: Butterworth-Heinemann; 1994:189-196.
51 Kutsy RL. Focal extratemporal epilepsy: clinical features, EEG patterns and surgical approach. J Neurol Sci. 1999;166:1-15.
52 Kuruvilla A, Flink R. Intraoperative electrocorticography in epilepsy surgery: useful or not? Seizure. 2003;12:577-584.
53 Picard C, Olivier A. Sensory cortical tongue representation in man. J Neurosurg. 1983;59:781-789.
54 Penfield W, Jasper H. Epilepsy and the Functional Anatomy of the Human Brain. Boston: Little Brown & Co; 1954.
55 Engel J, Ness PCV, Rasmussen TB, et al. Outcome with respect to epileptic seizures. In: Engel J, editor. Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993:609-621.
56 Elsharkawy AE, Behne F, Oppel F, et al. Long-term outcome of extratemporal epilepsy among 154 adult patients. J Neurosurg. 2008;108:676-686.
57 Kral T, von Lehe M, Podlogar M, et al. Focal cortical dysplasia: long term seizure outcome after surgical treatment. J Neurol Neurosurg Psychiatry. 2007;78:853-856.
58 Bauman JA, Feoli E, Romanelli P, et al. Multistage epilepsy surgery: safety, efficacy and utility of a novel approach in pediatric extratemporal epilepsy. Neurosurgery. 2005;56:318-334.
59 Tellez-Zenteno JF, Dhar R, Wiebe S. Long term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128:1188-1198.
60 Chapman K, Wyllie E, Najm I, et al. Seizure outcome after epilepsy surgery in patients with normal preoperative MRI. J Neurol Neurosurg Psychiatry. 2005;76:710-713.
61 Morrell F, Whisler WW, Bleck T. Multiple subpial transection: a new approach to the surgical treatment of focal epilepsy. J Neurosurg. 1989;70:231-239.
62 Mountcastle VB. Modality and topographic properties of single neurons of cat’s somatic sensory cortex. J Neurophysiol. 1957;20:408-434.
63 Mountcastle VB. The columnar organization of the neocortex. Brain. 1957;120:701-722.
64 Asanuma H. Recent developments in the study of the columnar arrangement of neurons within the motor cortex. Physiol Rev. 1975;55:143-156.
65 Merritt HH, Putnam TJ. Sodium diphenylhydantoinate in treatment of convulsive disorders. JAMA. 1938;111:1068-1073.
66 Feindel W. Development of surgical therapy of epilepsy at the Montreal Neurological Institute. Can J Neurol Sci. 1991;18:549-553.
67 Bailey P, Gibbs FA. The surgical treatment of psychomotor epilepsy. JAMA. 1951;145:365-370.
68 Sperry RW. Physiological plasticity and brain circuit theory. In: Harlow HF, Woolsey CN, editors. Biological and Biochemical Bases of Behavior. Madison: University of Wisconsin Press; 1958:33-37.
69 Sperry RW, Miner N. Pattern perception following insertion of mica plates into visual cortex. J Comp Physiol Psychol. 1955;48:463-469.
70 Sperry RW, Miner N, Myers RE. Visual pattern perception following subpial slicing and tantalum wire implantation in visual cortex. J Comp Physiol Psychol. 1955;48:50-58.
71 Morrell F. Microelectrode studies in chronic epileptic foci. Epilepsia. 1961;2:81-88.
72 Morrell F. Cellular pathophysiology of focal epilepsy. Epilepsia. 1969;10:495-505.
73 Pacia SV, Devinsky O, Perrine K, et al. Multiple subpial transections for intractable partial seizures: seizure outcome. J Epilepsy. 1997;10:86-91.
74 Wyler AR, Wilkus RJ, Retard SW, et al. Multiple subpial transection for partial seizures in sensorimotor cortex. Neurosurgery. 1995;37:1122-1128.
75 Rougier A, Sundstrom L, Claverie B, et al. Multiple subpial transection: report of 7 cases. Epilepsy Res. 1996;24:57-63.
76 Patil AA, Andrews RV, Torkelson R. Surgical treatment of intractable seizures with multilobar or bihemispheric seizure foci (MLBHSF). Surg Neurol. 1997;47:72-78.
77 Devinsky O, Perrine K, Vazquez B, et al. Multiple subpial transections in the language cortex. Brain. 1994;117:255-265.
78 Sawhney IMS, Robertson JA, Polkey CE, et al. Multiple subpial transection: a review of 21 cases. J Neurol Neurosurg Psychiatry. 1995;58:344-349.
79 Hufnagel A, Zenter J, Fernandez G, et al. Multiple subpial transection for control of epileptic seizures: effectiveness and safety. Epilepsia. 1997;38:678-688.
80 Shimizu H, Suzuki I, Ishijima B, et al. Multiple subpial transection (MST) for the control of seizures that originated in unresectable cortical foci. Jpn J Psychiatr Neurol. 1991;45:354-356.
81 Honovar M, Janota I, Polkey CE. Rasmussen’s encephalitis in surgery for epilepsy. Dev Med Child Neurol. 1992;34:1-4.
82 Zonghui L, Quanjun Z, Shiyue L, et al. Multiple subpial transection for treatment of intractable epilepsy. Chin Med J. 1995;108:539-541.
83 Tanaka T, Yonemasu Y. Basic and clinical approaches for surgical treatment of intractable epilepsies. Clin Neurol. 1994;34:1237-1239.
84 Neville BRG, Harkness WFJ, Cross JH. Surgical treatment of severe autistic regression in childhood epilepsy. Pediatr Neurol. 1997;16:137-140.
85 Patil AA, Andrews R, Torkelson R. Minimally invasive surgical approach for intractable seizures. Stereotact Funct Neurosurg. 1995;65:86-89.
86 Mulligan LP, Spencer DD, Spencer SS. Multiple subpial transections: the Yale experience. Epilepsia. 2001;42:226-229.
87 Schramm J, Aliashkevich AF, Grunwald T. Multiple subpial transections: outcome and complications in 20 patients who did not undergo resection. J Neurosurg. 2002;97:39-47.
88 Benifla M, Otsubo H, Ochi A, et al. Multiple subpial transections in pediatric epilepsy: indications and outcomes. Childs Nerv Syst. 2006;22:992-998.
89 Chuang MF, Harnod T, Wang PJ, et al. Effect of multiple subpial transection on patients with uncontrolled atypical infantile spasms. Epilepsia. 2006;47:659-660.
90 Cohen-Gadol AA, Stoffman MR, Spencer DD. Emerging surgical and radiotherapeutic techniques for treating epilepsy. Curr Opin Neurol. 2003;16:213-219.
91 Faught E. Collective data supports efficacy of multiple subpial transection. Epilepsy Curr. 2002;2(4):108.
92 Patil AA, Andrews RV, Johnson M, et al. Is epilepsy surgery on both hemispheres effective? Stereotact Funct Neurosurg. 2004;82:214-221.
93 Polkey CE. Multiple subpial transection. Adv Tech Stand Neurosurg. 2000;26:3-23.
94 Polkey CE. Multiple subpial transection: a clinical assessment. Int Rev Neurobiol. 2001;45:547-569.
95 Polkey CE. Alternative surgical procedures to help drug-resistant epilepsy—a review. Epileptic Disord. 2003;5:63-75.
96 Polkey CE. Clinical outcome of epilepsy surgery. Curr Opin Neurol. 2004;17:173-178.
97 Shimizu H, Kawai K, Sunaga S, et al. Hippocampal transection for treatment of left temporal lobe epilepsy with preservation of verbal memory. J Clin Neurosci. 2006;13:322-328.
98 Shimizu H, Kawai K, Sunaga S, et al. [Surgical treatment for temporal lobe epilepsy with preservation of postoperative memory function.]. Rinsho Shinkeigaku. 2004;44:868-870.
99 Zhao Q, Tian Z, Liu Z, et al. Evaluation of the combination of multiple subpial transection and other techniques for treatment of intractable epilepsy. Chin Med J. 2003;116:1004-1007.
100 Smith MC, Byrne R. Multiple subpial transection in neocortical epilepsy: part I. Adv Neurol. 2000;84:621-634.
101 Zhang YH, Pu LH, Liu XY, et al. [Clinical characteristics and treatment of Rasmussen syndrome in 16 children.]. Zhonghua Er Ke Za Zhi. 2007;45:697-702.
102 Morrell F, Whisler WW, Smith MC. Multiple subpial transection in Rasmussen’s encephalitis. In: Andermann F, editor. Chronic Encephalitis and Epilepsy: Rasmussen’s Syndrome. Boston: Butterworth-Heinemann; 1991:219-234.
103 D’Giano CH, Del CGM, Pomata H, et al. Treatment of refractory partial status epilepticus with multiple subpial transection: case report. Seizure. 2001;10:382-385.
104 Hufnagel A, Burr W, Elger CE, et al. Localization of the epileptic focus during methohexital-induced anesthesia. Epilepsia. 1992;33:271-284.
105 Wyler AR, Richey ET, Atkinson RA, et al. Methohexital activation of epileptogenic foci during acute electrocorticography. Epilepsia. 1987;28:490-494.
106 Hardiman O, Coughlan A, O’Moore B, et al. Interictal spike localization with methohexital: preoperative activation and surgical follow-up. Epilepsia. 1987;28:335-339.
107 Lesser RP, Luders H, Klem G, et al. Extraoperative cortical functional localization in patients with epilepsy. J Clin Neurophysiol. 1987;4:27-53.
108 Ojemann G, Whitaker HA. Language localization and variability. Brain Lang. 1978;81:239-260.
109 Whisler WW. Multiple subpial transection. In: Rengachary SS, editor. Neurosurgical Operative Atlas, Vol 6. Park Ridge, IL: American Association of Neurological Surgeons; 1997:125-129.
110 Morrell F, Kanner AM, Whisler WW. Multiple subpial transection. In: Stefan H, Andermann F, editors. Plasticity in Epilepsy. New York: Lippincott-Raven, 1998.
111 Whisler WW. Multiple subpial transection. In: Kaye A, Black P, editors. Operative Neurosurgery. London, United Kingdom: Churchill Livingstone, 1997.
112 Pierre-Louis SJC, Smith MC, Morrell F, et al. Anatomical effects of multiple subpial transection. Epilepsia. 1993;34(suppl):104.
113 Kaufmann W, Kraus G, Uematsu S, et al. Treatment of epilepsy with multiple subpial transections: an acute histological analysis in human subjects. Epilepsia. 1996;37:342-352.
114 Hashizume K, Tanaka T. Multiple subpial transection in kainic acid–induced focal cortical seizure. Epilepsy Res. 1998;32:389-399.
115 Leonhardt G, Spiekermann G, Muller S, et al. Cortical reorganization following multiple subpial transection in human brain—a study with positron emission tomography. Neurosci Lett. 2000;292:63-65.
116 Moo LR, Slotnick SD, Krauss G, et al. A prospective study of motor recovery following multiple subpial transections. Neuroreport. 2002;13:665-669.
117 Spencer SS, Schramm J, Wyler A, et al. Multiple subpial transection for intractable partial epilepsy: an international meta-analysis. Epilepsia. 2002;43:141-145.
118 Orbach D, Romanelli P, Devinsky O, et al. Late seizure recurrence after multiple subpial transections. Epilepsia. 2001;42:1316-1319.