Chapter 228 Timing of Decompression Surgery for Traumatic Spinal Cord Injury in a Patient with an Incomplete Myelopathy
Postural Nonoperative Management
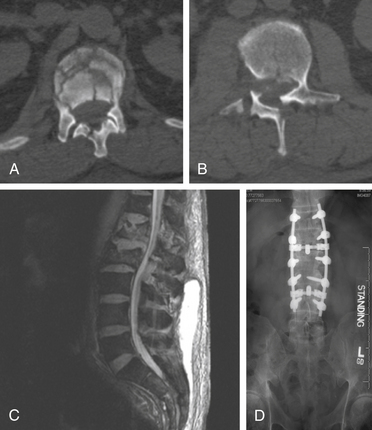
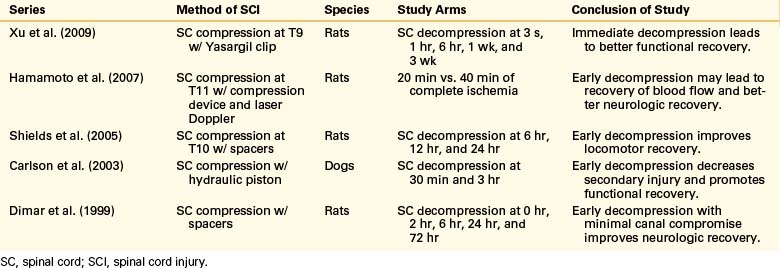
The management of complex spine fractures with incomplete spinal cord injury (SCI) in the severely injured polytrauma patients presents the spine surgeon with a difficult clinical scenario.1 These patients often have severe comorbid conditions such as pulmonary contusions, metabolic acidosis, long bone fractures, or acute respiratory distress syndrome. Controversy exists as to the optimal treatment and timing of intervention for unstable spine fractures with neurologic involvement in the severely injured.2–9 For example, on June 30, 2006, a 74-year-old man was the pilot of a helicopter that crashed after striking power lines. He suffered severe injuries including an open tibia/fibular fracture, complex pelvic fractures, abdominal compartment syndrome requiring emergent laparotomy, bilateral hemopneumothoraxes, traumatic subarachnoid hemorrhage, burst fracture of the L1 and L2 vertebrae, and T12 vertebral body fracture (Fig. 228-1). Neurologically, the patient had no rectal tone but was able move his feet. The patient presented to the ICU after his laparotomy in acute respiratory distress syndrome and was placed on a RotoRest bed (KCI, San Antonio, TX) for ventilatory support. The patient was eventually paralyzed, and an intracranial pressure monitor was placed for declining neurologic exam. The patient underwent tracheostomy and bilateral chest tube placement for pulmonary effusions. He remained in the ICU for nearly 4 weeks. He was not thought to be stable enough to tolerate a prolonged prone procedure for internal reduction and fixation of his thoracolumbar fractures. This scenario is all too common in modern level 1 trauma centers.
Early reports from Frankel et al.,9 Guttmann,10 and Bedbrook11,12 were heavily weighted toward nonoperative treatment. In recent years, nonoperative treatment of intact thoracolumbar fractures has been shown to be associated with excellent results.13–19 However, there have been few randomized studies comparing early versus late surgery in SCI. The study by Vaccaro et al.8 involved 34 patients with cervical spine fractures that were randomized to early (<72 hours) surgery and 28 that were randomized to late (>5 days). Unfortunately, the follow-up at 1 year was available on only 23 and 19 patients, respectively. The unanticipated finding by the authors was that there was no significant difference in outcome between the two groups. A retrospective review by Mirza et al.6 involved 43 patients with cervical spine injuries treated at two facilities. Their findings demonstrated improved outcome with early surgery (<72 hours) compared to delayed surgery performed 10 to 14 days after injury. No increased complications were noted in the early surgery group.
Similarly, conflicting results with timing of surgery have been encountered with thoracolumbar fractures. A retrospective study involving thoracolumbar fractures divided patients into three surgical groups: early surgery (<8 hours), more than 8 hours after surgery, and after 10 days.2 There were 26, 50, and 12 patients, respectively, in the three groups with an average follow-up of 5.6 years. Using the Frankel score, this study revealed better outcomes in the early surgery group compared to late surgical groups. There was also better pain control with early rather than delayed surgery. Complication rates did not differ between the three groups. McLain and Benson5 conducted a nonrandomized prospective study in thoracolumbar fractures. Fourteen patients underwent urgent surgery within 24 hours, and 13 patients underwent delayed surgery between 24 and 72 hours following injury. At 2-year follow-up, no significant difference in neurologic outcome between the two groups was noted. There was also no difference between groups in rates of complications, mortality, or morbidity. Cengiz et al.,20 in a prospective, nonrandomized study, compared early stabilization (<8 hours) in 12 patients with thoracolumbar fractures with late (>3 days) stabilization in 15 patients. A statistically significant improvement in motor recovery was noted with early surgery in partial SCI patients. Hospital stays were shorter with early surgery, and the rate of complications appeared higher in the delayed-surgery group.
With incomplete SCI, neurologic improvement has been reported with both operative21–25 and nonoperative13–19,26,27 techniques, although no consistent improvement has been reported with either modality. Neurologic deficit is more likely due to contusion of the spinal cord at the time of injury and not from persistent bony compression of neural structures. The complications of surgical intervention are by no means negligible and include infection, hardware failure, the need for subsequent surgery, and deep vein thrombosis. These complications in general have exceeded those encountered in recumbency.17,22,23,25,27,28
Owing to the severe pulmonary injury sustained by the patient discussed previously, spine surgery was delayed. The patient was immobilized throughout his intubation period and, once extubated, was fitted with a thoracolumbar spine orthosis and transferred to a rehabilitation facility. On follow-up, the patient remained paraparetic and incontinent of urine with poor rectal tone; he also complained of radicular pain bilaterally. Nearly 3 months following injury, when he had failed to regain the ability to walk, the patient was evaluated by neurosurgery and underwent L2-3 laminectomy, repair of the dural rent, and reduction of the herniated roots, with T12-L4 pedicle screw and rod fixation. Eighteen months postoperatively, the patient had improvement in his pain, had regained bladder but not bowel control, and was ambulatory with ankle braces and the assistance of one person.
The management of complex spine fractures, be they cervical, thoracic, or lumbar, in polytrauma patients remains a challenging arena for the spine surgeon.1,7 The choice of treatment should be individualized to the patient’s medical condition and ability to undergo often lengthy prone spine procedures in the face of multiple comorbidities. Though class I evidence in favor of early surgery for spine fractures is sparse,29 our policy has been to proceed with decompression and stabilization of spine fractures, particularly with deficits, as soon as the patient’s condition allows. Recumbency for thoracolumbar fractures and bracing or traction for cervical fractures can be used as a bridge to surgical stabilization when it is deemed safe and appropriate.
Cengiz S.L., Kalkan E., Bayir A., et al. Timing of thoracolumbar spine stabilization in trauma patients—impact on neurological outcome and clinical course. A real prospective randomized controlled study. Arch Orthop Trauma Surg. 2008;128:959-966.
Dai L.Y., Jiang L.S., Jiang S.D. Conservative treatment of thoracolumbar burst fractures: a long-term follow-up results with special reference to the load sharing classification. Spine (Phila Pa 1976). 2008;33(23):2536-2544.
Fehlings M.G., Perrin R.G. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine (Phila Pa 1976). 2006;31(Suppl 11):S28-S35.
Gaebler C., Maier R., Kutscha-Lissberg F., et al. Results of spinal cord decompression and thoracolumbar pedicle stabilization in relation to the time of operation. Spinal Cord. 1999;37:33-39.
Harris M.B., Sethi R.K. The initial assessment and management of the multiple-trauma patient with an associated spine injury. Spine (Phila Pa 1976). 2006;31(Suppl 11):S9-S15.
Hitchon P.W., Torner J.C., Haddad S.S., Follett K.F. Management options in thoracolumbar burst fractures. Surg Neurol. 1998;49:619-627.
Kerwin A.J., Frykberg E.R., Schinco M.A., et al. The effect of early spine fixation on non-neurological outcome. J Trauma. 2005;58:15-21.
McKinley W., et al. Outcomes of early surgical management versus late or no surgical intervention after acute spinal cord injury. Arch Phys Med Rehabil. 2004;85:1818-1825.
McLain R.F., Benson D.R. Urgent surgical stabilization of spinal fractures in the polytrauma patient. Spine (Phila Pa 1976). 1999;24:1646-1654.
Mirza S.K., Krengel W.F.III, Chapman J.R., et al. Early versus delayed surgery for acute cervical spinal cord injury. Clin Orthop Relat Res. 1999;359:104-114. 1999
Rechtine G.R.2nd. Nonoperative management and treatment of spinal injuries. Spine (Phila Pa 1976). 2006; May 15;31(Suppl 11):S22-S27.
Rechtine G.R.2nd, Cahill D., Chrin A.M. Treatment of thoracolumbar trauma: comparison of complications of operative versus nonoperative treatment. J Spinal Disorders. 1999;12(5):406-409.
Vaccaro A.R., Daugherty R.J., Sheehan T.P., et al. Neurological outcome of early versus late surgery for cervical spinal cord injury. Spine (Phila Pa 1976). 1997;22:2609-2613.
Wood K., Butterman G., Mehbod A., et al. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit: a prospective randomized study. J Bone Joint Surgery [Am]. 2003;85:773-781.
1. Harris M.B., Sethi R.K. The initial assessment and management of the multiple-trauma patient with an associated spine injury. Spine (Phila Pa 1976). 2006;31(Suppl 11):S9-S15.
2. Gaebler C., Maier R., Kutscha-Lissberg F., et al. Results of spinal cord decompression and thoracolumbar pedicle stabilization in relation to the time of operation. Spinal Cord. 1999;37:33-39.
3. Kerwin A.J., Frykberg E.R., Schinco M.A., et al. The effect of early spine fixation on non-neurological outcome. J Trauma 2005. 2005;58:15-21.
4. McKinley W., Meade M.A., Kirshblum S., et al. Outcomes of early surgical management versus late or no surgical intervention after acute spinal cord injury. Arch Phys Med Rehabil. 2004;85:1818-1825.
5. McLain R.F., Benson D.R. Urgent surgical stabilization of spinal fractures in the polytrauma patient. Spine (Phila Pa 1976). 1999;24:1646-1654.
6. Mirza S.K., Krengel W.F.III, Chapman J.R., et al. Early versus delayed surgery for acute cervical spinal cord injury. Clin Orthop Relat Res. 1999;359:104-114.
7. Rechtine G.R.2nd. Nonoperative management and treatment of spinal injuries. Spine (Phila Pa 1976). 2006;31(Suppl 11):S22-S27.
8. Vaccaro A.R., Daugherty R.J., Sheehan T.P., et al. Neurological outcome of early versus late surgery for cervical spinal cord injury. Spine (Phila Pa 1976). 1997;22:2609-2613.
9. Frankel H.L., Hancock D.O., Hyslop G., et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia, Part I. Paraplegia. 1969;7:179-192.
10. Guttmann L. Surgical aspects of the treatment of traumatic paraplegia. J Bone Joint Surg [Br]. 1949;31:399-403.
11. Bedbrook G.M. Treatment of thoracolumbar dislocation and fractures with paraplegia. Clin Orthop. 1975;112:27-43.
12. Bedbrook G.M. Spinal injuries with tetraplegia and paraplegia. J Bone Joint Surg [Br]. 1979;61(3):267-284.
13. Reid D.C., Hu R., Davis L.A., Saboe L.A. The nonoperative treatment of burst fractures of the thoracolumbar junction. J Trauma. 1988;28(8):1188-1194.
14. Cantor J.B., Lebwohl N.H., Garvey T., Eismont F.J. Nonoperative management of stable thoracolumbar burst fractures with early ambulation and bracing. Spine (Phila Pa 1976). 1993;18(8):971-976.
15. Mumford J., Weinstein J.N., Spratt K.F., Goel V.K. Thoracolumbar burst fractures. The clinical efficacy and outcome of nonoperative management. Spine (Phila Pa 1976). 1993;18(8):955-970.
16. Tropiano P., Huang R.C., Louis C.A., et al. Functional and radiographic outcome of thoracolumbar and lumbar burst fractures managed by closed orthopaedic reduction and casting. Spine (Phila Pa 1976). 2003;28(21):2459-2465.
17. Wood K., Butterman G., Mehbod A., et al. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit: a prospective randomized study. J Bone Joint Surg [Am]. 2003;85:773-781.
18. Thomas K.C., Bailey C.S., Dvorak M.F., et al. Comparison of operative and nonoperative treatment for thoracolumbar burst fractures in patients without neurological deficit: a systematic review. J Neurosurg Spine. 2006;4:351-358.
19. Dai L.Y., Jiang L.S., Jiang S.D. Conservative treatment of thoracolumbar burst fractures: a long-term follow-up results with special reference to the load sharing classification. Spine (Phila Pa 1976). 2008;33(23):2536-2544.
20. Cengiz S.L., Kalkan E., Bayir A., Ilik K., et al. Timing of thoracolumbar spine stabilization in trauma patients—impact on neurological outcome and clinical course. A real prospective randomized controlled study. Arch Orthop Trauma Surg. 2008;128:959-966.
21. Jacobs R., Asher M., Snider R. Thoracolumbar spinal injuries. A comparative study of recumbent and operative treatment in 100 patients. Spine (Phila Pa 1976). 1980;5:463-477.
22. Gertzbein S.D. Scoliosis Research Society. Multicenter spine fracture study. Spine (Phila Pa 1976). 1992;17(5):528-540.
23. Hitchon P.W., Torner J.C., Haddad S.S., Follett K.F. Management options in thoracolumbar burst fractures. Surg Neurol. 1998;49:619-627.
24. Seybold E.A., Sweeney C.A., Fredrickson B.E., et al. Functional outcome of low lumbar burst fractures. A multicenter review of operative and nonoperative treatment of L3-5. Spine (Phila Pa 1976). 1999;24(20):2154-2161.
25. Tator C.H., Duncan E.G., Edmonds V.E., et al. Comparison of surgical and conservative management in 208 patients with acute spinal cord injury. Can J Neurol Sci. 1987;14:60-69.
26. Weninger P., Schultz A., Harald H. Conservative management of thoracolumbar and lumbar spine compression and burst fractures: functional and radiographic outcomes in 136 cases treated by closed reduction and casting. Arch Orthop Trauma Surg. 2009;129:207-219.
27. Hitchon P.W., Torner J.C. Recumbency in thoracolumbar fractures. Neurosurg Clin North Am. 1997;8(4):509-517.
28. Rechtine G.R.2nd, Cahill D., Chrin A.M. Treatment of thoracolumbar trauma: comparison of complications of operative versus nonoperative treatment. J Spinal Disorders. 1999;12(5):406-409.
29. Fehlings M.G., Perrin R.G. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine (Phila Pa 1976). 2006;31(Suppl 11):S28-S35.
Emergent Surgery
Acute spinal cord injury (SCI) has momentous repercussions in our society. Every year, more than 10,000 Americans are victims of SCI and become physically disabled and psychologically affected.1 Without consideration for loss of productivity and income, the estimated cost of care of complete and incomplete SCI patients in the United States is estimated at $28,000 and $17,000 per patient per year,2 respectively. Thus, the optimization of SCI treatment may imply a greater number of SCI patients with timely neurologic recovery, potential return to work, and reinstatement of wages.
Patients with incomplete SCI generally have a higher functional recovery rate than do patients with complete SCI.3–5 Current neurosurgical and orthopedic practices therefore favor a more aggressive approach and early surgical intervention in this subset of SCI patients.3,6–8 However, because of the lack of well-designed prospective randomized control trials, there remains a controversy in determining the optimal timing of surgery for SCI patients3,7–9 Given the emotional driving forces surrounding the treatment of SCI patients, we strongly recommend early surgical intervention, as it comes with minimal risk but high hope of recovery.
Recent laboratory studies have established that SCI is a dynamic process with temporal evolution of pathophysiologic processes.10–13 Thus, there are primary and secondary injuries that contribute to the progression of acute SCI. The primary injury corresponds to the immediate effect of trauma with the mechanical spinal cord compression and immediate cellular and axonal damage. The secondary injury refers to the effect of the inflammatory cascades, ischemia, and oxidative damage on the spinal cord parenchymae.14
Rationale for Early Surgery
Temporal Pathophysiologic Evolution of Spinal Cord Injury
SCI is a dynamic process with temporal evolution of pathophysiologic processes at the cellular level. Fehlings et al.15 characterized five phases of SCI, based on studies conducted on a mouse model. The phases are (1) immediate at less than 2 hours, (2) early acute at less than 48 hours, (3) subacute at less than 14 days, (4) intermediate at less than 6 months, and (5) chronic beyond 6 months. The primary or initial mechanical injury takes place immediately after the injury, resulting in traumatic transection and injury of the axons, hemorrhage, and activation of immunologic cascades. The secondary injury begins during the early acute phase at less than 48 hours. It is thought to result from persistent cord compression and hypoperfusion of the spinal cord resulting in the development of vasogenic and cytotoxic edema, continued hemorrhage, cell death, and necrosis. This continuum of the secondary cascade is aggravated in patients with multiple traumas, who are more likely to become hypotensive and hypoxic from other injuries.11 The current emphasis on the development of neuroprotective pharmacologic therapies in the setting of acute SCI accentuates the importance of a timely intervention to prevent secondary injuries.15–17 Thus, there is a potential that early surgical decompression, performed within a therapeutic window, may prevent or revert further damages to the spinal cord.
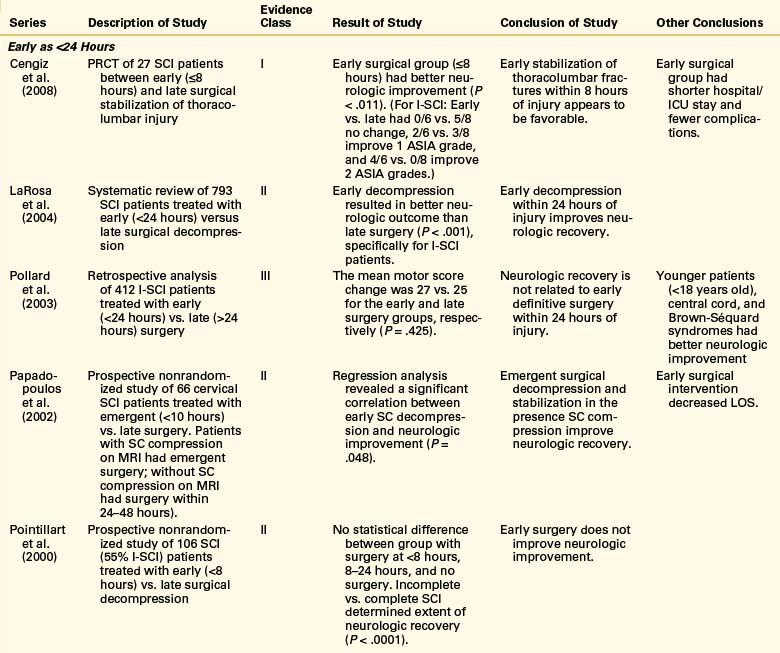
Experimental Studies with Animal Models: Timing of Decompression
Although clinical results have not shown a dramatic effect on the timing of intervention, animal and laboratory research of acute SCI have consistently shown that secondary injury to the spinal cord is potentially preventable or reversible with timely decompression.18–22 These results from 1999 to 2009 on rat and dog models of SCI are reviewed in Table 228-1. Hamamoto et al.19 conducted a study on mouse models of acute SCI evaluating the real-time changes in thoracic spinal cord blood flow as a result of compression forces. They were able to establish that the duration of ischemia resulting from spinal cord compression and the percentage of blood flow recovery following decompression were important factors in the determination of neurologic recovery. They also demonstrated that cases with longer period of spinal cord compression had less recovery of blood flow, more breakdown of the blood–spinal cord barrier, more apoptotic cell death, and less motor function recovery.
Early versus Late Surgery
Early Decompression for Incomplete Spinal Cord Injury
Controversy remains regarding the proper timing of surgical decompression and stabilization in the setting of acute SCI. Studies and reviews conducted to date offer largely class III evidence and some class II evidence either defending or refuting the benefits of early surgical decompression. Table 228-2 provides a summary of literature published in 1999 to 2009 on early decompression of SCI, with a focus on incomplete injuries. Over the past 10 years, several class III evidence studies have reported that early surgical decompression was not associated with improved neurologic recovery.4,23,24 Pointillart et al.23 conducted a nonrandomized prospective study of 66 cervical SCI patients (class II evidence); their study showed no statistically significant difference between groups of patients who had decompression within 8 hours of injury, decompression between 8 and 24 hours after injury, and conservative management. In this study, the lack of randomization constitutes a significant flaw, as a general tendency in current practice is to favor surgical intervention in certain groups of patients who the authors believe have a lower chance of recovery without such intervention. In addition, the time intervals of less than 8 hours versus 8 to 24 hours might show no difference, as they still fall in the therapeutic window of early surgery as defined by newer studies. Pollard and Apple’s25 conclusion that surgery within 24 hours of injury versus late surgery did not improve neurologic recovery was also inferred from a retrospective review of nonrandomized data; however, they did show that patients with young ages and those with incomplete injury had higher recovery rates.
TABLE 228-2 Summary of Studies Conducted on Early Decompressive Surgery Following Incomplete Spinal Cord Injury in 1999–2009
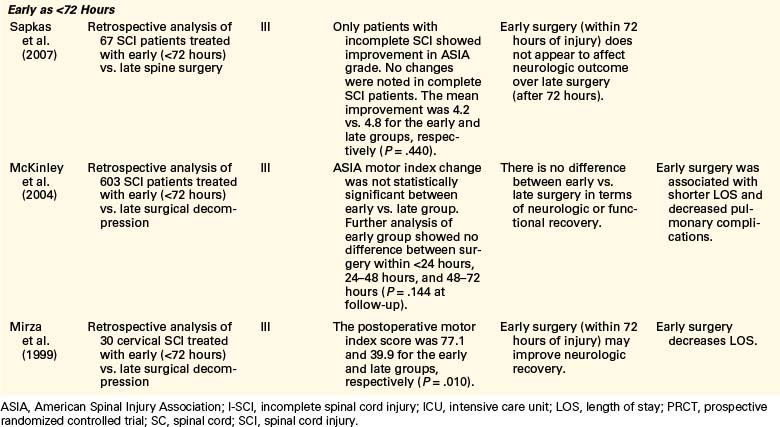
Conversely, several other studies have reported significant improvement in functional recovery with early surgical decompression.3,24,26,27 La Rosa et al.3 conducted a systematic review of the literature on early surgical decompression for acute SCI published between 1966 and 2000. This study analyzed data from 409 patients who underwent decompression within 24 hours of injury, 827 patients who were treated with late surgery, and 1335 patients who were managed conservatively. The authors concluded that early surgical decompression resulted in significantly better outcomes compared to late decompression (P < .001 for complete and incomplete SCI) and conservative management (P = .002 for complete SCI; P < .001 for incomplete SCI). However, on the basis of homogeneity analysis, only the data for incomplete SCI patient were found to be reliable. These findings were further reiterated by Fehlings et al.,7 in a review of 66 articles published between 1995 and 2005.
Fehlings et al.9 recently presented an interim report on the prospective multicenter cohort study STASCIS (Surgical Treatment of Acute Spinal Cord Injury Study), which evaluates the role and timing of decompression in patients with SCI. This study represents the first successful attempt to design and execute an investigation that would provide class I evidence data for the treatment of acute SCI. Preliminary results already suggest a more favorable outcome with early surgical intervention; these results show that in patients with isolated cervical SCI, early decompression within 24 hours of injury may improve neurologic recovery (P = .015) and decrease complication rates (P = .059) at 1-year follow-up.
Special Considerations for Urgent Surgery
There are a few clinical scenarios that exist in which urgent surgical decompression and stabilization are strongly recommended. One such scenario involves patients with incomplete SCI who have a highly unstable spinal column injury with a progressive neurologic deficit.7 Patients with spinal instability have the potential of a progression of their neurologic deficit with even logroll, transfer, and spine motion.28,29 Incomplete SCI patients with unstable subaxial cervical facet dislocation injuries may also be candidates for urgent surgical treatment. These can be initially managed by closed reduction; however, 26% of patients fail reduction by traction, an additional 28% do not maintain reduction with traction only, and there is a potential risk of further cord injury by ventral disc herniation with closed reduction alone.30
One high-risk population is patients with ankylosing spondylitis, since they have altered anatomy and biomechanics of the spine, which makes them prone to SCI following minor trauma.31 They have a high degree of acute spinal instability after a fracture, a higher rate of progressive neurologic deficit, and an increased mortality rate.31–34 There are varying treatment strategies with these patients; some groups have argued that ankylosing spondylitis patients with cervical SCI should be treated with halo immobilization alone.35,36 However, owing to the high rate of nonunion, patients’ inability to tolerate the devices, progressive myelopathy while in halo brace, and skin breakdown under the brace due to severe kyphosis, other groups recommend early decompression and internal fixation.37–40
Safety and Benefits of Early Surgery
There has been a concern that early surgical intervention in the acute trauma patient is associated with a higher complication rate in SCI patients, especially those with high cervical injuries and significant multiorgan trauma. These acute trauma patients often have concurrent cardiopulmonary compromise from hemorrhage, lung contusions, and loss of respiratory drive and vascular tone. For many years, several spine groups advocated against early surgical intervention in this subset of patients, owing to the higher incidence of neurologic deterioration and potential for postoperative complications.41–44 More recent studies have demonstrated that acute surgical intervention in the SCI population is not associated with an increased complication rate in comparison to conservative treatment groups.45–49 This upturn could be attributed to advances in the medical management of critically ill patients, neuroanesthesia, and spine surgery techniques.50 In fact, the recent series by McKinley et al.,24 as well as Cengiz et al.,26 illustrated decreased systemic complication rates with early surgical intervention.
A further concern surrounding early surgical intervention for acute SCI is whether or not this treatment causes an increase in patients’ length of hospital stay. A prospective randomized control trial study by Vaccaro et al.51 reported that there was no significant difference in length of ICU and inpatient rehabilitation length of stay for early versus late surgical groups. Other clinical series have documented a decrease in length of ICU and hospital stays with early surgery intervention.27,46
Obstacles of Early Surgery
Assessment and Stabilization of Spinal Cord Injury Patients
Every trauma patient should be approached and initially managed in accordance with the Advanced Trauma Life Support guidelines, which include establishment and maintenance of airway, breathing, circulation and proper spine immobilization. Cardiovascular and respiratory stability are cardinal to the management of SCI patients.52,53 Several human studies have shown that aggressive management of blood pressure for spinal cord hyperperfusion and adequate oxygenation have a significant effect on neurologic recovery.45,50,54 Conversely, hypotension and hypoxemia are associated with worse neurologic outcomes, independent of other interventions.10,55,56 Before any consideration for operative intervention, SCI patients need to be stable and optimized from a cardiovascular and pulmonary standpoint.
Logistical Obstacles to Emergency Surgery
Despite the controversy surrounding the timing of surgery in the setting of acute SCI, many centers have opted for aggressive early decompression protocols. Clinical data from the NASCIS-2 report on the use of methylprednisolone suggests that the therapeutic window for humans might be within 8 hours of injury.16 However, there are significant logistical obstacles that make it extremely challenging to minimize the transportation time of SCI patients from the injury site to the operating room to less than 8 hours. In their series, Ng et al.47 were able to perform surgery within 8 hours in only 8% of patients. Tator et al.57 accomplished decompression in 23.5% of patients within 24 hours. In view of these limitations, most groups adopted a time to treatment window of 24 hours; however this is inferred from laboratory data. In a recent study, Papadopoulos et al.27 established that a 24-hour window to treat was feasible in a tertiary treatment center.
Conclusion
A traumatic SCI is a devastating experience for a patient and has a significant socioeconomic impact. Patients with incomplete myelopathy or neurologic injury generally have better neurologic recovery and therefore are usually treated more aggressively than patients with complete injury. Owing to a deficiency of class I evidence, there are currently no standard or established guidelines regarding the role and timing of decompression in the setting of acute SCI. However, on the basis of reviews of published literature, La Rosa et al.3 and Fehlings et al.7 recommend as a practice guideline that early surgery (within 72 hours) can be performed safely in patients with SCI if they are hemodynamically optimized. These authors also recommend as a practice guideline that urgent decompression can be performed for acute SCI patients with neurologic deterioration. In addition, they suggest urgent decompression as a reasonable practice option in acute cervical SCI within 24 hours of injury, as it may reduce length of stay and complication rates. We strongly advocate for early surgical intervention (within 24 hours), as it has been shown to potentially improve functional outcomes of patients with incomplete SCI and because recent studies have shown that it is associated with lower complication rates and a shorter hospital length of stay.
Fehlings M.G., Perrin R.G. The role and timing of early decompression for cervical spinal cord injury: update with a review of recent clinical evidence. Injury. 2005;36(Suppl 2):B13-B26.
Fehlings M.G., Perrin R.G. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine (Phila Pa 1976). 2006;31:S28-S35. discussion S36
Fehlings M.G., Vaccaro A.R., Aarabi B., et al. One year outcomes of the STASCIS study: a prospective, multicenter trial to evaluate the role and timing of decompression in patients with cervical spinal cord injury. 2009. Available from http://aans.org/library/article.aspx?ArticleId=54358 Accessed May 12, 2009
La Rosa G., Conti A., Cardali S., et al. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using a meta-analytical approach. Spinal Cord. 2004;42:503-512.
McKinley W., Meade M.A., Kirshblum S., Barnard B. Outcomes of early surgical management versus late or no surgical intervention after acute spinal cord injury. Arch Phys Med Rehabil. 2004;85:1818-1825.
Sapkas G.S., Papadakis S.A. Neurological outcome following early versus delayed lower cervical spine surgery. J Orthop Surg (Hong Kong). 2007;15:183-186.
Papadopoulos S.M., Selden N.R., Quint D.J., et al. Immediate spinal cord decompression for cervical spinal cord injury: feasibility and outcome. J Trauma. 2002;52:323-332.
1. National Spinal Cord Injury Statistical Center. Spinal cord injury. Facts and figures at a glance. J Spinal Cord Med. 2005;28:379-380.
2. French D.D., Campbell R.R., Sabharwal S., et al. Health care costs for patients with chronic spinal cord injury in the veterans health administration. J Spinal Cord Med. 2007;30:477-481.
3. La Rosa G., Conti A., Cardali S., et al. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using a meta-analytical approach. Spinal Cord. 2004;42:503-512.
4. Sapkas G.S., Papadakis S.A. Neurological outcome following early versus delayed lower cervical spine surgery. J Orthop Surg (Hong Kong). 2007;15:183-186.
5. Geisler F.H., Coleman W.P., Grieco G., et al. Measurements and recovery patterns in a multicenter study of acute spinal cord injury. Spine (Phila Pa 1976). 2001;26:S68-S86.
6. Geisler F.H., Coleman W.P., Grieco G., et al. Recruitment and early treatment in a multicenter study of acute spinal cord injury. Spine (Phila Pa 1976). 2001;26:S58-S67.
7. Fehlings M.G., Perrin R.G. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine (Phila Pa 1976). 2006;31:S28-S35. discussion S36
8. Fehlings M.G., Perrin R.G. The role and timing of early decompression for cervical spinal cord injury: update with a review of recent clinical evidence. Injury. 2005;36(Suppl 2):B13-B26.
9. Fehlings M.G., Vaccaro A.R., Aarabi B., et al. One year outcomes of the STASCIS study: a prospective, multicenter trial to evaluate the role and timing of decompression in patients with cervical spinal cord injury. 2009. Available from http://aans.org/library/article.aspx?ArticleId=54358 Accessed May 12, 2009
10. Amar A.P., Levy M.L. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999;44:1027-1039. discussion 1039–1040
11. Tator C.H., Fehlings M.G. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15-26.
12. Fehlings M.G., Sekhon L.H. Cellular, ionic, and biomolecular mechanisms of the injury process. In: Benzel E., Tator C.H., editors. Contemporary management of spinal cord injury: from impact to rehabilitation. Chicago: American Association of Neurological Surgeons; 2000:33-50.
13. Tator C.H. Pathophysiology and pathology of spinal cord injury. In: Wilkins R.H., Rengachary S.S., editors. Neurosurgery. ed 2. New York: McGraw Hill; 1996:2847-2859.
14. Rowland J.W., Hawryluk G.W., Kwon B., Fehlings M.G. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25:E2.
15. Fehlings M.G., Baptiste D.C. Current status of clinical trials for acute spinal cord injury. Injury. 2005;36(Suppl 2):B113-B122.
16. Bracken M.B., Holford T.R. Effects of timing of methylprednisolone or naloxone administration on recovery of segmental and long-tract neurological function in NASCIS 2. J Neurosurg. 1993;79:500-507.
17. Bracken M.B., Shepard M.J., Holford T.R., et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. results of the third National Acute Spinal Cord Injury randomized controlled trial. J Neurosurg. 1998;89:699-706.
18. Xu K., Chen Q.X., Li F.C., et al. Spinal cord decompression reduces rat neural cell apoptosis secondary to spinal cord injury. J Zhejiang Univ Sci B. 2009;10:180-187.
19. Hamamoto Y., Ogata T., Morino T., et al. Real-time direct measurement of spinal cord blood flow at the site of compression: relationship between blood flow recovery and motor deficiency in spinal cord injury. Spine (Phila Pa 1976). 2007;32:1955-1962.
20. Shields C.B., Zhang Y.P., Shields L.B., et al. The therapeutic window for spinal cord decompression in a rat spinal cord injury model. J Neurosurg Spine. 2005;3:302-307.
21. Carlson G.D., Gorden C.D., Oliff H.S., et al. Sustained spinal cord compression: Part I: Time-dependent effect on long-term pathophysiology. J Bone Joint Surg [Am]. 2003;85-A:86-94.
22. Dimar J.R.2nd, Glassman S.D., Raque G.H., et al. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine (Phila Pa 1976). 1999;24:1623-1633.
23. Pointillart V., Petitjean M.E., Wiart L., et al. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord. 2000;38:71-76.
24. McKinley W., Meade M.A., Kirshblum S., Barnard B. Outcomes of early surgical management versus late or no surgical intervention after acute spinal cord injury. Arch Phys Med Rehabil. 2004;85:1818-1825.
25. Pollard M.E., Apple D.F. Factors associated with improved neurologic outcomes in patients with incomplete tetraplegia. Spine (Phila Pa 1976). 2003;28:33-39.
26. Cengiz S.L., Kalkan E., Bayir A., et al. Timing of thoracolumbar spine stabilization in trauma patients—impact on neurological outcome and clinical course. A real prospective randomized controlled study. Arch Orthop Trauma Surg. 2008;128:959-966.
27. Papadopoulos S.M., Selden N.R., Quint D.J., Patel N., Gillespie B., Grube S. Immediate spinal cord decompression for cervical spinal cord injury: feasibility and outcome. J Trauma. 2002;52:323-332.
28. Richter D., Latta L.L., Milne E.L., et al. The stabilizing effects of different orthoses in the intact and unstable upper cervical spine: a cadaver study. J Trauma. 2001;50:848-854.
29. Conrad B.P., Horodyski M., Wright J., et al. Log-rolling technique producing unacceptable motion during body position changes in patients with traumatic spinal cord injury. J Neurosurg Spine. 2007;6:540-543.
30. Treatment of subaxial cervical spinal injuries. Neurosurgery. 2002;50:S156-S165.
31. Westerveld L.A., Verlaan J.J., Oner F.C. Spinal fractures in patients with ankylosing spinal disorders: a systematic review of the literature on treatment, neurological status and complications. Eur Spine J. 2009;18:145-156.
32. Whang P.G., Goldberg G., Lawrence J.P., et al. The management of spinal injuries in patients with ankylosing spondylitis or diffuse idiopathic skeletal hyperostosis: a comparison of treatment methods and clinical outcomes. J Spinal Disord Tech. 2009;22:77-85.
33. Altenbernd J., Bitu S., Lemburg S., et al. Vertebral fractures in patients with ankylosing spondylitis: a retrospective analysis of 66 patients [in German]. Rofo. 2009;181:45-53.
34. Thumbikat P., Hariharan R.P., Ravichandran G., et al. Spinal cord injury in patients with ankylosing spondylitis: a 10-year review. Spine (Phila Pa 1976). 2007;32:2989-2995.
35. Murray G.C., Persellin R.H. Cervical fracture complicating ankylosing spondylitis: a report of eight cases and review of the literature. Am J Med. 1981;70:1033-1041.
36. Weinstein P.R., Karpman R.R., Gall E.P., Pitt M. Spinal cord injury, spinal fracture, and spinal stenosis in ankylosing spondylitis. J Neurosurg. 1982;57:609-616.
37. Taggard D.A., Traynelis V.C. Management of cervical spinal fractures in ankylosing spondylitis with posterior fixation. Spine (Phila Pa 1976). 2000;25:2035-2039.
38. Einsiedel T., Schmelz A., Arand M., et al. Injuries of the cervical spine in patients with ankylosing spondylitis: experience at two trauma centers. J Neurosurg Spine. 2006;5:33-45.
39. Cornefjord M., Alemany M., Olerud C. Posterior fixation of subaxial cervical spine fractures in patients with ankylosing spondylitis. Eur Spine J. 2005;14:401-408.
40. Kanter A.S., Wang M.Y., Mummaneni P.V. A treatment algorithm for the management of cervical spine fractures and deformity in patients with ankylosing spondylitis. Neurosurg Focus. 2008;24:E11.
41. Bedbrook G.M., Sakae T. A review of cervical spine injuries with neurological dysfunction. Paraplegia. 1982;20:321-333.
42. Wilmot C.B., Hall K.M. Evaluation of the acute management of tetraplegia: conservative versus surgical treatment. Paraplegia. 1986;24:148-153.
43. Marshall L.F., Knowlton S., Garfin S.R., et al. Deterioration following spinal cord injury. A multicenter study. J Neurosurg. 1987;66:400-404.
44. Tator C.H., Duncan E.G., Edmonds V.E., et al. Comparison of surgical and conservative management in 208 patients with acute spinal cord injury. Can J Neurol Sci. 1987;14:60-69.
45. Vale F.L., Burns J., Jackson A.B., Hadley M.N. Combined medical and surgical treatment after acute spinal cord injury: results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J Neurosurg. 1997;87:239-246.
46. Chen T.Y., Dickman C.A., Eleraky M., Sonntag V.K. The role of decompression for acute incomplete cervical spinal cord injury in cervical spondylosis. Spine (Phila Pa 1976). 1998;23:2398-2403.
47. Ng W.P., Fehlings M.G., Cuddy B., et al. Surgical treatment for acute spinal cord injury study pilot study #2: evaluation of protocol for decompressive surgery within 8 hours of injury. Neurosurg Focus. 1999;6:E3.
48. Mirza S.K., Krengel W.F.3rd, Chapman J.R., et al. Early versus delayed surgery for acute cervical spinal cord injury. Clin Orthop Relat Res. 1999;359:104-114.
49. Waters R.L., Meyer P.R.Jr., Adkins R.H., Felton D. Emergency, acute, and surgical management of spine trauma. Arch Phys Med Rehabil. 1999;80:1383-1390.
50. Levi L., Wolf A., Belzberg H. Hemodynamic parameters in patients with acute cervical cord trauma: description, intervention, and prediction of outcome. Neurosurgery. 1993;33:1007-1016. discussion 1016–1017
51. Vaccaro A.R., Daugherty R.J., Sheehan T.P., et al. Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine (Phila Pa 1976). 1997;22:2609-2613.
52. Blood pressure management after acute spinal cord injury. Neurosurgery. 2002;50:S58-S62.
53. Management of acute spinal cord injuries in an intensive care unit or other monitored setting. Neurosurgery. 2002;50:S51-S57.
54. Wolf A., Levi L., Mirvis S., et al. Operative management of bilateral facet dislocation. J Neurosurg. 1991;75:883-890.
55. King B.S., Gupta R., Narayan R.K. The early assessment and intensive care unit management of patients with severe traumatic brain and spinal cord injuries. Surg Clin North Am. 2000;80:855-870. viii–ix
56. Tator C.H. Experimental and clinical studies of the pathophysiology and management of acute spinal cord injury. J Spinal Cord Med. 1996;19:206-214.
57. Tator C.H., Fehlings M.G., Thorpe K., Taylor W. Current use and timing of spinal surgery for management of acute spinal surgery for management of acute spinal cord injury in North America: results of a retrospective multicenter study. J Neurosurg. 1999;91:12-18.
Nonemergent Surgery
Despite significant interest and research in the treatment of traumatic spinal cord injury (SCI), including advances in modern surgical and nonsurgical techniques to manage SCI, the prognosis for neurologic recovery in patients with SCI is still poor. In the United States alone, SCI affects 10,000 to 12,000 people annually.1–3 Tragically, these injuries, which are the result of motor vehicle accidents, falls, and athletic injuries, disproportionately affect younger patients.4 SCI shortens the lives of those that it affects in proportion to the degree of injury.5 Economically, SCI contributes to the loss of many productive years by affected individuals and society at large with significant direct costs, estimated at $80,000 to $120,000 per patient annually for an estimated yearly direct liability of $4 billion in the United States.6
Indirect evidence provided by pharmacologic studies, postmortem analysis, and animal models have been the key to our basic understanding the mechanisms of SCI. As early as 1911, Allen, in a dog model for traumatic SCI, demonstrated that drainage of traumatic hematomyelia via myelotomy led to improved neurologic recovery in injured animals.7 By demonstrating postoperative neurologic recovery in the context of traumatic cord injury, this study suggested that secondary, reversible mechanisms of SCI exist beyond the injury associated with the initial neurologic insult. Since its publication, multiple mechanisms of secondary SCI have been proposed, including spinal cord ischemia, oxidative injury from free radical formation, catecholamine release, cytokine-mediated inflammatory responses, and calcium ion–mediated cell injury and death.8–10 Indeed, the results of limited and often controversial pharmacologic studies aimed at targeting these various mechanisms have reported improved outcome in patients with incomplete SCI and have largely been the rationale for early interventions in affected patients.11–13
Anatomic postmortem studies have also shed light onto SCI mechanisms. Analysis of spinal cords from deceased patients with incomplete and complete injuries has demonstrated greater structural continuity in some cases of complete injury.13 This finding has led some researchers to the conclusion that mechanisms beyond structural white matter disruption contribute to the overall disability of affected patients and suggests that even in the context of structural discontinuity, reversible mechanisms of injury might still exist.14
A host of animal studies have been used to delineate mechanisms of SCI. In these studies, two major factors have been identified that determine the reversibility of SCI: the magnitude of impact force at the time of injury and the persistence of compressive forces after injury.15–17 While from a postinjury clinical treatment perspective, impact forces are unalterable, surgical and nonsurgical interventions can be utilized to treat persistent spinal cord compression. Multiple animal studies support the idea that early decompression of the spinal cord, within minutes to hours, may maximize neurologic outcomes.15,18,19 These findings are the basis for the concept of rapid decompression of the spinal cord after trauma.
Indications for Emergent and Nonemergent Interventions
Indications exist for which urgent surgery for decompression and correction of alignment can be rationalized. The hemodynamically stable patient with incomplete SCI with active, rapid neurologic decline and patients with bilateral locked cervical facets are appropriate candidates for emergent surgery. These indications have some support in the published literature20–21 and generally represent clinical scenarios in which withholding treatment would pose significant ethical dilemmas that would be unacceptable. With this moral constraint, it would be impossible to design well-controlled class I studies to determine the benefit of delaying surgery in these patients.
Clinical scenarios also exist that strongly favor nonemergent intervention. Traumatic central cord syndrome is the most common incomplete SCI.22 This entity manifests as disproportionate upper-extremity weakness with variable sensory deficits and bladder dysfunction. It has multiple causes, the most common of which is trauma in the setting of congenital cervical stenosis without fracture or dislocation. Other causes include disc, bone, or ligamentous disruption with subsequent cord compression. While the latter patients may benefit from decompressive surgery, no benefit has been demonstrated with surgical decompression in patients with spinal stenosis alone.23 The natural history of patients with traumatic central cord syndrome in the context of stenosis is favorable with a significant number of patients improving.24 Given that many of these patients are elderly with multiple comorbidities, the conservative tack toward treatment allows for medical optimization prior to any surgical intervention. A course of conservative treatment, including physical rehabilitation, may help to mobilize these patients and allow them to maximize the opportunity to spontaneously recover prior to surgery.
The second set of patients that do not qualify for urgent surgery are those with overt hemodynamic instability and/or trauma patients with concurrent life-threatening injuries to visceral organs. Standard trauma protocols mandate respiratory and hemodynamic stabilization of patients prior to interventions for neurologic issues. A significant number of patients with SCI are hemodynamically unstable. Sixty-eight percent of patients with severe cervical spine injuries are hypotensive.25
There is a substantial need, in particular, for cardiovascular interventions in patients with high cervical injuries.26 Hypotension, especially in the context of spinal cord compression, may compromise spinal cord perfusion and worsen outcome after SCI. Treatment of hypotension is therefore necessary to maximize neurologic outcome. Indeed, it has been demonstrated that the severity of SCI is related to delayed time to surgery secondary to increased hemodynamic instability in such patients.27
Rationale for Emergency Treatment (and Criticism of Such Treatment)
The most common rationale for emergency treatment of SCI lies in the results of the second and third National Acute Spinal Cord Injury Study (NASCIS II and III). NASCIS II demonstrated a modest outcome benefit of high-dose methylprednisolone therapy for patients with incomplete and complete SCI.28 NASCIS III suggested a trend to better outcomes in patients who were treated between 3 and 8 hours after trauma.12 Related to the potential window of opportunity for emergency intervention that these studies suggest are the known complications related to high-dose steroid therapy. Increased rates of stress ulceration and infective complications occur with use of high-dose steroids. Interestingly, the evidence demonstrating adverse side effects from high-dose steroid therapy is stronger than the evidence suggesting benefits of their use in patients with SCI. The wide usage of these data to justify early intervention is controversial and potentially injurious to patients. Significant criticisms of the NASCIS studies exist in the literature focusing on questionable statistical methodology, results, and lack of reviewable data for verification of findings.29–32 Evidence-based reviews of the literature about high-dose steroid therapy in nonpenetrating SCI have concluded that the recommendations suggested by NASCIS II and III should be considered investigational, possibly damaging, and certainly not a standard of care.33
Rationale for Delayed Treatment
The patient with incomplete SCI without traumatic central cord syndrome is the exception and not the rule. It is these patients, however, that are the pivotal focus of the literature on the search for optimal surgical timing. For the majority of the history of modern spine surgery, a delay of surgery in these patients has been the dominant practice. The concept of delayed surgery is grounded in the notion of preventing operating on the newly injured patient (akin to opening Pandora’s box), to prevent complications related to unknown medical problems and physiologic changes related to acute injury that might potentiate neurologic decline. The concept of conservative early management originated from the Stoke Mandeville Hospital experience, championed by Sir Ludwig Guttman in the 1940s. These treatment options, grounded in bedrest and postural stability, were the basis of landmark work by Frankel and associates in 196934 demonstrating good results in conservatively managed SCI patients, with a minimal rate of neurologic decline. Indeed nonoperative postural stability and reduction of deformity have been associated with neurologic improvement.4 This was further supported by Larson et al. in 1976, who advocated operating a week or more after SCI for medical optimization and neurologic stabilization and evolved the conservative concept into a modern standard of care.35
One of the most significant contributions to our understanding of the effects of early versus late surgical management is the work of Marshall et al. in 1987.36 Their multicenter study demonstrated two key findings in SCI: (1) neurologic decline in SCI is an uncommon event, usually related to treatment, and (2) decline in SCI is related to cervical surgery performed earlier than 5 days postoperatively. Both findings support the rationale of delaying surgery in SCI until the patient has been medically optimized and stabilized neurologically.
There are multiple studies in the literature both advocating and opposing nonemergent operative treatment in SCI. The data are generally limited to small prospective studies or retrospective studies, underlining the difficulty of performing rigorous studies of interventions for SCI. An important, isolated prospective randomized study focusing on neurologic outcome in cervical SCI was performed.37 Unfortunately, this study’s methodology precludes conclusions about whether emergent surgery (<24 hours after injury) is superior to delayed surgery. Nonetheless, despite these methodologic limitations, the findings of this study are important. The authors reported no difference in neurologic outcome in patients treated in an early fashion (<72 hours after injury) compared to patients treated after 5 days. While this does not definitively resolve the question of the necessity of emergent intervention in the stable, incompletely injured SCI patient, it certainly suggests that neurologic function is not adversely affected by delaying surgery. Furthermore, neurologic recovery has been reported after delaying surgical decompression of the spinal cord months to years after injury.36,38 Even the most exhaustive recent metareview of the surgical timing literature could recommended emergent decompression of the injured spine only at the level of option.20
Larson S.J., Holst R.A., Hemmy D.C., Sances A.Jr. Lateral extracavitary approach to traumatic lesions of the thoracic and lumbar spine. J Neurosurg. 1976;45:628-637.
Marshall L.F., Knowlton S., Garfin S.R., et al. Deterioration following spinal cord injury. A multicenter study. J Neurosurg. 1987;66:400-404.
Tator C.H., Fehlings M.G. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15-26.
Transfeldt E.E., White D., Bradford D.S., Roche B. Delayed anterior decompression in patients with spinal cord and cauda equina injuries of the thoracolumbar spine. Spine (Phila Pa 1976). 1990;15:953-957.
Vaccaro A.R., Daugherty R.J., Sheehan T.P., et al. Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine (Phila Pa 1976). 1997;22:2609-2613.
1. DeVivo M.J. Causes and costs of spinal cord injury in the United States. Spinal Cord. 1997;35:809-813.
2. Kraus J.F., Franti C.E., Riggins R.S., et al. Incidence of traumatic spinal cord lesions. J Chronic Dis. 1975;28:471-492.
3. Sekhon L.H., Fehlings M.G. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976). 2001;26:S2-S12.
4. Brunette D.D., Rockswold G.L. Neurologic recovery following rapid spinal realignment for complete cervical spinal cord injury. J Trauma. 1987;27:445-447.
5. Soden R.J., Walsh J., Middleton J.W., et al. Causes of death after spinal cord injury. Spinal Cord. 2000;38:604-610.
6. Ball J.R., Sekhon L.H. Timing of decompression and fixation after spinal cord injury: when is surgery optimal? Crit Care Resusc. 2006;8:56-63.
7. Allen A.R. Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column: a preliminary report. JAMA. 1911;57:878-880.
8. Hall E.D., Yonkers P.A., Horan K.L., Braughler J.M. Correlation between attenuation of posttraumatic spinal cord ischemia and preservation of tissue vitamin E by the 21-aminosteroid U74006F: evidence for an in vivo antioxidant mechanism. J Neurotrauma. 1989;6:169-176.
9. Tator C.H., Fehlings M.G. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15-26.
10. Young W. Secondary CNS injury. J Neurotrauma. 1988;5:219-221.
11. Bracken M.B., Holford T.R. Effects of timing of methylprednisolone or naloxone administration on recovery of segmental and long-tract neurological function in NASCIS 2. J Neurosurg. 1993;79:500-507.
12. Bracken M.B., Shepard M.J., Holford T.R., et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury: results of the third National Acute Spinal Cord Injury randomized controlled trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597-1604.
13. Bracken M.B., Shepard M.J., Holford T.R., et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. J Neurosurg. 1998;89:699-706.
14. Kulkarni M.V., Bondurant F.J., Rose S.L., Narayana P.A. 1.5 tesla magnetic resonance imaging of acute spinal trauma. Radiographics. 1988;8:1059-1082.
15. Guha A., Tator C.H., Endrenyi L., et al. Decompression of the spinal cord improves recovery after acute experimental spinal cord compression injury. Paraplegia. 1987;25:324-339.
16. Kobrine A.I., Evans D.E., Rizzoli H.V. Correlation of spinal cord blood flow, sensory evoked response, and spinal cord function in subacute experimental spinal cord compression. Adv Neurol. 1978;20:389-394.
17. Tarlov I.M., Klinger H. Spinal cord compression studies. II. Time limits for recovery after acute compression in dogs. AMA Arch Neurol Psychiatry. 1954;71:271-290.
18. Dolan E.J., Tator C.H., Endrenyi L. The value of decompression for acute experimental spinal cord compression injury. J Neurosurg. 1980;53:749-755.
19. Nystrom B., Berglund J.E. Spinal cord restitution following compression injuries in rats. Acta Neurol Scand. 1988;78:467-472.
20. Fehlings M.G., Perrin R.G. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine (Phila Pa 1976). 2006;31:S28-S35. discussion S36
21. Sonntag V.K. Management of bilateral locked facets of the cervical spine. Neurosurgery. 1981;8:150-152.
22. McKinley W., Santos K., Meade M., Brooke K. Incidence and outcomes of spinal cord injury clinical syndromes. J Spinal Cord Med. 2007;30:215-224.
23. Aito S., D’Andrea M., Werhagen L., et al. Neurological and functional outcome in traumatic central cord syndrome. Spinal Cord. 2007;45:292-297.
24. Aarabi B., Koltz M., Ibrahimi D. Hyperextension cervical spine injuries and traumatic central cord syndrome. Neurosurg Focus. 2008;25:E9.
25. Lehmann K.G., Lane J.G., Piepmeier J.M., Batsford W.P. Cardiovascular abnormalities accompanying acute spinal cord injury in humans: incidence, time course and severity. J Am Coll Cardiol. 1987;10:46-52.
26. Bilello J.F., Davis J.W., Cunningham M.A., et al. Cervical spinal cord injury and the need for cardiovascular intervention. Arch Surg. 2003;138:1127-1129.
27. Tuli S., Tuli J., Coleman W.P., et al. Hemodynamic parameters and timing of surgical decompression in acute cervical spinal cord injury. J Spinal Cord Med. 2007;30:482-490.
28. Bracken M.B., Shepard M.J., Collins W.F., et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury: results of the second National Acute Spinal Cord Injury study. N Engl J Med. 1990;322:1405-1411.
29. Coleman W.P., Benzel D., Cahill D.W., et al. A critical appraisal of the reporting of the National Acute Spinal Cord Injury studies (II and III) of methylprednisolone in acute spinal cord injury. J Spinal Disord. 2000;13:185-199.
30. Hurlbert R.J. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Neurosurg. 2000;93:1-7.
31. Nesathurai S. Steroids and spinal cord injury: revisiting the NASCIS 2 and NASCIS 3 trials. J Trauma. 1998;45:1088-1093.
32. Short D.J., El Masry W.S., Jones P.W. High dose methylprednisolone in the management of acute spinal cord injury: a systematic review from a clinical perspective. Spinal Cord. 2000;38:273-286.
33. Hurlbert R.J. The role of steroids in acute spinal cord injury: an evidence-based analysis. Spine (Phila Pa 1976). 2001;26:S39-S46.
34. Frankel H.L., Hancock D.O., Hyslop G., et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7:179-192.
35. Larson S.J., Holst R.A., Hemmy D.C., Sances A.Jr. Lateral extracavitary approach to traumatic lesions of the thoracic and lumbar spine. J Neurosurg. 1976;45:628-637.
36. Marshall L.F., Knowlton S., Garfin S.R., et al. Deterioration following spinal cord injury. A multicenter study. J Neurosurg. 1987;66:400-404.
37. Vaccaro A.R., Daugherty R.J., Sheehan T.P., et al. Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine (Phila Pa 1976). 1997;22:2609-2613.
38. Transfeldt E.E., White D., Bradford D.S., Roche B. Delayed anterior decompression in patients with spinal cord and cauda equina injuries of the thoracolumbar spine. Spine (Phila Pa 1976). 1990;15:953-957.