Thyroid testing
1. What is the single best test to screen for abnormal thyroid gland function?
Serum thyroid-stimulating hormone (TSH) measurement is the best test for assessing thyroid function because the vast majority of cases of thyroid dysfunction are due to primary thyroid disease, to which the pituitary gland responds with predictable changes in TSH secretion. TSH levels are misleading, however, when thyroid dysfunction results from pituitary or hypothalamic disease and in patients with non-thyroidal illnesses. Measurement of serum thyroxine (T4) and triiodothyronine (T3) are useful when the TSH level is outside the reference range.
2. How do you interpret the serum TSH level?
When the TSH is elevated, the patient almost always has primary hypothyroidism; when the TSH is low, the patient usually has primary hyperthyroidism. Abnormal serum TSH values reflect mild thyroid dysfunction long before serum T4 and T3 levels are outside their reference ranges. Exceptions to these rules occur in patients who have pituitary-hypothalamic disorders or non-thyroidal illnesses. Measurement of serum free T4 should be performed whenever the TSH level is high; both free T4 and total T3 (or free T3 by equilibrium dialysis) values are often informative when the TSH is low.
3. Explain how the serum TSH is used to manage patients undergoing thyroid hormone therapy.
Thyroid hormone therapy is usually given to patients for one of two purposes, replacement therapy for hypothyroidism or suppression therapy for thyroid cancer. When replacement is the goal, the dosage should be adjusted to maintain the serum TSH level within the reference range. When suppression is the goal, the dosage should be adjusted to keep the serum TSH level low normal or slightly low for most patients and to keep it “undetectable” for those with aggressive or metastatic thyroid cancer.
4. Discuss the advantages of free thyroid hormone assays.
Free T4 and T3 assays determine the amounts of unbound, bioactive thyroid hormones in the circulation. Free thyroid hormone measurements fall into two main categories: equilibrium dialysis and analog assays. Equilibrium dialysis methods are more accurate because they are not affected by serum thyroid hormone–binding protein abnormalities. Analog methods, which are used by most commercial laboratories, are variably affected by protein binding. Currently, free T4 assays are considered reasonably good, but the accuracy of commercially available free T3 assays remains questionable. This is why many experts still prefer total T3 over free T3 measurements.
5. What do total T4 and T3 assays measure?
These assays measure the total T4 and T3 concentrations in the circulation. More than 99% of circulating T4 and approximately 98% of T3 are bound to proteins, such as thyroxine-binding globulin (TBG), thyroxine-binding prealbumin (TBPA or transthyretin), and albumin. Serum total T4 and T3 levels can therefore be altered by protein-binding disorders.
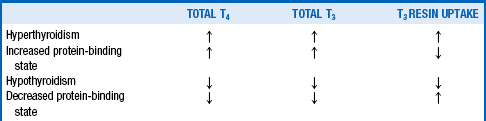
6. Name the major disorders of thyroid hormone-binding proteins.
Pregnancy, estrogen use, congenital TBG excess, and familial dysalbuminemic hyperthyroxinemia (FDH) are the most common. FDH is an inherited disorder in which albumin has enhanced affinity for T4, resulting in increased levels of total T4 but not T3. Protein binding of T4 and T3 is reduced by androgens and congenital TBG deficiency.
A T3 resin uptake (T3RU) measurement helps distinguish protein-binding disorders from true thyroid diseases. The T3RU is inversely proportional to the protein-binding capacity; accordingly, T3RU is low when T4 protein binding is increased and high when T4 protein binding is reduced. Table 32-1 indicates how these tests are used to make the correct diagnosis.
7. What antithyroid antibody tests are clinically useful?
Anti–thyroid peroxidase (TPO) and antithyroglobulin antibodies are present in the sera of most patients with Hashimoto’s thyroiditis. Either test can establish a diagnosis of Hashimoto’s disease, but the TPO antibodies are more sensitive. TSH receptor antibodies (TRAbs) and thyroid-stimulating immunoglobulins (TSIs) are detectable in the sera of most patients with Graves’ disease; their measurement is not necessary when the diagnosis of Graves’ disease is obvious but may be helpful when the diagnosis is in question.
8. How useful are thyroglobulin measurements?
Thyroglobulin (TG) is the major iodoprotein constituent of thyroid follicles. Serum TG levels are mildly increased in many thyroid diseases, but marked elevations are seen mainly with active thyroid cancer and in destructive thyroiditis (subacute, postpartum, or silent thyroiditis). TG measurements are useful for monitoring patients with thyroid cancer. When a patient has been treated and is cancer-free, the serum TG value should be undetectable. Normal or elevated serum TG values in such patients suggest the presence of residual or metastatic thyroid cancer. Most TG assays are not reliable in patients who have anti-TG antibodies because these antibodies interfere with the method of TG measurement.
9. When should a serum calcitonin level be measured?
Calcitonin is made by thyroid parafollicular C cells rather than by follicular cells. Serum calcitonin is elevated in medullary carcinoma of the thyroid (MCT) and in its familial precursor lesion, C-cell hyperplasia. Because MCT is an uncommon thyroid neoplasm, serum calcitonin measurement should not be used in the routine evaluation of most thyroid nodules. It is indicated, however, in a patient who exhibits a feature characteristic of MCT, such as familial occurrence or associated diarrhea.
10. Discuss the utility and interpretation of the radioactive iodine uptake (RAIU) test.
Thyroid follicular cells have iodine symporters or pumps that bring iodine into the cells for thyroid hormone synthesis. The activity of these iodine pumps can be assessed by measuring the radioactive iodine uptake (RAIU). The normal 24-hour RAIU is approximately 10% to 25% in the United States, but this value varies according to location because of geographic differences in dietary iodine intake. The RAIU is most useful in the differential diagnosis of thyrotoxicosis by separating disorders into two distinct categories: high-RAIU thyrotoxicoses and low-RAIU thyrotoxicoses (Table 32-2).
TABLE 32-2.
CLASSIFICATION OF THYROTOXICOSIS AS HIGH OR LOW RADIOACTIVE IODINE UPTAKE (RAIU)
| High-RAIU thyrotoxicosis | Graves’ disease Toxic multinodular goiter Solitary toxic adenoma Thyroid-stimulating hormone (TSH)–secreting tumor Human chorionic gonadotropin (hCG)–induced thyrotoxicosis |
| Low-RAIU thyrotoxicosis | Factitious thyrotoxicosis Iodine-induced thyrotoxicosis Subacute thyroiditis Postpartum thyroiditis Silent thyroiditis Amiodarone-induced thyrotoxicosis |
11. When and why should a thyroid scan be ordered?
A thyroid scan helps distinguish the three most common types of high-RAIU thyrotoxicosis. Graves’ disease is characterized by diffuse tracer uptake; toxic multinodular goiter by multiple discrete areas of increased uptake; and the solitary toxic adenoma by a single area of intense uptake. The scan is not helpful in low-RAIU thyrotoxicosis. A thyroid scan is no longer recommended in the evaluation of thyroid nodules unless the serum TSH level is low, in which case the scan can detect the presence of functioning (hot) nodules.
12. What is recombinant human TSH, and how is it used?
Recombinant human TSH (rhTSH) (Thyrogen) is a synthetic human TSH molecule. Thyrogen can be used to stimulate neoplastic thyroid tissue to absorb radioiodine for an imaging procedure. Thyroid cancer tissue ordinarily traps iodine poorly and can be imaged only if the serum TSH is elevated. This elevation can be accomplished either by stopping levothyroxine treatment for 3 to 6 weeks or by giving Thyrogen injections. After the serum TSH level has been increased by either method, serum TG is measured and radioiodine (131I or 123I) is given for whole-body scanning. A positive scan result or detectable TG level indicates the presence of residual or metastatic thyroid cancer. A Thyrogen-stimulated scan with TG measurement has the same accuracy as a levothyroxine withdrawal scan and has the advantage of not causing symptoms of hypothyroidism.
13. How can heterophile antimouse antibodies interfere with assessment of thyroid function?
Heterophile antimouse antibodies (HAMAs) sometimes develop in people who are regularly exposed to rodents, such as laboratory workers, farm workers, and other people who spend a lot of time outdoors, including homeless people. HAMAs can interfere with the measurement of several hormones, including TSH and thyroglobulin. When TSH or thyroglobulin values are not consistent with the clinical picture, interference by HAMAs should be suspected, and the patient questioned about possible exposure to rodents. When a laboratory is alerted to the possibility of HAMA interference, assay conditions can be altered to minimize or eliminate the misleading results.
Andersen, S, Pedersen, KM, Bruun, NH, et al, Narrow individual variations in serum T4 and T3 in normal subjects. a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab 2002;87:1068–1072.
Cavaleri, R, Thyroid radioiodine uptake. indications and interpretation. Endocrinologist 1992;2:341.
Demers, LM, Spencer, CA, Laboratory medicine practice guidelines. laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):2–126.
Haugen, BR, Pacini, F, Reiners, C, et al. A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancer. J Clin Endocrinol Metab. 1999;84:3877–3885.
Nelson, JC, Wang, R, Asher, DT, et al. The nature of analogue-based free thyroxine estimates. Thyroid. 2004;14:1030–1036.
Nicoloff, JT, Spencer, CA. The use and misuse of the sensitive thyrotropin assays. J Clin Endocrinol Metab. 1990;71:553–558.
Preissner, CM, Dodge, LA, O’Kane, DJ, et al. Prevalence of heterophilic antibody interference in eight automated tumor marker immunoassays. Clin Chem. 2005;51:208–210.
Preissner, CM, O’Kane, DJ, Singh, RJ, et al, Phantoms in the assay tube. heterophile antibody interference with serum thyroglobulin assays. J Clin Endocrinol Metab 2003;88:3069–3074.
Smith, SA. Commonly asked questions about thyroid function. Mayo Clin Proc. 1995;70:573–577.
Wang, R, Nelson, JC, Weiss, RM, et al. Accuracy of free thyroxine measurements across natural ranges of thyroxine binding to serum proteins. Thyroid. 2000;10:31–39.