Thyroid nodules and goiter
A goiter is a visible swelling in the anterior neck from an enlarged thyroid gland. Derivation of the term can be traced to the French goitre, the Middle French goitron, a vulgate Latin term guttrion, and the Latin terms guttrio and guttur. Most of the terms designate the throat.
The pathogenesis for euthyroid goiter remains an enigma. Proposed mechanisms include:
 Thyroid-stimulating hormone (TSH)–dependent thyroid enlargement to compensate for diminished thyroid hormone production due to environmental goitrogens
Thyroid-stimulating hormone (TSH)–dependent thyroid enlargement to compensate for diminished thyroid hormone production due to environmental goitrogens
 Inherited biosynthetic defects
Inherited biosynthetic defects
Regression of goiter after iodine supplementation and with thyroxine suppression of TSH supports these mechanisms. However, TSH values are not elevated in endemic goiter. Inherited biosynthetic genetic defects for thyroglobulin, thyroperoxidase, intracellular signaling pathways affecting cell life cycles, and the sodium/iodide (Na+/I−) symporter have been described.
3. Describe the natural history of diffuse nontoxic goiter.
Simple goiter tends to become multinodular over time. The nodules are heterogeneous in both morphology and function. Autonomous function, defined as TSH-independent production and secretion of thyroid hormone, can evolve. Supplementation programs in iodine-deficient populations clearly decrease the incidence of cretinism and goiter but have also increased the incidence of iodine-associated hyperthyroidism. This Jod-Basedow hyperthyroidism is more likely to occur in older people with autonomous adenomatous goiters. In the United States, this form of hyperthyroidism usually results from iodine excess due to radiographic contrast agents or medications rich in iodine. The thyroid hormone excess may be transient and may not require treatment. When it is severe, antithyroid medications and thyroidectomy can be used. Iodine excess usually precludes radioiodine as a treatment option.
4. How does lithium affect thyroid function?
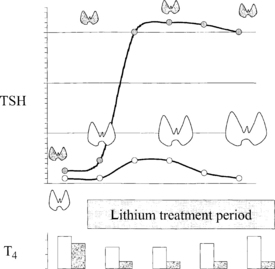
Lithium has diverse effects on thyroid function. It inhibits iodine uptake, dampens iodotyrosine coupling, alters thyroglobulin structure, blocks thyroid hormone secretion, and has mitogenic effects. Both goiter and hypothyroidism can appear during prolonged exposure to lithium (Fig. 36-1).
5. Describe the mechanism by which lithium produces goiter and hypothyroidism.
The inhibitory effect of lithium on thyroid hormone release provokes an increase in TSH secretion even in patients free of thyroid disease. Compensatory thyroid enlargement then occurs without the development of hypothyroidism. However, patients who have an underlying decrease in thyroid functional reserve, even if baseline thyroid hormone levels are normal, may demonstrate hypothyroidism after lithium initiation. This most commonly occurs in patients with chronic lymphocytic thyroiditis, a past history of subacute thyroiditis, or partial thyroidectomy. Because hypothyroid signs and symptoms may be difficult to decipher in the presence of depression or bipolar disorder, TSH testing before and during lithium treatment is recommended.
6. How common are thyroid nodules?
Thyroid nodules are common. Prevalence increases linearly with age. The cumulative lifetime chance of a palpable thyroid nodule approaches 6%. The prevalence at autopsy in 90-year-old subjects is about 60%. The vast majority of thyroid nodules are benign. The yield of thyroid cancer in surgical series before the widespread use of fine-needle aspiration (FNA) biopsy averaged about 10%.
7. List the differential diagnosis for a thyroid nodule.
8. Can the nature of a thyroid nodule be determined from family history?
Family history is usually not helpful. An exception is medullary thyroid cancers associated with the multiple endocrine neoplasia syndromes. Inheritance of these tumors is autosomal dominant with almost complete penetrance for the abnormal ret oncogene.
9. Do personal history and physical examination help define the nature of a thyroid nodule?
In general, no. Most patients with thyroid nodules have no symptoms and normal thyroid function. Thyroid cancer grows without causing pain. Hoarseness, dysphagia, dyspnea, and hemoptysis are rare features that suggest malignancy but also occur in benign thyroid disorders. Report of any of these symptoms by a patient with a visible goiter suggests either rapid growth or involvement of the recurrent laryngeal nerve. An aggressive form of thyroid malignancy, such as lymphoma or anaplastic thyroid cancer, is a consideration. However, both thyroid lymphoma and anaplastic thyroid cancer are rare. Other traits of nodules that suggest malignancy include size greater than 3 cm, fixation to adjacent structures, and palpable cervical lymph nodes.
10. How are most thyroid cancers discovered?
Most thyroid cancers are now discovered by chance. Neck ultrasonography, magnetic resonance imaging (MRI), and computed tomography (CT) studies for myriad indications may first detect a thyroid nodule. Because they are recognized incidentally to the purpose of the procedure, these nodules are termed thyroid incidentalomas. In comparison with the past, when medical imaging was less frequent, it is now unusual for thyroid nodules to be found first by the patient or during routine physical examination.
11. What diagnosis should be suspected when a thyroid nodule is first discovered after sudden onset of neck pain?
Hemorrhagic degeneration of a previously unknown benign adenoma is not infrequent. The thyroid capsule is innervated by sensory pain fibers, whereas only autonomic sympathetic and parasympathetic nerves innervate the substance of the thyroid gland. Sudden expansion of a nodule stretches the thyroid capsule, resulting in deep, aching pain that may radiate to the jaw or ear. This clinical presentation may be mistaken for dental abscess, otitis media, or otitis externa. Aspiration of hemorrhagic fluid often relieves the discomfort and confirms the diagnosis. Other thyroid disorders with palpable enlargement and thyroid tenderness to palpation to consider include thyroid neoplasms and de Quervain’s subacute thyroiditis.
12. If a nodule is cancer, what kind is it likely to be?
A papillary thyroid cancer or variant of papillary carcinoma is the most common by far (Table 36-1).
TABLE 36-1.
FREQUENCY OF THYROID CANCER TYPES
| Papillary | 50%-70% |
| Follicular | 10%-15% |
| Medullary | 1%-2% |
| Anaplastic | Rare |
| Primary thyroid lymphoma | Rare |
| Metastatic to thyroid | Rarely diagnosed |
13. Does the character of cyst fluid define the etiology of the cyst?
Simple thyroid cysts have yellow, burgundy, or chocolate-colored fluid and are generally benign. Complex thyroid nodules with both cystic and solid components contain brown or hemorrhagic fluid. Complex cysts have a higher risk of malignancy than simple cysts. Cytology of cyst fluid is almost always nonspecific, and often only histiocytes and crenated erythrocytes are seen without thyroid epithelial cells. If the fluid is crystal-clear, like tap water, the lesion is a parathyroid cyst. Serum calcium should be measured to exclude hyperparathyroidism.
14. What should be done if a thyroid cyst recurs after being drained?
One third of thyroid cysts reappear days to weeks after aspiration. If the volume on sequential aspirations does not decrease or the aspirated fluid is grossly bloody, surgical removal of the cyst should be considered.
15. Is the risk of cancer less in multinodular goiter or Hashimoto’s disease than in solitary thyroid nodules?
Although autopsy series indicate that up to 75% of thyroid nodules are multiple and that malignancy is rare, any thyroid nodule can be cancerous. Contrary to old axioms, a palpable nodule in the presence of multinodular goiter or lymphocytic thyroiditis seems to have the same risk of cancer as a solitary palpable nodule. There is evidence that TSH levels are a bit higher in patients found to have thyroid cancer than in those with benign nodules. There are even some reports now that thyroid hormone therapy may be a factor associated with a nodule’s being malignant rather than benign. Size does matter. Palpable nodules are generally at least 1 cm in greatest dimension. Nodules smaller than 1 cm are often not palpable and have a lower risk of malignancy than larger nodules. The decision to monitor nonpalpable thyroid nodules or perform fine-needle aspiration (FNA) should incorporate ultrasound features including size and other features more often seen with malignant than benign nodules.
16. Summarize the role of FNA in the evaluation of thyroid nodules
FNA is a safe, outpatient procedure with an accuracy of 90% to 95% in adequate specimens interpreted by experienced cytopathologists. FNA should be performed on all readily palpable solitary thyroid nodules, on dominant nodules in a multinodular goiter, and for sub-centimeter nodules with ultrasonographic characteristics suspicious for thyroid cancer. Suspicious ultrasonographic features include hypoechogenicity, ragged borders, stippled calcifications, internal vascularity, and “taller than wide” (meaning the anterior-posterior dimension of the nodule is greater than its tranverse dimension). Malignancy in multinodular goiter may be missed, and sampling multiple nodules or those nodules shown to be suspicious on ultrasound or to be photopenic on thyroid scintigraphy is a consideration. After a serum TSH level is shown to be normal, an FNA is the next evaluation for a thyroid nodule. Most FNAs return benign diagnoses, including adenomatous hyperplasia (benign multinodular goiter), colloid adenoma, and autoimmune thyroiditis. A reading of papillary thyroid cancer, seen in 3% to 5% of FNAs, helps guide planning for thyroid resection.
When the FNA is nondiagnostic, an ultrasound-guided FNA should be done. For indeterminate cytology categories, the revised American Thyroid Association guideline recommends the use of molecular markers to guide management. Currently, this recommendation is based on expert opinion grade evidence. The use of molecular markers is the most exciting addition to the evaluation and management of thyroid nodules in years. Its role is evolving and being defined but shows promise as a cost-effective test by reducing unnecessary thyroidectomies with their attendant cost and morbidity for patients in whom lesions suspicious for cancer prove to be benign and for guiding surgical and medical management decisions for patients with thyroid cancer.
17. Is FNA helpful in diagnosing follicular neoplasms?
Follicular neoplasms are more vexing. FNA cannot reliably differentiate adenoma from carcinoma because features of capsular or vascular invasion that define follicular carcinoma can be determined only on surgical pathology. Aspirates are inadequate for interpretation in about 15% of cases. This rate can be reduced by using ultrasound guidance, especially for the lesion with a cystic component.
18. Should an FNA be performed for a palpable nodule if the TSH is low?
19. If the TSH is found to be low, what is the next step?
A thyroid scan, to rule in solitary toxic nodule or toxic multinodular goiter, should be the next test. Although the scan is ordered with the anticipation of finding lesions with autonomous function, a photopenic (cold) nodule may sometimes be encountered.
20. Explain the distinction between cold and hot nodules.
A cold nodule has lower uptake of the radioactive agent than surrounding normal thyroid tissue. Most cold nodules are benign, but virtually all thyroid cancers are cold on scan. The solitary toxic or hot nodule avidly absorbs tracer, whereas uptake in the remainder of the thyroid is suppressed. Solitary toxic nodules are usually more than 3 cm in diameter; most occur in patients older than 40 years. Most solitary toxic thyroid nodules have gain-of-function mutations in the thyrotropin receptor gene. Toxic adenomas are never cancerous.
21. What is the significance of a warm nodule?
In contrast, a warm nodule may be malignant. Some hyperfunctional or isofunctional nodules are really cold nodules that appear to concentrate tracer because they are invested by normal thyroid tissue. Other autonomous nodules fail to secrete sufficient thyroid hormone to suppress TSH to dampen tracer uptake by surrounding normal thyroid tissue. Thyroid scanning after the patient takes a TSH-suppressive dose of thyroid hormone can define the autonomous nature of such a nodule. Autonomous nodules may be monitored by observation alone, whereas all others deserve FNA to exclude thyroid cancer.
22. Who invented the incision used for thyroidectomy?
Theodor Kocher (1841-1917), a Swedish surgeon, devised the incision. He was an innovator, so the physician should be cautious when asking for a “Kocher” in the operating room. Kocher’s name is also associated with a surgical forceps, a wrist operation, and a right subcostal incision for cholecystectomy.
23. Which treatment was used first for diffuse toxic goiter (Graves’ disease), radioactive iodine or antithyroid medications?
Both methods were developed in the early 1940s. Thiourea, the first goitrogenic substance to be used, had undesirable toxicities and was soon replaced by methimazole and propylthiouracil. Of the fission products developed during World War II, radioactive iodine (radioiodine) 130I was used before 131I. Radioiodine became widely available in about 1946.
24. What goitrous thyroid conditions are treated with radioactive iodine?
Radioiodine treatment is effective for diffuse toxic goiter, toxic nodular goiter, and solitary toxic nodules. Compressive symptoms from benign multinodular goiters in patients judged to be poor surgical risks can also be relieved by radioactive iodine. Although the goiter shrinks only about 30% or less, relief of symptoms is common.
25. What is the role of suppression therapy with thyroxine?
Although thyroxine suppression therapy was widely used in the past on the basis of the belief that it reduced the size of thyroid nodules, randomized controlled studies, including some with objective measurements by ultrasound, indicate that suppression therapy is ineffective. This finding suggests that the apparent reduction in the size of solitary nodules when judged only by palpation probably represented regression of surrounding thyroid rather than of the nodule itself. For euthyroid patients, thyroid hormone is ineffective except in iodine deficiency and for prevention of new nodules after lobectomy in radiation-exposed patients. These exceptions are almost never seen anymore. Routine treatment with TSH-suppressive doses of thyroid hormone for thyroid nodules or goiter probably has more iatrogenic side effects than benefits and is now discouraged.
Chudova, D, Wilde, JI, Wang, ET, et al. Molecular classification of thyroid nodules using high-dimensionality genomic data. J Clin Endocrinol Metab. 2010;12:5301–5309.
Cooper, DS, Doherty, GM, Haugen, BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214.
Dremier, S, Coppee, F, Delange, F, et al, Thyroid autonomy. mechanism and clinical effects. J Clin Endocrinol Metab 1996;81:4187–4193.
Lazarus, JH. The effects of lithium therapy on thyroid and thyrotropin-releasing hormone. Thyroid. 1998;8:909–913.
Li, H, Robinson, KA, Anton, B, et al. Cost-effectiveness of a novel molecular test for cytologically indeterminate thyroid nodules. J Clin Endocrinol Metab. 2011;11:E1719–E1726.
Mortensen, JD, Woolner, LB, Bennett, WA. Gross and microscopic findings in clinically normal thyroid glands. J Clin Endocrinol Metab. 1955;15:1270–1280.
Nikiforov, YE, Ohori, NP, Hodak, SP, et al, Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules. a prospective analysis of 1056. FNA samples. J Clin Endocrinol Metab 2011;96:3390–3397.
Oertel, YC, Miyahara-Felipe, L, Mendoza, MG, et al, Value of repeated fine needle aspirations of the thyroid. an analysis of over ten thousand FNAs. Thyroid 2007;17:1061–1066.