Chapter 37 Thyroid hormones, antithyroid drugs
• Thyroid hormones (thyroxine/levothyroxine T4, liothyronine T3).
• Use of thyroid hormone: treatment of hypothyroidism.
• Antithyroid drugs and hyperthyroidism: thionamides, drugs that block sympathetic autonomic activity, iodide and radio-iodine 131I, preparation of patients for surgery, thyroid storm (crisis), exophthalmos.
Thyroid hormones
L-Thyroxine (T4 or tetra-iodo-l-thyronine) and lio-l-thyronine (T3 or tri-iodo-l-thyronine) are the natural hormones of the thyroid gland. T4 is a less active precursor of T3, which is the major mediator of physiological effect. In this chapter, T4 for therapeutic use is referred to as levothyroxine (the rINN; see p. 69).
Levothyroxine for hypothyroidism
Levothyroxine is used in some countries for the treatment of non-toxic nodular goitre, on the assumption that nodular thyroid tissue growth is dependent on TSH. The treatment is not curative. Levothyroxine should not be used to treat obesity (see Obesity, p. 602).
Antithyroid drugs and hyperthyroidism
Drugs used for the treatment of hyperthyroidism include:
• Thionamides, which block the synthesis of thyroid hormone.
• Iodine: radioiodine, which destroys the cells that make thyroid hormone; iodide, an excess of which reduces the production of thyroid hormone temporarily (it is also necessary for the formation of hormone, and both excess and deficiency can cause goitre).
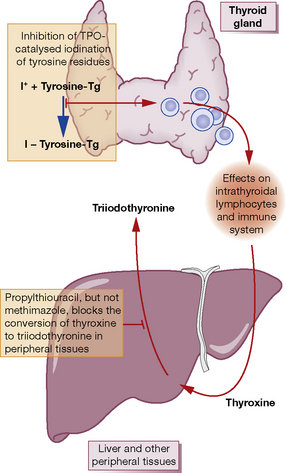
Thionamides (thiourea derivatives) carbimazole, methimazole, propylthiouracil
Mode of action (Fig. 37.1)
Propylthiouracil
(PTU) differs from other members of the group in that it also inhibits peripheral conversion of T4 to T3, but only at high doses used in treatment of thyroid storm (see p. 594). PTU differs from the other thionamides in its apparent radio-protective effect when used prior to radioiodine treatment.
Doses
• Carbimazole 40 mg total/day is given orally (or methimazole 30 mg) until the patient is euthyroid (usually 4–6 weeks). Then either titrate (titration regimen) by decrements initially of 10 mg every 4–6 weeks to a maintenance dose of 5–10 mg/day, or continue (block–replace regimen) 40 mg once daily, and add levothyroxine 100 micrograms/day, with monitoring of free T4 and TSH.
• Propylthiouracil 300–450 mg total/day is given orally until the patient is euthyroid: maintenance 50–100 mg total/day. Much higher doses (up to 2.4 g/day) with frequent administration are used for thyroid storm.
Use
• As adjuvant to radioiodine, before and after administration, to control the disease until the radiation achieves its effect.1
Clinical improvement is noticeable in 2–4 weeks, and the patient should be euthyroid in 4–6 weeks.
Control of antithyroid drug therapy
β-Adrenergic blockade
Quick relief can be obtained with a β-adrenoceptor blocking drug (judge the dose by heart rate), although these do not block all the metabolic effects of the hormone, e.g. on the myocardium, and the basal metabolic rate is unchanged. For this reason, β-blockade is not used as sole therapy except in mild thyrotoxicosis in preparation for radioiodine treatment, and in these patients it should be continued until the radioiodine has taken effect. β-Blockers do not alter the course of the disease, or affect biochemical tests of thyroid function. Any effect on thyroid hormonal action on peripheral tissues is clinically unimportant. Although atenolol is widely used, it is preferable to choose a drug that is non-selective for β1 and β2 receptors and lacks partial agonist effect (e.g. propranolol 20–80 mg 6–8-hourly, or timolol 5 mg once daily). The usual contraindications to β-blockade (see p. 403) apply, especially asthma.
Iodine (iodide and radioactive iodine)
Iodide is well absorbed from the intestine, distributed like chloride in the body, and rapidly excreted by the kidney. It is selectively taken up and concentrated (about × 25) by the thyroid gland, more in hyperthyroidism and less in hypothyroidism. A deficiency of iodide reduces the amount of thyroid hormone produced; this stimulates the pituitary to secrete TSH. The result is hyperplasia and increased vascularity of the gland, with eventual goitre formation.2
Effects
The effects of iodide are complex and related to the dose and thyroid status of the subject.
A euthyroid subject with an autonomous adenoma (hot nodule) becomes hyperthyroid if given iodide.
Uses
Potassium iodate
in doses of 85 mg orally 8-hourly (longer intervals allow some escape from the iodide effect) produces some effect in 1–2 days, maximal after 10–14 days, after which the benefit declines as the thyroid adapts. A similar dose used for 3 days covers administration of some 131I- or 123I-containing preparations, for instance meta-iodobenzylguanidine (123I-MIBG) (see p. 409).
Bronchial secretions
Iodide is concentrated in bronchial and salivary secretions. It acts as an expectorant (see Cough, p. 467).
Organic compounds
containing iodine are used as contrast media in radio-imaging. It is essential to ask patients specifically whether they are allergic to iodine before such contrast media are used. Severe anaphylaxis, even deaths, occur every year in busy imaging departments; iodine-containing contrast media are being superseded by so-called non-ionic preparations.3
Radioiodine (131I)
131I is treated by the body just like the ordinary non-radioactive isotope, so that when swallowed it is concentrated in the thyroid gland. It emits mainly β radiation (90%), which penetrates only 0.5 mm of tissue and thus allows therapeutic effects on the thyroid without damage to the surrounding structures, particularly the parathyroids. It also emits some γ-rays, which are more penetrating and are detectable with a radiation counter.4 131I has a physical (radioactive) t½ of 8 days.
In hyperthyroidism, the beneficial effects of a single dose may be felt in 1 month, and patients should be reviewed at 6 weeks to monitor for onset of hypothyroidism. The maximal effect of radioiodine may take 3 months to achieve. β-Adrenoceptor blockade and, in severe cases, an antithyroid drug (but see footnote 1) will be needed to render the patient comfortable while waiting; this is more likely when radioiodine is used for patients with relapsing thyrotoxicosis. Very rarely radiation thyroiditis causes excessive release of hormone and thyroid storm. Repeated doses may be needed.
Preparation for surgery
Routine preparation of hyperthyroid patients for surgery can be achieved satisfactorily by making them euthyroid with one of the above drugs plus a β-adrenoceptor blocker for comfort (see below) and safety,5 and adding iodate for 7–10 days before operation (not sooner) to reduce the surgically inconvenient vascularity of the gland.
In an emergency, the patient is prepared with a β-adrenoceptor blocker (e.g. propranolol 6-hourly, with dose titration to eliminate tachycardia) for 4 days, continued through the operation and for 7–10 days afterwards. Iodide is also given (see p. 591). The important differences with this second technique are that the gland is smaller and less friable but the patient’s tissues are still hyperthyroid and, to avoid a hyperthyroid crisis or storm, it is essential that the adrenoceptor blocker continue as above without the omission of even a single 6-hourly dose of propranolol.
Treatment of subclinical hyperthyroidism
Subclinical hyperthyroidism is defined biochemically by normal serum free T4 and free T3 but suppressed TSH concentrations. Some 5% of patients per annum progress to frank hyperthyroidism, and there is an increased risk of atrial fibrillation, stroke and osteoporosis. The treatment of endogenous subclinical hyperthyroidism should be considered when the TSH level is less than 0.1 mU/L, especially in patients aged over 60 years, those with an increased risk for heart disease, osteopenia or osteoporosis, or with clinical symptoms suggestive of hyperthyroidism. Other patients should be monitored 6-monthly; eventually 50% become euthyroid.6
Drugs that cause hypothyroidism
Amiodarone
Some 90% of patients receiving amiodarone remain euthyroid. Despite the exposure of the thyroid gland to an extraordinary load of iodine, important adjustments are made in thyroidal iodine handling and hormone metabolism; these are shown in Figure 37.2, and the consequences for thyroid function tests summarised in Table 37.1.
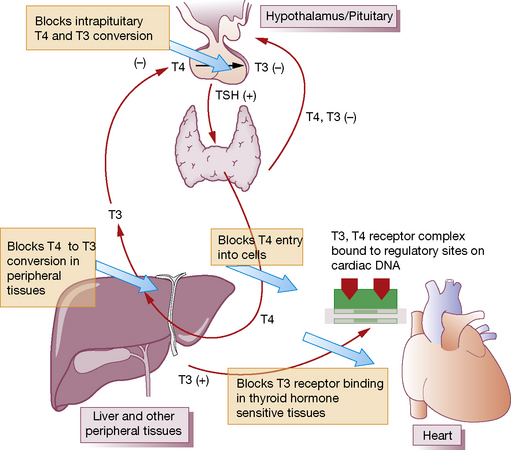
Fig. 37.2 Mechanisms by which amiodarone affects thyroid hormone metabolism. TSH, thyroid stimulating hormone.
(Adapted from Basaria S, Cooper D S 2005 Amiodarone and the thyroid. American Journal of Medicine 118:706–714.)
Table 37.1 Effects of amiodarone on thyroid function tests in euthyroid subjects
| Acute effects | Chronic effects | |
|---|---|---|
| Thyroid hormone | (≤ 3 months) | (> 3 months) |
| Total and free T4 | ↑ 50% | Remains ↑ 20–40% of baseline |
| T3 | ↓ 15–20%, remains in low-normal range | Remains ↓ 20%, remains in low-normal range |
| Reverse T3 | ↑ > 200% | Remains ↑ > 150% |
| TSH | ↑ 20–50%, transient, generally remains < 20 mU/L | Normal |
Miscellaneous
Calcitonin
• Autoimmune disease of the thyroid can cause over- or under-production of thyroid hormone.
• Hypothyroidism is readily treated with levothyroxine 50–200 micrograms daily orally, continued indefinitely.
• The treatment of hyperthyroidism due to Graves’ disease is either 12–18 months’ treatment with carbimazole or propylthiouracil, or a (usually) single dose of 131I.
• The natural history of Graves’ disease is of alternating remission and relapse. Progression to hypothyroidism can occur, especially after 131I treatment. All patients require long-term follow-up.
• Severe forms of thyroid eye disease require adrenal steroid and immunosuppressants, or low-dose radiotherapy. Urgent surgical decompression is required for optic nerve compression.
Bahn R.S. Graves’ ophthalmopathy. N. Engl. J. Med.. 2010;362:726–738.
Bartalena L., Tanda M.L. Clinical practice. Graves’ ophthalmopathy. N. Engl. J. Med.. 2009;360:994–1001.
Basaria S., Cooper D.S. Amiodarone and the thyroid. Am. J. Med.. 2005;118:706–714.
Biondi B., Palmieri E.A., Klain M., et al. Subclinical hyperthyroidism: clinical features and treatment options. Eur. J. Endocrinol.. 2005;152:1–9.
Chan G.W., Mandel S.J. Therapy insight: management of Graves’ disease during pregnancy. Nat. Clin. Pract. Endocrinol. Metab.. 2007;3:470–478.
Cooper D.S. Antithyroid drugs. N. Engl. J. Med.. 2005;352:905–917.
Mitchell A.L., Pearce S.H. How should we treat patients with low serum thyrotropin concentrations? Clin. Endocrinol. (Oxf.). 2010;72:292–296.
Pearce E.N., Farwell A.P., Bravermen L.E., et al. Thyroiditis. N. Engl. J. Med.. 2003;348:2646–2655.
Roberts C.G.P., Ladenson P.W. Hypothyroidism. Lancet. 2004;363:793–803.
Ross D.S. Radioiodine therapy for hyperthyroidism. N. Engl. J. Med.. 2011;364:542–550.
1 Use of a thionamide during the week before and after radioiodine therapy may impair the response to radiation (Velkeniers B, Cytryn R, Vanhaelst L, Jonckheer M H 1988 Treatment of hyperthyroidism with radioiodine: adjunctive therapy with antithyroid drugs reconsidered. Lancet i: 1127–1129) (see Mode of action of thionamides, above).
2 Apparently from the beginning of time: Michelangelo’s image of the separation of light from darkness on the ceiling of the Sistine Chapel in the Vatican depicts the creator with a multinodular goitre (Bondeson L, Bondeson A-G 2003 Michelangelo’s divine goitre. Journal of the Royal Society of Medicine 96:609–611).
3 The newer preparations approximately triple the cost of diagnostic investigations requiring contrast media. With a fatality rate of about 1 per 50 000 in patients receiving the older agents, hospitals are faced with an interesting cost–benefit equation.
4 And emissions can be sufficient to activate airport radiation alarms. One victim was detained, strip-searched and interrogated, but released on producing his radionucleotide card (Gangopadhyay K K, Sundram F, De P 2006 Triggering radiation alarms after radioiodine treatment. British Medical Journal 333:293–294).
5 No patient should be operated on with a resting pulse of 90 beats/min or above, and no dose of β-adrenoceptor blocker, including the important postoperative dose, should be omitted (Toft A D, Irvine W J, Sinclair I et al 1978 Thyroid function after surgical treatment of thyrotoxicosis. A report of 100 cases treated with propranolol before operation. New England Journal of Medicine 298:643–647).
6 Consensus statement: Surks M I, Ortiz E, Daniels G H et al 2004 Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. Journal of the American Medical Association 291:228–238.