166 Thyroid Gland Disorders
 Normal Thyroid Hormone Economy
Normal Thyroid Hormone Economy
Regulation
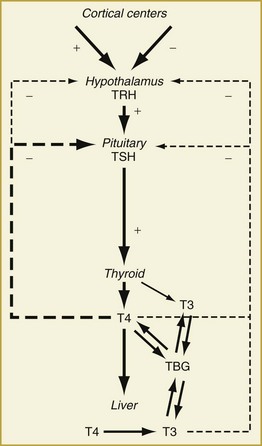
Synthesis and secretion of thyroid hormone is under the control of the anterior pituitary hormone, thyrotropin (or thyroid-stimulating hormone [TSH]). Following a classic negative feedback system, TSH secretion increases when serum thyroid hormone levels fall and decreases when they rise (Figure 166-1). TSH secretion is also under the regulation of the hypothalamic hormone, thyrotropin-releasing hormone (TRH). The negative feedback of thyroid hormone is targeted mainly at the pituitary level but likely affects TRH release from the hypothalamus as well. In addition, input from higher cortical centers can affect hypothalamic TRH secretion.
Under the influence of TSH, the thyroid gland synthesizes and releases thyroid hormone. Thyroxine (T4, 65% iodine by weight) is the principal secretory product of the thyroid gland, comprising about 90% of secreted thyroid hormone under normal conditions.1 Whereas T4 may have direct actions in some tissues, it primarily functions as a hormone precursor that is metabolized in peripheral tissues to the transcriptionally active 3,5,3′-triiodothyronine (T3, 59% iodine by weight).
Metabolic Pathways
The major pathway of metabolism of T4 is by sequential monodeiodination.2 At least three deiodinases, each with its unique expression in different organs, catalyze the deiodination reactions involved in the metabolism of T4. Removal of the 5′-, or outer ring, iodine by type I iodothyronine 5′-deiodinase (D1) or type II iodothyronine 5′-deiodinase (D2) is the “activating” metabolic pathway leading to formation of T3. Removal of the inner ring, or 5-, iodine by type III iodothyronine deiodinase D3 is the “inactivating” pathway producing the metabolically inactive hormone, 3,3′,5′-triiodothyronine (reverse T3, rT3). D1 is found most abundantly in the liver, kidneys, and thyroid. It is up-regulated in hyperthyroidism and down-regulated in hypothyroidism. D2 is found primarily in the brain, pituitary, and skeletal muscle and is down-regulated in hyperthyroidism and up-regulated in hypothyroidism. D3 is expressed primarily in the brain, in skin, and in placental and chorionic membranes. The actions of D3 also include inactivation of T3 to form T2, another inactive metabolite. Under normal conditions, about 41% of T4 is converted to T3, about 38% is converted to rT3, and about 21% is metabolized via other pathways, such as conjugation in the liver and excretion in bile.4,5
T3 is the metabolically active thyroid hormone and exerts its actions via binding to chromatin-bound nuclear receptors and regulating gene transcription in responsive tissues.3 Important in understanding the alterations in circulating thyroid hormone levels seen in critical illness is the fact that only around 10% of circulating T3 is secreted directly by the thyroid gland while more than 80% of T3 is derived from conversion of T4 in peripheral tissues.1,2 Thus, factors that affect peripheral T4-to-T3 conversion will have significant effects on circulating T3 levels. Serum levels of T3 are approximately 100-fold less than those of T4, and like T4, T3 is metabolized by deiodination to form diiodothyronine (T2) and by conjugation in the liver. The half-lives of circulating T4 and T3 are 5 to 8 days and 1.3 to 3 days, respectively.4
Serum Binding Proteins
Both T4 and T3 circulate in the serum as hormones bound to several proteins synthesized by the liver.5 Thyroid-binding globulin (TBG) is the predominant transport protein and binds roughly 80% of the circulating serum thyroid hormones. The affinity of T4 for TBG is about 10-fold greater than that of T3 and is part of the reason circulating T4 levels are higher than T3 levels. Other serum binding proteins include transthyretin,6 which binds some 15% of T4 but little if any T3, and albumin, which has a low affinity but a very large binding capacity for T4 and T3. Overall, 99.97% of circulating T4 and 99.7% of circulating T3 is bound to plasma proteins.
 Thyroid Hormone Economy in Critical Illness
Thyroid Hormone Economy in Critical Illness
Peripheral Metabolic Pathways
One of the initial alterations in thyroid hormone metabolism in acute illness is the acute inhibition of D1, resulting in the impairment of T4-to-T3 conversion in peripheral tissues.7 D1 is inhibited by a wide variety of factors, including acute illness (Box 166-1),2 resulting in the acute decrease in T3 production in critically ill patients. In contrast, inner ring deiodination by D3 may be increased by acute illness, resulting in increased levels of rT3.8 Additionally, because rT3 is subsequently deiodinated by D1, degradation of rT3 decreases, and levels of this inactive hormone rise in proportion to the fall in T3 levels. Finally, there is impaired transport of T4 to peripheral tissues such as the liver and kidney, where much of the circulating T3 is produced, further contributing to the decrease in production of T3.9
Thyrotropin Regulation
Serum TSH levels are usually normal early in acute illness.10 Decreased TRH secretion due to inhibitory signals from higher cortical centers, impaired TRH metabolism,11 the alteration of pulsatile TSH,12 and the decrease or absence of a nocturnal TSH surge12,13 may all further lower TSH levels. Serum levels of leptin, the ob gene product that has been shown to vary directly with thyroid hormone levels,14 also falls as illness progresses15 and hypothalamic TRH secretion falls, which in turn leads to lowered TSH levels.16
The decrease of hypothalamic TRH gene expression in animal models is, however, not associated with increased serum T4 and T3 levels.17 Finally, certain thyroid hormone metabolites that are increased during acute nonthyroidal illness may play a role in the inhibition of TSH and TRH secretion.18
Common medications used in the treatment of the critically ill patient may also have inhibitory effects on serum TSH levels (Box 166-2). Van den Berghe et al.19 reported that intravenous (IV) administration of dopamine for as short a time as 15 to 21 hours can acutely decrease TSH levels, and its withdrawal results in a 10-fold increase in serum TSH levels. In one study, children who received dopamine infusions during a pediatric ICU admission for meningococcal sepsis had lower TSH levels than those who did not.20,21 Increased levels of glucocorticoids, whether from endogenous or exogenous sources, also have direct inhibitory effects on TSH secretion.
Serum Binding Proteins
The affinity of thyroid hormones binding to transport proteins and the concentrations of serum binding proteins are altered with acute illness (Table 166-1). Serum levels of transthyretin and albumin decrease, especially during prolonged illness, malnutrition, and in high catabolic states. TBG levels may be increased, as seen with liver dysfunction and human immunodeficiency virus (HIV) infection, or decreased, as seen with severe or prolonged illness.5 TBG may also be rapidly degraded by protease cleavage during cardiac bypass, thereby partially explaining the rapid fall of serum T3 levels in patients undergoing cardiac surgery.22
TABLE 166-1 Factors That Alter Binding of T4 to Thyroid-Binding Globulin
| Increase Binding | Decrease Binding | |
|---|---|---|
| Drugs | Estrogens | Glucocorticoids |
| Methadone | Androgens | |
| Clofibrate | L-Asparaginase | |
| 5-Fluorouracil | Salicylates | |
| Heroin | Mefenamic acid | |
| Tamoxifen | Antiseizure medications (phenytoin, Tegretol) | |
| Raloxifene | Furosemide | |
| Heparin | ||
| Anabolic steroids | ||
| Systemic Factors | Liver disease | Inherited |
| Porphyria | Acute illness | |
| HIV infection | Nonesterified free fatty acids (NEFAs) | |
| Inherited |
An acquired binding defect of T4 to TBG is commonly seen in patients with critical illness. This is thought to result from the release of some as yet unidentified factor from injured tissues that has the characteristics of unsaturated nonesterified fatty acids (NEFA),23 which also inhibit T4-to-T3 conversion.24 In systemically ill patients, NEFA levels rise in parallel with the severity of the illness,25 and drugs such as heparin stimulate the generation of NEFA.26 Many drugs including high-dose furosemide, antiseizure medications, and salicylates also alter binding of T4 to TBG. The alterations in serum binding proteins in critical illness make estimating free hormone concentrations difficult (see later).
 Evaluation of Thyroid Function in the Critically Ill Patient
Evaluation of Thyroid Function in the Critically Ill Patient
Diagnostic Tests
Thyrotropin Assays
Abnormal thyroid function tests have been reported in 20% to 40% of acutely ill patients, more than 80% of whom have no intrinsic thyroid dysfunction after resolution of the illness.27–29 In a study of 1580 hospitalized patients, only 24% of patients with suppressed TSH values (TSH < assay limit of detection) and 50% of patients with TSH values over 20 mU/L were found to have thyroid disease.27,28 More importantly, none of the patients with subnormal but detectable TSH values and only 14% of patients with elevated TSH values less than 20 mU/L were subsequently diagnosed with intrinsic thyroid dysfunction. The development of sensitive third-generation TSH assays have led to small improvements in discerning between overt hyperthyroidism and nonthyroidal illness.27 Overall, however, while a normal TSH level has a high predictive value of normal thyroid function, an abnormal TSH value alone is not helpful in evaluating thyroid function in the critically ill patient.
Serum T4 and T3 Concentrations
Measurement of free thyroid hormone concentrations in the patient with nonthyroidal illness is fraught with difficulty.30 The gold standard for determination of free hormone levels is equilibrium dialysis. However, this technique is labor intensive and time consuming and thus is rarely used. The most commonly available laboratory tests of thyroid hormone concentrations, the free T4 index, free T4, and free T3, are measured by analog methods which represent estimates of the free hormone concentration and are therefore subject to inaccuracies.31,32
The free T4 index is determined by multiplying the total T4 concentration by the T3 or T4 resin uptake, which is an inverse estimate of serum TBG concentrations.32 Recent developments have allowed the measurement of free T4 levels by the analog method, a less expensive alternative to the free T4 index,33 but the two tests are likely comparably accurate.34 In a healthy population, there is a close correlation between the free T4 index and free T4 levels. In the critically ill patient, this association is no longer seen, mainly because of difficulties in estimating TBG binding with resin uptake tests. In spite of this, the sensitivity of the free T4 index in a large study of hospitalized patients was 92.3%, compared to 90.7% for the sensitive TSH test.27
Serum T3 concentrations are affected to the greatest degree by alterations in thyroid hormone economy resulting from acute illness. Therefore, there is no indication for routine measurement of serum T3 levels in the initial evaluation of thyroid function in the critically ill patient. This test should only be obtained if thyrotoxicosis is clinically suspected in the presence of a suppressed sensitive TSH and elevated (or high normal) free T4 index or free T4 values. The total T3 assay is preferable to the free T3 (analog) assay, owing to the variability between laboratories with the latter test.32
Although some investigators have reported that serum rT3 levels are a significant prognostic indicator of mortality in the ICU,35 rT3 levels are generally unreliable and should not be used to distinguish between intrinsic thyroid dysfunction and nonthyroidal illness.36
Serum Thyroid Autoantibodies
Autoantibodies to thyroglobulin and thyroid peroxidase (TPO), two intrinsic thyroid proteins, are commonly ordered tests.32 Significant titers of either or both of these antibodies indicate the presence of autoimmune thyroid disease, but the presence of thyroid autoantibodies alone does not necessary indicate thyroid dysfunction, as they are present in approximately 12% to 26% of the general population.37 Thyroid autoantibodies do, however, add to the sensitivity of abnormal TSH and FTI values in diagnosing known intrinsic thyroid disease.27,28
 Sick Euthyroid Syndrome
Sick Euthyroid Syndrome
As discussed earlier, critical illness causes multiple nonspecific alterations in thyroid hormone concentrations in patients without intrinsic thyroid dysfunction that relate to the severity of the illness.18,38,39 One author has postulated that sick euthyroid syndrome may be a compensatory mechanism in response to the oxidative stress of acute illness.40 Whatever the underlying cause, these alterations in thyroid hormone parameters represent a continuum of changes that depends on the severity of the illness and can be categorized into several distinct stages (Figure 166-2).18 The wide spectrum of changes observed often results from the differing points in the course of the illness when the thyroid function tests were obtained. Importantly, these changes are rarely isolated and often associated with alterations of other endocrine systems, such as decreases in serum gonadotropin and sex hormone concentrations41 and increases in serum ACTH and cortisol levels.42 Thus, the sick euthyroid syndrome should not be viewed as an isolated pathologic event but as part of a coordinated systemic reaction to illness involving both the immune and endocrine systems.
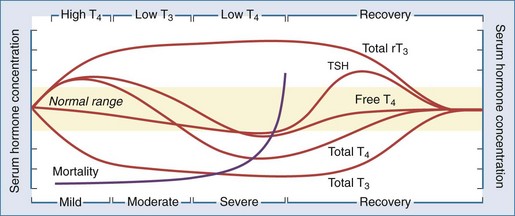
Figure 166-2 Alterations in thyroid hormone concentrations with critical illness.
(From Farwell AF. Sick euthyroid syndrome in the intensive care unit. In: Irwin RS, Rippe JM, editors. Intensive care medicine. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2003.)
Low T3 State
Common to all of the abnormalities in thyroid hormone concentrations seen in critically ill patients is a substantial depression of serum T3 levels, which can occur as early as 24 hours after the onset of illness. Over half of patients admitted to the medical service will demonstrate depressed serum T3 concentrations.27,28 Development of the low T3 state arises from impairment of peripheral T4-to-T3 conversion through inhibition of type 1 deiodinase (discussed earlier). This results in marked reduction of T3 production and rT3 degradation,43 thereby leading to reciprocal changes in serum T3 and serum rT3 concentrations. Low T3 levels are also found in peripheral tissues.35 Thyroid hormone receptor expression is also decreased in acute nonthyroidal illness,44 possibly in response to the decrease in tissue T3 levels.
High T4 State
Serum T4 levels may be elevated early in acute illness due to either the acute inhibition of type 1 deiodinase or increased TBG levels. This is seen most often in the elderly and in patients with psychiatric disorders. As the duration of illness increases, non-deiodinative pathways of T4 degradation increase serum T4 levels to the normal range.28
Low T4 State
As the severity and duration of the illness increases, serum total T4 levels decrease into the subnormal range. Contributors to this decrease in serum T4 levels are (1) a decrease in the binding of T4 to serum carrier proteins, (2) a decrease in serum TSH levels, leading to decreased thyroidal production of T4, and (3) an increase in non-deiodinative pathways of T4 metabolism. The decline in serum T4 levels correlates with prognosis in the ICU, with mortality increasing as serum T4 levels drop below 4 µg/dL and approaching 80% in patients with serum T4 levels below 2 µg/dL.45–47 Despite marked decreases in serum total T4 and T3 levels in the critically ill patient, free hormone levels have been reported to be normal or even elevated,30,31 providing a possible explanation for why most patients appear eumetabolic despite thyroid hormone levels in the hypothyroid range. Thus, the low T4 state is unlikely to be a result of a hormone-deficient state and is probably more of a marker of multisystem failure in these critically ill patients.
Recovery State
As acute illness resolves, so do the alterations in thyroid hormone concentrations. This stage may be prolonged and is characterized by modest increases in serum TSH levels.48 Full recovery with restoration of thyroid hormone levels to the normal range may require several weeks49 or months after hospital discharge.27 One study reported that 35 of 40 patients with nonthyroidal illness after coronary artery bypass grafting were able to regain normal thyroid function 6 months after surgery.50
Treatment Of The Sick Euthyroid Syndrome
The question of whether the sick euthyroid syndrome in critically ill patients represents pathologic alterations in thyroid function that negatively impact these patients or simply reflects the multisystem failure (i.e., respiratory, cardiac, renal, hepatic failure) that occurs in critically ill patients is still debatable.51–54 What is not debatable is that thyroid hormone replacement therapy has not been shown to be of benefit in the vast majority of these patients in the published studies to date (Box 166-3).54 Evidence does suggest a beneficial effect of liothyronine (L-T3) on increasing organs available for harvest from brain-dead organ donors. While L-T3 appears to slightly improve hemodynamic and neurohumoral parameters in patients with congestive heart failure, these benefits may represent a pharmacologic effect of T3 rather than a physiologic replacement hormonal effect. Further, the studies involving patients with congestive heart failure are more remarkable for a lack of deleterious effect of L-T3 treatment then for any sustained clinical benefit. However, future studies do appear to be warranted in this patient population. At the present time, in the absence of any clinical evidence of hypothyroidism, there does not appear to be any compelling evidence for the use of thyroid hormone therapy in any patient with decreased thyroid hormone parameters due to the sick euthyroid syndrome.
Box 166-3
Summary of Clinical Trials on the Effects of Treatment of Sick Euthyroid Syndrome with Thyroid Hormone*
General ICU Patients
Premature Infants
Cardiac Donors
Congestive Heart Failure
* Refer to Reference 54 for detailed citations.
 Thyroid Storm
Thyroid Storm
Thyroid storm is an acute, life-threatening complication of hyperthyroidism and represents the extreme manifestation of the disease.55–57 Historically, thyroid storm was frequently associated with surgery for hyperthyroidism and approached an incidence of 10% in some series, depending upon the diagnostic criteria employed. Currently, because of better recognition of the disease and improved perioperative management, thyroid storm is rare, accounting for less than 2% of all hospital admissions related to thyrotoxicosis.58 Most often, thyroid storm is precipitated by an intercurrent medical problem in untreated or partially treated hyperthyroid patients.55–57 The diagnosis of thyroid storm is a clinical one; there are no distinctive laboratory features, and thyroid hormone concentrations are similar to those observed in uncomplicated thyrotoxicosis. Although the cause of the rapid clinical decompensation is unknown, a sudden inhibition of thyroid hormone binding to plasma proteins by the precipitating factor, causing a rise in free hormone concentrations in the already elevated free hormone pool, may play a role in the pathogenesis of thyroid storm.59
Clinical Manifestations
Thyroid storm is primarily a clinical diagnosis; as such, the varying incidence of this disorder in patient series likely results from how strict the diagnostic criteria employed are. Clinical features are similar to those of thyrotoxicosis but more exaggerated (Box 166-4). Cardinal features of thyroid storm include fever (temperature usually > 38.5°C), tachycardia out of proportion to the fever, and mental status changes.60 Tachyarrhythmias, especially atrial fibrillation in the elderly, are common. Nausea, vomiting, diarrhea, agitation, and delirium are frequent presentations. Vascular collapse and shock due to dehydration and cardiac decompensation are poor prognostic signs, as is the presence of jaundice.61 Multiorgan failure has been reported.62 Coma and death may ensue in up to 20% of patients, frequently due to cardiac arrhythmias, congestive heart failure, hyperthermia, or the precipitating illness.63
Most patients display the classic signs of Graves disease, the most common cause of thyrotoxicosis, with ophthalmopathy and a diffusely enlarged goiter as the usual manifestations.56 Thyroid storm has also been associated with toxic nodular goiters. In the elderly, atypical signs and symptoms may include severe myopathy, profound weight loss, apathy, and a minimally enlarged goiter.64
Precipitating Factors
In the past, thyroid storm was frequently associated with surgery for hyperthyroidism (Box 166-5), with symptoms beginning a few hours after thyroidectomy in patients prepared for surgery with potassium iodide alone. Most of these cases occurred in patients who were not appropriately prepared for surgery by current standards. Certain clinical and socioeconomic factors have also been suggested to be associated with complicated hyperthyroidism, including the lack of insurance, age younger than 30 or older than 50 , and serum T4 concentrations greater than twice the upper limit of normal.65 Because of better recognition of the disease, preoperative treatment with thionamides to deplete the gland of thyroid hormone prior to surgery, and improved perioperative management with β-blockade, thyroid storm now is rarely a postoperative complication of thyroid surgery.
Currently, thyroid storm appears most commonly following infection, causing the thyrotoxic state to decompensate.56 Pneumonia, upper respiratory tract infections, and enteric infections are common precipitating infections. Other precipitating factors include stress, trauma, nonthyroidal surgery, diabetic ketoacidosis, labor, heart disease, and iodinated contrast studies in the unrecognized or partially treated hyperthyroid patient.66–69 Iatrogenic thyroid storm has been reported due to thyroid hormone overdose.70,71 Thyroid storm occurring after 131I therapy is extremely rare,72–74 especially considering the frequency of the use of radioiodine in the definitive treatment of hyperthyroidism. When reported, radioiodine-induced thyroid storm usually occurs if there was no pretreatment with antithyroid drugs.72
Diagnosis
As mentioned earlier, the diagnosis of thyroid storm is a clinical one. To emphasize this point, Wartofsky et al.55 developed a modified Acute Physiology and Chronic Health Evaluation (APACHE) score with criteria including temperature, central nervous system effects, gastrointestinal effects, cardiovascular effects, and precipitant history to assist in the diagnosis. There are no distinct laboratory abnormalities outside of elevated thyroid hormone concentrations, which are similar to those found in uncomplicated thyrotoxicosis. Serum T3 concentrations are often elevated to a greater degree than serum T4 concentrations, owing to the preferential secretion of T3 in the hyperthyroid gland.56 There is little correlation between the degree of elevation of thyroid hormones and the presentation of thyroid storm. Serum TSH concentrations are typically undetectable; however, because of the influence of nonthyroidal illness on TSH secretion (see earlier), a low TSH by itself is insufficient to make a diagnosis of thyroid storm. Serum T4 and T3 concentrations in the normal range, regardless of the TSH concentration, effectively eliminate thyroid storm as a tenable diagnosis.
Treatment
It should be emphasized that a thyroid storm is a major medical emergency that must be treated in an ICU.55–57 Therapy can divided into two major categories (Box 166-6): (1) thyroid-directed treatment aimed at decreasing thyroid hormone production, conversion, and secretion and blocking the peripheral manifestations of thyroid hormone; and (2) supportive treatment aimed at controlling the fever, stabilizing the cardiovascular system, and managing the precipitating cause.
Box 166-6
Treatment of Thyroid Storm
Thyroid-Directed Therapy
Thyroid-Directed Treatment
Prompt inhibition of thyroid hormone synthesis and secretion is essential. Antithyroid drugs are given in large doses to both inhibit synthesis of thyroid hormones and block the uptake of iodine. Propylthiouracil (PTU) is preferred over methimazole, given its greater efficacy when used in large doses, in reducing T3 levels during severe hyperthyroidism (by inhibition of type 1 deiodinase), and impairing peripheral conversion of T4 to T3.75 However, since other more powerful inhibitors of type 1 deiodinase are usually part of the therapeutic regimen in thyroid storm, the main beneficial effects of PTU are its inhibition of iodide uptake and hormone synthesis. PTU and methimazole can be administered by nasogastric tube or rectally if necessary.76 Neither of these preparations is available for parenteral administration, although a protocol has been reported for the reconstitution of methimazole to be given IV.77
Iodides, the most effective drugs to block release of thyroid hormone from the thyroid gland, should be used only after antithyroid drugs have been administered. Monotherapy with iodides will actually increase the synthesis of new thyroid hormones and markedly worsen the hyperthyroidism when the gland escapes from the initial iodide-induced blockade of hormone secretion (acute Wolf-Chaikoff effect).78 Previously, the iodide preparation of choice was the radiographic contrast dye, iopanoic acid (Telepaque), because of its high iodine content (0.6 mg iodine/g dose) and the ability for the drug to directly inhibit type 1 deiodinase and thus block T4-to-T3 conversion.2 However, this drug is largely unavailable worldwide. Lugol’s solution or saturated solution of potassium iodide (SSKI) are currently the main source of therapeutic iodides.79,80 It is important to realize that use of iodides preclude the use of radioactive iodine as a definitive therapy for hyperthyroidism for several months. Lithium has also been reported to be effective in inhibiting thyroid hormone release to a similar degree as iodides.
High-dose dexamethasone is recommended as supportive therapy, both as an inhibitor of T4-to-T3 conversion and as management of possible coexistent adrenal insufficiency. β-Adrenergic blockers, specifically propranolol, are also weak inhibitors of T4-to-T3 conversion, although their main beneficial effect is on heart rate control.81 Orally administered ion-exchange resin (colestipol or cholestyramine) can trap hormone in the intestine and prevent recirculation.82,83 Plasmapheresis, peritoneal dialysis, and charcoal hemoperfusion have also been used in severe cases.84
Long-Term Therapy
Once the acute phase of thyroid storm is controlled, antithyroid drug therapy should be continued until euthyroidism is achieved, while the adjunctive therapy can be discontinued. Definitive therapeutic options for hyperthyroidism include radioactive iodine (after a few months to allow excretion of the excess iodides used during the acute management of thyroid storm) and surgery.85–87 Long-term (1-2 years) treatment with antithyroid drugs in hopes of achieving a remission is an option for the patient with Graves disease,88 although this is best achieved using methimazole because of the concern of the rare complication of severe liver injury with PTU.89
 Myxedema Coma
Myxedema Coma
Myxedema coma is a rare syndrome that represents the extreme expression of severe long-standing hypothyroidism.57,90,91 It is a medical emergency, and even with early diagnosis and treatment, the mortality can be as high as 60%.92 The name is somewhat of a misnomer, as actual coma is rare.90 The syndrome includes decompensated hypothyroidism, central nervous system impairment, and cardiovascular compromise. Myxedema coma occurs most often in the elderly and during the winter months; in one series, 9 of 11 cases of myxedema coma were admitted in late fall or winter. As with thyroid storm, myxedema coma is usually caused by a precipitating event in the untreated or partially treated hypothyroid patient.
Clinical Manifestations
The cardinal features of myxedema coma are: (1) hypothermia, which can be profound, (2) altered mental status, (3) cardiovascular depression, and (4) a precipitating cause(s) (Box 166-7). The severely hypothyroid patient essentially becomes poikilothermic due to disordered thermoregulation. This is the reason many cases occur in the winter months. Body temperatures as low as 23.3°C have been reported; thus, rectal temperatures are essential to making the diagnosis. Excessive lethargy and sleepiness may have been present for weeks to months, often interfering with meals. Decreased consciousness has been found to be an important adverse prognostic indicator for mortality.93 Rarely, psychosis and delirium have been reported. Bradycardia and hypotension may be profound, and the respiratory rate is often depressed. Since intrinsic hypothyroidism by itself is insufficient to produce the clinical syndrome of myxedema coma, a precipitating cause must be assumed to be present.90
In addition to the noted features, most patients have the physical features of severe hypothyroidism,91 including macroglossia, delayed reflexes, dry, rough skin and myxedematous facies, which results from periorbital edema, pallor, hypercarotinemia, and patchy hair loss. Hypotonia of the gastrointestinal tract is common and often so severe as to suggest an obstructive lesion.94 Urinary retention due to a hypotonic bladder is related but less frequent. Pleural, pericardial, and peritoneal effusions may be present. Severe airway obstruction has been reported.95
Precipitating Factors
As mentioned, cold stress is a common precipitant to myxedema coma (Box 166-8). Other common precipitating factors include pulmonary and urinary tract infections, cerebrovascular accidents, trauma, surgery, congestive heart failure, and intravascular volume loss from acute or chronic gastrointestinal bleeding or overuse of diuretics.57,90,91 The clinical course of lethargy proceeding to stupor and then coma is often hastened by drugs, especially sedatives, narcotics, antidepressants, and tranquilizers.96 Indeed, many cases of myxedema coma have occurred in the undiagnosed hypothyroid patient who has been hospitalized for other medical problems.
Diagnosis
Like the diagnosis of thyroid storm, myxedema coma is a clinical diagnosis. Elderly patients may present with particularly subtle findings.97 Even though rare, the diagnosis of myxedema coma should be considered in any hypothermic, obtunded patient. Medical history in these patients, including a prior history of hypothyroidism, may only be able to be confirmed from other sources. Friends, relatives, and acquaintances might have noted increasing lethargy, complaints of cold intolerance, and changes in the voice. Clues to the diagnosis include an outdated container of L-T4 discovered with the patient’s belongings, which suggests that he or she has been remiss in taking medication. The medical record may also indicate thyroid hormone use, previous referral to treatment with radioactive iodine, or a history of thyroidectomy. Finally, the physical exam finding of a thyroidectomy scar should raise suspicion as to the diagnosis.
Because more than 95% of cases of myxedema coma are due to primary hypothyroidism,57,90,91 the laboratory findings include an elevated serum TSH and low or undetectable total and free serum T4 concentrations. These thyroid hormone abnormalities are similar to those in uncomplicated overt hypothyroidism. In the patient with central hypothyroidism, the diagnosis of myxedema coma may be very difficult, as serum TSH concentrations will be normal or low. However, other symptoms of pituitary dysfunction are usually present in these rare patients.
Dilutional hyponatremia is common and may be severe. Elevated creatine kinase concentrations, sometimes markedly so, are encountered frequently and may misdirect the clinical picture towards cardiac ischemia.98,99 However, the MB fraction in most of these cases is normal, and an electrocardiogram (ECG) often demonstrates low voltage and loss of T waves that is characteristic of severe hypothyroidism. Elevated lactate dehydrogenase (LDH) concentrations, acidosis, and anemia are common findings. Lumbar puncture reveals increased opening pressure and high protein content in the cerebrospinal fluid.
Treatment
Treatment of myxedema coma is a medical emergency and should be managed in an ICU setting. The mainstays of therapy are: supportive care with ventilatory and hemodynamic support, rewarming, correction of hyponatremia and hypoglycemia, treatment of the precipitating incident, and administration of thyroid hormone (Box 166-9).57,90,91 Sedatives, hypnotics, narcotics, and anesthetics must be minimized or avoided altogether because of their extended duration of action and exacerbation of obtundation in the hypothyroid patient.
Hypothermia is one of the hallmarks of myxedema coma, and its severity may be underestimated if the thermometer used does not register below 30°C. At core temperatures below 28°C, ventricular fibrillation is a significant life-threatening risk. Despite its gravity, the management of the hypothermia of myxedema coma differs from the treatment of exposure-induced hypothermia in euthyroid subjects. In myxedema coma, the patient should be kept in a warm room and covered with blankets. Active heating should be avoided, since it increases oxygen consumption and promotes peripheral vasodilation and circulatory collapse. Active heating is recommended only for situations of severe hypothermia where ventricular fibrillation is an immediate threat. In these cases, the rate of rewarming should not exceed 0.5°C per hour, and the core temperature should be raised to approximately 31°C.57,90,91
Because of a 5% to 10% incidence of coexisting adrenal insufficiency in patients with myxedema coma,100 IV steroids (i.e., hydrocortisone, 100 mg IV every 8 hours) are indicated before initiating T4 therapy. Parenteral administration of thyroid hormone is necessary owing to uncertain absorption through the gut.101–103 A reasonable approach is an initial IV loading dose of 200 to 300 µg L-T4. If there is inadequate improvement in the state of consciousness, blood pressure, or core temperature during the first 6 to 12 hours after administration, another dose of L-T4 should be given to bring the total dose during the first 24 hours to 0.5 mg. This should be followed by 50 to 100 µg IV every 24 hours until the patient is stabilized. Alternatively, in the most severe cases, some clinicians recommend using L-T3 at a dosage of 12.5 to 25 µg IV every 6 hours until the patient is stable and conscious. Caution must be used to avoid overstimulation of the cardiovascular system. Once stable, the patient should be switched to L-T4. The dose of thyroid hormone should be adjusted on the basis of hemodynamic stability, the presence of coexisting cardiac disease, and the degree of electrolyte imbalance.104
Although myxedema coma is associated with a high mortality, which may be as high as 60%,92,105 survival can be maximized by correcting the secondary metabolic disturbances and reversing the hypothyroid state in a sustained but gradual fashion, since an effort to correct hypothyroidism too rapidly may completely negate the beneficial effects of the initial treatment.
Midgley JE. Direct and indirect free thyroxine assay methods: theory and practice. Clin Chem. 2001;47:1353-1363.
Hennemann G, Krenning EP. The kinetics of thyroid hormone transporters and their role in non-thyroidal illness and starvation. Best Pract Res Clin Endocrinol Metab. 2007;21:323-338.
Plikat K, Langgartner J, Buettner R, Bollheimer LC, Woenckhaus U, Scholmerich J, et al. Frequency and outcome of patients with nonthyroidal illness syndrome in a medical intensive care unit. Metabolism. 2007;56:239-244.
This paper provides an in-depth review of the mortality associated with the sick euthyroid syndrome.
Farwell AP. Thyroid hormone therapy is not indicated in the majority of patients with the sick euthyroid syndrome. Endocr Pract. 2008;14:1180-1187.
Nayak B, Burman K. Thyrotoxicosis and thyroid storm. Endocrinol Metab Clin North Am. 2006;35:663-686.
This is the most recent review of thyroid storm.
Wartofsky L. Myxedema coma. Endocrinol Metab Clin North Am. 2006;35:687-698.
This is the most recent review of myxedema coma.
Dutta P, Bhansali A, Masoodi SR, Bhadada S, Sharma N, Rajput R. Predictors of outcome in myxoedema coma: a study from a tertiary care centre. Crit Care. 2008;12:R1.
1 Larsen PR, Silva JE, Kaplan MM. Relationships between circulating and intracellular thyroid hormones: Physiological and clinical implications. Endocr Rev. 1981;2:87-102.
2 Bianco AC, Larsen PR. Intracellular Pathways of Iodothyronine Metabolism. In Braverman LE, Utiger RD, editors: Werner and Ingbar’s The Thyroid, 9th ed, Philadelphia: Lippincott-Williams and Wilkins, 2005. In Press
3 Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097-1142.
4 Zimmermann MB. Iodine deficiency. Endocr Rev. 2009;30:376-408.
5 Benvenga S. Thyroid hormone transport proteins and the physiology of hormone binding. In: Braverman LE, Utiger RD, editors. The Thyroid. 9th ed. Philadelphia: Lippincott Williams and Wilkins; 2005:97-108.
6 Palha JA. Transthyretin as a thyroid hormone carrier: function revisited. Clin Chem Lab Med. 2002;40:1292-1300.
7 Koenig RJ. Regulation of type 1 iodothyronine deiodinase in health and disease. Thyroid. 2005;15:835-840.
8 Huang SA, Bianco AC. Reawakened interest in type III iodothyronine deiodinase in critical illness and injury. Nat Clin Pract Endocrinol Metab. 2008;4:148-155.
9 Hennemann G, Krenning EP. The kinetics of thyroid hormone transporters and their role in non-thyroidal illness and starvation. Best Pract Res Clin Endocrinol Metab. 2007;21:323-338.
10 Faber J, Kirkegaard C, Rasmussen B, Westh H, Busch SM, Jensen IW. Pituitary-thyroid axis in critical illness. J Clin Endocrinol Metab. 1987;65:315-320.
11 Duntas LH, Nguyen TT, Keck FS, Nelson DK, Iii JJ. Changes in metabolism of TRH in euthyroid sick syndrome. Eur J Endocrinol. 1999;141:337-341.
12 Adriaanse R, Romijin JA, Brabant G, Endert E, Wiersinga WM. Pulsatile thyrotropin secretion in nonthyroidal illness. J Clin Endocrinol Metab. 1993;77:1313-1317.
13 Romijin JA, Wiersinga WM. Decreased nocturnal surge of thyrotropin in nonthyroidal illness. J Clin Endocrinol Metab. 1990;70:35-42.
14 Hsieh CJ, Wang PW, Wang ST, Liu RT, Tung SC, et al. Serum leptin concentrations of patients with sequential thyroid function changes. Clin Endocrinol (Oxf). 2002;57:29-34.
15 Corsonello A, Buemi M, Artemisia A, Giorgianni G, Mauro VN, Corica F. Plasma leptin concentrations in relation to sick euthyroid syndrome in elderly patients with nonthyroidal illnesses. Gerontology. 2000;46:64-70.
16 Warner MH, Beckett GJ. Mechanisms behind the non-thyroidal illness syndrome: an update. J Endocrinol. 2010;205:1-13.
17 Mebis L, Debaveye Y, Ellger B, Derde S, Ververs EJ, Langouche L, et al. Changes in the central component of the hypothalamus-pituitary-thyroid axis in a rabbit model of prolonged critical illness. Crit Care. 2009;13:R147.
18 Farwell AP. Sick euthyroid syndrome in the intensive care unit. In: Irwin RS, Rippe JM, editors. Intensive care medicine. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2008:1309-1322.
19 Van den Berghe G, de Zegher F, Lauwers P. Dopamine and the sick euthyroid syndrome in critical illness. Clin Endocrinol. 1994;41:731-737.
20 den Brinker M, Dumas B, Visser TJ, Hop WC, Hazelzet JA, Festen DA, et al. Thyroid function and outcome in children who survived meningococcal septic shock. Intensive Care Med. 2005;31:970-976.
21 den Brinker M, Joosten KF, Visser TJ, Hop WC, de Rijke YB, Hazelzet JA, et al. Euthyroid sick syndrome in meningococcal sepsis: the impact of peripheral thyroid hormone metabolism and binding proteins. J Clin Endocrinol Metab. 2005;90:5613-5620.
22 Afandi B, Schussler GC, Arafeh AH, Boutros A, Yap MG, Finkelstein A. Selective consumption of thyroxine-binding globulin during cardiac bypass surgery. Metabolism. 2000;49:270-274.
23 Lim CF, Munro SL, Wynne KN, Topliss DJ, Stockigt JR. Influence of non-esterified fatty acids and lysolecithins on thyroxine binding to thyroxine-binding globulin and transthyretin. Thyroid. 1995;4:319-324.
24 Chopra IJ, Huang T-S, Solomon DH, Chaudhuri G, Chua Teco GN. The role of T4-binding serum proteins in oleic acid-induced increase in free T4 in nonthyroidal illnesses. J Clin Endocrinol Metab. 1986;63:776-779.
25 Lim CF, Docter R, Visser TJ, Krenning EP, Bernard B, van Toor H, et al. Inhibition of thyroxine transport into cultured rat hepatocytes by serum of nonuremic critically ill patients: effects of bilirubin and non-esterified fatty acids. J Clin Endocrinol Metab. 1993;76:1165-1172.
26 Stockigt JR, Lim CF. Medications that distort in vitro tests of thyroid function, with particular reference to estimates of serum free thyroxine. Best Pract Res Clin Endocrinol Metab. 2009;23:753-767.
27 Spencer C, Elgen A, Shen D, Duda M, Qualls S, Weiss S, Nicoloff J. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem. 1987;33:1391-1396.
28 Spencer CA. Clinical utility and cost-effectiveness of sensitive thyrotropin assays in ambulatory and hospitalized patients. Mayo Clin Proc. 1988;63:1214-1222.
29 Plikat K, Langgartner J, Buettner R, Bollheimer LC, Woenckhaus U, Scholmerich J, et al. Frequency and outcome of patients with nonthyroidal illness syndrome in a medical intensive care unit. Metabolism. 2007;56:239-244.
30 Chopra IJ. Simultaneous measurement of free thyroxine and free 3,5,3′-triiodothyronine in undiluted serum by direct equilibrium dialysis/radioimmunoassay: evidence that free triiodothyronine and free thyroxine are normal in many patients with the low triiodothyronine syndrome. Thyroid. 1998;8:249-257.
31 Nelson JC, Weiss RM. The effect of serum dilution on free thyroxine concentration in the low T4 syndrome of nonthyroidal illness. J Clin Endocrinol Metab. 1985;61:239-246.
32 Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:3-126.
33 Midgley JE. Direct and indirect free thyroxine assay methods: theory and practice. Clin Chem. 2001;47:1353-1363.
34 Liewendahl K, Mahonen H, Tikanoja S, Helenius T, Turula M, Valimaki M. Performance of direct equilibrium dialysis and analogue-type free thyroid hormone assays, and an immunoradiometric TSH method in patients with thyroid dysfunction. Scan J Clin Lab Invest. 1987;47:421-428.
35 Peeters RP, van der Geyten S, Wouters PJ, Darras VM, van Toor H, Kaptein E, et al. Tissue thyroid hormone levels in critical illness. J Clin Endocrinol Metab. 2005;90:6498-6507.
36 Burmeister LA. Reverse T3 does not reliably differentiate hypothyroid sick syndrome from euthyroid sick syndrome. Thyroid. 1995;5:435-441.
37 Prummel MF, Wiersinga WM. Thyroid peroxidase autoantibodies in euthyroid subjects. Best Pract Res Clin Endocrinol Metab. 2005;19:1-15.
38 DeGroot LJ. Dangerous dogmas in medicine: the nonthyroidal illness syndrome. J Clin Endocrinol Metab. 1999;84:151-164.
39 Burman KD, Wartofsky L. Thyroid function in the intensive care unit setting. Crit Care Clin. 2001;17:43-57.
40 Selvaraj N, Bobby Z, Sridhar MG. Is euthyroid sick syndrome a defensive mechanism against oxidative stress? Med Hypotheses. 2008;71:404-405.
41 Woolf PD, Hamill RW, McDonald JV, Lee LA, Kelly M. Transient hypogonadism caused by critical illness. J Clin Endocrinol Metab. 1985;60:444-450.
42 Parker LN, Levin ER, Lifrak ET. Evidence for adrenaocortical adaptation to severe illness. J Clin Endocrinol Metab. 1985;60:947-952.
43 Rodriguez-Perez A, Palos-Paz F, Kaptein E, Visser TJ, Dominguez-Gerpe L, Alvarez-Escudero J, et al. Identification of molecular mechanisms related to nonthyroidal illness syndrome in skeletal muscle and adipose tissue from patients with septic shock. Clin Endocrinol (Oxf). 2008;68:821-827.
44 Beigneux AP, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Sick euthyroid syndrome is associated with decreased TR expression and DNA binding in mouse liver. Am J Physiol Endocrinol Metab. 2003;284:E228-E236.
45 Kaptein EM, Weiner JM, Robinson WJ, Wheeler WS, Nicoloff JT. Relationship of altered thyroid hormone indices to survival in nonthyroidal illness. Clin Endocrinol. 1982;16:565-574.
46 Slag MF, Morley JE, Elson MK, Crowson TW, Nuttall FQ, Shafer RB. Hypothyroxinemia in critically ill patients as a predictor of high mortality. JAMA. 1981;245:43-45.
47 Maldonado LS, Murata GH, Hershman JM, Braunstein GD. Do thyroid function tests independently predict survival in the critically ill? Thyroid. 1992;2:119-123.
48 Hamblin S, Dyer SA, Mohr VS, Le Grand BA, Lim C, Tuxen DV, et al. Relationship between thyrotropin and thyroxine changes during recovery from severe hypothyroxinemia of critical illness. J Clin Endocrinol Metab. 1986;62:717-722.
49 Iglesias P, Munoz A, Prado F, Guerrero MT, Macias MC, Ridruejo E, et al. Alterations in thyroid function tests in aged hospitalized patients: prevalence, aetiology and clinical outcome. Clin Endocrinol (Oxf). 2009;70:961-967.
50 Cerillo AG, Storti S, Mariani M, Kallushi E, Bevilacqua S, Parri MS, et al. The non-thyroidal illness syndrome after coronary artery bypass grafting: a 6-month follow-up study. Clin Chem Lab Med. 2005;43:289-293.
51 Bello G, Paliani G, Annetta MG, Pontecorvi A, Antonelli M. Treating nonthyroidal illness syndrome in the critically ill patient: still a matter of controversy. Curr Drug Targets. 2009;10:778-787.
52 Lechan RM. The dilemma of the nonthyroidal illness syndrome. Acta Biomed. 2008;79:165-171.
53 De Groot LJ. Non-thyroidal illness syndrome is a manifestation of hypothalamic-pituitary dysfunction, and in view of current evidence, should be treated with appropriate replacement therapies. Crit Care Clin. 2006;22:57-86. vi
54 Farwell AP. Thyroid hormone therapy is not indicated in the majority of patients with the sick euthyroid syndrome. Endocr Pract. 2008;14:1180-1187.
55 Burch HB, Wartofsky L. Life-threatening thyrotoxicosis. Thyroid storm. Endocrinol Metab Clin North Am. 1993;22:263-277.
56 Nayak B, Burman K. Thyrotoxicosis and thyroid storm. Endocrinol Metab Clin North Am. 2006;35:663-686. vii
57 Farwell AP, Emerson CH. Thyroid emergencies. In: Rippe JM, Irwin RS, editors. Manual of Intensive Care Medicine. Philadelphia: Lippincott Williams & Wilkins; 2010:533-538.
58 Ringel MD. Management of hypothyroidism and hyperthyroidism in the intensive care unit. Crit Care Clin. 2001;17:59-74.
59 Brooks MH. Free thyroxine concentrations in thyroid storm. Ann Intern Med. 1980;93:694-697.
60 Harris C. Recognizing thyroid storm in the neurologically impaired patient. J Neurosci Nurs. 2007;39:40-42. 57
61 Hull K, Horenstein R, Naglieri R, Munir K, Ghany M, Celi FS. Two cases of thyroid storm-associated cholestatic jaundice. Endocr Pract. 2007;13:476-480.
62 Chong HW, See KC, Phua J. Thyroid storm with multiorgan failure. Thyroid. 2010;20:333-336.
63 Chen YT, Yang GG, Hsu YH. Thyroid storm and lymphocytic myocarditis. Intern Med. 2010;49:593-596.
64 Ghobrial MW, Ruby EB. Coma and thyroid storm in apathetic thyrotoxicosis. South Med J. 2002;95:552-554.
65 Sherman SI, Simonson L, Ladenson PW. Clinical and socioeconomic predispositions to complicated thyrotoxicosis: a predictable and preventable syndrome? Am J Med. 1996;101:192-198.
66 Vora NM, Fedok F, Stack BCJr. Report of a rare case of trauma-induced thyroid storm. Ear Nose Throat J. 2002;81:570-572. 574
67 Naito Y, Sone T, Kataoka K, Sawada M, Yamazaki K. Thyroid storm due to functioning metastatic thyroid carcinoma in a burn patient. Anesthesiology. 1997;87:433-435.
68 Abalovich M, Amino N, Barbour LA, Cobin RH, De Groot LJ, Glinoer D, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2007;92:S1-47.
69 Weber C, Scholz GH, Lamesch P, Paschke R. Thyroidectomy in iodine induced thyrotoxic storm. Exp Clin Endocrinol Diabetes. 1999;107:468-472.
70 Yoon SJ, Kim DM, Kim JU, Kim KW, Ahn CW, Cha BS, Lim SK, et al. A case of thyroid storm due to thyrotoxicosis factitia. Yonsei Med J. 2003;44:351-354.
71 Hartung B, Schott M, Daldrup T, Ritz-Timme S. Lethal thyroid storm after uncontrolled intake of liothyronine in order to lose weight. Int J Legal Med. 2010;124:637-640.
72 McDermott MT, Kidd GS, Dodson LE, Hofeldt FD. Radioiodine-induced thyroid storm. Am J Med. 1980;75:353.
73 Kadmon PM, Noto RB, Boney CM, Goodwin G, Gruppuso PA. Thyroid storm in a child following radioactive iodine (RAI) therapy: a consequence of RAI versus withdrawal of antithyroid medication. J Clin Endocrinol Metab. 2001;86:1865-1867.
74 Thebault C, Leurent G, Potier J, Bedossa M, Bonnet F. A case of thyroid storm following radioiodine therapy underlying usefulness of cardiac MRI. Eur J Intern Med. 2009;20:e136-e137.
75 Laurberg P, Vestergaard H, Nielsen S, Christensen SE, Seefeldt T, Helleberg K, et al. Sources of circulating 3,5,3′-triiodothyronine in hyperthyroidism estimated after blocking of type 1 and type 2 iodothyronine deiodinases. J Clin Endocrinol Metab. 2007;92:2149-2156.
76 Yeung SC, Go R, Balasubramanyam A. Rectal administration of iodide and propylthiouracil in the treatment of thyroid storm. Thyroid. 1995;5:403-405.
77 Hodak SP, Huang C, Clarke D, Burman KD, Jonklaas J, Janicic-Kharic N. Intravenous methimazole in the treatment of refractory hyperthyroidism. Thyroid. 2006;16:691-695.
78 Roti E, Colzani R, Braverman LE. Adverse effects of iodine on the thyroid. Endocrinologist. 1997;7:245-254.
79 Roti E, Robuschi G, Gardini E, Montermini M, Salvi M, Manfredi A, et al. Comparison of methimazole, methimazole and sodium ipodate, and methimazole and saturated solution of potassium iodide in the early treatment of hyperthyroid Graves’ disease. Clin Endocrinol (Oxf). 1988;28:305-314.
80 Philippou G, Piperingos G, Souvatzoglou A, Koutras DA, Moulopoulos SD. Treatment of hyperthyroidism with potassium iodide. Exp Clin Endocrinol. 1991;97:308-311.
81 Ashikaga H, Abreu R, Schneider RF. Propanolol administration in a patient with thyroid storm. Ann Intern Med. 2000;132:681-682.
82 Mercado M, Mendoza-Zubieta V, Bautista-Osorio R, Espinoza-de los Monteros AL. Treatment of hyperthyroidism with a combination of methimazole and cholestyramine. J Clin Endocrinol Metab. 1996;81:3191-3193.
83 Shakir KM, Michaels RD, Hays JH, Potter BB. The use of bile acid sequestrants to lower serum thyroid hormones in iatrogenic hyperthyroidism. Ann Intern Med. 1993;118:112-113.
84 Petry J, Van Schil PE, Abrams P, Jorens PG. Plasmapheresis as effective treatment for thyrotoxic storm after sleeve pneumonectomy. Ann Thorac Surg. 2004;77:1839-1841.
85 Brent GA. Clinical practice. Graves’ disease. N Engl J Med. 2008;358:2594-2605.
86 Allahabadia A, Daykin J, Sheppard MC, Gough SC, Franklyn JA. Radioiodine treatment of hyperthyroidism-prognostic factors for outcome. J Clin Endocrinol Metab. 2001;86:3611-3617.
87 Regalbuto C, Marturano I, Condorelli A, Latina A, Pezzino V. Radiometabolic treatment of hyperthyroidism with a calculated dose of 131-iodine: results of one-year follow-up. J Endocrinol Invest. 2009;32:134-138.
88 Cooper DS. Antithyroid drugs. N Engl J Med. 2005;352:905-917.
89 Bahn RS, Burch HS, Cooper DS, Garber JR, Greenlee CM, Klein IL, et al. The role of propylthiouracil in the management of Graves’ disease in adults: report of a meeting jointly sponsored by the American Thyroid Association and the Food and Drug Administration. Thyroid. 2009;19:673-674.
90 Fliers E, Wiersinga WM. Myxedema coma. Rev Endocr Metab Disord. 2003;4:137-141.
91 Wartofsky L. Myxedema coma. Endocrinol Metab Clin North Am. 2006;35:687-698. vii-viii
92 Dutta P, Bhansali A, Masoodi SR, Bhadada S, Sharma N, Rajput R. Predictors of outcome in myxoedema coma: a study from a tertiary care centre. Crit Care. 2008;12:R1.
93 Rodriguez I, Fluiters E, Perez-Mendez LF, Luna R, Paramo C, Garcia-Mayor RV. Factors associated with mortality of patients with myxoedema coma: prospective study in 11 cases treated in a single institution. J Endocrinol. 2004;180:347-350.
94 Ebert EC. The thyroid and the gut. J Clin Gastroenterol. 2010;44:40206.
95 Lee CH, Wira CR. Severe angioedema in myxedema coma: a difficult airway in a rare endocrine emergency. Am J Emerg Med. 2009;27:e1021-e1022. 1021
96 Church CO, Callen EC. Myxedema coma associated with combination aripiprazole and sertraline therapy. Ann Pharmacother. 2009;43:2113-2116.
97 Rehman SU, Cope DW, Senseney AD, Brzezinski W. Thyroid disorders in elderly patients. South Med J. 2005;98:543-549.
98 Hickman PE, Silvester W, Musk AA, McLellan GH, Harris A. Cardiac enzyme changes in myxedema coma. Clin Chem. 1987;33:622-624.
99 Nee PA, Scane AC, Lavelle PH, Fellows IW, Hill PG. Hypothermic myxedema coma erroneously diagnosed as myocardial infarction because of increased creatine kinase MB. Clin Chem. 1987;33:1083-1084.
100 Matsuda T, Abe H, Takase M, Arakawa A, Matsumoto T, Fujime M, et al. Case of combined adrenal cortical adenoma and myelolipoma. Pathol Int. 2004;54:725-729.
101 Arlot S, Debussche X, Lalau JD, Mesmacque A, Tolani M, Quichaud J, et al. Myxoedema coma: response of thyroid hormones with oral and intravenous high-dose L-thyroxine treatment. Intensive Care Med. 1991;17:16-18.
102 Pereira VG, Haron ES, Lima-Neto N, Medeiros-Neto GA. Management of myxedema coma: report on three successfully treated cases with nasogastric or intravenous administration of triiodothyronine. J Endocrinol Invest. 1982;5:331-334.
103 Liwanpo L, Hershman JM. Conditions and drugs interfering with thyroxine absorption. Best Pract Res Clin Endocrinol Metab. 2009;23:781-792.
104 Kwaku MP, Burman KD. Myxedema coma. J Intensive Care Med. 2007;22:224-231.
105 Beynon J, Akhtar S, Kearney T. Predictors of outcome in myxoedema coma. Crit Care. 2008;12:111.