Thyroid cancer
1. What are the different types of thyroid cancers?
Thyroid cancer can be divided into three broad subtypes: (1) differentiated thyroid cancer (DTC), which includes papillary thyroid cancer (PTC) and its variants, follicular thyroid cancer (FTC) and Hürthle cell carcinoma; (2) medullary thyroid cancer (MTC); and (3) anaplastic thyroid cancer (ATC), which is a form of undifferentiated thyroid cancer. Other miscellaneous types of thyroid cancers include thyroid lymphoma, mucoepidermoid carcinoma, and metastases to the thyroid gland.
2. Describe the epidemiology of thyroid cancer.
Thyroid cancer is one of the few cancers that have increased in both absolute incidence and mortality over the past several decades; an estimated 56,400 new cases were diagnosed and 1780 deaths occurred in 2012. However, the relative survival is actually improved compared with the 1970s, with an average 5-year survival in 97% of patients. Many new thyroid cancer diagnoses have resulted from increased imaging. The detection of tumors smaller than 1.0 cm has accounted for 50% of the increase since the late 1990s. However, up to 20% of the increase in diagnoses is for tumors larger than 2.0 cm, a finding suggesting that enhanced detection of incidental cancers is not the sole cause of the increased incidence. Thyroid cancer is the fifth most common cancer in women, and it affects women three times as often as it does men. However, the mortality rate in men and women is similar, indicating that thyroid cancer tends to be more aggressive in men.
3. What are the risk factors for thyroid cancer?
In the absence of an inheritable genetic syndrome such as multiple endocrine neoplasia type 2 (MEN2), the main risk factors include a family history and exposure to ionizing radiation, especially at a young age (< 15 years old). This latter factor was demonstrated in the aftermath of the Chernobyl nuclear accident, studies of which suggested a dose-related radiation exposure risk of thyroid cancer that was 5- to 20-fold greater than in unexposed children. Relative iodine deficiency may also have been a predisposing factor.
Differentiated thyroid cancer
4. What are the different forms of DTC?
PTC, the most common form of DTC, encompasses about 80% of all thyroid cancer cases. Included under PTC are the follicular variant of PTC (FVPTC) and rarer aggressive subtypes, such as tall cell variant, sclerosing variant, and other poorly differentiated forms of PTC. FTC, the next most common type of DTC, accounts for about 10% to 15% of thyroid cancer cases overall but perhaps fewer in iodine-replete areas of the world. Finally, pure Hürthle cell carcinomas are composed of invasive follicular cells that have a distinctive oxyphilic change and that may be relatively radioiodine resistant compared with other forms of DTC.
5. Which is easier to diagnose based on thyroid fine-needle aspiration (FNA), PTC or FTC?
PTC has distinctive nuclear features such as enlarged, overlapping nuclei with nuclear grooves and intranuclear pseudoinclusions that allow for a cytopathology diagnosis with a positive predictive value (PPV) greater than 95%. In contrast, FTC is functionally defined as the invasion of largely normal-appearing follicular cells through a tumor capsule. Because this cannot be ascertained on a cytology aspirate, pure FTC is difficult to diagnose based on thyroid FNA.
6. How do molecular markers play a role in the diagnosis or prognosis of thyroid cancer?
The discovery and utilization of molecular markers that can be assessed in thyroid biopsy aspirates have enhanced the ability of practitioners to predict malignancy in thyroid nodules that have indeterminate FNA cytology (average malignancy risk ∼25%). Two commercial tests that use different analyses to predict malignancy risk are available. The Afirma test by Veracyte, Inc., uses a microarray analysis on a gene set that has a high negative predictive value (NPV) of 93% and a 40%-50% PPV for suspicious nodules. This test is very useful in predicting benign lesions and for avoiding unnecessary diagnostic surgery. The miRInform test by Asuragen, Inc., evaluates thyroid nodule aspirates for specific DNA mutation markers (KRAS, HRAS, NRAS, and BRAF mutations) and RNA fusion transcripts (RET/PTC1, RET/PTC3, and PAX8/PPARγ) that are specific for thyroid cancer. RAS mutations carry about an 85% PPV for thyroid cancer, but they can also be present in benign follicular adenomas. The other markers of this panel are functionally 100% predictive of malignancy. Their sensitivity is relatively poor, however, because thyroid cancers may harbor genetic alterations not found in this test. BRAF is present in approximately 30% to 60% of PTCs and predicts greater local invasion, lymph node metastases, radioiodine resistance, and an overall worse prognosis than do other mutations found in DTC.
7. Describe the staging of DTC.
The American Thyroid Association (ATA) recommends the American Joint Commission on Cancer (AJCC) staging system. Thyroid cancer is the only cancer that has age as a component of stage (Table 37-1). According to the AJCC system, if a patient is less than 45 years old, stage II disease is the highest stage possible, and then only if distant metastases are present outside the neck. Conversely, in patients who are 45 years old or older, intrathyroidal tumors up to 2 cm are stage I and tumors 2 to 4 cm are stage II. Any locoregional metastases raise the stage to so-called high-risk disease at stage III. Stage IV tumors either have gross invasion into extrathyroidal neck structures or distant metastases.
TABLE 37-1.
STAGING SYSTEM FOR DIFFERENTIATED THYROID CANCER
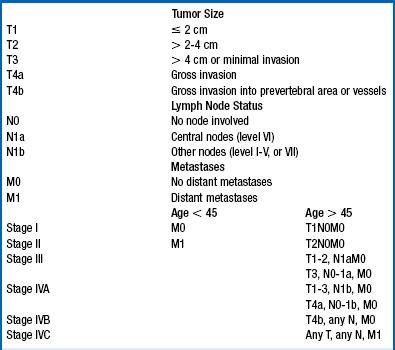
From American Joint Committee on Cancer (AJCC): Cancer staging manual, ed 6, New York, 2002, Springer.
8. How do PTC and FTC generally metastasize?
PTC typically spreads to locoregional lymph nodes. Outside the neck, the most common site of distant metastases is the lungs, with basilar miliary spread. FTC classically has hematogenous spread and a relatively high rate of bone metastases. Aggressive thyroid cancer of any type can have direct extension or invasion into extrathyroidal soft tissue, fascia, and muscle.
9. How often do metastases occur?
In adults, locoregional lymph node metastases occur as often as 30% to 60% of the time, whereas distant metastases are much rarer, occurring in only 2% to 4% of patients. In pediatric thyroid cancer, the presentation is more aggressive. Regional lymph metastases occur in 60% to 80% of these patients, and distant metastases occur in 10% to 20%.
10. What is the primary treatment for thyroid cancer?
Surgical resection is the primary treatment. The greatest predictor of a successful operation (cancer removal and avoidance of complications) is the surgeon’s experience. High-volume thyroidectomy practices consistently have excellent outcomes with minimal complication rates.
11. What determines the extent of the initial surgical procedure?
Ideally, the first thyroid cancer operation is the last. Preoperative neck ultrasound (US) is an invaluable tool for identifying the extent of lymph node metastases in the anterior lateral cervical lymph node chains. US is superior to computed tomography (CT) and magnetic resonance imaging (MRI) because it identifies malignant features of lymph nodes beyond size alone. For known thyroid cancer larger than 1 cm, or when lymph node metastases are detected preoperatively, a near-total thyroidectomy with lymph node resection is the procedure of choice. If the primary tumor is smaller than 1 cm, a hemithyroidectomy may be adequate. The need for prophylactic central neck lymph node dissection (prophylactic because central neck lymph nodes cannot be visualized with an intact thyroid in place) is controversial because of the lack of studies demonstrating improved survival with prophylactic central neck dissection.
12. What is the role of radioactive iodine in thyroid cancer therapy?
Radioiodine can be used for ablating any remnant thyroid tissue after thyroidectomy and can be considered adjuvant therapy after surgery for any locoregional lymph node or distant metastases.
13. Should all patients with thyroid cancer receive radioiodine?
No. Radioiodine therapy is indicated for patients with distant metastases, tumors with gross extrathyroidal extension, and tumors larger than 4.0 cm. Radioiodine therapy is not indicated for unifocal tumors smaller than 1.0 cm because there is no known survival advantage or decrease of recurrence. For thyroid cancers of medium size, patient-specific selection is indicated for determining whether to use radioiodine therapy.
14. How are patients prepared for radioactive iodine therapy?
Thyroid-stimulating hormone (TSH) stimulates iodine uptake into thyroid cells; therefore, TSH should be elevated to enhance uptake into functional thyroid tissue (remnant or DTC tissue). TSH can be elevated by withdrawing thyroid hormone therapy and thereby rendering the patient hypothyroid (usually a 3-week withdrawal from levothyroxine [LT4] alone without a triiodothyronine [T3] bridge is adequate) or by using recombinant human TSH (rhTSH [Thyrogen]), which is approved for remnant ablation and has efficacy similar to that of withdrawal preparation. Additionally, a low-iodine diet is recommended to decrease competition of dietary or “cold iodine” with radioiodine for uptake into thyroid follicular cells. In the absence of other forms of contamination (contrast agents or iodine-containing medications such as amiodarone), 1 week of a strict low-iodine diet is usually adequate to deplete the body of competing nonradioactive iodine.
15. What is the proper dose of radioactive iodine?
No large-scale randomized trial has been conducted to answer this question. For remnant ablation, recent prospective data demonstrates that a dose of 30 mCi has efficacy similar to 100 mCi. For locally invasive and distant metastatic tumors, larger doses may be indicated. The ATA guidelines recommend the following: “The minimum activity (30-100 mCi) necessary to achieve successful remnant ablation should be utilized, particularly for low-risk patients.”
16. Is radioactive iodine therapeutic or diagnostic?
Both. The treatment is designed to eradicate residual thyroid cancer after surgery, but patients should have a scan approximately 3 to 10 days after treatment to visualize radioiodine uptake and rule out or detect metastatic disease.
17. What are the complications of radioiodine therapy?
The main acute complications are dry mouth, transient taste alteration, and sialadenitis. Excess tearing from tear duct blockage can also occur. Up to 40% of patients will experience at least one side effect, but these effects generally resolve more than 90% of the time. There is a statistically significant, dose-dependent increased risk of secondary malignancy with radioiodine, but the absolute risk is very small (∼10 cases/10,000 patient-years).
18. What is the role of TSH in thyroid cancer therapy?
TSH is trophic to thyroid follicular cells; therefore, TSH suppression therapy is indicated to minimize its growth signal. Excess thyroid hormone is prescribed to target TSH goals, which are generally lower than 0.1 mU/L for high-risk disease and 0.1 to 0.5 mU/L for low-risk disease. TSH suppression therapy provides an overall survival benefit for patients with active thyroid cancer, but this benefit must be balanced with the potential toxicity of thyroid hormone excess, mainly increased bone turnover or osteoporosis and the risk of atrial fibrillation. In the absence of detectable thyroid cancer, the TSH target is the low normal range (e.g., 0.5-2.5 mU/L).
19. What is thyroglobulin (Tg), and how is it assessed?
Tg is a precursor protein for thyroid hormone that is released into the blood by the thyroid gland and by most differentiated thyroid cancer cells. Thus, in the absence of a thyroid gland, Tg is an excellent thyroid cancer tumor marker. It correlates roughly with the mass of thyroid cancer present and can be followed for evidence of tumor growth or stability. Tg antibodies (TgAbs) are reflexively measured with Tg because up to 20% of patients with DTC have detectable TgAbs that interfere with most commercial Tg assays, thus causing false lowering of the reported Tg level. Therefore, in the presence of TgAbs, assessment of disease presence based on Tg measurements should be made with caution. Tg can also be measured from lymph node aspirates after washing the needle with saline solution and running the wash in the Tg assay. Even in the absence of cytologically detectable thyroid cancer in a lymph node aspirate, a positive Tg wash indicates thyroid cancer metastasis to that lymph node.
20. What is a diagnostic radioiodine whole-body scan (WBS)?
Approximately 12 months after initial therapy (surgery and radioiodine therapy), selected patients can receive a low dose of iodine-123 (123I) (∼1-5 mCi) under TSH stimulation (ideally with rhTSH preparation), and the next day a scan can assess radiotracer uptake sites. This procedure should always be performed with a Tg measurement under TSH stimulation to correlate the tumor marker with imaging. Visualized WBS uptake is likely to be falsely positive if the Tg level is undetectable. If the WBS is negative, it reduces the likelihood that a future dose of radioiodine will result in a clinically acceptable response (decrease in tumor mass and/or Tg level).
21. What is the most sensitive combination of tests for detecting residual thyroid cancer?
Persistent thyroid cancer is usually present in the neck; therefore, a sensitive neck US examination, in combination with a stimulated Tg measurement, is more sensitive than a diagnostic WBS. Up to 20% of patients with suppressed TSH will have undetectable Tg that becomes positive under TSH stimulation, a finding indicating the presence of occult disease.
22. What is the relevance of fluorodeoxyglucose (FDG) avidity in thyroid cancer metastases?
FDG uptake on a positron emission tomography (PET) scan indicates increased thyroid cancer aggressiveness and a decreased likelihood of radioiodine uptake in those lesions. In metastatic disease, this is a poor prognostic sign that suggests a need for aggressive therapy, especially when lesions are growing on cross-sectional imaging. A few PET-positive lesions are stable, so clinical criteria should always be assessed before initiating treatment, even with FDG-avid lesions.
23. What are the indications for external beam radiation therapy (EBRT)?
Treatment of gross, unresectable residual disease in the neck, painful bone metastases, or other metastases that threaten critical structures (e.g., vertebral metastases) are indications to consider EBRT.
24. Is there an indication for chemotherapy in thyroid cancer management?
Doxorubicin (Adriamycin) is the only Food and Drug Administration (FDA)–approved chemotherapy for thyroid cancer management, but it is rarely indicated. This drug has relatively high toxicity and low efficacy, and in the era of targeted therapies, such as tyrosine kinase inhibitors, it is rarely prescribed. Indications would include rapidly growing lesions not amenable to surgery, radioiodine, or EBRT. Most clinical trials today use multikinase inhibitors that target the mitogen-activated protein (MAP) kinase pathway and vascular endothelial growth factor (VEGF) receptors, among others. Examples of agents studied include sorafenib, pazopanib, and levatinib, although at the time of this writing, none is FDA approved for DTC.
Medullary thyroid cancer
MTC is a carcinoma of the calcitonin-secreting parafollicular cells (also known as C-cells), whose primary function is calcitonin secretion. It really is more of a neuroendocrine tumor that arises within the thyroid.
26. What is the epidemiology of MTC?
MTC accounts for 2% to 5% of thyroid cancer cases. There is a slight female predominance, and the mean age at diagnosis is approximately 50 years. Approximately 75% to 80% of cases are sporadic, and the others are heritable.
27. Describe the staging of MTC.
Unlike in DTC, staging of MTC does not use age as a criterion. MTC has a more standard TNM staging scheme in the AJCC classification in which stages I and II are small and larger tumors (< 4 cm) confined to the thyroid gland, and stages III and IV involve extrathyroidal extension of the primary tumor, lateral lymph node metastases, and distant metastases.
28. How does MTC clinically manifest?
Typically, MTC manifests as a painless thyroid nodule or cervical lymphadenopathy that is detected either on examination or incidentally in the setting of imaging for another indication.
29. Is calcitonin a useful marker for the diagnosis of MTC?
Yes. It is extremely specific for the parafollicular cells that give rise to MTC. As neuroendocrine tumors, MTCs also express carcinoembryonic antigen (CEA), thus making this another useful marker. Other neuroendocrine markers, such as chromogranin A, have been used clinically, but to a lesser extent than calcitonin and CEA.
30. What are the hereditary forms of MTC?
Among MTC cases, 20% to 25% are hereditary, occurring in autosomal dominant forms in the setting of MEN2A, MEN2B, and familial MTC (FMTC, which does not have other MEN clinical sequelae). Also see Chapter 51.
31. Do RET proto-oncogene mutations predict disease severity?
Yes. The ATA MTC guidelines rank MTC risk based on genotype-phenotype correlations. For some mutations, such as M918T, thyroidectomy is recommended as soon as possible or prophylactically within the first year of life if there are known MEN2B-affected family members (MTC in MEN2B tends to be very aggressive).
32. What is the primary treatment of MTC?
Surgery is the primary treatment for MTC, thus highlighting the importance of preoperative diagnosis and planning before the initial operation. The use of calcitonin as a preoperative diagnostic test in nodular goiters is controversial; the ATA currently cannot recommend or advise against its routine use preoperatively. A preoperative full neck US scan should be evaluated in all patients with suspected MTC, to optimize the initial surgical procedure.
33. What is the role of radioiodine and TSH suppression for MTC therapy?
None. Parafollicular cells do not contain the sodium-iodine symporter (NIS) protein or TSH receptors and therefore do not respond to either therapy. Radioiodine should not be given. The TSH goal after thyroidectomy is generally in the low normal range.
34. Describe the monitoring for MTC.
Biochemically, the best serum markers for MTC are calcitonin and CEA. These should not be monitored more often than every 3 months because fluctuations in these levels can cause false reassurance or panic if they are checked too often. Based on marker levels, other imaging may be recommended. Generally, if the calcitonin is detectable but lower than 150 pg/mL, a neck US scan is sufficient. If calcitonin levels are higher than 150 pg/mL, a neck US scan and cross-sectional imaging that may include neck and chest CT with contrast, three-phase contrast-enhanced liver CT, or bone MRI of the spine and pelvis can be considered (the liver and bone are common sites of distant MTC metastases).
35. How do the calcitonin and CEA doubling times predict MTC outcomes?
A doubling of the value of these markers in less than or more than 24 months generally predicts progressive disease or general stability of lesions, respectively. A doubling time of less than 6 months indicates a very poor prognosis, with increased mortality and 10-year survival of less than 10%. A doubling time of more than 24 months predicts no or rare deaths in up to 10 years of follow-up. Doubling times between 6 and 24 months have intermediate mortality risks (∼25% to 35%).
36. What are the treatment options for metastatic MTC?
Currently, vandetanib and cabozantinib are FDA-approved oral medications for metastatic, progressive MTC. They are multi-targeted tyrosine kinase inhibitors that are generally well tolerated and result in stasis of previously progressive lesions in the majority of patients. Common side effects are skin reactions, hypertension and rare, severe reactions including QT prolongation and sudden death for vandetanib and GI perforatons for cabozantinib; prospective patients should therefore be screened and monitored with an electrocardiogram before and during treatment with vandetanib. Standard chemotherapies tend to be relatively toxic with limited efficacy but are reasonable treatment options if vandetanib is ineffective.
Anaplastic thyroid carcinoma
ATC is a completely dedifferentiated form of thyroid cancer. It is not responsive to radioiodine or TSH suppression therapies and by definition always is considered stage IV thyroid cancer.
38. Does ATC arise de novo or from well-differentiated thyroid cancer?
The balance of evidence favors that ATC devolves from DTC. This is based on the discovery of ATC mixed with PTC in numerous cases, as well as cases in patients who developed ATC after initial therapy for PTC. Presumably, the ATC arose from persistent, poorly differentiated PTC that was not successfully treated by the initial therapy.
39. What is the epidemiology of ATC?
Fortunately, ATC is a very rare tumor, accounting for only 1.3% of all thyroid cancer cases. Most diagnoses occur in the sixth and seventh decades of life.
40. What is the prognosis for ATC?
The prognosis is as poor as the worst of all forms of cancer. Most patients die within 6 months of diagnosis despite aggressive treatment. One-year survival rates range from 10% to 20%, although there are some long-term survivors.
41. How does ATC clinically manifest?
The classic presentation is a rapidly expanding neck mass, usually associated with pathologically enlarged lymph nodes, and often with hoarseness, dysphagia, and possibly hemoptysis. Cross-sectional imaging is appropriate for evaluation of distant metastases because these are often evident at initial presentation.
42. What are the treatment strategies for ATC?
A careful staging assessment is critical for patients with newly diagnosed ATC because a palliative approach may be the most appropriate and humane option. In patients who are candidates, complete surgical resection of the primary tumor results in prolonged survival. EBRT has also been shown to improve survival time. In general, multimodality therapy is optimal with complete surgical resection, EBRT, and chemotherapy (taxane-based therapies show the most promise). Clinical trials are focusing on the use of multitargeted kinase inhibitors after or in combination with EBRT and chemotherapy.
Alexander, EK, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012;367:705–715.
Aschebrook-Kilfoy, B, et al. Thyroid cancer incidence patterns in the United States by histologic type, 1992-2006. Thyroid. 2011;21:125–134.
Ball, DW, Medullary thyroid cancer. monitoring and therapy. viii. Endocrinol Metab Clin North Am 2007;36:823–837.
Baloch, ZW, et al, The National Cancer Institute Thyroid fine needle aspiration state of the science conference. a summation. Cytojournal 2008;5:6.
Biondi, B, Cooper, DS. Benefits of thyrotropin suppression versus the risks of adverse effects in differentiated thyroid cancer. Thyroid. 2010;20:135–146.
Brown, AP, et al. The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab. 2008;93:504–515.
Cardis, E, Hatch, M, The Chernobyl accident. an epidemiological perspective. Clin Oncol (R Coll Radiol) 2011;23:251–260.
Cooper, DS, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214.
Elisei, R, et al. Follow-up of low-risk differentiated thyroid cancer patients who underwent radioiodine ablation of postsurgical thyroid remnants after either recombinant human thyrotropin or thyroid hormone withdrawal. J Clin Endocrinol Metab. 2009;94:4171–4179.
Enewold, L, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980-2005. Cancer Epidemiol Biomarkers Prev. 2009;18:784–791.
Foote, RL, et al, Enhanced survival in locoregionally confined anaplastic thyroid carcinoma. a single-institution experience using aggressive multimodal therapy. Thyroid 2011;21:25–30.
Frasoldati, A, et al. Role of thyroglobulin measurement in fine-needle aspiration biopsies of cervical lymph nodes in patients with differentiated thyroid cancer. Thyroid. 1999;9:105–111.
Grewal, RK, et al. Salivary gland side effects commonly develop several weeks after initial radioactive iodine ablation. J Nucl Med. 2009;50:1605–1610.
Haas, V, et al. Unusual manifestation of anaplastic thyroid cancer. Acta Medica (Hradec Kralove). 2008;51:233–236.
Kim, HK, et al. Daily urine iodine excretion while consuming a low-iodine diet in preparation for radioactive iodine therapy in a high iodine intake area. Clin Endocrinol (Oxf). 2011;75:851–856.
Kloos, RT, et al, Medullary thyroid cancer. management guidelines of the American Thyroid Association. Thyroid 2009;19:565–612.
Klopper, J, Haugen, B. Can calcitonin and carcinoembryonic antigen doubling times predict progression of thyroid carcinoma. Nat Clin Pract Endocrinol Metab. 2008;4:428–429.
Kurzrock, R, Sherman, SI, Ball, DW, et al. Activity of XL184 (Cabozantinib), an oral thyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011 Jul 1;29(19):2660–2666.
La Quaglia, MP, et al. Differentiated thyroid cancer: clinical characteristics, treatment, and outcome in patients under 21 years of age who present with distant metastases. A report from the Surgical Discipline Committee of the Children’s Cancer Group. J Pediatr Surg. 2000;35:955–959.
Mallick, U, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. 2012;366:1674–1685.
McIver, B, et al, Anaplastic thyroid carcinoma. a 50-year experience at a single institution. Surgery 2001;130:1028–1034.
Nikiforov, YE, et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009;94:2092–2098.
Schlumberger, M, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012;366:1663–1673.
Serhal, DI, et al. Rapid rise in serum thyrotropin concentrations after thyroidectomy or withdrawal of suppressive thyroxine therapy in preparation for radioactive iodine administration to patients with differentiated thyroid cancer. J Clin Endocrinol Metab. 2004;89:3285–3289.
Siegel, R, et al. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29.
Spencer, CA, Lopresti, JS. Measuring thyroglobulin and thyroglobulin autoantibody in patients with differentiated thyroid cancer. Nat Clin Pract Endocrinol Metab. 2008;4:223–233.
Stavrakis, AI, et al. Surgeon volume as a predictor of outcomes in inpatient and outpatient endocrine surgery. Surgery. 2007;142:887–899.
Stulak, JM, et al. Value of preoperative ultrasonography in the surgical management of initial and reoperative papillary thyroid cancer. Arch Surg. 2006;141:489–494.
Thornton, K, et al, Vandetanib for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease. U.S. Food and Drug Administration drug approval summary. Clin Cancer Res 2012;18:3722–3730.
Xing, M, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–6379.
Zhang, L, et al, Risk factors for neck nodal metastasis in papillary thyroid microcarcinoma. a study of 1066 patients. J Clin Endocrinol Metab 2012;97:1250–1257.

