43 Thyroid and parathyroid disorders
Thyroid physiology
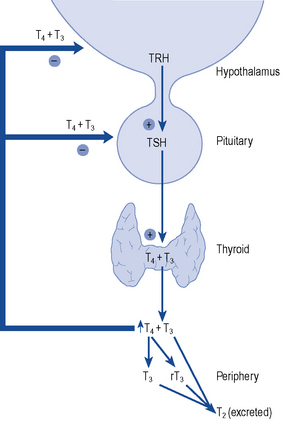
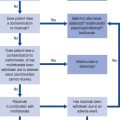
The thyroid gland consists of two lobes and is situated in the lower neck. The gland synthesises, stores and releases two major metabolically active hormones: Tetra-iodothyronine (Thyroxine, T4) and tri-iodothyronine (T3). Regulation of hormone synthesis is by variable secretion of the glycoprotein hormone TSH from the anterior pituitary. In turn, TSH is regulated by hypothalamic secretion of the tripeptide thyrotrophin-releasing hormone (TRH) (Fig. 43.1). Low circulating levels of thyroid hormones initiate the release of TSH and probably also TRH. Rising levels of TSH promote increased iodide trapping by the gland and a subsequent increase in thyroid hormone synthesis. The increase in circulating hormone levels feeds back on the pituitary and hypothalamus, shutting off TRH, TSH and further hormone synthesis.
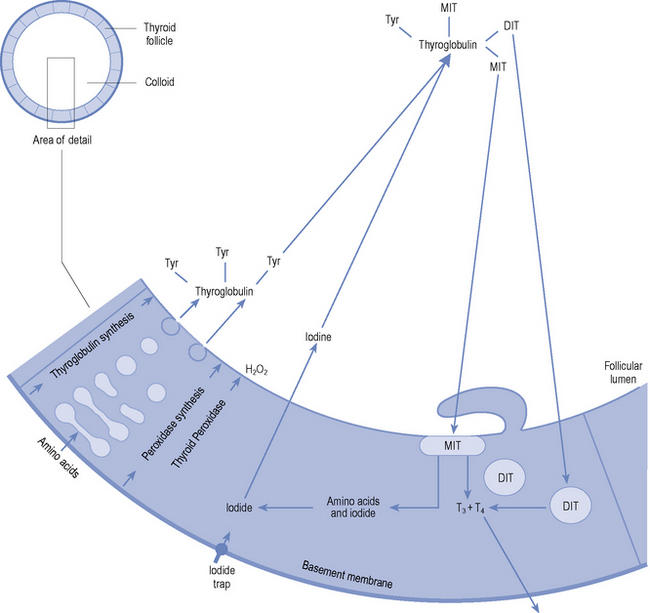
Both T4 and T3 are produced within the follicular cells in the thyroid. The stages in synthesis are shown in Fig. 43.2. In summary:
The ratio of T4:T3 secreted by the thyroid gland is approximately 10:1. Consequently, the gland secretes approximately 80–100 μcg of T4 and 10 μcg of T3 per day. However, only 10% of circulating T3 is derived from direct thyroidal secretion, the remaining 90% being produced by peripheral conversion from T4. T4 can therefore be considered a prohormone that is converted in the peripheral tissues (liver, kidney and brain) either to the active hormone T3 or to the biologically inactive reverse T3 (rT3). In the circulation, the hormones exist in both the active free and inactive protein-bound forms. T4 is 99.98% bound, with only 0.02% circulating free. T3 is slightly less protein bound (99.8%), resulting in a considerably higher circulating free fraction (0.2%). Details of protein binding are shown in Table 43.1.
| Carrier protein | Plasma concentration | Proportion of T4 and T3 bound (%) |
|---|---|---|
| Thyroid-binding globulin (TBG) | 15 mg/L | 75 |
| Transthyretin (formerly thyroid-binding prealbumin) | 250 mg/L | 10 |
| Albumin | 40 g/L | 15 |
Hypothyroidism
Aetiology
Primary hypothyroidism accounts for more than 95% of adult cases. It is usually due to a failure of the thyroid gland itself as a result of autoimmune destruction, or the effects of treatment of thyrotoxicosis. Hypothyroidism may be drug induced. Amiodarone and lithium cause hypothyroidism in around 10% of patients treated (see later). Secondary disease is due to hypopituitarism, and tertiary disease due to failure of the hypothalamus. Peripheral hypothyroidism is due to tissue insensitivity to the action of thyroid hormones. A more extensive classification is shown in Box 43.1.
Clinical manifestations
Hypothyroidism can affect multiple body systems, but symptoms are mainly non specific and gradual in onset (Box 43.2). Symptoms are frequently vague especially in the early stages. It is common for symptoms to be incorrectly attributed by patients and their relatives to increasing age. The reverse is also common in that patients who have read about, or have friends/family with, hypothyroidism will assume that it is responsible for symptoms of fatigue and weight gain. Thus, hypothyroidism is often confused with simple obesity and depression. Thyroid function tests give accurate diagnosis in all cases.
Box 43.2 Signs and symptoms of hypothyroidism
| Skin and appendages | Dry, cool, flaking, thickened skin |
| Reduced sweating | |
| Yellowish complexion. Puffy facies and eyes | |
| Sparse, coarse, dry hair | |
| Brittle nails | |
| Neuromuscular system | Slow speech |
| Poor memory and reduced cognitive function | |
| Somnolence | |
| Carpal tunnel syndrome | |
| Psychiatric disturbance | |
| Hearing loss | |
| Depression | |
| Muscle pain and weakness | |
| Delayed deep tendon reflexes | |
| Metabolic abnormalities | Raised total and LDL cholesterol |
| Macrocytic anaemia | |
| Gastro-intestinal | Weight gain with decreased appetite |
| Abdominal distension and ascites | |
| Constipation | |
| Cardiovascular | Reduced cardiac output |
| Bradycardia | |
| Cardiac enlargement |
Investigations
Testing thyroid function
As indicated earlier (and later in the section on thyrotoxicosis), a clinical assessment and measurement of free T4 and TSH are usually all that are necessary to arrive at an accurate diagnosis of thyroid state. All modern TSH assays now employ double antibody immunometric techniques, which are robust and highly reliable. Moreover, these assays are now so sensitive that they are able to identify thyrotoxic patients with TSH levels below the normal euthyroid range. Commercial free T4 and free T3 assays, however, are all indirect methods and are subject to interference from drugs and other disease states. As such, both T3 and T4 can be decreased as a non-specific consequence of systemic illness (‘sick euthyroid’ syndrome) and depression along with a host of drugs (Surks and Sievert, 1995), which can interfere with thyroid hormone metabolism and free hormone assays (Table 43.2). Such patients require specialist assessment and collaboration with the local laboratory to rule out confounding disease and pituitary failure.
| Clinical/biochemical effects | |
|---|---|
| Decrease TSH secretion | |
| Dopamine | Hypothyroidism (rarely clinically important) |
| Glucocorticoids | |
| Octreotide | |
| Alter thyroid hormone secretion | |
| Iodide (amiodarone, contrast agents) | Both hyper- and hypothyroidism |
| Lithium | Hypothyroidism |
| Decrease T4 absorption | |
| Colestyramine/colestipol | Increased thyroxine dose requirement |
| Aluminium hydroxide | |
| Ferrous sulphate | |
| Calcium carbonate | |
| Multivitamins | |
| Sevelamer | |
| Protein pump inhibitors | |
| Sucralfate | |
| Alter T4 and T3 metabolism | |
| Increased hepatic metabolism | |
| Phenobarbital | Low T4 and T3 levels |
| Phenytoin | Normal or increased TSH |
| Rifampicin | |
| Carbamazepine | |
| Reduce conversion of T4 to T3 | |
| B-blockers | Lower T3 levels |
| Propylthiouracil | Normal or increased TSH |
| Amiodarone | |
| Glucocorticoids | |
| Reduce T4 and T3 binding | |
| Furosemide | Increased measured free T4 in some assays |
| Salicylates and NSAIDs | |
| Heparin | |
| Increase thyroglobulin levels | |
| Oestrogen and tamoxifen | Increased total T4 |
| Opiates and methadone | |
| Others | |
| Cytokines – interferon and interleukin 2 | Thyroiditis. Can produce hypothyroidism and thyrotoxicosis |
Treatment
The aims of treatment with thyroxine are to ensure that patients receive a dose that will restore well-being and that usually returns the TSH level to the lower end of the normal range (Vanderpump et al., 1996). All patients with symptomatic hypothyroidism require replacement therapy. T4 is usually the treatment of choice except in myxoedema coma where T3 may be used in the first instance. Before commencing T4 replacement, the diagnosis of glucocorticoid deficiency must be excluded to prevent precipitation of a hypoadrenal crisis. If in doubt, hydrocortisone replacement should be given concomitantly until cortisol deficiency is excluded.
It is important to avoid both under- and overtreatment. Hypothyroidism is very rarely life threatening, but adverse effects may result from prolonged overtreatment (which is indicated by a TSH level suppressed below the normal range). Though T4 exerts an effect on many organs and tissues, it is the effect on bone and the heart that give the greatest cause for concern. There is evidence that bone density is reduced in patients taking excessive T4 replacement therapy (Faber and Galloe, 1994; Uzzan et al., 1996), and that atrial fibrillation is more common if TSH is suppressed (Sawin et al., 1994). In order to minimise the risk of development of these complications, the dose of T4 should be carefully tailored to the needs of each individual patient. Some patients will have undetectable serum TSH levels while taking thyroxine and may complain of recurrent fatigue if the dose is reduced to permit the TSH to rise. In these patients, it may be permissible to leave the dose unchanged if levels of free T4 and T3 are normal, after a discussion of the relative risks and benefits with the patient (Vanderpump et al., 1996).
Patient care
Hypothyroidism requires lifelong treatment with T4. Patients on long-term drug therapy are recognised to have a low adherence with their medication regimen. Treatment with T4 is often terminated because patients feel well and think that treatment is no longer required. Patients should understand the effects of drug holidays on their health and thyroid function tests and should know that a normal TSH indicates adequate dosage. Written advice should be provided and monitoring of dosage should continue annually. There are a series of excellent patient information leaflets available on the British Thyroid Association website at www.british-thyroid-association.org
Despite adequate counselling, some patients persistently forget to take their tablets reliably, leading to variable thyroid state and wildly fluctuating test results. Other patients lack capacity to self-medicate reliably. There is evidence to show that weekly dosing with T4 is a safe and acceptable way to manage this type of patient, in whom family members or community staff can supervise treatment (Grebe et al., 1997; Rangan et al., 2007). There are no guidelines yet published, but in practice, patients are normally started on 500–700 μcg T4 weekly. Dose changes are made in exactly the same way by assessing TSH levels after 6 weeks of stable dosing.
Rarely, patients are seen in whom TSH levels fluctuate or remain elevated despite high doses of thyroxine and in whom adherence seems to be very good. There are a number of possible causes for this, including malabsorption of thyroxine which can be due to coeliac or inflammatory bowel disease or a number of commonly prescribed drugs (Table 43.2). Such patients will need a careful sequential assessment by an endocrine service (Morris, 2009).
Prevention
At present, nothing can be done to prevent autoimmune thyroid failure from developing; however, much can be done to ensure early detection and treatment. Careful follow-up of patients who have undergone radioiodine treatment, subtotal thyroidectomy or completed a course of treatment for thyrotoxicosis is essential along with monitoring of those prescribed amiodarone and lithium. An increase in TSH with normal concentrations of T3 and T4 will indicate the onset of hypothyroidism before the patient becomes symptomatic. Box 43.3 shows the prevalence of hypothyroidism after treatment for thyrotoxicosis.
Hyperthyroidism/thyrotoxicosis
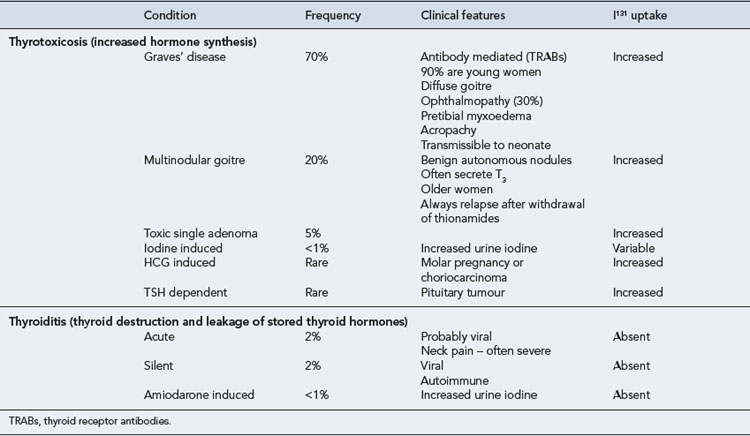
Hyperthyroidism is defined as the production by the thyroid gland of excessive amounts of thyroid hormones. Thyrotoxicosis refers to the clinical syndrome associated with prolonged exposure to elevated levels of thyroid hormone. This distinction is important when evaluating thyroid function tests (Table 43.3).
Epidemiology
Hyperthyroidism is a common condition. It has been estimated that there are 4.7/1000 women with active disease. When previously treated cases were included, the population prevalence rose to 20/1000 in women. As for hypothyroidism, it is much less common in men who have a lifetime prevalence of around 2/1000 (Tunbridge et al., 1977).
Clinical manifestations
Thyrotoxicosis is characterised by increases in metabolic rate and activity of many systems due to excessive circulating quantities of thyroid hormones. There is a wide spectrum of clinical disturbances. The signs and symptoms reflect increased adrenergic activity, especially in the cardiovascular and neurological systems (Box 43.4). Not all manifestations will be seen in every patient. Additional clinical features will depend on the underlying cause of the thyrotoxicosis (Table 43.3).
Box 43.4 Signs and symptoms of thyrotoxicosis
| Skin and appendages | Warm, moist skin |
| Thinning or loss of hair | |
| Increased sweating | |
| Heat intolerance | |
| Nervous system | Insomnia |
| Irritability, nervousness | |
| Lid retraction – staring eyes | |
| Symptoms of an anxiety state | |
| Psychosis | |
| Musculoskeletal | Fine motor tremor |
| Proximal muscle weakness | |
| Rapid deep tendon reflexes | |
| Osteoporosis | |
| Gastro-intestinal | Weight loss despite increased appetite |
| Thirst | |
| Diarrhoea | |
| Cardiovascular | Palpitations, tachycardia |
| Shortness of breath on exertion | |
| Atrial fibrillation | |
| Congestive cardiac failure | |
| Worsening angina |
Treatment
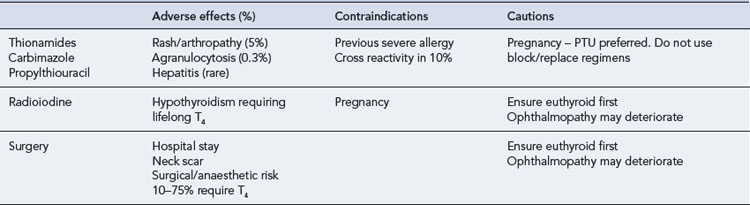
A number of factors need to be considered when choosing the most appropriate form of therapy for an individual patient (Table 43.4). There are usually a number of therapeutic options available, and the patient should be involved in the deciding on treatment. The decision may also be influenced by physician preference, which in turn can depend on the facilities available. Three forms of therapy are available, including anti-thyroid drugs, surgery and radioactive iodine. There is no general agreement as to the specific indications for each form of therapy, and none of them is ideal, all being associated with both short- and long-term sequelae. Neither surgery nor radioactive iodine should be given until the patient has been rendered euthyroid due to the risk of inducing a thyroid crisis.
Graves’ disease
A proportion (40–50%) of patients with Graves’ disease will achieve a long-lasting remission after a period of euthyroidism on thionamides. The optimal duration of anti-thyroid treatment is unknown and remains a controversial issue (Maugendre et al., 1999), but in most units, the length of the treatment course has fallen to between 6 and 12 months. It is not appropriate to discuss complex treatment decisions with a thyrotoxic patient, so most are well into this period when discussions of their options occur. Remission of Graves’ disease is much less likely in those with very large goitres, those who require high-dose thionamide treatment to maintain euthyroidism, those with high TRAB titres and patients who have relapsed once after a course of drug treatment. Such patients should therefore be rendered euthyroid and then have a discussion about either surgical or radioiodine thyroid ablation.
Anti-thyroid drugs
Adverse effects
The most common adverse effect of anti-thyroid treatment is rash and arthropathy (5%) and less commonly agranulocytosis, hepatitis, aplastic anaemia and lupus-like syndromes (Table 43.5). Overall, serious effects such as these occur in approximately 0.3% of patients treated. These side effects usually occur during the first 6 weeks of treatment, but this is not invariable. Cross-sensitivity between carbimazole and PTU is around 10%, and the patient can often be safely changed to the alternative agent if an adverse event occurs.
| Adverse effect | Comments | |
|---|---|---|
| Skin | Pruritic, maculopapular rash | Most common in first 6 weeks |
| May disappear spontaneously with continued treatment | ||
| Can be treated with an antihistamine | ||
| Change to alternative agent | ||
| Occurs in 5% of patients | ||
| Urticarial rash with systemic symptoms, that is fever, arthralgia | Discontinue drug | |
| Alternative treatment required | ||
| Haematological | Agranulocytosis | Most common in first 6 weeks |
| Incidence increases with age | ||
| Discontinue drug | ||
| Reversible | ||
| Consider alternative treatment | ||
| Occurs in 0.3% of patients | ||
| Leucopoenia | Transient | |
| Continue treatment | ||
| Does not predispose to agranulocytosis | ||
| Other | Hepatitis | Rare |
| Vasculitis | Discontinue drug | |
| Hypoprothrombinaemia | ||
| Aplastic anaemia | ||
| Thrombocytopenia |
Patient counselling
Patients should be advised of the importance of regular clinic attendance. This is necessary to monitor both therapeutic outcome and the development of adverse effects. The development of skin rashes, mouth ulcers or a sore throat should be reported by the patient, immediately investigated and a full blood count performed (see Box 43.5). It may be dangerous to treat these symptoms with over-the-counter medication before carrying out further investigations.
Thyroid ablative therapy
Radioactive iodine
Radioiodine therapy is extremely easy to administer by mouth and is very effective for a large majority of patients. It is contraindicated in pregnancy and breastfeeding and is usually avoided in children. It is known to make ophthalmopathy worse in some patients with Graves’ disease, but giving prednisolone 0.5 mg/kg for 3 weeks and commencing thyroxine replacement early (3 weeks after radioiodine) can mitigate this. Despite public concern in relation to radioactivity, accumulated experience over more than 60 years has not demonstrated any discernible risk of genetic, leukaemic or lymphoma risk (Vanderpump et al., 1996).
Drugs and the thyroid
Drugs and thyrotoxicosis
Table 43.2 indicates drug treatments associated with thyrotoxicosis. Amiodarone-induced thyrotoxicosis (AIT) is caused by two entirely different mechanisms. Type 1 AIT is similar to iodide-induced thyrotoxicosis and results from activation of nodular disease or of latent Graves’ disease in patients with thyroid autoimmunity. In this condition, the thyroid is actively synthesising hormone and treatment is with thionamides. Type 2 AIT has features similar to thyroiditis with leakage of pre-formed thyroid hormone, low uptake of radiolabel on scanning and is treated with glucocorticoids. AIT is an extremely challenging condition to manage for multiple reasons. These include difficulty in discrimination between type 1 and type 2 disease, each of which have different treatments, and the facts that most patients are taking amiodarone for serious cardiac dysrhythmias and amiodarone has a very long tissue half-life. These patients should be under the care of a specialist endocrinology team.
A recent observation has been the increased frequency of Graves’ disease in patients undergoing bone marrow transplantation, after administration of alemtuzumab (CAMPATH, a monoclonal antibody to CD52 cells), or alpha-interferon for multiple sclerosis and during Highly Active Anti-Retroviral Treatment (HAART) of HIV infection. It is thought that these cases all have immunological reconstitution as an underlying factor in their aetiology (Weetman, 2009).
Drugs and hypothyroidism
Lithium inhibits T4 and T3 release from the thyroid (making it a useful adjunctive treatment for thyrotoxicosis in patients who react to thionamides) and causes a goitre in 40% of patients and hypothyroidism in 20%, again more commonly in those with positive TPO antibodies. Like amiodarone, lithium is usually continued and these patients are treated with thyroxine. Drug-related interference with absorption of thyroxine is one of the causes of hypothyroidism in patients treated with thyroxine. Table 43.2 indicates the agents which may be implicated.
Calcium and parathyroid hormone
Hypoparathyroidism/hypocalcaemia
Aetiology
Hypoparathyroidism most commonly occurs as a result of surgery for thyroid disease or after neck exploration and resection of adenoma causing hyperparathyroidism. In experienced hands, the incidence of permanent hypoparathyroidism is less than 1% for all thyroid and parathyroid surgery. Other causes include autoimmune parathyroid destruction either as an isolated idiopathic disorder or as part of a multiple endocrine deficiency characterised by hyposecretion of several endocrine glands. Transient hypoparathyroidism with symptomatic hypocalcaemia can occur in neonates. The condition pseudohypoparathyroidism occurs in patients with defects of the PTH receptor such that though PTH levels are normal (or raised), calcium is low. Increasingly, reports are identifying acute symptomatic hypocalcaemia and hypomagnesaemia complicating the use of omeprazole and other protein pump inhibitors. These patients are severely magnesium depleted and have an acquired hypoparathyroidism which is reversible on stopping the offending drug (Cundy and Dissanayake, 2008).
Clinical manifestations
Most of the clinical features of hypoparathyroidism are due to hypocalcaemia. The decrease in ionised plasma calcium levels leads to increased neuromuscular excitability. The major signs and symptoms are shown in Box 43.6.
Investigations
Hyperphosphataemia is often present. It should be noted that there are many other causes of hypocalcaemia (Box 43.7). Pseudohypoparathyroidism is easily distinguished as it is associated with excessive PTH secretion and reduced target organ responsiveness. Drugs that may produce hypocalcaemia include calcitonin, plicamycin (formerly mithramycin), phosphate, bisphosphonates, phenytoin, phenobarbital, colestyramine, cisplatin, 5-FU and high-dose i.v. citrate or lactate.
Treatment
For chronic treatment, PTH therapy is not currently a practical option as the hormone has to be administered parenterally, and the current high cost is prohibitive. Maintenance treatment for hypoparathyroidism is easily achieved with a vitamin D preparation to increase intestinal calcium absorption, often in conjunction with calcium supplementation. Details of the preparations available are given in Table 43.6. Ergocalciferol (vitamin D2) is difficult to use and is not recommended. It has a long pharmacological and biological half-life, takes 4–8 weeks to restore normocalcaemia and its effects can persist for up to 4 months following withdrawal. In contrast, calcitriol and its synthetic analogue alfacalcidol are much easier to use. Alfacalcidol restores normocalcaemia within 1 week and its effects only persist for 1 week following withdrawal, permitting greater flexibility in dosage manipulation. The usual daily dose is 0.5–2 μcg. Patients need close monitoring initially until stable normocalcaemia is achieved and thereafter at a minimum of 6-month intervals indefinitely.
| Drug | Preparations | Activity |
|---|---|---|
| Ergocalciferol (calciferol, vitamin D2) | Calciferol injection 7.5 mg (300,000 units/mL) | Requires renal and hepatic activation |
| Calciferol tablets 250 μcg (10,000 units) and 1.25 mg (50,000 units) | ||
| Calcium and ergocalciferol tablets (2.4 mmol of calcium + 400 units of ergocalciferol) | ||
| Colecalciferol (vitamin D3) | A range of preparations containing calcium (500–600 mg) and colecalciferol (200–440 units) | Requires renal and hepatic activation |
| Alfacalcidol (1 α-hydroxycolecalciferol) | Alfacalcidol capsules, 250 ng, 500 ng and 1 μcg | Requires hepatic activation |
| Alfacalcidol injection, 2 μcg/mL | ||
| Calcitriol (1,25-dihydroxycholecalciferol) | Calcitriol capsules, 250 ng and 500 ng | Active |
| Calcitriol injection, 1 μcg/mL | ||
| Dihydrotachysterol | Dihydrotachysterol oral solution, 250 mg/ml | Requires hepatic activation |
Hyperparathyroidism
Clinical manifestations
The clinical features of primary hyperparathyroidism are shown in Box 43.8. These are related to the effects of hypercalcaemia itself, plus the effects of mobilisation of calcium from the skeleton and excretion in the urine. With increasingly early recognition of the biochemical abnormalities of primary hyperparathyroidism largely due to automated measurement of plasma calcium, most patients are identified at an early stage with mild or asymptomatic disease. The classical presenting features of bone disease and renal stones are now relatively uncommon. Although radiological evidence of bone disease is now rare in these patients, measurement of bone mineral content by densitometry (DEXA) scanning usually indicates that bone loss is accelerated and the risk of osteoporotic fractures later in life is increased.
Treatment of hypercalcaemia
Severe hypercalcaemia is a common medical emergency. It must be corrected whilst investigation continues to identify the cause. Table 43.7 shows the treatments available. In practice, rehydration and parenteral bisphosphonates, for example, pamidronate 60 mg in 250 mL normal saline over 30 min, will normalise calcium over 72 h in most patients.
| Mechanism | Treatment |
|---|---|
| Increase urinary calcium excretion | Normal saline plus loop diuretic |
| Reduce bone resorption | Bisphosphonates |
| Calcitonin | |
| Gallium, mithramycin | |
| Reduce gastro-intestinal absorption | Glucocorticoids in calcitriol dependent (vitamin D excess, sarcoid and some lymphoma patients) |
| Chelation | Intravenous EDTA or phosphate |
| Dialysis |
Questions
Answers
Answers
Answers
Answers
Answers
Cundy T., Dissanayake A. Severe hypomagnesaemia in long-term users of proton-pump inhibitors. Clin. Endocrinol. (Oxf.). 2008;69:338-341.
Faber J., Galloe M. Changes in bone mass during prolonged treatment subclinical hyperthyroidism due to L-thyroxine treatment: a meta analysis. Eur. J. Endocrinol.. 1994;130:350-356.
Grebe S.K., Cooke R.R., Ford H.C., et al. Treatment of hypothyroidism with once weekly thyroxine. J. Clin. Endocrinol. Metab.. 1997;82:870-875.
Maugendre D., Gatel A., Campion L., et al. Antithyroid drugs and Graves’ disease: prospective randomised assessment of long-term treatment. Clin. Endocrinol. (Oxf.). 1999;50:127-132.
Morris J.C. How do you approach the problem of TSH elevation in a patient on high-dose thyroid hormone replacement? Clin. Endocrinol. (Oxf.). 2009;70:671-673.
Rangan S., Tahrani A., Macleod A., Moulik P. Once weekly thyroxine treatment as a strategy to treat non-compliance. Postgrad. Med. J.. 2007:83. e3 Available at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2600132/pdf/e3.pdf
Sawin C.T., Geller A., Wolf P.A., et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N. Engl. J. Med.. 1994;331:1249-1252.
Surks M.I., Sievert R. Drugs and thyroid function. N. Engl. J. Med.. 1995;333:1688-1694.
Tunbridge W.M., Evered D.C., Hall R., et al. The spectrum of thyroid disease in a community: the Wickham survey. Clin. Endocrinol. (Oxf.). 1977;7:481-493.
Uzzan B., Campos J., Cicherat M., et al. Effects on bone mass of long-term treatment with thyroid hormones: a meta-analysis. J. Clin. Endocrinol. Metab.. 1996;81:4278-4289.
Vanderpump M.P.J., Alquist J.A.O., Franklyn J.A., et al. Consensus statement for good practice and audit measures in the management of hypo and hyperthyroidism. Br. Med. J.. 1996;313:539-544.
Weetman A. Immune reconstitution syndrome and the thyroid. Clin. Endocrinol. Metab.. 2009;23:693-702.
Beckerman P., Silver J. Vitamin D and the parathyroid. Am. J. Med. Sci.. 1999;317:363-369.
Brown A.J. Vitamin D analogues. Am. J. Kidney Dis.. 1998;32(2 Suppl. 2):S25-S39.
Hanna F.W.F., Lazarus J.H., Scanlon M.F. Controversial aspects of thyroid disease. Br. Med. J.. 1999;319:894-899.
Lourwood D.L. The pharmacology and therapeutic utility of bisphosphonates. Pharmacotherapy. 1998;18:779-789.
Newman C.M., Price A., Davies D.W., et al. Amiodarone and thyroid: a practical guide to the management of thyroid dysfunction induced by amiodarone therapy. Heart. 1998;79:121-127.
Woeber K. Update on the management of hyperthyroidism and hypothyroidism. Arch. Intern. Med.. 2000;160:1067-1071.