Thyroid and Parathyroid
Thyroid Gland
The term “thyroid” is derived from the Greek word for “shield” because of the gland’s shape and relationship to the laryngeal thyroid cartilage. The thyroid gland has a dual embryonic origin.1–3 The two thyroid cell types, thyroid follicular cells (thyrocytes) and parafollicular (C-cells), are derived from all three germ cell layers.3
The most abundant cells, the follicular cells, arise from the thyroid anlage. The development of the thyroid gland begins as a bud of epithelial proliferation in the floor of the primitive pharynx between the developing tuberculum impar and copula of the tongue anlage, around 24 days’ gestation.4 This thyroid anlage soon forms a ventral outgrowth known as the thyroid diverticulum. The progenitor follicular cells proliferate distally and then laterally, leading to the characteristic bilobed appearance of the gland connected by an isthmus.
As the embryo grows, the developing thyroid gland descends anterior to the hyoid bone and larynx, forming the thyroglossal duct. Because of the close association of the developing thyroid gland and embryonic heart, it is thought that the descent of the heart results in the thyroid gland being pulled.3,5 The thyroid gland remains connected to the tongue by the thyroglossal duct. At approximately 7 weeks’ gestation, the thyroid gland reaches its final site in front of the trachea and the thyroglossal duct disappears.4 The original opening of the thyroglossal duct persists as a vestigial pit at the base of the tongue called the foramen cecum.6,7 About 15% to 75% of people have a pyramidal lobe, which is derived from the lower part of the thyroglossal duct and extends upward from the isthmus.8
Around the time the thyroid gland reaches its final position, it merges with the two lateral anlagen or ultimobranchial bodies, resulting in the incorporation of the C-cells (parafollicular cells) into the thyroid gland. The ultimobranchial bodies are a pair of transient embryonic structures derived from the endoderm of the fourth pharyngeal pouch and the ectoderm of the fifth pharyngeal pouch, into which the C-cell precursors migrate from the neural crest.9,10 The thyroid follicular cells continue to organize the thyroid follicles. As the ultimobranchial bodies merge with the thyroid, their C-cells disperse within the interfollicular space.4 Remnants of the ultimobranchial bodies, or solid cell nests, are seen postnatally and are usually located in the middle third of the thyroid lateral lobes.11
Physiology
The hypothalamus synthesizes and secretes thyrotropin-releasing hormone (TRH), which is carried to the pituitary gland by the hypothalamic-pituitary portal venous system.12–14 Once in the pituitary gland, TRH stimulates the synthesis and secretion of thyrotropin (thyroid-stimulating hormone [TSH]) from the anterior pituitary gland. TSH binds to receptors in the thyroid gland, stimulating follicular cell production and secretion of T4 and T3. Thyroid secretion and serum concentrations of T4 and T3 are maintained by a negative feedback loop involving inhibition of TSH and TRH secretion by T4 and T3.15,16
Iodide is actively transported into the follicular cells by the sodium-iodide symporter at the basolateral membrane.17–21 Thyroid peroxidase (TPO) oxidizes iodide into its chemically active form. Thyroglobulin in the follicular lumen serves as a matrix for the synthesis of T4 and T3. First, TPO catalyzes the iodination of selected tyrosyl residues in thyroglobulin in a process known as iodination and organification. This results in the formation of monoiodotyrosine (MIT) and diiodotyrosine (DIT). TPO then catalyzes a coupling reaction in which two iodotyrosines are coupled to form T4 or T3. Iodinated thyroglobulin is stored as colloid in the follicular lumen. When needed, thyroglobulin is internalized into the follicular cell and digested in lysosomes. Subsequently, T4 (80%) and T3 (20%) are released into the bloodstream. MIT and DIT are deiodinated and released iodide is recycled for hormone synthesis.3
C-cells produce thyrocalcitonin, which is important in calcium homeostasis.
Anatomy
The thyroid gland is highly vascular being supplied by paired superior thyroidal arteries (first anterior branches of the external carotid arteries) and inferior thyroidal arteries (branches of the thyrocervical trunks that originate from the subclavian arteries). The thyroidea ima is an inconstant single vessel that has a variable origin but usually arises directly from the aortic arch or innominate artery and helps supply the inferior thyroid gland. Venous drainage is via the superior and middle thyroid veins, which drain into the internal jugular veins, and the inferior thyroid veins, which often join to form a single trunk draining to the left brachiocephalic vein. Lymphatic drainage is extensive and multidirectional. The thyroid gland is innervated by the vagus nerve and the cervical sympathetic neural plexus.22
Normal Findings
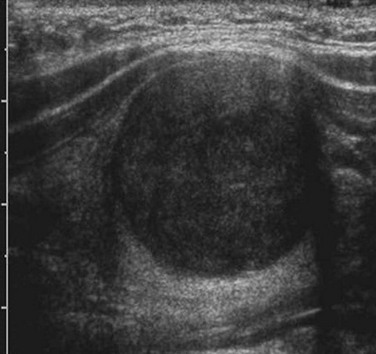
Ultrasonography is usually the first choice of imaging in pediatrics because it is noninvasive, is readily available, and does not utilize radiation. A normal thyroid gland will have homogeneous echotexture, which is slightly hyperechoic relative to adjacent neck muscles.23,24 Colloid follicles are commonly seen as small (less than 3 millimeters [mm] in diameter) anechoic cystic areas. Occasionally, the follicles contain inspissated colloid, which appear as punctate echogenic foci (Fig. 17-1).25
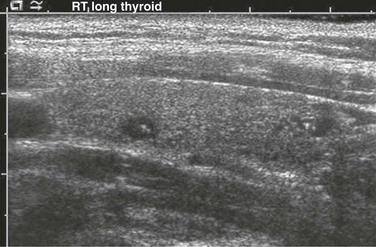
Figure 17-1 Normal thyroid ultrasound.
Small anechoic foci with a central hyperechoic focus represent colloid follicles, which are a normal finding.
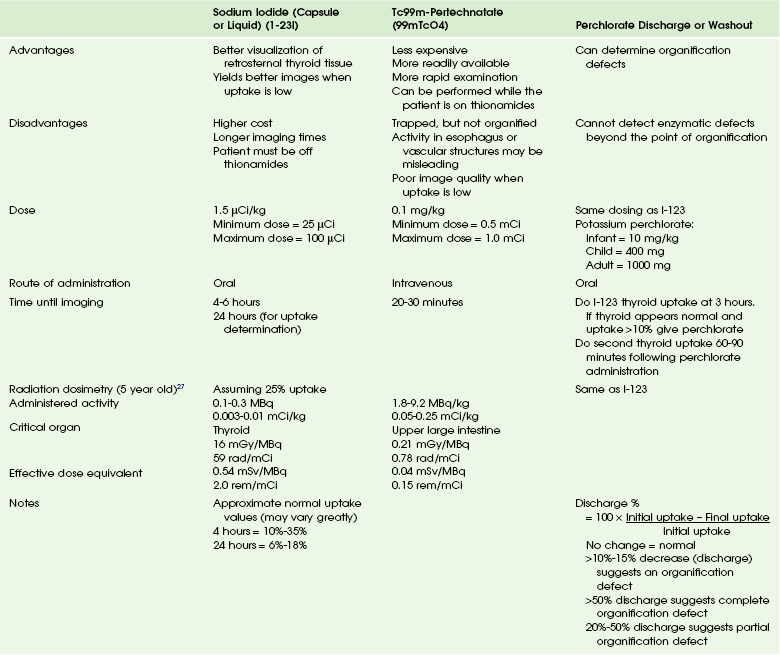
Nuclear scintigraphy provides morphologic and functional information about the thyroid gland. Thyroid scintigraphy is performed using intravenous Tc-99m pertechnetate (99mTcO4) or oral Na I-123 (I-123) (Table 17-1). Because of the large radiation dose to the thyroid gland (approximately 0.01 to 0.03 gray [Gy] per microCurie [uCi] administered), I-131 is not used for routine diagnostic imaging.26,27 The normal thyroid gland shows homogeneous radiopharmaceutical uptake and distribution in both lobes. The isthmus of the thyroid gland often demonstrates slightly less activity than the right and left thyroid lobes. Normal I-123 24-hour uptake ranges from 10% to 30%.
The normal thyroid gland (because of its iodide content) has a density of approximately 80 to 100 Hounsfield units on CT. A well-visualized gland usually indicates a normally functioning thyroid, whereas a poorly seen gland correlates with poor thyroid function. The injection of iodinated contrast material diffusely and homogeneously enhances the gland.22 The use of iodinated contrast agents will alter radioactive iodine uptake, whereas gadolinium contrast material will not. The normal thyroid gland shows homogeneous signal intensity slightly greater than muscle on T1-weighted images. On T2-weighted images, the thyroid gland is relatively hyperintense to muscle. Following contrast administration, the gland enhances diffusely and homogeneously.
Hypothyroidism
Hypothyroidism is the most common disturbance of thyroid function in children. It may be congenital (Box 17-1) or may be acquired in childhood or adolescence (Box 17-2). The thyroid gland produces hormones that play a vital role in regulating many cellular and physiologic activities. Untreated congenital hypothyroidism in early infancy results in profound retardation of growth and neurocognitive development (cretinism). Untreated hypothyroidism in older children leads to growth failure as well as slowed metabolism and impaired memory.
Congenital Hypothyroidism
Hypothyroidism in the newborn may be permanent or transient. Congenital hypothyroidism with lower than normal T4 causes retardation of growth and neurocognitive development if left untreated. The incidence of congenital hypothyroidism in the United States has dramatically increased over the last two decades, from 2.9 cases per 10,000 births in 1991 to nearly 4 cases per 10,000 births in 2000.28,29 All states now require all newborns to be screened for hypothyroidism.
Etiologies, Pathophysiology, and Clinical Presentation: The majority of cases of congenital hypothyroidism are caused by thyroid gland dysgenesis (see Box 17-1). Thyroid dysgenesis refers to a developmental defect of thyroid morphogenesis. The three types are (1) ectopia, (2) aplasia (athyrosis), and (3) hypoplasia.
Imaging: Imaging is not routinely used to diagnose congenital hypothyroidism. According to the most recent recommendations for congenital hypothyroidism in newborns by the American Academy of Pediatrics, the American Thyroid Association, and the Lawson Wilkins Pediatric Endocrine Society, thyroid imaging in congenital hypothyroidism is optional because of controversy regarding the risk-benefit ratio and uncertainty whether imaging findings have any bearing on patient management.30
Diagnostic studies for congenital hypothyroidism can include ultrasonography and thyroid scintigraphy with 1-123 or 99mTcO4. Use of both ultrasonography and thyroid scintigraphy has been shown to provide a more complete depiction of congenital hypothyroidism in the newborn than either study performed alone.31
99mTcO4 or I-123 can be used to help determine if thyroid dysgenesis is the cause of hypothyroidism (see Table 17-1). In patients with thyroid agenesis, the test fails to demonstrate functional thyroid tissue. It is important that the images include the oropharynx and upper neck as well as the upper portion of the chest so that an ectopic thyroid gland can be excluded.
99mTcO4 scintigraphy demonstrates a round or oval area of uptake in the midline of the upper neck in most cases of ectopia (Fig. 17-2). The ectopic gland may occupy a lingual (most common), sublingual, or prelaryngeal location. Mediastinal and lateral locations are rare. Functional thyroid tissue may be identified in more than one location, most commonly in the lingual and sublingual regions. It is unusual to identify thyroid tissue in its normal location in the presence of an ectopic gland. Patients with an ectopic thyroid gland will usually have hypothyroidism. In some unusual cases, the ectopic gland is capable of secreting sufficient thyroid hormone such that hypothyroidism is not apparent on neonatal screening. These patients often present with signs of ectopia later in life when the hyperstimulated gland enlarges and causes local symptoms.
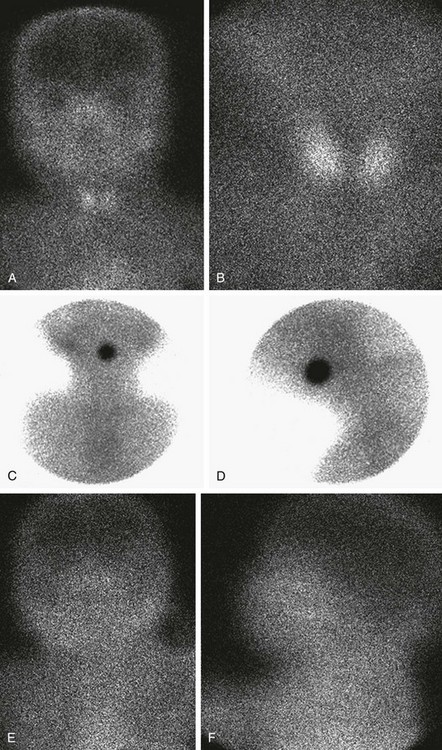
Figure 17-2 Normal and abnormal thyroid scans.
A and B, Normal thyroid anatomy. Anterior and close-up (pinhole) views show normal bilobate architecture and normal position. Thyroid function cannot be meaningfully estimated from these images in an infant with known thyroid insufficiency. C and D, Thyroid ectopia. Anterior and lateral views of the neck show a solitary rounded focus of radiopharmaceutical trapping at the base of the tongue. No clinical difference exists between the terms lingual and sublingual, and they are used interchangeably. E and F, No thyroid is identified. Anterior and lateral views show no functioning thyroid tissue. Although this finding suggests thyroid agenesis, it is nonspecific because severely decreased thyroid function, particularly resulting from maternal thyrotropin receptor–blocking antibody, also may have this appearance.
In cases of dyshormonogenesis, 99mTcO4 scintigraphy will demonstrate a normally positioned thyroid gland that may or may not be enlarged (e-Fig. 17-3). If dyshormonogenesis is suspected, a perchlorate washout test may be performed. Perchlorate is actively transported into the thyroid gland with a greater affinity than iodide and is, therefore, a competitive inhibitor of the thyroid iodide trap. During unimpaired thyroid hormonogenesis, iodide entering the thyroid gland is rapidly oxidized and iodinates tyrosine, forming MIT and DIT, with subsequent coupling of MIT and DIT to generate T4 and T3. Intrathyroid deiodination of the iodinated tyrosines and thyronines results in a very small pool of thyroidal inorganic iodide. Any congenital or acquired condition associated with a defect in iodide organification may yield a higher intrathyroidal inorganic iodide concentration. The perchlorate discharge or washout test is a means of estimating the size of this intrathyroidal “free” iodide pool, thereby detecting and roughly quantifying disturbances in iodide organification. The perchlorate discharge or washout test is performed by giving the patient an oral dose of I-123, followed by a dose of perchlorate and measuring the “washout” (see Table 17-1). The perchlorate test will be negative in patients who do not have an organification defect and also when enzymatic defects are present in the synthetic pathway beyond the point of organification.32–34
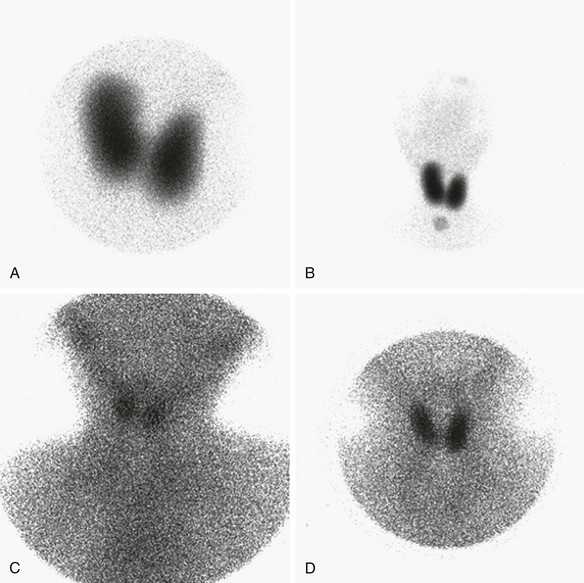
e-Figure 17-3 Abnormal thyroid scans.
A and B, Organification defect. A, Anterior pinhole view. A bilobed thyroid is present in the neck. It shows diffusely increased uptake. B, Anterior prominently sized thyroid compared with the neck and the head. C and D, Anterior pinhole views of poorly functioning thyroid from two patients. A bilobed thyroid is normally located within the neck. Trapping by the thyroid is poor with high body background. The thyroid may appear small (C) or normal (D). This pattern can be seen in patients with thyroid gland hypoplasia or a transient insult, such as maternal antibodies. Scintigraphically, these entities cannot be distinguished.
Hypothyroidism in Children and Adolescents
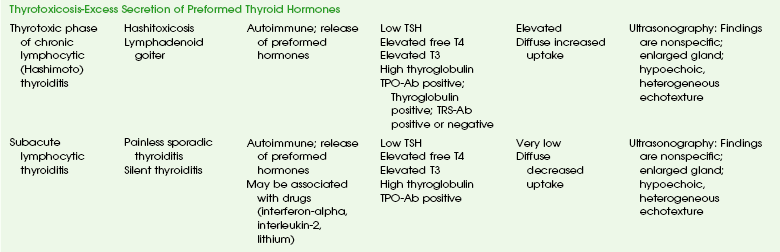
Chronic Autoimmune (Hashimoto) Thyroiditis: Acquired hypothyroidism is caused by many factors in the pediatric population (see Box 17-1). Chronic autoimmune (Hashimoto) thyroiditis is the most common cause of acquired hypothyroidism in children and adolescents in iodine sufficient areas. It is more common in girls than in boys and increases in frequency with age during childhood and adolescence.35–37
Etiologies, Pathophysiology, and Clinical Presentation: Chronic autoimmune thyroiditis is a complex, thyroid-specific T-cell mediated disease with a strong genetic component.38–40 It often coexists with other autoimmune diseases and may also be expressed as part of an autoimmune polyendocrine syndrome type 2.41,42 The two major forms of the disorder are goitrous autoimmune thyroiditis and atrophic autoimmune thyroiditis with the common pathologic feature being lymphocytic infiltration and the common serologic feature being the presence of high serum concentrations of antibodies to TPO and thyroglobulin. Approximately 2% of all surveyed adolescents have serum TSH levels indicating hypothyroidism.43
The most common physical finding at presentation is a goiter, along with growth retardation and short stature.35 The growth delay is usually insidious in onset and may be present for several years before other symptoms occur.44 Other common symptoms include changes in school performance, sluggishness, lethargy, cold intolerance, constipation, dry skin, brittle hair, facial puffiness, and muscle aches. If the cause is from hypothalamic or pituitary disease, the patients may have headaches, visual symptoms, or pituitary disease manifestations.
Imaging: Most physicians consider the presence of serum antithyroid antibodies as sufficient evidence for chronic autoimmune thyroiditis, and thyroid ultrasonography or radionuclide scanning are rarely indicated. Children with central hypothyroidism should undergo cranial imaging, preferably MR (with contrast), and tests for other pituitary hormone deficiencies.
Ultrasound findings are nonspecific but include an enlarged relatively hypoechoic gland with coarse heterogeneous echotexture. Less commonly, the echogenicity of the gland is increased relative to adjacent muscle. Fibrotic septations in the chronic form may produce a pseudolobulated appearance of the parenchyma. Multiple discrete, hypoechoic, 1 to 6 mm micronodules may also be seen (Fig. 17-4, A to C).
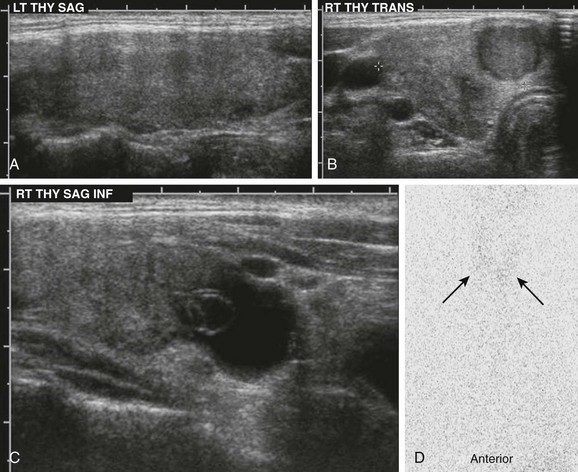
Figure 17-4 Chronic autoimmune (Hashimoto) thyroiditis.
A, Ultrasonography demonstrates an enlarged gland that is relatively hypoechoic with heterogeneous echotexture. B and C, The right lobe of the same patient has a hypoechoic nodule and cystic changes. D, The I-123 scan shows diffuse decreased uptake (arrows). The 24-hour uptake was only 0.5%.
In the early (preclinical) stage of Hashimoto thyroiditis, elevated I-123 uptake values with diffusely increased radionuclide activity may be seen. This happens because the initial mild decline in circulating thyroid hormone causes a compensatory rise in TSH secretion that stimulates the gland. Thyroid follicles may demonstrate a variable response to the chronic TSH stimulation, leading to patchy follicular proliferation. On the thyroid scan, this phenomenon manifests as patchy areas of increased activity (follicles that respond to TSH) and of decreased activity (those that do not respond). As more thyroid parenchyma is replaced by fibrous tissue, the radionuclide uptake becomes nonuniformly decreased (see Fig. 17-4, D).45
Hypertyhroidism and Thyrotoxicosis
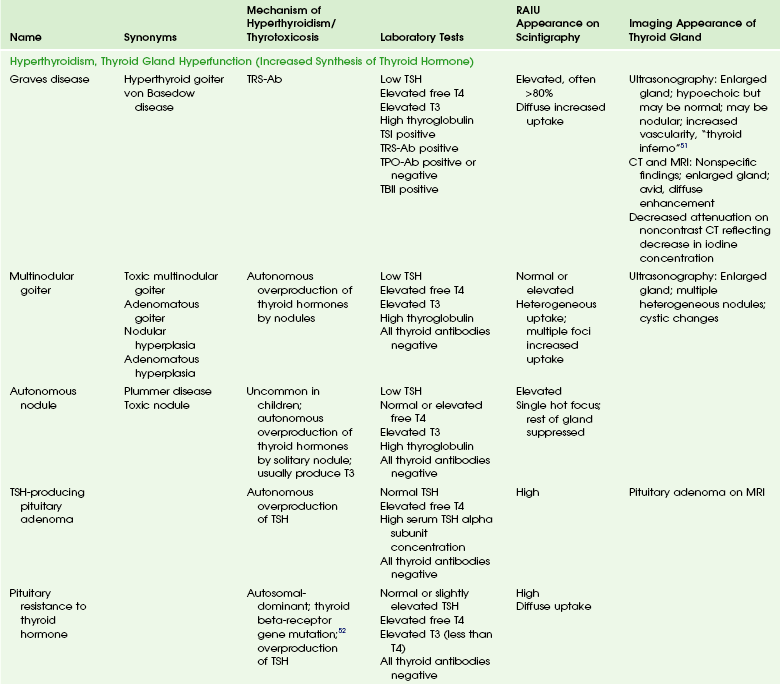
Hyperthyroidism refers to overproduction of thyroid hormone by the thyroid gland. Thyrotoxicosis refers to the clinical and biochemical manifestations of excess thyroid hormones. Hyperthyroidism and thyrotoxicosis in children have multiple causes (Table 17-2). Most cases of thyrotoxicosis in children are associated with hyperthyroidism. Graves disease is the most common cause of hyperthyroidism in the pediatric population.
A 2008 study estimated the incidence of hyperthyroidism by using the number of new prescriptions of thionamides and data from the 2008 U.S. census and concluded that the incidence among individuals aged 0 to 11 years was 0.44 cases per 1000 population; in those aged 12 to 17 years, 0.26 cases per 1000; and in those aged 12-17 years, 0.59 cases per 1000.46
Graves Disease
Etiologies, Pathophysiology, and Clinical Presentation: Graves disease is the most common cause of hyperthyroidism in children and adolescents. The general cause is thyrotropin receptor-stimulating antibodies (TRS-Ab), which activate the TSH receptor.
Many of the clinical features of hyperthyroidism are similar in children, adolescents, and adults. Most children with Graves disease have a diffuse goiter.47 Like hypothyroidism, hyperthyroidism also has an effect on growth and pubertal development. Acceleration of growth with advanced epiphyseal maturation may be seen in untreated hyperthyroidism, although changes may be subtle and the degree depends on the duration of hyperthyroidism before diagnosis. Pubertal development, in contrast, tends to be delayed or slowed in children with untreated hyperthyroidism.
Graves disease causes other unique problems not associated to the high serum thyroid hormone concentrations. They include Graves ophthalmopathy and pretibial myxedema. Graves ophthalmopathy is common in children but is generally less severe than in adults. The clinical manifestations of Graves ophthalmopathy stem from a combination of increased orbital fat and extraocular muscle volume. The exact etiology is unknown; however, it may result from antibodies against a TSH receptor–like protein in retro-orbital connective tissue leading to adipogenesis. Although originally thought to represent another causative agent, antibodies to extraocular muscles are now generally thought to be secondary to extraocular muscle inflammation and damage.48,49
Imaging: In the majority of patients, no imaging is needed. The diagnosis may be made through physical examination, laboratory tests, and the onset or chronicity of symptoms. If thyrotoxicosis has been present for less than 8 weeks, transient thyrotoxicosis secondary to subacute thyroiditis or the thyrotoxic phase of autoimmune or silent thyroiditis should be considered. These conditions are self-limiting and refractory to therapy with thionamides. Thyrotoxicosis that has been present for more than 8 weeks suggests true hyperthyroidism thyrotoxicosis. However, if the diagnosis cannot be made clinically, an I-123 uptake, with or without a scan, should be performed (see Table 17-2).50–52
Treatment: Graves disease can be treated pharmacologically, surgically, or with radioiodine ablation. The thionamide antithyroid drugs propylthiouracil and methimazole continue to be the most commonly used medications in the treatment of Graves disease in the United States.53 Thionamides exert their antithyroid effects primarily by inhibiting thyroid hormone synthesis through interference with the oxidation and binding of iodide into thyroglobulin.54 PTU also inhibits the peripheral conversion of T4 to T3 by type 1 deiodinase.55,56 In resistant disease or noncompliant patients, treatment is by radioiodine ablation or thyroidectomy. Concerns over the potential long-term complications of pediatric radiation exposure have traditionally made endocrinologists hesitant about using radioiodine in the treatment of Graves disease. The use of radioactive iodine has now been detailed for more than 1000 children, with remission rates over 95% and very few complications.57 Total-body radiation doses after I-131 therapy vary with age, and the same absolute dose of I-131 will result in more radiation exposure to a young child than to an adolescent or adult.58–61 Thus, in addition to selecting a dose that will achieve adequate thyroid tissue destruction, the age of the patient, and the total I-131 dose need to be considered.
Infection
Acute suppurative thyroiditis is an infection of the thyroid gland that is rarely seen during childhood but is potentially life threatening.62,63
Etiologies, Pathophysiology, and Clinical Presentation: Acute suppurative thyroiditis is usually caused by a bacterial infection. Staphylococcus aureus, Streptococcus pyogenes, Streptococcus epidermidis, and Streptococcus pneumoniae are the most common aerobic bacteria. The predominant anaerobic bacteria are gram-negative bacilli and Peptostreptococcus spp.64
Suppurative thyroiditis may be related to a pyriform sinus fistula or thyroglossal duct remnant, especially when recurrent infections occur.62,65,66
Benign Lesions or Masses
Thyroid nodules and cysts are uncommon in children before puberty.67–70 Most thyroid nodules are benign, although an increased incidence of malignancy exists in pediatric thyroid nodules with an overall 26.4% risk.68
Benign Nodules
The most common cause of benign solitary thyroid nodules is follicular adenoma (hyperplastic nodule).71
Etiologies, Pathophysiology, and Clinical Presentation: Follicular adenomas are thought to be the result of cycles of hyperplasia and colloid involution of thyroid nodules.24 These are encapsulated lesions, which are usually solitary and nonfunctioning. Follicular adenomas are usually asymptomatic or may present as a palpable nodule. Sudden enlargement and pain are usually related to spontaneous hemorrhage within the lesion. With the increasing number of cross-sectional studies being performed, thyroid nodules are often found incidentally.72
Imaging: The findings of ultrasonography, CT, and MRI are nonspecific and may be seen in both benign and malignant nodules.
On ultrasonography, follicular adenomas are usually hypoechoic relative to normal thyroid tissue, although some are hyperechoic and a few are isoechoic. A thin hypoechoic “halo” or rim around the lesion may be seen. The cause of the “halo” is unknown, but the fibrous capsule, compressed thyroid parenchyma, or pericapsular inflammatory infiltration may be the cause.24,73 Adenomas may also contain hypoechoic or anechoic areas from internal hemorrhage and necrosis. Calcifications may also be present (e-Fig. 17-5).
Cysts
Cysts are often thought to be caused by benign degenerative thyroid diseases.71 However, as with thyroid nodules, a great heterogeneity exists in these disease processes in children, ranging from benign pure cysts to malignant lesions.70
Etiologies, Pathophysiology, and Clinical Presentation: True simple cysts lined by epithelium are rare. The majority of benign thyroid cysts are felt to be the result of cystic degeneration of a follicular adenoma.71 Hemorrhagic cysts are usually the result of bleeding into a follicular adenoma.
Imaging: Simple benign cysts are anechoic on ultrasonography and hypodense on CT. On MRI, they will follow the signal characteristics of water, demonstrating low signal on T1-weighted images and high signal on T2-weighted images. Hemorrhagic cysts will have high signal on T1-weighted images and will be hyperdense on CT.
Treatment: Thyroid cysts and thyroglossal duct cysts may be excised for diagnosis or if they become secondarily infected, cause mass effect resulting in pain or dysphagia, or for cosmesis. The treatment of choice for TDC is the Sistrunk operation, which entails complete removal of the cyst and tract. This procedure also includes removal of the central portion of the hyoid bone.
Malignant Lesions
Thyroid malignancies are rare in children. The incidence of thyroid carcinomas is roughly 4.9 per million in children, with the peak incidence being in the 15- to 19-year-old age group (1.8 per 100,000). Papillary, follicular, and medullary carcinomas are seen in children, whereas anaplastic and poorly differentiated carcinomas are very rare.75 Other rare thyroid neoplasms include teratomas76 and non-Hodgkin lymphoma.77 Most childhood thyroid malignancies are of the papillary type.
Etiologies, Pathophysiology, and Clinical Presentation: Exposure to head and neck irradiation is associated with an increased risk for the development of thyroid carcinoma.78,79 Some thyroid cancers have a genetic predisposition and may be associated with certain syndromes. A positive family history is seen in medullary carcinoma and in 3% of papillary carcinomas (chromosome 19p13.2).80,81 A high incidence of papillary thyroid carcinomas is seen in familial adenomatosis polyposis coli and Cowden disease.82,83 Inherited medullary carcinoma is seen in multiple endocrine neoplasia type 2a and 2b or as part of familial medullary carcinoma.84
Thyroid carcinoma in children is biologically and clinically different from that seen in adults. The most common clinical presentation is a solitary thyroid nodule.85 Other presenting manifestations such as dysphonia or dysphagia caused by local invasion of surrounding structures are rare in children. Children present with cervical node involvement (60%) and pulmonary metastases (13%) more often than adults.86,87 Children also have more advanced disease and a higher rate of recurrence.87 The pulmonary metastases are almost always functional and tend to be miliary.88 Lymph nodes are the most common site of dissemination, followed by the lungs. Bone metastases are rare.88
Imaging: The imaging appearance of papillary carcinoma is variable. At ultrasonography, the presence of indistinct margins, hypoechogenicity, predominantly solid composition, vascularity, absence of a hypoechoic halo, and calcifications are suggestive of malignancy.89 Ultrasonography also helps guide fine-needle aspiration, recommended for all thyroid nodules.90 Although a solitary nodule is most common in children, multifocal nodules, diffuse infiltration with heterogeneous hypodensity, or a normal-appearing thyroid gland may be found on CT.22 Metastatic lymph nodes are usually enlarged; may be calcified, cystic, or hemorrhagic; or contain colloid (Fig. 17-6).91
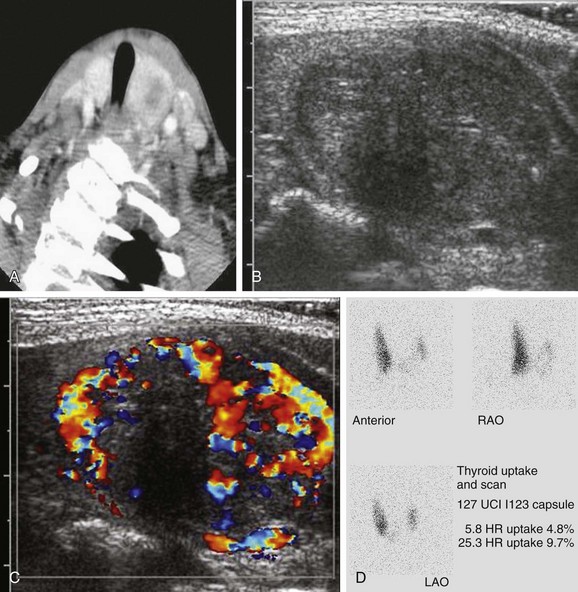
Figure 17-6 Papillary carcinoma.
This patient was found to have a thyroid nodule on contrast-enhanced computed tomography being performed to assess for infection. A, The computed tomography scan shows a low attenuation lesion in the left lobe of the thyroid gland. B and C, Ultrasound images demonstrate a relatively heterogeneous nodule with punctate echogenicities and an eccentric round region of hypoechogenicity. Peripherally the lesion has increased vascularity. D, The I-123 uptake and scan demonstrates decreased uptake in the left gland and a focal “cold” lesion.
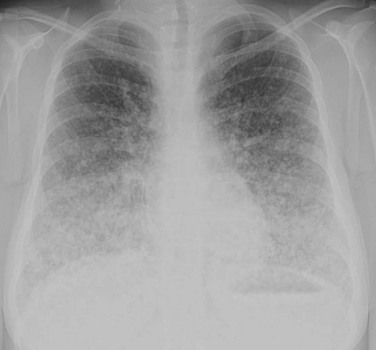
MRI is helpful in evaluating and delineating the extent of local invasion and lymph node metastases. Chest CT is more sensitive for pulmonary metastatic disease and detects micronodular and interstitial patterns of metastases much better than conventional chest radiography (Fig. 17-7).92
Treatment: A total or near-total thyroidectomy is recommended by most experts and is believed to decrease the incidence of recurrence.86,93,94 Thyroidectomy also enables the use of thyroglobulin levels and whole body radioiodine scans for monitoring disease persistence and recurrence. Modified lateral neck dissection is recommended when lateral node involvement is seen on clinical examination, preoperative ultrasonography, or intraoperative biopsy.88,95
Routine postoperative radioiodine ablation is recommended in children. Remnant thyroid tissue (greater than 0.3% uptake on radioiodine scan) is seen in most cases following thyroidectomy.92 This tissue can interfere with the detection of residual or recurrent disease on whole body radioiodine scans or by thyroglobulin measurements. Prior to radioablation, a diagnostic whole body scan with I-123 or 0.5 to 2 milliCurie (mCi) of I-131 is obtained.96 Images are obtained 24 to 48 hours after administration of the radioisotope. The preablation I-131 scan shows the extent of thyroid remnant as well as disease burden after thyroidectomy. Although some experts believe that preablation imaging may cause decreased I-131 uptake during ablation, no unequivocal evidence exists to suggest that this occurs when a low-dose (<2 mCi) diagnostic scan is performed.97,98
The uptake of radioiodine is dependent on TSH stimulation of both normal and malignant thyroid tissue. The TSH level should be greater than 30 microunits per milliliter (uU/mL) for optimal radioiodine uptake. A diagnostic scan followed by ablation is performed 6 weeks after thyroidectomy. Thyroid hormone medications must be withheld for a sufficient time to permit an adequate rise in the TSH level. T3 has a short half-life and can be given until 2 weeks before the scan.92 Additionally, the patient is placed on a low iodine diet for 2 weeks prior to the scan to increase the avidity of remnant tissue for iodine.88
The maximally safe radioiodine dose in children is calculated on the basis of quantitative blood and whole body dosimetry, and the minimally effective dose is calculated on the basis of lesion dosimetry.99 Since this is complicated, most centers use a fixed dose of 30 mCi.100 Rarely, a second treatment may be needed for complete ablation. Much higher doses of up to 200 mCi are required to ablate pulmonary metastases.101 It is customary to obtain a scan 5 to 7 days after radioablation, which will demonstrate avid uptake in any thyroid remnant and may show metastases not apparent on the previous diagnostic scan.92
On the basis of the belief that thyroid carcinoma cells are dependent on TSH stimulation for growth, TSH suppressive therapy with thyroxine is also used to suppress tumor growth. The optimal TSH level that needs to be maintained is not known. Initially, TSH levels of less than 0.1 uU/mL are recommended, but once remission has been achieved, levels of less than 0.5 uU/mL may be acceptable.93
Parathyroid Glands
The third pharyngeal pouch gives rise to the thymus and the inferior parathyroids. As both primitive inferior parathyroid glands lose their connection with the pharyngeal wall, they descend with the thymus. This migration of the inferior parathyroid glands with the thymus accounts for their lower position than the superior parathyroid glands that are derived from the fourth pharyngeal pouches.102 The glands are usually distributed evenly between the lower pole of the thyroid gland and isthmus but may be found anywhere along their course of descent.103
The fourth pharyngeal pouch gives rise to the superior parathyroid glands, which attach to the posterior surface of the descending thyroid. They have a much shorter migration distance than the inferior parathyroid glands, which accounts for their more predictable location. Generally, the superior parathyroid glands are located posterior at the level of the upper two thirds of the thyroid gland, approximately 1 centimeter above the crossing point of the recurrent laryngeal nerve and inferior thyroid artery.104
Physiology
The function of the parathyroid glands is to produce parathyroid hormone (PTH), which is one of two major hormones involved in calcium and phosphate homeostasis. PTH is produced by chief cells within the parathyroid glands. Oxyphil cells may also secret PTH, but their true function is unknown. PTH closely maintains serum ionized calcium in a narrow range through stimulation of renal tubular calcium resorption and bone resorption.105 PTH also stimulates the conversion of calcidiol (25-hydroxyvitamin D) to calcitriol in renal tubular cells, thereby stimulating intestinal calcium absorption. PTH secretion is, in turn, regulated via a calcium-sensing receptor on the surface of parathyroid cells.106 When the calcium-sensing receptor is activated by an increase in calcium, PTH secretion is inhibited. Conversely, deactivation of the receptor by decreases in calcium stimulates PTH secretion.
Anatomy
The paired superior parathyroid glands are fairly constant in their position near the upper surface of the thyroid lobes. The inferior parathyroid glands are found in proximity to the lower pole of the thyroid gland. Ectopic superior parathyroid glands may be found at the level of the upper pole of the thyroid gland (2%) and above the upper pole (0.8%). Other ectopic positions of the superior parathyroid glands in the posterior neck, retropharyngeal, retroesophageal, or intrathyroid regions are even rarer (1% total).103,104 Ectopic inferior parathyroid glands may be found anywhere along their area of descent up to the superior border of the pericardium.103 Supernumerary glands, when present, are often found in the mediastinum associated with the thymus.
The vascular supply of the parathyroid glands is predominately the inferior thyroid artery, although the superior parathyroid glands may also be supplied by the superior thyroid artery.104,107,108 The venous drainage is predominately to the inferior thyroid veins.109
The parathyroids are scantily supplied with vasomotor nerve fibers from the superior, middle, or inferior cervical sympathetic ganglia.110,111
Normal Findings
Normal glands are difficult to visualize because of their small size (less than 5 mm in length). On ultrasonography, a normal parathyroid gland will have an echotexture similar to the adjacent thyroid parenchyma, which adds to the difficulty in identifying the normal glands.24
Hyperparathyroidism
Primary hyperparathyroidism is rare in children, with an estimated incidence of 2 to 5 in 100,000.112,113 Primary hyperparathyroidism is caused by overproduction of PTH by one or more parathyroid glands.
Etiologies, Pathophysiology, and Clinical Presentation: Primary hyperparathyroidism is most often caused in children by a parathyroid adenoma.112–114 Multiple endocrine neoplasia (MEN)-I or MEN-II syndromes or familial non-MEN hyperparathyroidism have also been documented and can constitute as much as 30% to 50% of pediatric hyperparathyroid disease.115,116
The symptoms of hyperparathyroidism may be nonspecific and include joint aches, fatigue, weakness, loss of appetite, depression, and difficulty concentrating. The commonest clinical signs in children with primary hyperparathyroidism is skeletal (bone resorption) and renal disease (hematuria, nephrocalcinosis, or nephrolithiasis).115
Imaging: Imaging is rarely used to diagnose hyperparathyroidism. Evaluation of serum calcium and PTH levels is diagnostic. In most patients with hyperparathyroidism, both serum calcium and PTH levels are higher than normal. Occasionally, a patient may have an elevated calcium level and a normal or minimally elevated PTH level. Since PTH should normally be low when calcium is elevated, a minimally elevated PTH is considered abnormal and indicates hyperparathyroidism.
Other reliable studies include 99mTcO4 or 201Tl subtraction imaging and 99mTc-sestamibi scans, which have been reported to have a sensitivity of 67% to 80% for localizing adenomas and a sensitivity of 45% to 60% for hyperplasia in adults; however, data in children are not well established (Fig. 17-8).117,118 Scintigraphy, occasionally along with CT and MRI, is helpful in evaluating possible mediastinal location of adenomas or hyperfunctioning parathyroid tissue.
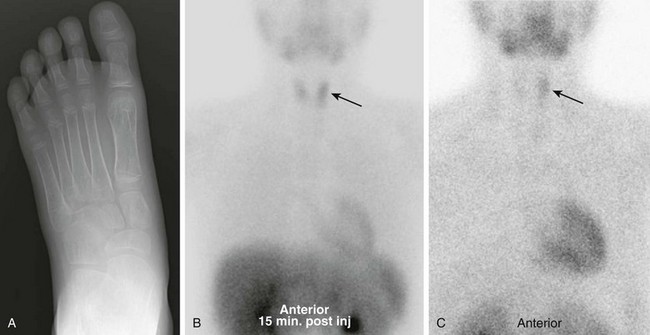
Figure 17-8 Parathyroid adenoma in a patient presenting with foot pain.
A, Radiograph of the foot demonstrates diffuse severe osteopenia. Laboratory studies revealed marked hypercalcemia and elevated parathyroid hormone. Initial ultrasonography failed to identify an adenoma. B, Nuclear scintigraphy shows increased uptake is noted in the left lobe of the thyroid gland (arrow) on the initial 99mTc-sestamibi images. C, Incomplete washout of the radiopharmaceutical is seen on 2-hour delayed images (arrow), suggesting a parathyroid adenoma. The lesion was surgically excised, and the patient’s symptoms resolved.
The most specific radiographic manifestation of hyperparathyroidism is subperiosteal bone resorption. Brown tumors (osteoclastomas) are rare sequelae of hyperparathyroidism occurring in fewer than 5% of all cases.119 The lesions localize in areas of intense bone resorption, and the bone defect becomes filled with fibroblastic tissue.
Braverman, LE, Utiger, RD. Werner and Ingbar’s the thyroid: a fundamental and clinical text, 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
Parekh, C, Jackson, HA. Thyroid cancer in children. In: Carroll WL, Finlay JL, eds. Cancer in children and adolescents. Sudbury, MA: Jones and Bartlett; 2010:459–466.
Smith, JR, Oates, ME. Radionuclide imaging of the parathyroid glands: patterns, pearls, and pitfalls. RadioGraphics. 2004;24(4):1101–1115.
Sofferman, RA, Ahuja, AT. Ultrasound of the thyroid and parathyroid glands. New York, NY: Springer; 2012.
References
1. Manley, NR, Cappecci, MR. Hox group 3 paralogs regulate the development and migration of the thymus, thyroid, and parathyroid. Dev Biol. 1998;195(1):1–15.
2. De Felice, M, De Lauro, R. Thyroid development and its disorders: genetics and molecular mechanisms. Endocrine Rev. 2004;25(5):722–746.
3. Santisteban, P. Development and anatomy of the hypothalamic-pituitary-thyroid axis. In: Braverman LE, Utiger RD, eds. Werner and Ingbar’s the thyroid a fundamental and clinical text. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:8–25.
4. VanVliet, G. Development of the thyroid gland: lessons from congenitally hypothyroid mice and men. Clin Genet. 2003;63:445–455.
5. Boyd JD. Development of the thyroid and parathyroid glands and the thymus. Lecture delivered at the Royal College of Surgeons of England, June 30, 1950:455-471.
6. Jinkins, JR. Atlas of neuroradiologic embryology, anatomy, and variants. Philadelphia, PA: Lippincott Williams & Wilkins; 2000.
7. Moore, KL. The developing human clinically oriented embryology. Philadelphia, PA: Saunders; 1973.
8. Braun, EM, Windisch, G, Wolf, G, et al. The pyramidal lobe: clinical anatomy and its importance in thyroid surgery. Surg Radiol Anat. 2007;29:21–27.
9. Stone, JA, Figuerora, RE. Embryology and anatomy of the neck. Neuroimaging Clin North Am. 2000;10:55–73.
10. LeDouarin, N, Fontaine, J, LeLievre, C. New studies on neural crest origin of the avian ultimobranchial glandular cells. Histochemistry. 1974;38(4):297–305.
11. Harach, HR. Solid cell nests of the thyroid. J Pathol. 1988;155(3):191–200.
12. Jackson, I. Thyrotropin-releasing hormone. N Engl J Med. 1982;306:145–155.
13. Gershengorn, MC. Thyrotropin releasing hormone. A review of the mechanisms of acute stimulation of pituitary hormone release. Mol Cell Biochem. 1982;45(2):163–179.
14. O’Leary, R, O’Connor, B. Thyrotropin-releasing hormone. J Neurochem. 1995;65(3):953–963.
15. Harris, ARC, Christianson, D, Smith, MS, et al. The physiological role of thyrotropin-releasing hormone in the regulation of thyroid-stimulating hormone and prolactin secretion in the rat. J Clin Invest. 1978;61(2):441–448.
16. Nikrodhanond, AA, Ortiga-Carvalho, TM, Shibusawa, N, et al. Dominant role of thyrotropin-releasing hormone in the hypothalamic-pituitary-thyroid axis. J Biochem. 2006;281(8):5000–5007.
17. Alexander, WD, Wolff, I. Cation requirements for iodide transport. Arch Biochem Biophys. 1964;106:525–528.
18. Bagchi, N, Fawcett, DM. Role of sodium ion in active transport of iodide by cultured thyroid cells. Biochem Biophys Acta. 1973;318:235–251.
19. O’Neill, B, Magnolato, D, Semenza, G. The electrogenic, Na+-dependent IJ transport system in plasma membrane vesicles from thyroid glands. Biochim Biophys Acta. 1987;896:263–274.
20. Weiss, SJ, Philip, NJ, Ambesi-Impiombato, FS, et al. Thyrotropin-stimulated iodide transport mediated by adenosine 3′,5′-monophosphate and dependent on protein synthesis. Endocrinology. 1984;114:1099–1107.
21. De la Vieja, A, Dohan, O, Levy, O, et al. Molecular analysis of the sodium/iodide symporter: impact on thyroid and extrathyroid pathophysiology. Physiol Rev. 2000;80(3):1083–1105.
22. Loevner, LA. Thyroid and parathyroid glands: Anatomy and pathology. In: Som PM, Curtin HD, eds. Head and neck imaging. 4th ed. St. Louis, MO: Mosby; 2003:2134–2171.
23. James, EM, Charboneau, JW. High-frequency (10 MHz) thyroid ultrasonography. Semin Ultrasound CT MR. 1985;6:294–309.
24. Solbiati, L, Cioffi, V, Ballarati, E. Ultrasonography of the neck. Radiol Clin North Am. 1992;30:941–954.
25. Siegel, MJ. Face and neck. In: Siegel MJ, ed. Pediatric sonography. 3rd ed. Philadelphia, PA: Lippincott William & Wilkins; 2002:123–166.
27. Balon, HR, Silberstein, EB, Meier, DA, et al, Society of Nuclear Medicine procedure guideline for thyroid scintigraphy, 2006.
28. Harris, KB, Pass, KA. Increase in congenital hypothyroidism in New York State and in the United States. Mol Genet Metab. 2007;91(3):268–277.
29. Hinton, CF, Harris, KB, et al. Trends in incidence rates of congenital hypothyroidism related to select demographic factors: data from the United States, California, Massachusetts, New York, and Texas. Pediatrics. 2010;125:S37–S40.
30. American Academy of Pediatrics, American Thyroid Association and Lawson Wilkins Pediatric Endocrine Society. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics. 2006;117:2290–2303.
31. Chang, YW, Lee, DH, Hong, YH, et al. Congenital hypothyroidism: analysis of discordant US and scintigraphic findings. Radiology. 2011;258(3):872–879.
32. el-Deskouki, M, al-Juaryyan, N, al-Nuaim, A, et al. Thyroid scintigraphy and perchlorate discharge test in the diagnosis of congenital hypothyroidism. Eur J Nucl Med. 1995;22(9):1005–1008.
33. Frieta, JE, Gross, MD, Sarkar, SD. Laboratory (in vitro) assessment of thyroid function. In: Martin WH, Sandler MP, eds. Diagnostic nuclear medicine. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2003:611.
34. Wells, RG, Sty, JR. Thyroid scintigraphy. In: Miller JH, Gelfand MJ, eds. Pediatric nuclear imaging. 1st ed. Philadelphia, PA: Saunders; 1994:47.
35. De Vries, L, Bulvik, S, Phillip, M. Chronic autoimmune thyroiditis in children and adolescents: at presentation and during long-term follow up. Arch Dis Child. 2009;94(1):33–37.
36. Rallison, ML, Dobyns, BM, Meikle, AW, et al. Natural history of thyroid abnormalities: prevalence, incidence, and regression of thyroid diseases in adolescents and young adults. Am J Med. 1992;91(4):363–370. [Erratum in: Am J Med 92(5):582, 1992].
37. Demirbilek, H, Kandemir, N, Gonc, EN, et al. Hashimoto’s thyroiditis in children and adolescents: a retrospective study on clinical, epidemiological and laboratory properties of the disease. J Pediatr Endocrinol Metab. 2007;20(11):1199–1205.
38. Chistiakov, DA. Immunogenetics of Hashimoto’s thyroiditis. J Autoimmune Dis. 2005;2:1–21.
39. Vaidya, B, Kendall-Taylor, P, Pearce, SHS. The genetics of autoimmune thyroid disease. J Clin Endocrinol Metab. 2002;87(12):5385–5397.
40. Phillips, D, McLachlan, S, Stephenson, A, et al. Autosomal dominant transmission of autoantibodies to thyroglobulin and thyroid peroxidase. J Clin Endocrinol Metab. 1990;70:742–746.
41. Jenkins, RC, Weetman, AP. Disease associations with autoimmune thyroid disease. Thyroid. 2002;12:977–988.
42. Betterle, C, Zanchetta, R. Update on autoimmune polyendocrine syndromes (APS). Acta Biomed Ateneo Parmense. 2003;74:9–33.
43. Hollowell, JG, Staehling, NW, Flanders, WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–499.
44. Ozer, G, Yuksel, B, Kozanoglu, M, et al. Growth and development of 280 hypothyroidic patients at diagnosis. Acta Paediatr Jpn. 1995;37(2):145–149.
45. Intezno, CM, Capuzzi, DM, Jabbour, S, et al. Scintigraphic features of autoimmune thyroiditis. RadioGraphics. 2001;21:957–964.
46. Emiliano, AB, Governale, L, Parks, M, et al. Shifts in propylthiouracil and methimazole prescribing practices: antithyroid drug use in the United States from 1991 to 2008. J Clin Endocrinol Metab. 2010;95(5):2227–2233.
47. Nordyke, RA, Gilbert, FI, Jr., Harada, AS. Graves’ disease. Influence of age on clinical findings. Arch Intern Med. 1988;148:626–631.
48. Khoo, TK, Bahn, RS. Pathogenesis of Graves’ ophthalmopathy: the role of autoantibodies. Thyroid. 2007;17(10):1013–1018.
49. Bahn, RS, Heufelder, AE. Pathogenesis of Graves’ ophthalmopathy. N Engl J Med. 1993;329:1468–1475.
50. Huang, SA. Thyroid. In: Treves ST, ed. Pediatric nuclear medicine/PET. 3rd ed. New York, NY: Springer; 2007:57–71.
51. Ralls, PW, Mayekawa, DS, Lee, KP, et al. Color-flow Doppler sonography in Graves disease: “thyroid inferno.”. AJR Am J Roentgenol. 1988;150:781–784.
52. Refetoff, S. Resistance to thyroid hormone: an historical overview. Thyroid. 1994;4(3):345–349.
53. Cooper, DS. Hyperthyroidism. Lancet. 2003;362:459–468.
54. Cooper, DS. Antithyroid drugs. N Engl J Med. 2005;352:905–917.
55. Cooper, DS, Saxe, VC, Meskell, M, et al. Acute effects of propylthiouracil (PTU) on thyroidal iodide organification and peripheral iodothyronine deiodination: correlation with serum PTU levels measured by radioimmunoassay. J Clin Endocrinol Metab. 1982;54:101–107.
56. Abuid, J, Larsen, PR. Triiodothyronine and thyroxine in hyperthyroidism: comparison of the acute changes during therapy with antithyroid agents. J Clin Invest. 1974;54:201–208.
57. Rivkees, SA, Sklar, C, Freemark, M. Clinical review 99: the management of Graves’ disease in children, with special emphasis on radioiodine treatment. J Clin Endocrinol Metab. 1998;83:3767–3776.
58. Fahey, FH, Treves, ST, Adelstein, SJ. Minimizing and communicating radiation risk in pediatric nuclear medicine. J Nucl Med. 2011;52(8):1240–1251.
59. Rivkees, SA. The management of hyperthyroidism in children with emphasis on the use of radioactive iodine. Pediatr Endocrinol Rev. 2003;1(2):212–222.
60. Toohey RE, Stabin MG. Comparative analysis of dosimetry parameters for nuclear medicine. ORISE Report 99-1064, 1999. Proceedings of the Sixth International Radiopharmaceutical Dosimetry Symposium, Gatlinburg, TN, 1996:532-551.
61. Toohey, RE, Stabin, MG, Watson, EE. The AAPM/RSNA physics tutorial for residents: internal radiation dosimetry: principles and applications. Radiographics. 2000;20:533–546.
62. Szabo, SM, Allen, DB. Thyroiditis. Differentiation of acute suppurative and subacute case report and review of the literature. Clin Pediatr. 1989;28(4):171–174.
63. Rich, EJ, Mendelman, PM. Acute suppurative thyroiditis in pediatric patients. Pediatr Infect Dis J. 1987;6(10):936–940.
64. Brook, I. Microbiology and management of acute suppurative thyroiditis in children. Int J Ped Otorhinolaryngol. 2003;67(5):447–451.
65. Chi, H, Lee, YJ, Chiu, NC, et al. Acute suppurative thyroiditis in children. Pediatr Infect Dis J. 2002;21(5):384–387.
66. Lucaya, J, Berdon, WE, Enriquez, G, et al. Congenital pyriform sinus fistula: a cause of acute left-sided suppurative thyroiditis and neck abscess in children. Pediatr Radiol. 1990;21(1):27–29.
67. Rallison, ML, Dobyns, BM, Keating, FR, Jr., et al. Thyroid nodularity in children. JAMA. 1975;233(10):1069–1072.
68. Niedziela, M. Pathogenesis, diagnosis and management of thyroid nodules in children. Endocr Relat Cancer. 2006;13:427–453.
69. Yip, FW, Reeve, TS, Poole, AG, et al. Thyroid nodules in childhood and adolescence. Aust N Z J Surg. 1994;64:676–678.
70. Yoskovitch, A, Laberge, J-M, Rodd, C, et al. Cystic thyroid lesion in children. J Pediatr Surg. 1998;33:866–870.
71. Hung, W, Anderson, KD, Chandra, RS, et al. Solitary thyroid nodules in 71 children and adolescents. J Pediatric Surg. 1992;27(11):1407–1409.
72. Ahmed, S, Horton, KM, Jeffrey, BR, et al. Incidental thyroid nodules on chest CT: review of the literature and management suggestions. AJR Am J Roentgenol. 2010;195(5):1066–1071.
73. Propper, RA, Skolnick, ML, Weinstein, BJ, et al. The nonspecificity of the thyroid halo sign. J Clin Ultrasound. 1980;8(2):129–132.
74. Sidoti, M, Marino, G, Resemini, E, et al. The rational use of fine needle aspiration biopsy (FNAB) in diagnosing thyroid nodules. Minerva Endocrinol. 2006;31(2):159–172.
75. Bernstein L, Gurney JC. Carcinomas and other malignant epithelial neoplasms. Cancer incidence and survival among children and adolescents: United States SEER Program, 1975-1995.
76. Thompson, LD, Rosai, J, Heffess, CS. Primary thyroid teratomas: a clinicopathologic study of 30 cases. Cancer. 2000;88(5):1149–1158.
77. Hart, S, Horsman, JM, Radstone, CR, et al. Localised extranodal lymphoma of the head and neck: the Sheffield Lymphoma Group experience (1971-2000). Clin Oncol (R Coll Radiol). 2004;16(3):186–192.
78. Winship, T, Rosvoll, R. Thyroid carcinoma in children: final report of a 20-year study. Clin Proc Child Hosp D.C.. 1970;26:11.
79. Williams, ED. Biological mechanisms underlying radiation induction of thyroid carcinoma. In: Thomas G, Karaoglou A, Williams ED, eds. Radiation and thyroid cancer. Singapore: World Scientific; 1999:177–188.
80. Chi, DD, Moley, JF. Medullary thyroid carcinoma: genetic advances, treatment recommendations, and the approach to the patient with persistent hypercalcitoninemia. Surg Oncol Clin N Am. 1998;7(4):681–706.
81. Canzian, F, Amati, P, Harach, HR, et al. A gene predisposing to familial thyroid tumors with cell oxyphilia maps to chromosome 19p13.2. Am J Hum Genet. 1998;63(6):1743–1748.
82. Herraiz, M, Barbesino, G, Faquin, W, et al. Prevalence of thyroid cancer in familial adenomatous polyposis syndrome and the role of screening ultrasound examinations. Clin Gastroenterol Hepatol. 2007;5(3):367–373.
83. Haggitt, RC, Reid, BJ. Hereditary gastrointestinal polyposis syndromes. Am J Surg Pathol. 1986;10(12):871–887.
84. Marini, F, Falchetti, A, Del Monte, F, et al. Multiple endocrine neoplasia type 2. Orphanet J Rare Dis. 2006;1:45.
85. Harness, JK. Childhood thyroid carcinoma. In: Clark OH, Duh Q-Y, eds. Textbook of endocrine surgery. 2nd ed. Philadelphia, PA: Saunders; 1997:75–81.
86. Grigsby, PW, Gal-or, A, Michalski, JM, et al. Childhood and adolescent thyroid carcinoma. Cancer. 2002;95(4):724–729.
87. Zimmerman, D, Hay, ID, Gough, IR, et al. Papillary thyroid carcinoma in children and adults: long-term follow-up of 1039 patients conservatively treated at one institution during three decades. Surgery. 1988;104(6):1157–1166.
88. Jarzab, B, Handkiewicz-Junak, D, Wloch, J. Juvenile differentiated thyroid carcinoma and the role of radioiodine in its treatment: a qualitative review. Endocr Relat Cancer. 2005;12(4):773–803.
89. Frates, MC, Benson, CB, Charboneau, JW, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Ultrasound Q. 2006;22(4):231–238. [discussion 239-240].
90. Dinauer, C, Francis, GL. Thyroid cancer in children. Endocrinol Metab Clin North Am. 2007;36(3):779–806. [vii].
91. Som, PM, Brandwin, M, Lidov, M, et al. The varied appearance of papillary carcinoma cervical nodal disease: CT and MR findings. AJNR. 1994;15:1129–1138.
92. Piekarski, JD, Schlumberger, M, Leclere, J, et al. Chest computed tomography (CT) in patients with micronodular lung metastases of differentiated thyroid carcinoma. Int J Radiat Oncol Biol Phys. 1985;11(5):1023–1027.
93. Hung, W, Sarlis, NJ. Current controversies in the management of pediatric patients with well-differentiated non-medullary thyroid cancer: a review. Thyroid. 2002;12(8):683–702.
94. Harness, JK, Thompson, NW, McLeod, MK, et al. Differentiated thyroid carcinoma in children and adolescents. World J Surg. 1992;16(4):547–553. [discussion 553-554].
95. La Quaglia, MP, Black, T, Holcomb, GW, 3rd., et al. Differentiated thyroid cancer: clinical characteristics, treatment, and outcome in patients under 21 years of age who present with distant metastases. A report from the Surgical Discipline Committee of the Children’s Cancer Group. J Pediatr Surg. 2000;35(6):955–959.
96. Sisson, JC. Selection of the optimal scanning agent for thyroid cancer. Thyroid. 1997;7(2):295–302.
97. Muratet, JP, Daver, A, Minier, JF, et al. Influence of scanning doses of iodine-131 on subsequent first ablative treatment outcome in patients operated on for differentiated thyroid carcinoma. J Nucl Med. 1998;39(9):1546–1550.
98. Silberstein, EB. Comparison of outcomes after (123)I versus (131)I pre-ablation imaging before radioiodine ablation in differentiated thyroid carcinoma. J Nucl Med. 2007;48(7):1043–1046.
99. Maxon, HR. Quantitative radioiodine therapy in the treatment of differentiated thyroid cancer. Q J Nucl Med. 1999;43(4):313–323.
100. Yeh, SD, La Quaglia, MP. 131-I therapy for pediatric thyroid cancer. Semin Pediatr Surg. 1997;6(3):128–133.
101. Beierwaltes, WH, Nishiyama, RH, Thompson, NW, et al. Survival time and “cure” in papillary and follicular thyroid carcinoma with distant metastases: statistics following University of Michigan therapy. J Nucl Med. 1982;23(7):561–568.
102. Moore, KL. The developing human clinically oriented embryology. Philadelphia, PA: Saunders; 1973.
103. Wang, Y, Ji, Q, Li, D, et al. Preoperative CT diagnosis of right nonrecurrent inferior laryngeal nerve. Head Neck. 2010;33:232–238.
104. Akerstrom, G, Malmaeus, J, Bergstrom, R. Surgical anatomy of human parathyroid glands. Surgery. 1984;95:14–21.
105. Brown, EM. Four-parameter model of the sigmoidal relationship between parathyroid hormone release and extracellular calcium concentration in normal and abnormal parathyroid tissue. J Clin Endocrinol Metab. 1983;56(3):572–581.
106. Brown, EM, Pollak, M, Seidman, CE, et al. Calcium-ion-sensing cell-surface receptors. N Engl J Med. 1995;333(4):234.
107. Alveryd, A. Parathyroid glands in thyroid surgery. I. Anatomy of parathyroid glands. II. Postoperative hypoparathyroidism—identification and autotransplantation of parathyroid glands. Acta Chir Scand. 1968;389:1–120.
108. Nobori, M, Saiki, S, Tanaka, N, et al. Blood supply of the parathyroid gland from the superior thyroid artery. Surgery. 1994;115(4):417–423.
109. Shimkin, PM, Doppman, JL, Pearson, KD, et al. Anatomic considerations in parathyroid venous sampling. AJR Am J Roentgenol. 1973;118(3):654–662.
110. Rhinehart, DA. The nerve of the thyroid and parathyroid bodies. Am J Anat. 1912;13:91–99.
111. Raybuck, HE. The innervation of the parathyroid glands. Anat Rec. 1952;112(1):117–123.
112. Li, CC, Yang, C, Wang, S, et al. A 10-year retrospective study of primary hyperparathyroidism in children. Exp Clin Endocrinol Diabetes. 2012;120(4):229–233.
113. Kollars, J, Zarroug, AE, van Heerden, J, et al. Primary hyperparathyroidism in pediatric patients. Pediatrics. 2005;114(4):974–980.
114. Huang, CB, Huang, SC, Chou, FF, et al. Primary hyperparathyroidism in children: report of a case and a brief review of the literature. J Formos Med Assoc. 1993;92:1095–1098.
115. Allo, M, Thompson, NW, Harness, JK, et al. Primary hyperparathyroidism in children, adolescents, and young adults. World J Surg. 1982;6:771–776.
116. Marx, SJ. New insights into primary hyperparathyroidism. Hosp Pract (Off Ed). 1984;19:55–63.
117. Heller, KS, Attie, JN, Dubner, S. Parathyroid localization: inability to predict multiple gland involvement. Am J Surg. 1993;166:357–359.
118. Lee, VS, Wilkinson, RH, Jr., Leight, GS, Jr., et al. Hyperparathyroidism in high-risk surgical patients: evaluation with double-phase technetium-99m sestamibi imaging. Radiology. 1995;197:627–633.
119. Horowitz, M, Wishart, JM, Need, AG, et al. Primary hyperparathyroidism. Clin Geriatr Med. 1994;10:757–775.