Chapter 473 Thrombotic Disorders in Children
Epidemiology
The majority of children who develop a TE have multiple risk factors that may be acquired, inherited, and/or anatomic (Table 473-1). The presence of a central venous catheter (CVC) is the single most important risk factor for venous thromboembolism (VTE) in pediatric patients, associated with approximately 90% of neonatal VTE and 60% of childhood VTE. These catheters are often necessary for the care of premature neonates and children with acute and chronic diseases and are used for intravenous hyperalimentation, chemotherapy, dialysis, antibiotics, or supportive therapy. CVCs may damage the endothelial lining and/or cause blood flow disruption, increasing the risk of thrombosis. There are multiple other acquired risk factors that are associated with thrombosis, including trauma, infection, chronic medical illnesses, and medications. Cancer, congenital heart disease, and prematurity are the most common medical conditions associated with TEs.
Table 473-1 POTENTIAL PROTHROMBOTIC STATES
CONGENITAL
Deficiency of anticoagulants
AT-III, protein C or protein S, plasminogen
Resistance to cofactor proteolysis
Factor V Leiden
High levels of procoagulants
Prothrombin 20210 mutation
Elevated factor VIII levels
Damage to endothelium
Homocystinemia
ACQUIRED
Obstruction to flow
Indwelling lines
Pregnancy
Polycythemia/dehydration
Immobilization
Injury
Trauma, surgery, exercise
Inflammation
IBD, vasculitis, infection, Behçet syndrome
Hypercoagulability
Pregnancy
Malignancy
Antiphospholipid syndrome
Nephrotic syndrome
Oral contraceptives
L-Asparaginase
Elevated factor VIII levels
RARE OTHER ENTITIES
Congenital
Dysfibrinogenemia
Acquired
Paroxysmal nocturnal hemoglobinuria
Thrombocythemia
Vascular grafts
AT-III, antithrombin III; IBD, inflammatory bowel disease.
Antiphospholipid antibody syndrome (APS) is a well-described syndrome in adults characterized by recurrent fetal loss and/or thrombosis. Antiphospholipid antibodies (APA) are associated with both venous and arterial thrombosis. The mechanism by which these antibodies cause thrombosis is not well understood. A diagnosis of APS requires the presence of both clinical and laboratory abnormalities (see under Laboratory Testing). The laboratory abnormalities must be persistent for 12 wk. Because of the high risk of recurrence, patients with APS often require long-term anticoagulation. It is important to note that healthy children may have a transient lupus anticoagulant, often diagnosed because of a prolonged PTT on routine preoperative testing. These antibodies may be associated with a recent viral infection and are not a risk factor for thrombosis.
Clinical Manifestations
Pulmonary embolism (PE): Symptoms of PE include shortness of breath, pleuritic chest pain, cough, hemoptysis, fever, and, in the case of massive PE, hypotension and right heart failure. Based on autopsy studies, PEs are often not diagnosed, perhaps because young children are unable to accurately describe their symptoms and their respiratory deterioration may be masked by other conditions (Chapter 401.1).
Diagnosis
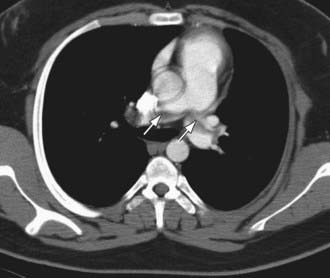
Ultrasound with Doppler flow is the most commonly employed imaging study due for the diagnosis of upper or more often lower extremity VTE. Spiral CT is used most frequently for the diagnosis of PE (Fig. 473-1). Other diagnostic imaging options include CT and MR venography, which are noninvasive, although the sensitivity and specificity of these studies is not known. They may be particularly helpful in evaluating proximal thrombosis. For the diagnosis of cerebral sinovenous thrombosis and acute ischemic stroke, the most sensitive imaging study is brain magnetic resonance imaging with venography (MRV) or diffusion weighted imaging.
Treatment
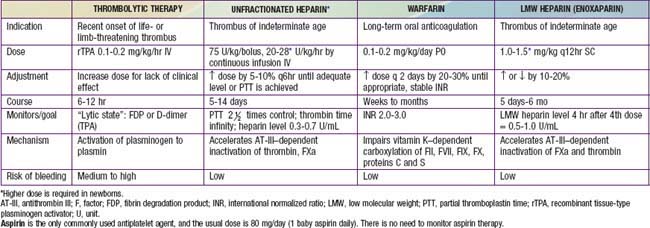
Therapeutic options for children with thrombosis include anticoagulation, thrombolysis, surgery, and observation. The goal of anticoagulation is to reduce the risk of embolism, halt clot extension, and prevent recurrence. In premature neonates and critically ill children who may have an increased risk of bleeding, the potential benefits must be weighed against the risks. Options for acute anticoagulation include unfractionated heparin (uFH) or low molecular weight heparin (LMWH), though LMWH is more frequently used because of the ease of dosing and need for less monitoring (Table 473-2). Both drugs act by catalyzing the action of antithrombin. Thrombolytic therapy, using a plasminogen activator, will hasten thrombus resolution but at the risk of increased bleeding. Surgery may be necessary for life- or limb-threatening thrombosis when there is a contraindication to thrombolysis. The optimal treatment for a child with acute ischemic stroke depends on the likely etiology and the size of the infarct. Children with sickle cell disease who develop stroke are treated with chronic red blood cell transfusions to reduce recurrence.
473.1 Anticoagulant and Thrombolytic Therapy
Leslie J. Raffini and J. Paul Scott
Table 473-2 provides an outline of commonly used anticoagulant agents.
Unfractionated (Standard) Heparin
Guidelines for therapy using unfractionated heparin are shown in Table 473-1. In newborns with low levels of clotting factors, in patients with a lupus inhibitor, or in patients with elevated levels of factor VIII (as a result of stress or surgery), PTT may not reflect the correct degree of anticoagulation, and specific heparin levels should be obtained so that the heparin level is 0.35-0.70 U/mL by anti–factor Xa assay or 0.2-0.4 U/mL by protamine sulfate assay.
Low Molecular Weight Heparin
LMWH is an effective, convenient alternative to standard heparin therapy, and its use is described in Table 473-1. Several heparins and heparinoids are undergoing clinical trials. Most pediatric experience is with enoxaparin. Adult patients receiving LMW heparin rarely need to have their heparin levels monitored, but in pediatric patients, there is more diversity of response. Monitoring is critical to ensure that a therapeutic level is achieved. PTT cannot be used to monitor LMW heparin; a specific assay should be used. Once a therapeutic range is achieved, routine monitoring is not required or is required only infrequently. When LMW heparin is used for prophylaxis against thrombosis, the dose is 0.5 mg/kg q12hr subcutaneously, with the goal of achieving a level of 0.3 U/mL 4 hr after injection.
Warfarin
Prothrombin time (PT) is the clotting test used to assess warfarin anticoagulation. Current recommendations are based on the International Normalized Ratio (INR), which permits comparison of PT using a wide variety of reagents or instruments. The INR for standard treatment of thrombosis is 2.0-3.0. Table 473-1 provides guidelines for the administration of warfarin to children. For patients with mechanical heart valves and those with homozygous protein C deficiency, the INR should be 3.0-4.0.
Thrombolytic Therapy
Thrombolytic agents, such as recombinant tissue-type plasminogen activator (rTPA), activate plasminogen to lyse blood clots by enzymatic digestion; rTPA is most often used in pediatrics for thrombolytic therapy, as described in Table 473-1. For this therapy to be effective, the patient should have a relatively fresh clot (<3-5 days old), the clot must be accessible to the lytic agent, and there must be an adequate amount of plasminogen. Once plasmin has been formed, it lyses fibrin. Relatively more fibrin-specific than the older thrombolytic agents urokinase and streptokinase, rTPA activates plasminogen within or on a fibrin clot. Clinical trials with rTPA suggest that it rarely produces a systemic hyperfibrinolytic state. The initial dose of rTPA is 0.1 mg/kg/hr. It may be useful to monitor for a therapeutic effect by looking for an increase in the concentration of D-dimers or fibrin degradation products. Higher doses or more prolonged courses of thrombolytic therapy are likely to be associated with an increased risk of bleeding complications. Low doses of rTPA have been efficacious in restoring patency in occluded vascular access catheters.
Baskin JL, Pui CH, Reiss U, et al. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet. 2009;374:159-169.
Brandão LR, Williams S, Kahr WHA, et al. Exercise-induced deep vein thrombosis of the upper extremity. Acta Haematol. 2006;115:221-229.
Büller HR, ten Cate-Hoek AJ, Hoes AW, et al. Safely ruling out deep venous thrombosis in primary care. Ann Intern Med. 2009;150:229-235.
Eikelboom JW, Weitz JI. Selective factor Xa inhibition for thromboprophylaxis. Lancet. 2008;372:6-8.
Fischer HD, Juurlink DN, Mamdani MM, et al. Hemorrhage during warfarin therapy associated with cotrimoxazole and other urinary tract anti-infective agents. Arch Intern Med. 2010;170:617-621.
Goldenberg NA. Long-term outcomes of venous thrombosis in children. Curr Opin Hematol. 2005;12:370-376.
Goldenberg NA, Durham JD, Knapp-Clevenger R, et al. A thrombolytic regimen for high-risk deep venous thrombosis may substantially reduce the risk of postthrombotic syndrome in children. Blood. 2007;110:45-53.
Goldenberg NA, Pounder E, Knapp-Clevenger R, et al. Validation of upper extremity post-thrombotic syndrome outcome measurement in children. J Pediatr. 2010;157(5):852-855.
Hunt BJ. Pediatric antiphospholipid antibodies and antiphospholipid syndrome. Semin Thromb Hemost. 2008;34:274-281.
2009 The International Warfarin Pharmacogenetics Consortium: Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009;360:753-764.
Landenfeld CS. Noninvasive diagnosis of deep vein thrombosis. JAMA. 2008;300:1696-1697.
Manco-Johnson MJ. How I treat venous thrombosis in children. Blood. 2007;107:21-29.
The Medical Letter. Dabigatran etexilate (Pradaxa)—a new oral anticoagulant. Med Lett. 2010;52(1351):89-90.
Monagle P, Chalmers E, Chan A, et al. Antithrombotic therapy in neonates and children: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:887S-968S.
Raffini L, Huang YS, Witmer C, et al. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124:1001-1008.
Roy PM. Diagnosis of venous thromboembolism. BMJ. 2009;339:412-413.
Ruiz-Irastorza G, Crowther M, Branch W, et al. Antiphospholipid syndrome. Lancet. 2010;376:1498-1506.
Schulman S, Kearon C, Kakkar AJ, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342-2352.
van Stralen KJ, Rosendaal FR, Doggne CJM. Minor injuries as a risk factor for venous thrombosis. Arch Intern Med. 2008;168:21-26.
Wadelius M, Chen LY, Lindh JD, et al. The largest prospective warfarin-treated cohort supports genetic forecasting. Blood. 2009;113:784-792.
Young G, Albisetti M, Bonduel M, et al. Impact of inherited thrombophilia on venous thromboembolism in children: a systematic review and meta-analysis of observational studies. Circulation. 2008;118:1373-1382.