Chapter 12 Thromboembolic Disease
Historical Background and Epidemiology of Acute Venous Thromboembolic Disease
The incidence of deep venous thrombosis (DVT) ranges from 5 to 9 per 10,000 person-years in the general population, and the incidence of venous thromboembolism (DVT and pulmonary embolism [PE] combined) (VTE) is about 14 per 10,000 person-years.1,2 This equates to more than 275,000 new cases of VTE per year in the United States.3 The number of identifiable risk factors that predispose to the development of VTE has steadily grown. This information has allowed physicians to provide more effective thromboembolism prophylaxis that is evidence-based and stratified according to the number of risk factors present. In patients with established DVT and PE, there has been a relatively recent emphasis evaluating the rate of recurrence and the clinical factors that predispose to recurrent VTE. In turn, the type and duration of long-term anticoagulation also continue to be stratified, based on the number of risk factors for recurrence.
Etiology and Natural History of Acute Venous Thromboembolism
Etiology
Venous thrombosis may develop as a result of endothelial damage, hypercoagulability, and venous stasis (Virchow triad). Of these risk factors, relative hypercoagulability appears most important in most cases of spontaneous DVT, whereas stasis and endothelial damage play a greater role in secondary DVT following immobilization, surgery, or trauma. Identifiable risk factors for VTE may be classified as inherited, acquired, and those with a mixed etiology (Table 12-1).
![]() TABLE 12–1 Common Inherited and Acquired Risk Factors for Venous Thrombosis
TABLE 12–1 Common Inherited and Acquired Risk Factors for Venous Thrombosis
| Acquired | Inherited |
APC, Activated protein C; HRT, hormone replacement therapy; OCP, oral contraceptives; VTE, venous thromboembolism.
When multiple inherited and acquired risk factors are present in the same patient, a synergistic effect may occur. Clinically manifest thrombosis most often occurs with the convergence of multiple genetic and acquired risk factors.4 Hospitalized patients have an average of 1.5 risk factors per patient, with 26% having three or more risk factors.5 Multiple risk factors often act synergistically to increase risk dramatically above the sum of individual risk factors. For example, patients who are heterozygous for factor V Leiden are at only moderately increased risk for VTE (4- to 8-fold). However, when combined with the additional risk of oral contraceptive use, the risk for VTE increases to approximately 35-fold, the same order of magnitude as for someone who is homozygous for factor V Leiden. The concomitant presence of obesity, advancing age, and factor V Leiden increases the thrombosis risk associated with hormone replacement therapy alone. In symptomatic outpatients, the odds ratio for an objectively documented DVT increases from 1.26 for one risk factor to 3.88 for three or more risk factors.6
Natural History
Overt venous injury appears to be neither a necessary nor sufficient condition for thrombosis, although the role of biologic injury to the endothelium is increasingly apparent. Under conditions favoring thrombosis, the normally antithrombogenic endothelium may become prothrombotic, producing tissue factor, von Willebrand factor, and fibronectin. Stasis alone is probably also an inadequate stimulus in the absence of low levels of activated coagulation factors.7
Diagnosis and Imaging
Imaging
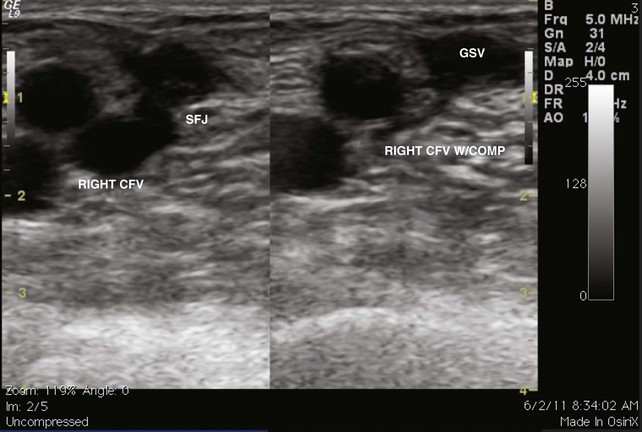
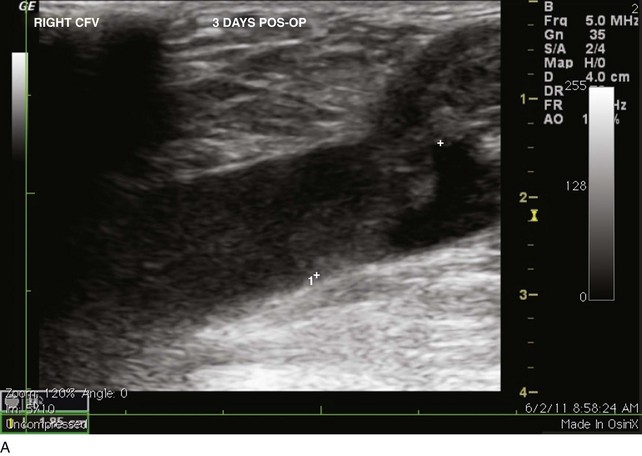
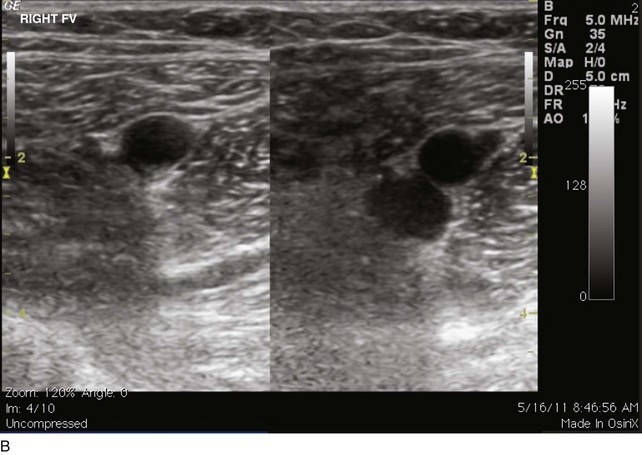
The venous duplex scan is the most commonly performed test for the detection of infrainguinal DVT, both above and below the knee, with sensitivity and specificity of greater than 95% in symptomatic patients. In a venous duplex examination performed with the patient supine, spontaneous flow, variation of flow with respiration, and response of flow to the Valsalva maneuver, all are assessed. Continued improvements in ultrasound technology also have improved the ability to visualize color flow within the tibial veins. However, the primary method of detecting DVT with ultrasound is demonstration of the lack of compressibility of the vein with probe pressure on B-mode imaging. Normally, in transverse section, the vein walls should coapt with pressure (Figs. 12-1 and 12-2). Lack of coaptation indicates thrombus.
Initial Antithrombotic Therapy for Acute Venous Thromboembolism
Once the diagnosis of VTE has been made, antithrombotic therapy should be initiated promptly. The following dosing protocols are the more commonly used treatment regimens, but they are not applicable to all patient populations. In patients who require anticoagulation for acute VTE disease, initial anticoagulation usually is administered parenterally with one of the medications listed in Table 12-2. Initial anticoagulation should be continued for at least 5 days, and most patients may begin warfarin therapy on the same day they begin parenteral anticoagulation. Parenteral anticoagulation may be discontinued once the prothrombin time–to–international normalized ratio (PT/INR) is in the therapeutic range (INR goal 2.5, range 2.0 to 3.0) for longer than 24 hours (i.e., two consecutive daily INR values of 2.0 to 3.0).
![]() TABLE 12–2 Approved Parenteral Medications for Initiation of Anticoagulation
TABLE 12–2 Approved Parenteral Medications for Initiation of Anticoagulation
| Medication | Route | Dose and Duration |
|---|---|---|
| Unfractionated heparin (UH) | IV SC |
5000 U or 80 U/kg bolus IV, then 1300 U/h or 18 U/kg/h IV continuous infusion adjusted to prolong the aPTT corresponding to a plasma heparin of 0.3-0.7 IU/mL Adjusted dose SC UH is an effective alternative |
| Low-molecular-weight heparin (LMWH) | SC | Once or twice daily, dose is brand dependent No monitoring required (usually) Renal excretion |
| Fondaparinux | SC | 5 mg daily if body weight <50 kg 7.5 mg daily if body weight 50-100 kg 10 mg daily if body weight >100 kg No monitoring required (usually) Renal excretion |
IV, Intravenous; SC, subcutaneous.
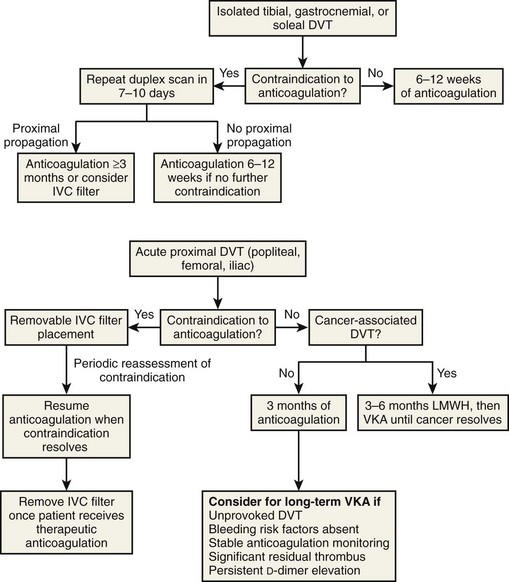
In patients who have contraindications to anticoagulation, the treatment algorithm will vary, depending upon the location of the original DVT (Fig. 12-3).
Long-Term Antithrombotic Therapy
The purpose of long-term anticoagulation is the prevention of recurrent VTE. The recommended duration of anticoagulation is outlined in Table 12-3.
| Clinical Subgroup | ACCP Treatment Recommendations |
|---|---|
| First episode LE DVT/transient risk | VKA for 3 months |
| First episode LE DVT/unprovoked |
This assumes that the patient has received a 5- to 10-day course of parenteral medications.
ACCP, American College of Chest Physicians; DVT, deep venous thrombosis; LE, lower extremity; LMWH, low-molecular-weight heparin; SC, subcutaneous; VTE, venous thromboembolism.
Adapted from Kearon C, et al. Antithrombotic therapy for venous thromboembolic disease. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133:454-545.
Thrombophilic Conditions: Inherited and Acquired
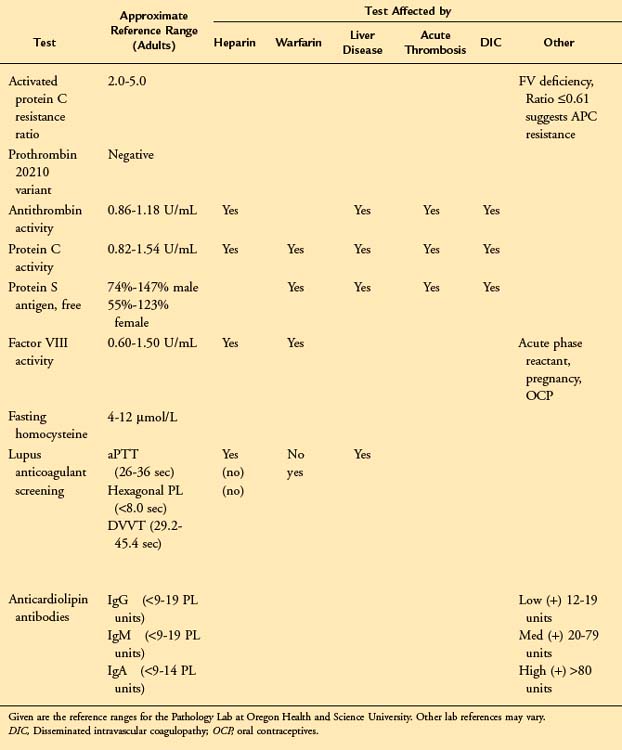
Testing for Thrombophilia
Most authorities include cerebral, renal, portal, or hepatic vein thrombosis as unusual sites. However, the association with upper extremity thrombosis or retinal vein thrombosis is less certain (Table 12-4).
Clinical Presentation
Approximately 35% to 46% of patients with SVT are males (average age 54 years). The average age for females is about 58 years.9,10 Factors associated with SVT include varicose veins (most frequent), age older than 60 years, obesity, tobacco use, and a history of DVT or SVT. Factors associated with extension of SVT include age older than 60 years, male sex, and a history of DVT. SVT of the great saphenous vein (GSV) is most common; however, SVT of the small saphenous vein (SSV) should not be overlooked because it may progress to popliteal DVT.
Etiology
To determine whether a hypercoagulable state contributes to the development of SVT, a number of markers were determined in a population of patients with acute SVT.11 All patients had a coagulation profile performed that included (1) protein C antigen and activity, (2) activated protein C (APC) resistance, (3) protein S antigen and activity, (4) antithrombin (AT), and (5) lupus-type anticoagulant. Among 29 enrolled patients, 12 (41%) were found to have abnormal results consistent with a hypercoagulable state. Five of the patients (38%) with combined SVT/DVT and seven of the patients (44%) with isolated SVT were found to be hypercoagulable. These findings suggest that patients with SVT are at an increased risk of having an underlying hypercoagulable state.
Diagnosis
Duplex imaging has shown concomitant DVT to be present in 5% to 40% of patients with SVT.12–14 It is important to note that up to 25% of these DVTs may not be contiguous with the SVT and may be in the contralateral lower extremity.
Treatment
The progression of isolated SVT to DVT has been evaluated. Among 263 patients with isolated SVT by duplex ultrasonography, 30 (11%) patients had documented progression to deep venous involvement.15 Progression from the GSV in the thigh into the common femoral vein (21 patients) was most common, with 18 of these extensions noted to be nonocclusive and 12 having a free-floating component.
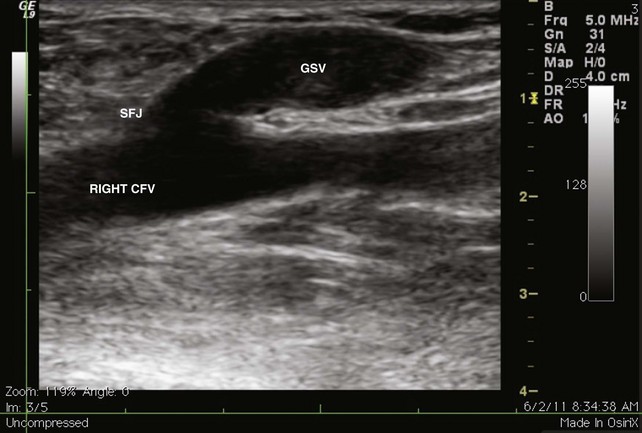
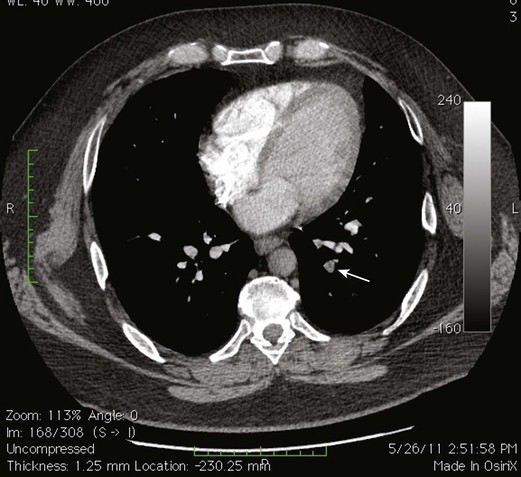
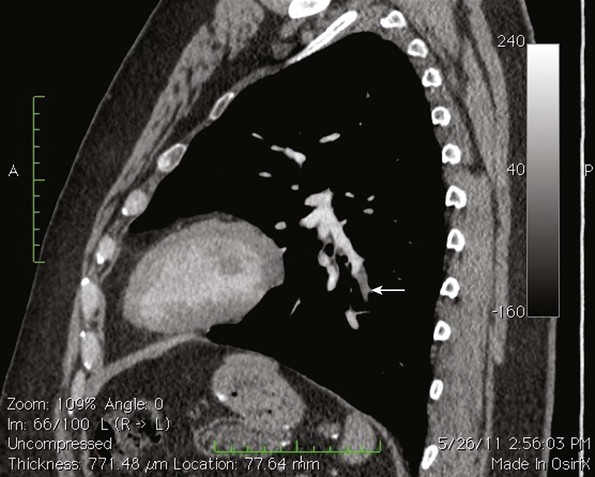
Because of the recognized potential for extension into the deep system and embolization, high saphenous ligation with or without stripping is often recommended for SVT within 1 cm of the SFJ. In a series of 43 patients who underwent ligation of the SFJ with and without local CFV thrombectomy and stripping of the GSV, two patients were found with postoperative contralateral DVT, one of whom had a PE16–19 (Figs. 12-4 through 12-6).
A prospective nonrandomized study was conducted to evaluate the efficacy of anticoagulation in the management of SFJ thrombophlebitis (SFJT).20 Duplex ultrasonography was performed before admission, both to establish the diagnosis and to evaluate the deep venous system. Patients were hospitalized, given a full course of heparin treatment, and evaluated with duplex ultrasound 2 to 4 days after admission. Patients with SFJT alone and resolution of SFJT as documented by duplex ultrasound scans were maintained on warfarin for 6 weeks. Those patients with SFJT and DVT were maintained on warfarin for 6 months. Among 20 enrolled patients, 8 (40%) had concurrent DVT, including 4 with unilateral DVT, 2 with bilateral DVT, and 2 with development of DVT despite anticoagulation. DVT was contiguous with SFJT in 5 patients and noncontiguous in 3 patients. Seven of 13 duplex ultrasound scans obtained at 2 to 8 months’ follow-up demonstrated partial resolution of SFJT, 5 had complete resolution, and 1 demonstrated no resolution. There were no episodes of PE, recurrence, or anticoagulation complications at maximum follow-up of 14 months. Anticoagulation therapy to manage SFJT was effective in achieving resolution, preventing recurrence, and preventing PE within the follow-up period.
Meta-analysis of surgical versus medical therapy for isolated above-knee SVT has been attempted but has not been feasible because of the paucity of comparable data between the two groups.21 This review suggested that although stripping provides superior symptomatic relief, medical management with anticoagulants is somewhat superior with respect to minimizing complications and preventing subsequent DVT and PE. Based on these data, the authors suggest that anticoagulation is appropriate in patients without contraindications.
Although proximal GSV SVT occurs frequently, the best treatment regimen based on its underlying pathophysiology and resolution rate remains controversial. More recent investigations do offer some guidelines suggesting that anticoagulation is more effective than venous ligation in preventing DVT and PE.22 Further examination of the unresolved issues involving SVT is fundamental.
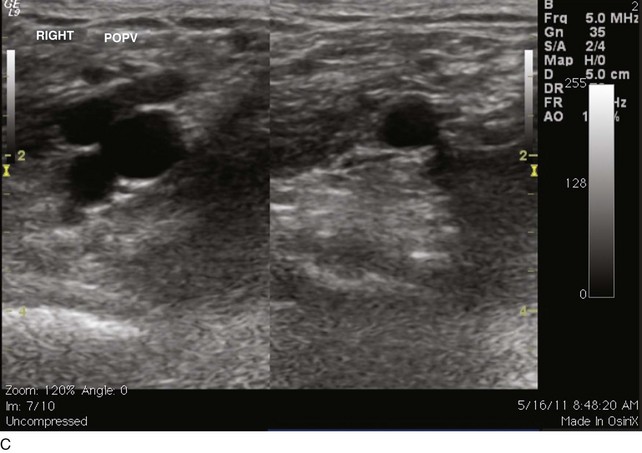
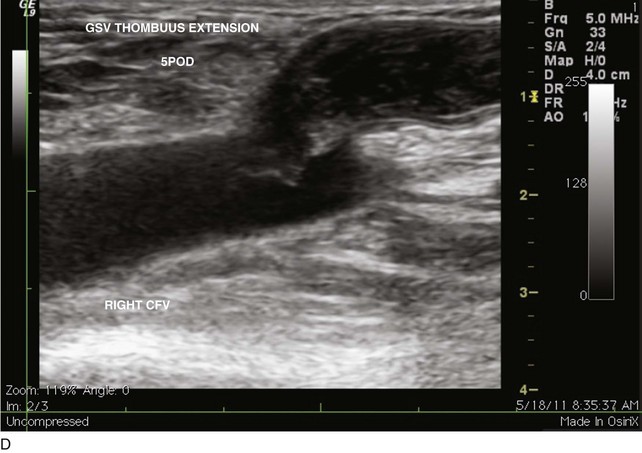
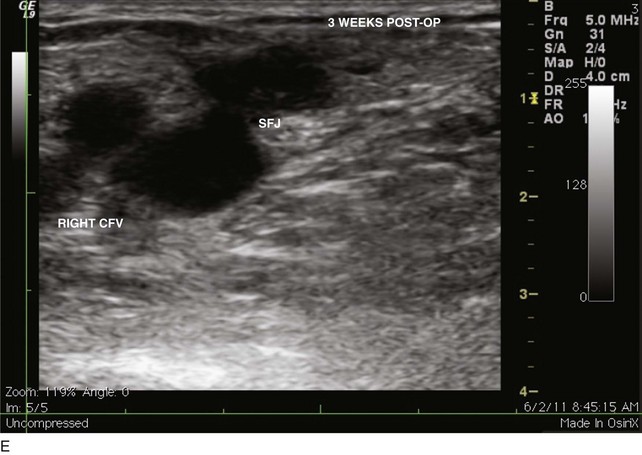
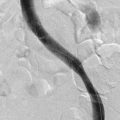
EHIT (endothermal heat-induced thrombosis) was briefly covered in Chapter 5. The following is a typical example of the natural course of an SFJT after thermal ablation treatment. This series of ultrasounds demonstrates a generous thrombus at SFJ retracting over time from postoperative day 3 (initial ultrasound) to postoperative day 5, where retraction has begun. At 3 weeks postoperative, the thrombus extension is no longer visualized. No anticoagulation or platelet therapy was used. Notice that no contiguous thrombotic lesions were identified in the deep system. This situation is very different from the ultrasound and CT scans shown previously (see Figs. 12-4 through 12-6), in which case the patient presented with spontaneous GSV thrombosis extending into the SFJ and developed a PE (Fig. 12-7).
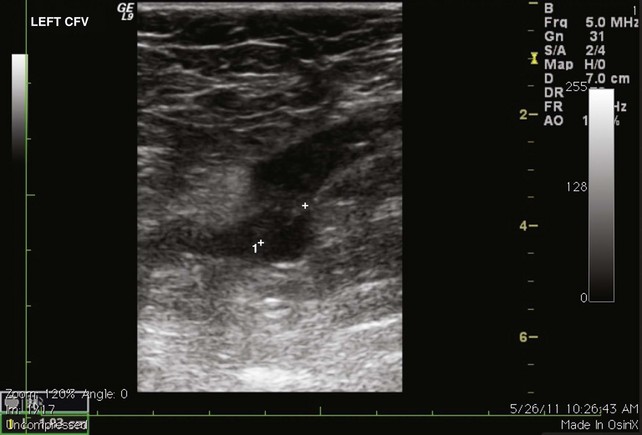
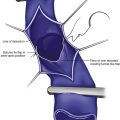
Fig 12–4 Superficial venous thrombosis: thrombus at saphenofemoral junction extends into common femoral vein.
1 Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:454-545.
2 Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107:I22-I30.
3 Decousus H, Prandoni P, Mismetti P, et al. Fondaparinux for the treatment of superficial-vein thrombosis in the legs. N Engl J Med. 2010;363:1222-1232.
4 Rosendaal FR. Venous thrombosis: a multicausal disease. Lancet. 1999;353:1167-1173.
5 Anderson FA, Wheeler HB, Goldberg RJ, et al. The prevalence of risk factors for venous thromboembolism among hospital patients. Arch Intern Med. 1992;152:1660-1664.
6 Oger E, Leroyer C, LeMoigne E, et al. The value of risk factor analysis in clinically suspected deep venous thrombosis. Respiration. 1997;64:326-330.
7 Thomas DP, Merton RE, Hockley DJ. The effect of stasis on the venous endothelium: an ultrastructural study. Br J Haematol. 1983;55:113-122.
8 De Weese MS. Nonoperative treatment of acute superficial thrombophlebitis and deep femoral venous thrombosis. In: Ernst CB, Stanley JC, editors. Current Therapy in Vascular Surgery. Philadelphia: BC Decker; 1991:952-960.
9 Lohr JM, McDevitt DT, Lutter KS, et al. Operative management of greater saphenous thrombophlebitis involving the saphenofemoral junction. Am J Surg. 1992;164:269-275.
10 Lutter KS, Kerr TM, Roedersheimer LR, et al. Superficial thrombophlebitis diagnosed by duplex scanning. Surgery. 1991;110:42-46.
11 Hanson JN, Ascher E, DePippo P, et al. Saphenous vein thrombophlebitis (SVT): a deceptively benign disease. J Vasc Surg. 1998;27:677-680.
12 Bjorgell O, Nilsson PE, Jarenros H. Isolated nonfilling of contrast in deep vein segments seen on phlebography, and a comparison with color Doppler ultrasound to assess the incidence of deep venous thrombosis. Angiology. 2000;51:451-461.
13 Jorgensen JO, Hanel KC, Morgan AM, et al. The incidence of deep venous thrombosis in patients with superficial thrombophlebitis of the lower limbs. J Vasc Surg. 1993;18:70-73.
14 Skillman JJ, Kent KC, Porter DH, et al. Simultaneous occurrence of superficial and deep thrombophlebitis in the lower extremity. J Vasc Surg. 1990;11:818-823. discussion 823-824
15 Chengelis DL, Bendick PJ, Glover JL, et al. Progression of superficial venous thrombosis to deep vein thrombosis. J Vasc Surg. 1996;24:745-749.
16 Gjores JE. Surgical therapy of ascending thrombophlebitis in the saphenous system. Angiology. 1962;13:241-243.
17 Husni EA, Williams WA. Superficial thrombophlebitis of lower limbs. Surgery. 1982;91:70-74.
18 Lofgren EP, Lofgren KA. The surgical treatment of superficial thrombophlebitis. Surgery. 1981;90:49-54.
19 Plate G, Eklof B, Jensen R, et al. Deep venous thrombosis, pulmonary embolism and acute surgery in thrombophlebitis of the long saphenous vein. Acta Chir Scand. 1985;151:241-244.
20 Leon L, Giannoukas AD, Dodd D, et al. Clinical significance of superficial vein thrombosis. Eur J Vasc Endovasc Surg. 2005;29:10-17.
21 Sullivan V, Denk PM, Sonnad SS, et al. Ligation versus anticoagulation: treatment of above-knee superficial thrombophlebitis not involving the deep venous system. J Am Coll Surg. 2001;193:556-562.
22 Neher JO, Safranek S, Greenwald JL. Clinical inquiries. What is the best therapy for superficial thrombophlebitis? J Fam Pract. 2004;53:583-585.