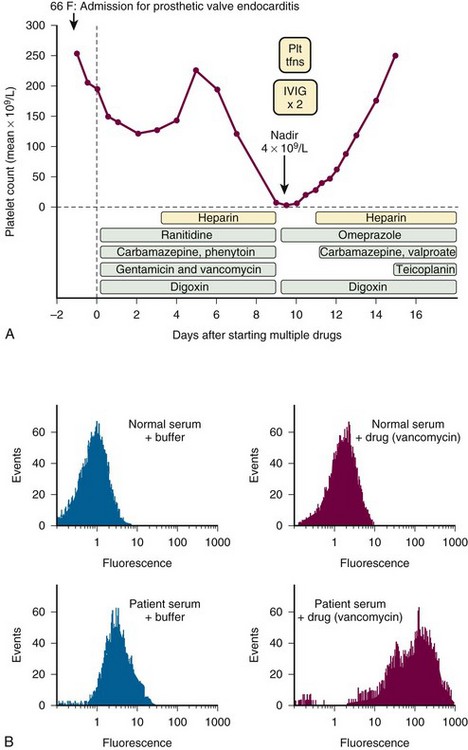
Chapter 57 Thrombocytopenia Caused by Platelet Destruction, Hypersplenism, or Hemodilution
Table 57-1 Mechanisms of Platelet Destruction or Consumption
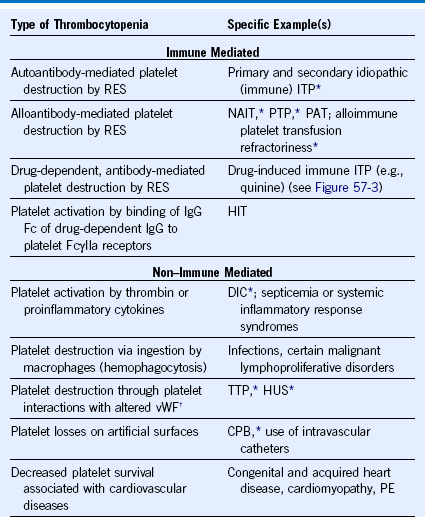
CPB, Cardiopulmonary bypass surgery; DIC, disseminated intravascular coagulation; HIT, heparin-induced thrombocytopenia; HUS, hemolytic uremic syndrome; IgG, immunoglobulin G; ITP, Idiopathic (immune) thrombocytopenic purpura; NAIT, Neonatal alloimmune thrombocytopenia; PAT, passive alloimmune thrombocytopenia; PE, pulmonary embolism; PTP, posttransfusion purpura; RES, reticuloendothelial system; TTP, thrombotic thrombocytopenic purpura; vWF, von Willebrand factor.
* See Chapter 56 for a discussion of thrombocytopenia in these disorders.
Table 57-2 Differential Diagnosis of Thrombocytopenia in Pregnancy
DIC, Disseminated intravascular coagulation; HUS, hemolytic uremic syndrome; TTP, thrombotic thrombocytopenic purpura.
*Preeclampsia or eclampsia usually is not associated with overt DIC.
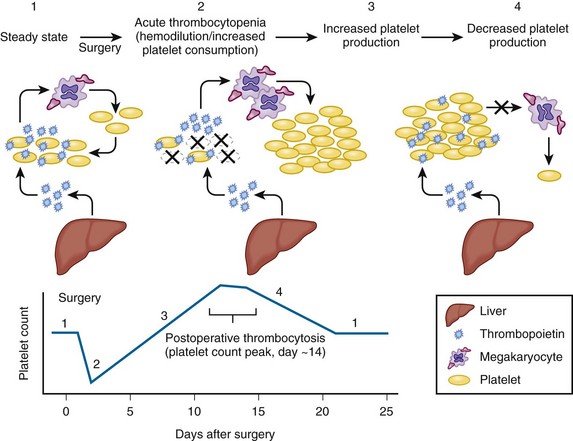
Figure 57-1 POSTSURGERY PLATELET COUNT CHANGES.
(Reprinted, with modifications, with permission, from Arnold DM, Warkentin TE: Thrombocytopenia and thrombocytosis. In Wilson WC, Grande CM, Hoyt DB, editors: Trauma: Critical care, vol 2. New York, 2007, Informa Healthcare, p 983.)
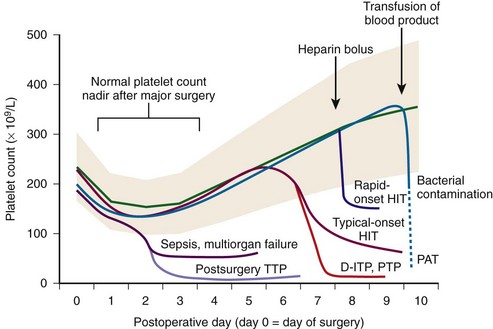
Figure 57-2 TIMING OF ONSET AND SEVERITY OF THROMBOCYTOPENIA: IMPLICATIONS FOR DIFFERENTIAL DIAGNOSIS.
(Reprinted, with permission, from Greinacher A, Warkentin TE: Acquired non-immune thrombocytopenia. In: Marder VJ, Aird WC, Bennett JS, et al, editors: Hemostasis and thrombosis: Basic principles and clinical practice, ed 6. Philadelphia, 2012, Lippincott Williams & Wilkins, in press.)
Table 57-3 Laboratory Tests Used to Investigate a Patient With Thrombocytopenia
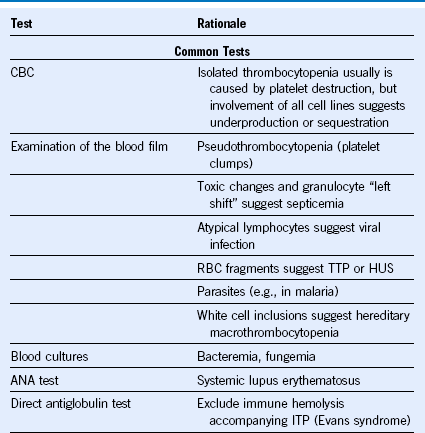
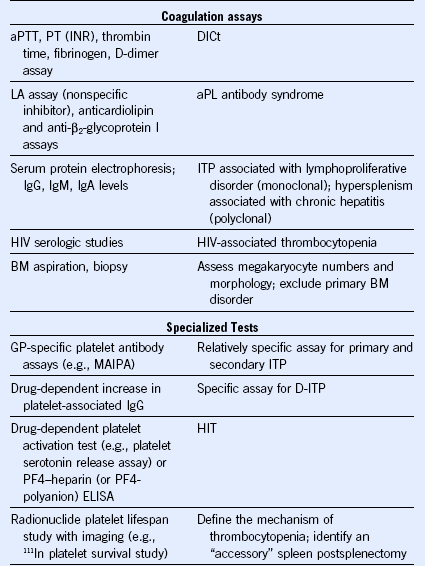
ANA, Antinuclear antibody; aPL, antiphospholipid; aPTT, activated partial thromboplastin time; CBC, complete blood count; DIC, disseminated intravascular coagulation; D-ITP, drug-induced immune thrombocytopenia; ELISA, enzyme-linked immunosorbent assay; GP, glycoprotein; HIT, heparin-induced thrombocytopenia; HIV, human immunodeficiency virus; HUS, hemolytic uremic syndrome; IgG, immunoglobulin G; INR, international normalized ratio; ITP, idiopathic (immune) thrombocytopenic purpura; LA, lupus anticoagulant; MAIPA, monoclonal antibody immobilization of platelet antigens; PF4, platelet factor 4; PT, prothrombin time; RBC, red blood cell; TTP, thrombotic thrombocytopenic purpura.
Table 57-4 Differential Diagnosis of Splenomegaly and Hypersplenism
| Infections |
|---|
| ACUTE |
| SUBACUTE AND CHRONIC |
| Inflammation |
| Congestive Splenomegaly |
| INTRAHEPATIC |
| EXTRAHEPATIC |
| CHRONIC PASSIVE CONGESTION |
| Hematologic Disorders |
| Neoplasia |
| MALIGNANT |
| BENIGN |
| Storage Diseases |
| Miscellaneous |
ALPS, Autoimmune lymphoproliferative syndrome; CMV, cytomegalovirus; MPD, myeloproliferative disorder; RBC, red blood cell; SLE, systemic lupus erythematosus.
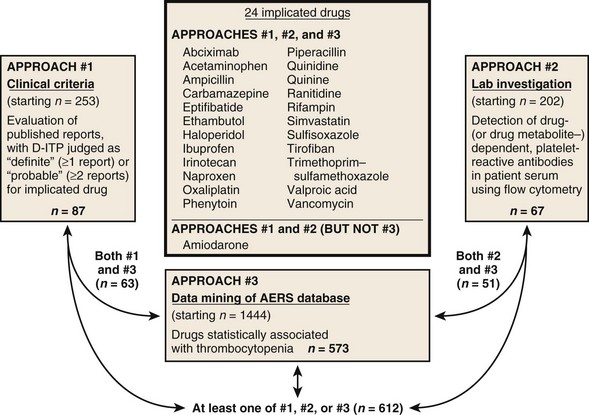
Figure 57-3 TWO DOZEN DRUGS WITH STRONG EVIDENCE FOR CAUSING DRUG-INDUCED IMMUNE THROMBOCYTOPENIA (D-ITP) SYNDROME.
(Adapted from Warkentin TE, Anderson JAM: DITP causation: 3 methods better than 1? Blood 116:2002, 2010.)
Search Strategies When Investigating a Patient With Possible Drug-Induced Immune Thrombocytopenic Purpura
Four sources of information as to whether a drug has been implicated as a cause of D-ITP:
• PubMed search (http://www.ncbi.nlm.nih.gov/pubmed): [name of drug] and [thrombocytopenia]. By way of example, the author encountered a patient who developed severe thrombocytopenia 5 days after starting treatment with mirtazapine. Searching [mirtazapine] and [thrombocytopenia] identified one report1 of mirtazapine-induced D-ITP syndrome.
• Drug-induced thrombocytopenia website (http://www.ouhsc.edu/platelets/ditp.html): Investigators at the University of Oklahoma published a comprehensive survey of drugs implicated in D-ITP using clinical criteria2; a website maintained by these investigators is updated every 2 years.
• Database from drug-dependent platelet-reactive antibody testing at the BloodCenter of Wisconsin, 1995-2010 (http://www.ouhsc.edu/platelets/InternetPostingLab2_18_11Frames.htm): the BloodCenter of Wisconsin maintains a website reporting its experience in detecting drug-dependent platelet-reactive antibodies.3
• Combined approach that uses clinical criteria,2 laboratory criteria,3 and Adverse Event Reporting System.4 Figure 57-3 lists two dozen drugs for which convincing clinical and laboratory evidence exists.4,5 To review the comprehensive list of all drugs investigated in this study,4 interested readers can consult the online supplemental table (http.bloodjournal.hematologylibrary.org/content/suppl/2010/06/08/blood-2010-03-276691.DC1/TableS1.pdf).
Approach to Patients With Thrombocytopenia Following Percutaneous Coronary Interventions
• GPIIb/IIIa inhibitor–induced pseudothrombocytopenia. The patient has no symptoms or signs of bleeding, and platelet aggregates are seen in the blood film. The platelet count is falsely reported as low by the automated particle counter, which fails to count aggregated platelets. No treatment is required.
• GPIIb/IIIa inhibitor–induced thrombocytopenia. The platelet count falls abruptly, often to profoundly reduced levels (typical nadir, <20 × 109/L). Hemostatic impairment is variable, ranging from no petechiae to fatal hemorrhages; occasionally, patients develop anaphylactoid reactions or even associated thrombosis. Treatment involves stopping all platelet antagonists and anticoagulants and giving platelets if the patient has signs of bleeding. Prophylactic platelet transfusions also can be considered if the platelet count is very low (e.g., <10 × 109/L). Testing for drug-dependent antibodies can be accomplished using flow cytometry or ELISA.
• Rapid-onset HIT. In patients who have preexisting HIT antibodies because of recent heparin exposure (generally within the past 100 days), rapid-onset HIT can occur when heparin is given during PCI. This is much less common than GPIIb/IIIa inhibitor–induced thrombocytopenia or pseudothrombocytopenia, so presumptive treatment of HIT with a nonheparin anticoagulant is rarely indicated in this situation. The platelet count nadir is usually much higher than with GPIIb/IIIa inhibitor–induced thrombocytopenia.
• Radiocontrast-induced ITP. Very rarely, patients who have previously received iodinated contrast can develop abrupt-onset, severe thrombocytopenia after exposure to contrast during PCI. Platelet transfusions (with or without high-dose IVIG) are appropriate for a bleeding patient.
Table 57-5 Mechanisms for Thrombocytopenia Complicating Infections
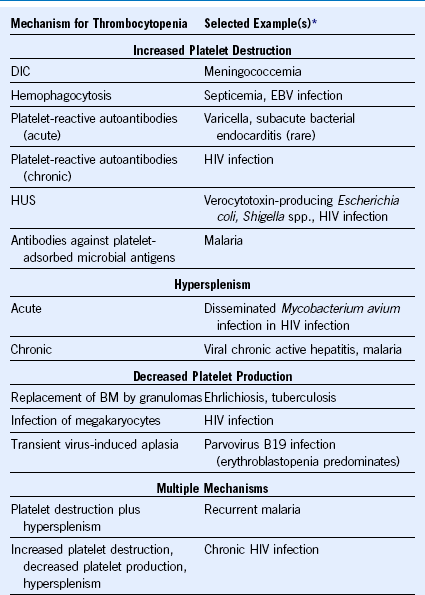
BM, Bone marrow; DIC, disseminated intravascular coagulation; EBV, Epstein-Barr virus; HUS, hemolytic uremic syndrome.
1 Liu X, Sahud MA. Glycoprotein IIb/IIIa complex is the target in mirtazapine-induced immune thrombocytopenia. Blood Cell Mol Dis. 2003;30:241.
2 George JN, Raskob GE, Shah SR, et al. Drug-induced thrombocytopenia: a systematic review of published case reports. Ann Intern Med. 1998;129:886.
3 Aster RH, Curtis BR, McFarland JG, et al. Drug-induced immune thrombocytopenia: pathogenesis, diagnosis, and management. J Thromb Haemost. 2009;7:911.
4 Reese JA, Li X, Hauben M, et al. Identifying drugs that cause immune thrombocytopenia: an analysis using 3 distinct methods. Blood. 2010;116:2127.
5 Warkentin TE, Anderson JAM. DITP causation: 3 methods better than 1? Blood. 2010;116:2002.