18 Thorax
Imaging Modalities
Although all existing imaging modalities have found application in the diagnosis of diseases of the chest, the chest radiograph—the oldest technique—remains the most commonly ordered initial radiologic study.1 In addition to conventional screen-film techniques, digital radiography, which captures images directly in computer format, is replacing the conventional screen-film chest radiograph. Cross-sectional imaging such as computed tomography (CT) or magnetic resonance imaging (MRI) often is the next step in the diagnostic workup. CT is the second most commonly used thoracic imaging technique offering the most comprehensive examination of the mediastinum, lung parenchyma, pleura, and chest wall. The advent of helical CT in the 1990s allowed faster scanning and volume acquisition of data with reformation options and three-dimensional (3D) reconstruction capabilities. Helical CT was made possible by the development of slip-ring technology, which allows the tube and detector to rotate around a continuously moving table on which a patient lies. Thus, the tube and detector acquire data in a corkscrew, or helical, path. The next generation of scanners, the multidetector-row CT (MDCT) scanners, took the helical technology one step further by simultaneously acquiring data through multiple detectors. In addition to further decreasing scanning time, multidetector technology reduces or even completely eliminates the mismatch between longitudinal (axial) and transverse resolution, which further improves 3D reconstruction capabilities. MDCT decreases motion artifact and improves the quality of images, particularly in a patient in distress or an uncooperative pediatric patient. Faster scanning also allows the entire examination to be performed during optimal contrast enhancement of the cardiovascular system. For example, faster imaging using an MDCT scanner allows CT angiography for evaluation of pulmonary embolism and vascular abnormalities. Three-dimensional reconstruction allows anatomic examination of the airway and virtual bronchoscopy.
Magnetic resonance imaging (MRI) is based on the magnetic properties of the proton (the nucleus of the hydrogen atom). MRI contrast depends on several factors, including the difference in the behavior of protons in different tissues in response to magnetization as well as proton density. MRI provides soft tissue contrast resolution superior to that of CT.2 MRI contrast agents, most of which are based on gadolinium, are widely used, with a safety record superior to that of iodinated contrast agents used in CT scanning.2
MRI offers the advantages of excellent soft tissue contrast, multiplanar capability, intrinsic flow sensitivity, and lack of ionizing radiation.2 In clinical applications, MRI of the thorax is reserved for (1) specific problematic cases where mediastinal, chest wall, vertebral body, or vascular invasion needs to be further assessed after a CT of the chest has been performed, or (2) evaluating the mediastinum of patients for whom the administration of iodinated contrast material is contraindicated. The multiplanar capability of MRI aids in the evaluation of chest wall and pleural abnormalities, particularly in the apical regions.3 CT and MRI may play complementary roles in evaluating chest wall disorders such as mesenchymal tumors, primary and secondary malignancies, inflammatory conditions, and infectious disease. CT more readily demonstrates soft tissue calcification and bone destruction, whereas MRI better delineates the extent of invasive tumors, soft tissue involvement, and infiltration of bone marrow as well as vascular involvement. The ability of MRI to evaluate the lung parenchyma is limited by artifact from multiple air–soft tissue interfaces, cardiac and respiratory motion, and poor signal-to-noise ratio.2
In addition to imaging based on depiction of anatomic and morphologic structures, functional imaging is widely used in thoracic imaging. 18F-fluorodeoxyglucose (FDG) is a D-glucose analog commonly used in positron emission tomography (PET) imaging of the thorax. The fluorine incorporated in the FDG undergoes decay with emission of a positron that after an annihilation reaction with an electron produces two photons that can be detected by a camera. Because malignant tissues have high rates of metabolism, they disproportionately accumulate FDG. Increased glucose metabolism by malignant cells may allow physiologic differentiation between benign and malignant abnormalities. As inflammatory lesions also can demonstrate increased uptake of FDG, differentiation between malignancy and inflammatory lesions may be difficult. At the same time, some malignant lesions such as bronchioloalveolar carcinoma may not demonstrate uptake on PET scan. Quantification of FDG uptake by a lesion may assist in this differentiation as well as affect prognosis. The standardized uptake value (SUV) is a widely used semiquantitative method for measuring FDG uptake.4–5
FDG PET is used in examination and staging of lung cancer, differentiating benign from malignant solitary pulmonary nodules, and in diagnosis and assessment of treatment response of lymphoma.6 In the staging of lung cancer, FDG PET is useful in evaluating local disease, lymph node involvement, and distant metastatic sites. The accuracy of FDG PET in demonstrating intrathoracic metastatic nodal disease is greater than that of CT or MRI. Whole-body PET is useful in detection of unsuspected extrathoracic metastases. Conventional imaging often does not allow differentiation of tumor from posttreatment scarring. Increased FDG uptake at the sites of residual radiographic abnormalities may suggest persistent or recurrent tumor.6 In addition to its role in the staging of lung cancer, whole-body PET scanning is used in the diagnosis and staging of many other neoplasms including malignant melanoma, lymphoma, colorectal cancer, and head and neck tumors. Combining the PET scan with its sensitivity to detect lesions with a CT scan’s high special resolution may increase the diagnostic accuracy of both modalities.7,8 An integrated PET/CT scanner combines a high-resolution, high-sensitivity PET scanner with a fast multidetector CT scanner. In addition to precise matching of PET and CT images, combining the two devices in one unit allows use of the CT data to measure the differences in the absorption of the PET emission by different parts of the patient’s body (attenuation correction).
Anatomy of the Thorax
Mediastinum
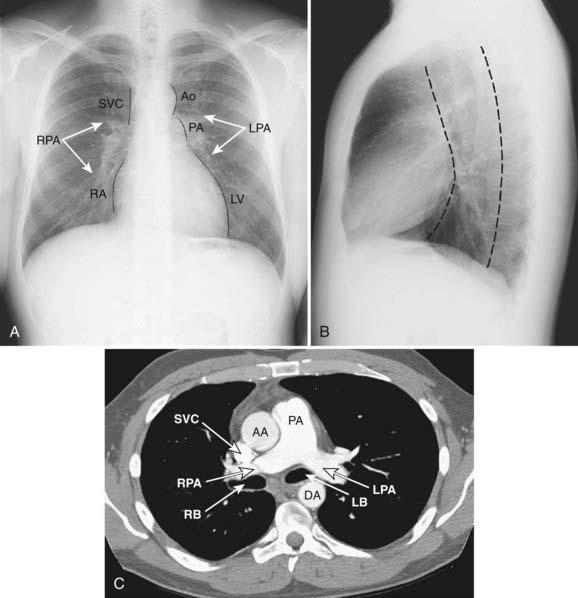
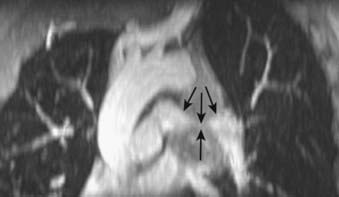
The mediastinum is commonly divided into anterior, middle, and posterior compartments based on the lateral chest radiograph. A line drawn along the back of the heart and front of the trachea divides the anterior and middle mediastinum, whereas the line connecting a point on each thoracic vertebra about a centimeter behind its anterior margin separates the middle and posterior compartments (Fig. 18-1).9,10 Neoplasms usually arise from normal structures contained in each compartment. The anterior mediastinum contains the thymus, fat, and lymph nodes, and the most common neoplasms found in that area are thymoma, lymphoma, and germ cell tumors. Thyroid abnormalities that extend from the neck into the mediastinum are most commonly found in the anterior mediastinum but may also extend to the middle and posterior mediastinum. The middle mediastinum contains the heart, pericardium, great vessels, trachea, bronchi, esophagus, and lymph nodes. Esophageal tumors, tracheal tumors, and lymph nodes are typically located in this compartment. The posterior mediastinum contains autonomic nerves, vessels, and lymph nodes. Neurogenic tumors and lymphadenopathy usually present in this location.10,11 Besides primary and secondary tumors of these compartments, other vascular and developmental lesions can occur, but they are beyond the scope of this chapter.
Diseases of the Thorax
Lung Malignancies
In 2008, there were an estimated 215,020 new cases of lung cancer in the United States: 114,690 in men and 100,330 in women. Lung cancer is the leading cause of cancer death in both men and women, accounting for 31% of all cancer deaths in men and 26% of all cancer deaths in women. Smoking is responsible for at least 30% of all cancer deaths and 87% of all lung cancers; compared to nonsmokers, lung cancer mortality rates are 22 times higher for male and 12 times higher for female smokers. In addition, an estimated 3000 nonsmoking adults die each year from lung cancer caused by secondhand smoking.12
Histologically, the most common subtypes of lung cancer, in order of decreasing frequency, include adeno, squamous cell, small cell, and large cell carcinoma. Adenocarcinomas are further subdivided into several histologic subtypes, including bronchioloalveolar carcinoma. There are major differences in therapeutic approaches to patients with small cell lung carcinoma and other types of lung cancer; therefore, all primary lung malignancies are usually divided into non–small cell and small cell categories.13,14
Non–Small Cell Lung Cancer
The most common cell types of lung cancer have a certain, typical radiographic presentation. It should be noted, however, that there is significant overlap between radiographic appearances of lung cancer. In addition, the relative frequency of different categories of this disease, as well as “typical” radiographic findings, have been changing in the past few decades.15
Adenocarcinoma
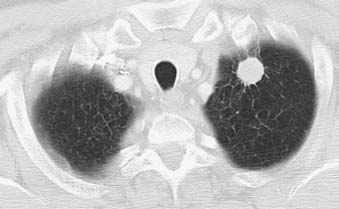
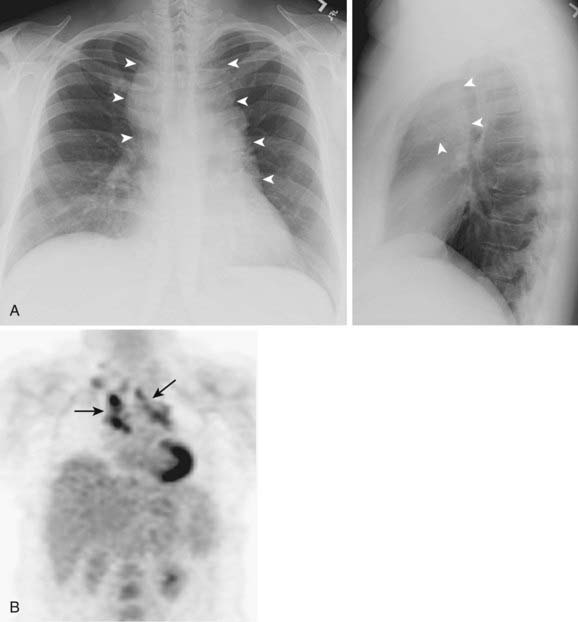
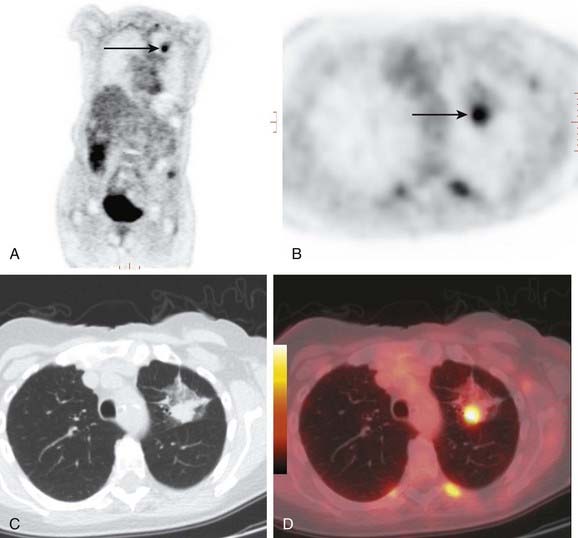
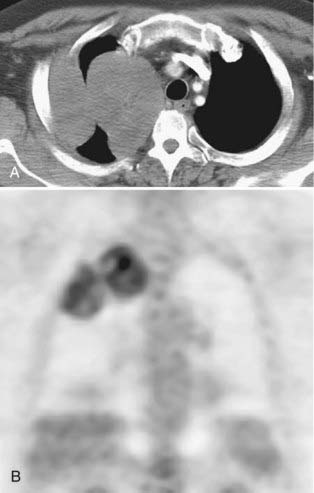
Adenocarcinoma is the most common cell type of bronchogenic carcinoma and accounts for approximately half of all cases. It typically presents as a small (often <3 cm), peripheral, round or oval solitary pulmonary nodule. It is commonly smoothly marginated, but spiculated margins can also occur (Figs. 18-2 to 18-4).14 Calcifications are rare but are occasionally seen on CT scans. Central lesions have a higher frequency of hilar and mediastinal metastases at presentation.14 Distant metastases are frequently present at the time of diagnosis. Peripheral tumors may directly invade the pleura and grow circumferentially around the lung, mimicking diffuse malignant mesothelioma.16 Central tumors may directly invade mediastinal structures or extend via the pulmonary veins to invade the left atrium.
Bronchioloalveolar Carcinoma
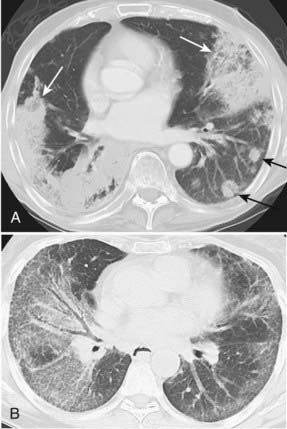
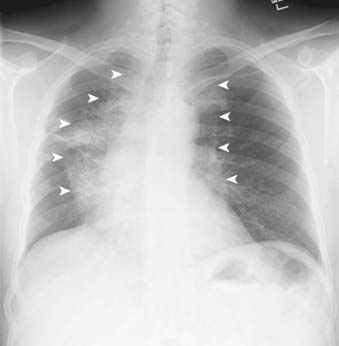
Bronchioloalveolar carcinoma, a subtype of adenocarcinoma, commonly presents as a well-circumscribed, peripherally located solitary nodule.14,17 CT scan may reveal surrounding ground glass opacity. Cavitation is an infrequent finding in adenocarcinoma.18 Bronchioloalveolar carcinoma can present as multifocal disease; several patterns have been described. It may present as multiple well-defined nodules of varying size involving one or both lungs. Another pattern of presentation can be focal, poorly defined or scattered lung opacities that may coalesce, causing opacification of a lobe or rarely the entire lung, and resemble pneumonia. Reticulonodular opacities similar in appearance to interstitial lung disease have been described (Fig. 18-5).14,18 High-resolution CT may demonstrate air attenuation and pseudocavitation within the nodules corresponding to small bronchi and cystic spaces.14,19 Unusual radiographic appearances include lobar atelectasis and expansile consolidation without air bronchograms.14,20,21 On FDG PET scans, bronchioalveolar cell carcinoma can result in a false-negative FDG scan.6,22
Squamous Cell Carcinoma
Squamous cell carcinoma is a slow-growing malignancy with late metastasis predominantly to the liver, adrenal glands, kidneys, and bones.14 Its typical presentation is described as a central endobronchial obstructing lesion with associated atelectasis or postobstructive pneumonia (Figs. 18-6 and 18-7). In addition, mucoid impaction or bronchiectasis may occur.14 With increasing frequency,15 these tumors present as a solitary peripheral nodule with or without cavitation.23 When the tumor cavitates, the inner wall is typically thick and irregular and, if secondarily infected, may develop an air-fluid level (Fig. 18-8).24 Extensive necrosis, however, may result in a thin-walled cavity. Squamous cell carcinoma is the most common histologic type found in Pancoast or superior sulcus tumors.14
Large Cell Carcinoma
The majority of large cell carcinomas present as a large (average size >4 cm) peripheral mass with poorly defined margins. Cavitation and calcification of the tumor may occur. These tumors grow rapidly and metastasize early via lymphatic and hematogenous routes, often presenting with hilar or mediastinal adenopathy.14 Giant cell carcinoma is a subtype with multiple giant cells and a more aggressive behavior and poorer prognosis.
A variant of large cell carcinoma is large cell neuroendocrine carcinoma (LCNEC), or intermediate cell neuroendocrine carcinoma. It has a poorer prognosis than classic large cell carcinoma.25 The ability to express neuroendocrine markers also places LCNEC into a broad category of neuroendocrine tumors of the lung, including atypical carcinoid, typical carcinoid, and small cell lung carcinoma.25,26
Multiple Lung Primary Tumors
Multiple lung carcinomas are present in less than 1% of all lung cancers. Synchronous lesions are defined as the simultaneous presence of tumors that are physically distinct and separate, and can have different histology. If the histology is the same, however, the tumors should be present in different segments or lobes of the lung and originate from carcinoma in situ. No carcinoma in lymphatics or extrapulmonary metastases should be present at the time of diagnosis. Simultaneous lesions not meeting these criteria most likely represent metastases.27 Metachronous lesions are defined as a second primary cancer appearing after a time interval in a patient considered cured of the initial cancer. Metachronous lesions comprise at least two thirds of multiple pulmonary neoplasms, and on average are recognized 4 to 5 years after the first primary. Up to one third of patients surviving resection for lung cancer may develop a second primary tumor.28 These lesions are regarded as metachronous primary tumors rather than metastases if they show unique histologic features. If histology is the same, then in order to meet the criteria for a metachronous lesion, the cancer-free interval should be at least 2 years and the tumor must originate from carcinoma in situ or in a different lobe or lung. There should be no carcinoma in lymphatics common to both the old and the new lesions, and no extrapulmonary metastasis should exist at the time of diagnosis.27 Squamous cell cancer is the most common histologic type of multiple carcinomas; adenocarcinoma, however, is currently the most common cell type. The reason for this trend is unknown, but the change in contents of cigarettes and filters that now contain more nitrates and less nicotine is suspected.29,30
Staging of Non–Small Cell Lung Cancer
Accurate preoperative staging in patients with non–small cell lung cancer is important for selecting those patients with localized disease who are likely to benefit from surgical resection.31,32 Cure rates and survival for non–small cell lung cancer are predicted by staging.33 The TNM staging system of the American Joint Committee on Cancer is the most widely accepted and used classification system for preoperative and postoperative staging. Mediastinal lymph nodes are involved with disease in approximately one quarter of newly diagnosed lung cancer patients.31,34 The evaluation of these lymph nodes is the primary goal of intrathoracic staging of non–small cell lung cancer. The current noninvasive imaging techniques evaluate lymph nodes based on either size (CT) or metabolism (PET).35 Conventional cross-sectional imaging techniques provide excellent anatomic information used in staging, but they have limitations differentiating benign and malignant lymph nodes based on size criteria. CT of the chest remains the most widely accepted imaging modality for evaluation of the primary tumor (the T classification); nodal metastases (the N classification); and distant metastases, especially the adrenal glands (the M classification).13
Primary Tumor Staging—T Classification
Even though it is not absolutely essential, intravenous contrast is usually administered when a CT scan of the chest is performed for staging. Intravenous contrast helps distinguish between normal vascular structures and lymph nodes and aids in evaluation of the degree of mediastinal invasion.33 PET/CT can be used in assessing the primary tumor however false-positive results can occur and may be caused by metabolically active infectious or inflammatory lesions. Granulomatous disease like sarcoidosis, tuberculosis or fungal granulomas can commonly produce significant FDG accumulation.36 Also FDG may not be useful if lesions are too small and below the threshold of PET resolution. FDG PET showed no demonstrable benefit in diagnosis and staging of non–small cell lung cancer 2 cm in size or less.37
Nodal Staging—N Classification
CT alone, however, often cannot accurately differentiate between benign lymph node enlargement and metastasis, and its accuracy for mediastinal staging has not improved over the past decade despite improvement in resolution.31 For example, normal-sized lymph nodes may contain metastases, whereas enlarged lymph nodes may have benign underlying etiology. The most commonly used criterion is a short axis diameter equal to or greater than 1 cm, even though up to 40% of lymph nodes identified as abnormal using this method can be benign. CT scanning, however, provides an extra benefit of guidance when selective biopsy is required.33 PET can be useful in suggesting metastatic involvement of lymph nodes, but it can be falsely positive in a number of inflammatory and infectious conditions. In addition, very small lesions, typically less than 1 to 1.2 cm, can be beyond the detection ability of PET. Current evidence indicates that PET is more sensitive and specific for evaluation of mediastinal lymph nodes.31,38,39 In addition, PET may be superior to CT in more accurately predicting long-term survival in non–small cell lung cancer patients.40 PET/CT is more accurate than PET alone for nodal staging of non–small cell lung cancer.41 Staging of non–small cell lung cancer is very important in guiding treatment modalities and predicting survival.42 FDG PET can identify involved nodal disease even when the nodes are normal sized on CT.43 In comparison to CT, FDG PET has been found to be more sensitive (57% versus 84%) and more specific (82% versus 89%) for detection of nodal disease in mediastinum in lung cancer.44 In nodal staging, FDG PET is cost-effective and can decrease the likelihood that a patient with mediastinal nodal metastases will undergo attempted surgery.45 Also, M and N staging with PET is superior compared to CT.46
The role of MRI currently remains limited to evaluation of superior sulcus tumors, brachial plexus, and vertebral invasion. Intravenous contrast may improve the accuracy of MRI in detection of abnormal mediastinal lymph nodes.33 MRI’s accuracy for anatomic imaging of mediastinal lymph nodes is comparable with that of CT and exceeds the accuracy of CT in evaluating hilar lymph nodes.47 PET/CT currently remains the study of choice for mediastinal staging of non–small cell lung cancer.33,47
The SUV provides quantitative measurement of positron activity in the region of interest. It has been shown that prognosis of non–small cell lung cancer depends on the SUVmax of the primary lesion.48 A SUVmax value greater than 2.5 in a pulmonary lesions in general is highly suggestive of malignancy.49 However, even active infection/inflammation can have a high SUV; therefore, interpretation of FDG PET scans in the proper clinical scenario is very important. As the mean SUV increases, there is a decrease in the median survival in a patients with non–small cell lung cancer.50
Distant Metastases—M Staging
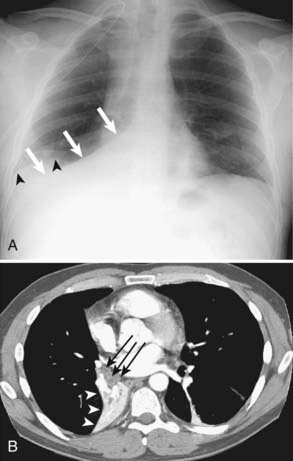
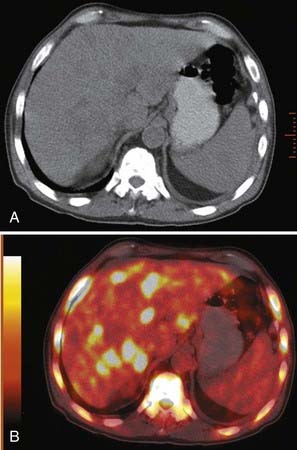
Extrathoracic metastases are found in almost half of the newly diagnosed lung cancer patients.31,34 The adrenal glands are one of the most common sites of metastases from lung cancer. At the time of presentation, 5% to 10% of patients with lung cancer have adrenal metastases (Fig. 18-9). Metastases to the liver, brain, and skeletal system are also common.31,33 (Figs. 18-10, 18-11) The more unusual sites of metastases include the kidneys, pancreas, and small bowel. Serosal and mesenteric implants may become quite large and invade and perforate the adjacent bowel.
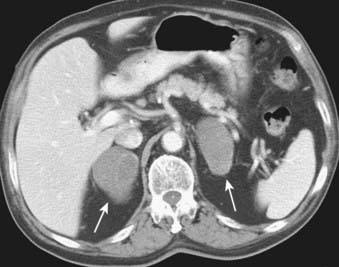
FIGURE 18-9 • Bilateral adrenal masses consistent with metastases from large cell carcinoma of the lung.
When a clinician suspects distant metastases (e.g., neurologic symptoms, abdominal or flank pain, pathologic fractures, or abnormal serum liver enzyme levels),31 the preferred workup includes CT or MRI scan with contrast of the brain and PET/CT. Inclusions of the adrenals and partial imaging of the liver on the chest CT scan are currently routine. When an adrenal lesion is greater than 3 cm in size, it likely represents a metastasis and warrants further workup with CT, MRI, or sometimes percutaneous biopsy.31 A liver lesion in an asymptomatic patient is relatively unlikely to represent a metastasis, with most incidentally discovered lesions being cysts or hemangiomas. When clinically indicated, evaluation with contrast-enhanced CT or MRI scan or occasionally percutaneous biopsy is performed.33 Distant metastases can still be missed because of the limited area of coverage by each technique. Whole-body PET scan can detect an additional 10% to 20% of distant metastases.31,33,51 MRI of the brain is more sensitive than CT and detects smaller lesions in greater numbers, but does not identify more patients with metastatic cancer or affect survival. There is normally intense physiologic brain activity on FDG PET which can be problematic in detecting focal uptake in brain metastases.52 Dedicated PET brain imaging is required to evaluate brain metastases, which require dedicated brain PET imaging protocols, equipment modifications and prolonged image-acquisition.53,54 In detection of metastatic disease to brain, a drawback can be the low sensitivity (60%). Even in sizable brain lesions, false-negative findings can be seen.52,55
FDG PET is useful in detection of abdominal metastatic disease specifically to the adrenal glands and liver. FDG PET has a high accuracy rate in detecting adrenal metastases from lung cancer.52,56 Although the data are limited at the present time, it has been shown that FDG PET can detect hepatic metastatic disease from lung cancer with an accuracy of 92% to 100%.53,52
FDG PET has specificity, sensitivity and accuracy exceeding 90% in the assessment of bone metastases from lung cancer.52,57 PET is useful in the detection of distant metastatic disease from lung cancer. Biopsy though is usually required and warranted to confirm a suspected metastasis.58
Invasive Staging
Noninvasive staging methods cannot provide tissue diagnosis that is often required for the evaluation of tumor resectability.35 Histologic evaluation of mediastinal lymph nodes is currently considered essential for accurate staging, but techniques such as bronchoscopy and mediastinoscopy also have limitations and, like any invasive procedures, are associated with morbidity.
Invasive techniques include needle biopsy such as transbronchial needle aspiration, transthoracic needle aspiration, or endoscopic ultrasound-guided needle aspiration, as well as open surgical biopsy performed by mediastinoscopy.35 Mediastinoscopy should be performed when the PET and CT findings indicate presence of nodal disease or when the results of the PET and CT scans are incongruent.59,60 To achieve consistent and reproducible classification of the mediastinal lymph nodes, the following schema describing 14 nodal stations has been adopted by the American Joint Committee on Cancer and other organizations.13
Small Cell Lung Cancer
Small cell lung cancer (SCLC) is a rapidly proliferating, biologically aggressive form of lung cancer that has a short survival without treatment.61 These tumors probably arise from neuroendocrine cells, which contain neurosecretory granules, and may secrete peptide hormones.14 SCLC is associated with tobacco use.62
The typical presentation of this disease is a centrally located tumor, frequently associated with mediastinal extension and often extensive encasement of mediastinal structures, as well as compression of the tracheobronchial tree. Rarely, SCLC may present as a peripheral tumor that is often associated with hilar adenopathy and atelectasis. The peripheral tumor itself may not be visible on radiographic studies, and extensive extrathoracic metastases to the liver, bone marrow, adrenal glands, and the brain may be the initial presentation.14 Rarer presentations of the disease also include a solitary lesion within the lung parenchyma that may mimic the appearance of non–small cell cancer. The appearance of SCLC may overlap with a more unusual presentation of non–small cell lung cancer that can manifest as a central mass with a similar metastatic pattern. A pleural effusion may be present, and when malignant, it affects the staging and patient’s outcome.63
Staging of Small Cell Lung Cancer
A two-stage classification developed by the Veterans Administration Lung Cancer Study Group is widely accepted for staging of SCLC. When the entire tumor is confined to the thorax and can be included in a single radiation therapy port, the disease is defined as limited stage. Most patients, however, present with extensive disease involving distant metastases and noncontiguous metastases to the contralateral lung, which requires systemic treatment.14,61,62
Imaging modalities currently used in initial staging of SCLC are CT of the chest and upper abdomen including the entire liver and adrenal glands, a bone scan, and CT or MRI examination of the brain. FDG PET can influence both the stage and the management of patients with SCLC and lead to a reduction in the number of tests and invasive procedure,64,65 although the data on the role of FDG PET in SCLC is limited.66
Metastatic Lung Lesions
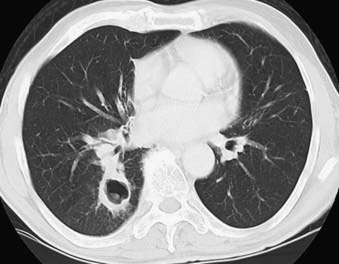
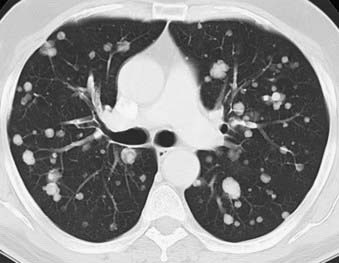
The lung is one of the most common sites for metastases that are found at autopsy.67 Breast, colon, kidneys, and uterus, as well as head and neck cancers, are the common malignancies with pulmonary metastases.68 Many other less common malignant neoplasms are known to frequently metastasize to the lung, including choriocarcinoma, osteosarcoma, testicular tumors, melanoma, Ewing’s sarcoma, and thyroid carcinoma. CT is the modality of choice for detection of pulmonary metastases. It typically demonstrates multiple peripherally located round nodules of various sizes indicative of the hematogenous route of spread (Fig. 18-12) or diffuse thickening of the interstitium, which represents lymphangitic carcinomatosis.69 Although the finding of multiple pulmonary nodules carries a significant chance of the diagnosis of metastases,70 not all pulmonary metastatic lesions have this typical appearance; several atypical findings have been described. These atypical appearances include cavitation, calcification, and hemorrhage around the metastatic nodule.67,71
Unusual Tumors
Although the great majority of primary lung malignancies fall into the category of adenocarcinoma, squamous cell carcinoma, large cell undifferentiated carcinomas and small cell carcinoma, a number of benign and malignant lesions of mesenchymal, epithelial, lymphoreticular, and vascular origin have been described. These lesions are rare and represent less than 1% of all lung neoplasms.72
Lung Cancer Screening
Screening of a high-risk population (primarily cigarette smokers) for lung cancer using a low-dose helical CT has gained popularity in recent years. CT is superior to plain film radiograph in the detection of small lung nodules, which may represent early-stage lung cancer. Its usefulness, however, remains controversial, since decrease in mortality associated with earlier detection has not been proven. In addition, it has not been determined whether an increase in the number of tumors detected at an early stage represents a true stage shift or just a statistic bias. A large number of false-positive results may also lead to unnecessary workup with associated patient morbidity. More clinical trials aimed at resolving the controversies that surround lung cancer screening programs are currently under way.73,74 It is may be that a large randomized trial of low dose CT versus chest x-ray will be able to answer the issues raised. A large randomized study sponsored by the National Cancer Institute and the American College of Radiology Imaging Network has enrolled, with initial examination, 53,000 individuals into a randomized study involving comparison of CT and chest x-ray screening. Initial accrual has been completed and results, including early mortality results should be available in the next several years.75
Mediastinal Masses
The organs and structures of the mediastinum are composed of a multitude of tissues that can give rise to both benign and malignant neoplasms. Significant differences exist between adults and children in incidence of mediastinal lesions. Primary thymic neoplasms, thyroid masses, and lymphomas are the most frequently encountered mediastinal neoplasms in adults, whereas in pediatric patients neurogenic tumors, germ cell neoplasms, and foregut cysts are most common, representing 80% of mediastinal tumors in that population.76,77 Overall, two thirds of mediastinal masses are benign. Most patients with mediastinal masses are asymptomatic, and 83% of asymptomatic mediastinal masses are benign. By contrast, in patients with symptoms caused by a mediastinal mass, the diagnosis of malignancy is made in 57% of cases.76 On cross-sectional imaging, invasion and obstruction of adjacent structures are suggestive of malignancy.76
Thymoma
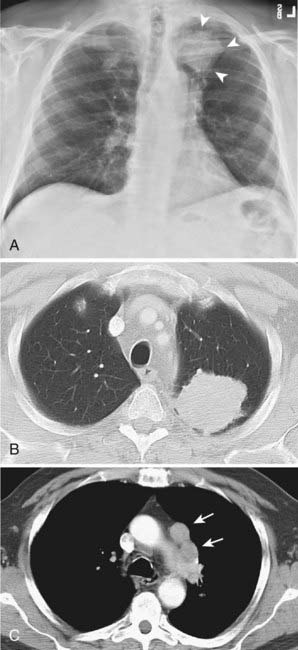
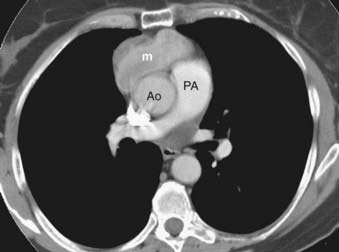
Thymoma is a rare tumor, but it is the most frequent lesion in the mediastinum and the most common primary tumor of the thymus. Most thymomas present in adults in the fifth and sixth decades of life. The majority of these tumors are found in the anterior mediastinum (Fig. 18-13), but occasionally they are encountered in the neck and posterior mediastinum. A number of classifications of thymomas exist based on histologic type and the ability to invade adjacent structures.78 Noninvasive thymomas are usually seen on CT as well-defined oval, rounded, or lobulated masses that can be partially or completely surrounded by fat. MRI demonstrates a homogenous mass with well-defined margins that is isointense with skeletal muscle on T1-weighted images and demonstrates high signal intensity on T2-weighted images. Homogeneous enhancement is typically seen upon administration of intravenous contrast, but cystic or hemorrhagic areas that do not enhance can be present occasionally. Invasive thymomas have ill-defined margins and a heterogeneous appearance with septations and intratumoral loculations seen especially well on T2-weighted images. Calcifications are occasionally seen in noninvasive thymomas; invasive thymomas rarely calcify.79,80 Thymomas are followed in frequency of incidence by neurogenic tumors and benign cysts, together representing 60% of mediastinal masses in all patients.
Germ Cell Tumor
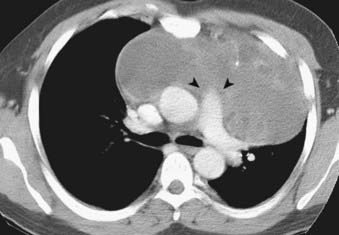
Malignant germ cell tumors grow rapidly and invade adjacent mediastinal structures. Unlike their benign counterparts, they usually have an imaging appearance of heterogeneous solid masses with irregular margins that only rarely show calcification (Fig. 18-14). CT and MRI play a complementary role in evaluating calcification and invasion of the chest wall and adjacent mediastinal structures.81
Lymphoma
Lymphoid neoplasms comprise a number of conditions that are classified based on morphology, immunophenotype, genetic, and clinical features.82 These neoplasms are commonly placed into two major categories: non-Hodgkin’s and Hodgkin’s disease. Hodgkin’s disease is a discrete type of lymphoma, whereas the non-Hodgkin’s lymphoma category includes a heterogeneous group of diseases that differ in histopathologic types as well as clinical manifestations.83 Non-Hodgkin’s lymphoma accounts for 4% of all cancer cases in adults in this country. The incidence of Hodgkin’s disease is almost eight times lower than the incidence of non-Hodgkin’s lymphoma. People infected with HIV and organ transplant recipients are at higher risk of developing the disease.12
Non-Hodgkin’s lymphoma and Hodgkin’s disease differ not only in their histologic characteristics, but also in demographics and clinical behavior. Thoracic involvement at presentation is more common in Hodgkin’s disease than in non-Hodgkin’s lymphoma.83
Hodgkin’s Disease
Hodgkin’s disease virtually always begins in lymph nodes and predictably spreads from one lymph node group to another, with radiographic evidence of intrathoracic disease seen in two thirds of patients at the time of presentation. The mediastinal lymphadenopathy most frequently involves anterior mediastinal and paratracheal lymph nodes followed by subcarinal, superior diaphragmatic, paraesophageal, and internal mammary groups. Extensive mediastinal lymphadenopathy can cause obstruction of the superior vena cava or compress the esophagus and major airways (Fig. 18-15).
When lung parenchymal involvement occurs, it is almost always associated with hilar or mediastinal lymphadenopathy. The most frequent pattern of lung parenchymal disease in Hodgkin’s lymphoma is multiple pulmonary nodules. The second pattern is reticular; it is thought to be caused by obstruction of lymphatic or venous drainage by enlarged hilar and mediastinal lymph nodes and presents with septal lines. Finally, Hodgkin’s lymphoma may present as lobar or segmental consolidation with air bronchograms indistinguishable from pneumonia. Pleural effusions may develop and are usually a consequence of lymphatic or venous obstruction.83
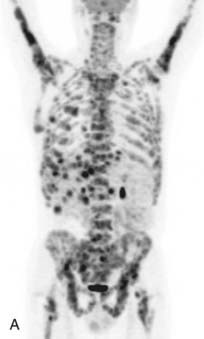
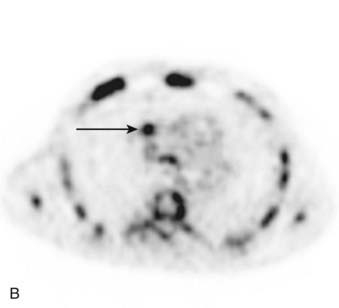
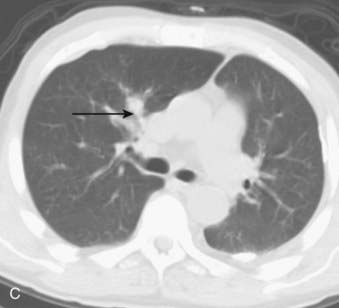
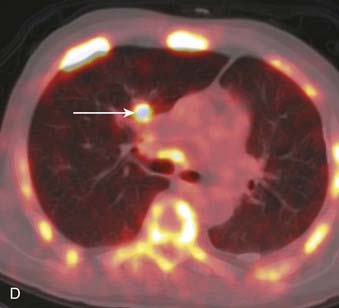
CT is the most common used modality in the initial staging of Hodgkin’s disease; however, FDG PET is also being used in the initial staging of Hodgkin’s disease. Upstaging of the disease has been reported in 29% of patients84 and alteration of the clinical stage based on PET has been shown in 20% of patients had.85 On the other hand, in cases of PET positive but CT negative lesions, downstaging should be done carefully and may involve biopsy confirmation to prove absence of disease.86 Note though that FDG PET, false-positive findings can be seen in cases of infection and/or inflammation. Also, evaluation of nodal disease can be limited in cases of intense physiologic uptake in brown fat and muscles especially in younger and leaner patients87,88 (Fig. 18-16).
Non-Hodgkin’s Lymphoma
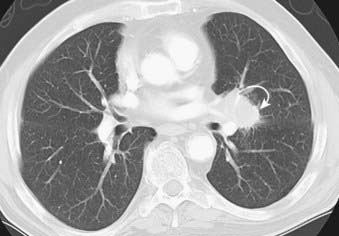
This condition usually presents at a more advanced stage than Hodgkin’s disease. Superior mediastinal lymphadenopathy is less frequently found. Lung parenchymal involvement of non-Hodgkin’s lymphoma is much less common at presentation and is found in less than 5% of patients, but, unlike Hodgkin’s disease, it may be isolated (Fig. 18-17). All three radiologic patterns of lung parenchymal involvement found in Hodgkin’s disease—pulmonary nodules, reticular pattern, and consolidation with air bronchograms—can be seen in non-Hodgkin’s lymphoma as well. Pleural effusions also occur and are usually associated with mediastinal lymphadenopathy.83
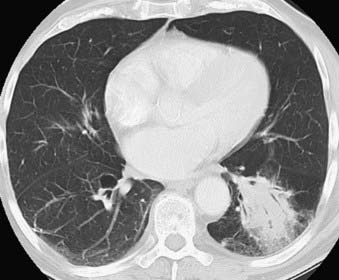
FIGURE 18-17 • Primary lymphoma of the lung presenting as a dense consolidation with air bronchograms.
Fusion PET/CT is useful in therapy monitoring and response to therapy as well radiation therapy planning or in planning of a surgical biopsy.86
Pleural Tumors
Among various pleural tumors, metastatic disease represents the most common neoplasm.89 Primary pleural neoplasms are less common than invasion of the pleura by a secondary malignancy such as bronchogenic carcinoma, breast cancer, lymphoma, and ovarian or gastric carcinoma.89,90 Primary pleural neoplasms are most commonly mesothelioma and less commonly localized fibrous tumor and pleural liposarcoma.90
Mesothelioma
Mesothelioma of the pleura is a rare, aggressive, and usually fatal malignant neoplasm that, in 80% of cases reported in the Western world, affects individuals occupationally exposed to asbestos through various industries.91–93 Mesothelioma appears to have a complex etiology in which environmental carcinogens (asbestos and erionite), ionizing radiation, viruses, and genetic factors act alone or in concert to cause malignancy.93,94 The tumor affects both visceral and parietal pleura. Its natural history involves aggressive local growth with encasement and invasion of the lung, vital mediastinal structures, and the chest wall.91,92
Difficulties in diagnosis, staging, and treatment set this disease apart from other malignancies.92 The pathologic diagnosis of mesothelioma is difficult because it may mimic metastatic adenocarcinoma91 and may have a variable radiologic presentation.91
Chest radiographs, CT, MRI, and FDG PET scanning are commonly used in the diagnosis and staging of malignant mesothelioma. The most common radiographic findings on CT include pleural thickening, including thickening of the pleural surfaces of the interlobar fissures, and pleural effusions (Fig. 18-18). Contraction of the involved hemithorax is frequently present.95 Mediastinal shift as well as disease beyond the parietal pleura, chest wall, mediastinum, lymph nodes, and diaphragm can also be seen.95
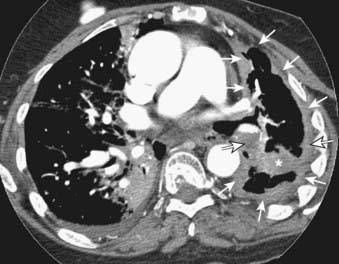
FIGURE 18-18 • Mesothelioma: circumferential left pleural mass extending into the major fissure (asterisk) and encasing the lung (arrows).
When pleural thickening is present, the main differential diagnosis in addition to mesothelioma includes pleural thickening due to benign causes (empyema, infections, fibrothorax, and idiopathic exudates) and adenocarcinoma involving the pleura.96 Mediastinal pleural involvement, circumferential pleural thickening, nodularity, irregularity of pleural contour, and infiltration of the chest wall or diaphragm, or both, are most suggestive of a malignant disease both on CT and MRI, whereas pleural calcification on CT is suggestive of a benign cause.96,97
Localized Tumor of the Pleura
Solitary fibrous tumor of the pleura is a nonepithelial neoplasm that is unrelated to asbestos exposure.98,99 These tumors arise from mesenchymal cells underlying the pleura100 and arise much more frequently from the visceral than the parietal or mediastinal pleura. The tumor may have benign or malignant histologic features, but these do not always predict the clinical behavior of the tumor. In most cases, the tumor appears pedunculated100 and has the appearance of a circumscribed, spheric, or ovoid noncalcified lesion arising along the pleural surface89 (Fig. 18-19). Fibrous tumors of the pleura are characterized by low signal intensity on all MRI sequences, which is explained by high collagen content within the tumors’ stroma and which might help include this in the preoperative differential diagnosis of a pleural-based mass.101
Chest Wall Masses
A multitude of disorders affect the chest wall, including congenital and infectious abnormalities, inflammatory and infectious conditions, and soft-tissue and bone tumors. Tumors may arise from any tissue found in the chest wall: muscles, bone, cartilage, fat, fibrous connective tissue, nerves, breast tissue, blood, and lymphatic vessels. As masses expand into the lung, they usually displace the pleura and produce an obtuse angle with the chest wall on radiographs, CT scans, and MR images. When rib changes are detected, a mass can be reliably diagnosed as extrapleural.102
Radiation Changes
Cancers in the chest treated with radiation are lung, breast, esophageal, mediastinal tumors (most commonly lymphoma), and head and neck tumors.103,104 Radiation therapy is used for cure, and palliation, either alone or in combination with surgery or chemotherapy.77
Radiation-induced thoracic injuries are classified as lung injuries, mediastinal injuries, cardiovascular injuries, and chest wall injuries, and their appearance varies with the angle of the beam and shape of the radiation portal. The severity or radiation damage to the lung is determined by technical factors (total dose, dose rate and fractionation, portals and beam arrangement) as well as patient factors such as preexisting lung condition and functional capacity of the irradiated portion of the lung. Chemotherapeutic agents potentate the effect of radiation injury, whereas steroids may alleviate the manifestations of radiation pneumonitis.104 Most manifestations of radiation-induced injury are subclinical, and the patients remain asymptomatic. The radiation-induced lung disease is usually, but not always, confined to the radiation volume.103,104
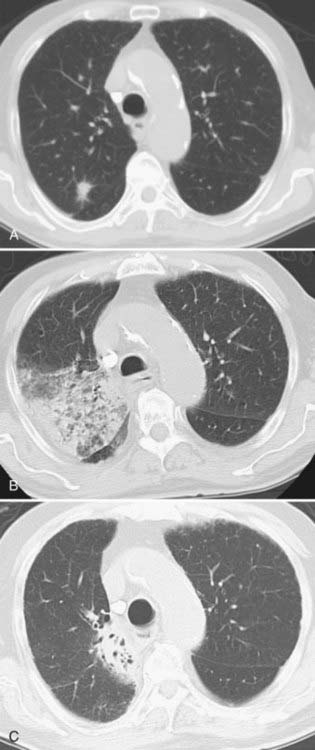
Radiation-induced lung disease occurs in two clinical stages: (1) the early stage, which usually occurs in the first 4 to 12 weeks, is characterized by radiation-induced pneumonitis and (2) the late stage, which represents radiation fibrosis. The evolution of the fibrosis extends over a period of 6 to 24 months and usually remains stable after 2 years (Fig. 18-20).104 CT is a sensitive and accurate method of evaluation of radiation-induced lung disease. Libshitz and Shuman described four patterns of findings in irradiated lung. The first two patterns are believed to reflect the acute exudative stage of radiation-induced injury. The first pattern is represented by ground-glass opacity or homogeneous consolidation, and the second involves patchy consolidation in the lung that does not conform to the shape of the portal. The third pattern, presumed to correspond to an organizing or proliferative phase of radiation-induced pneumonitis, is described as discrete consolidation that conforms to the shape of the portal but does not outline it uniformly. Finally, the fourth pattern that corresponds to the chronic fibrotic phase has the appearance of solid consolidation that conforms to the irradiated areas of the lung (Fig. 18-21).10,104,105 The usual findings of fibrosis are volume loss, architectural distortion, bronchiectasis, and pleural thickening. These bronchiectatic changes become stable 9 to 12 months after the completion of radiation therapy. Filling in of these bronchiectatic bronchi at a later stage may serve as an indicator of locally recurrent bronchogenic carcinoma.106 Stereotactic body radiotherapy (SBRT) using image guided radiation therapy techniques is a relatively new way of delivering high doses of radiation therapy in a few treatments to a small lung tumor.107 Fig. 18-22 shows an example of radiation changes after SBRT.
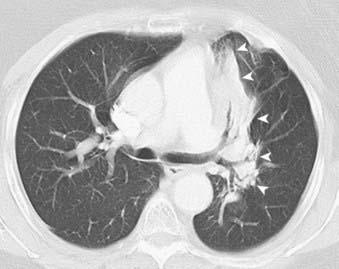
FIGURE 18-21 • Postradiation changes: left paramediastinal lung consolidation with sharp borders that conforms to the radiation port.
More uncommon complications of radiation treatment to the lung include pulmonary necrosis, and bronchiolitis obliterans with organizing pneumonia. In addition, spontaneous pneumothorax, mesothelioma, and lung cancer have been described as complications of radiation therapy.103
Radiation effects are not limited to the lungs. In the mediastinum, radiation effects include thymic cysts, calcified lymph nodes, and injuries to the esophagus. Cardiovascular complications have also been described; their onset is usually late and gradual. These injuries include premature coronary artery stenosis, calcifications of the ascending aorta, pericardial disease, valvular injuries, and conduction abnormalities. Breast cancer risk is increased in women who receive thoracic irradiation before the age of 30. Chest wall complications are also well-recognized albeit infrequent. They include radiation-induced sarcomas, osteochondromas, and rib or clavicle fractures.103
1 Frush DP, Donnelly LF, Chotas HG. Contemporary pediatric thoracic imaging. Am J Roentgenol. 2000;175:841.
2 Lufkin RB. The MRI manual, ed 2. St. Louis: Mosby; 1998. xiii, 466
3 Knisely BL, Broderick LS, Kuhlman JE. MR imaging of the pleura and chest wall. Magn Reson Imaging Clin N Am. 2000;8:125.
4 Pandit N, Gonen M, Krug L, Larson SM. Prognostic value of [(18)F]FDG-PET imaging in small cell lung cancer. Eur J Nucl Med Mol Imaging. 2003;30:78.
5 Jeong HJ, Min JJ, Park JM, et al. Determination of the prognostic value of [(18)F]fluorodeoxyglucose uptake by using positron emission tomography in patients with non–small cell lung cancer. Nucl Med Commun. 2002;23:865.
6 Erasmus JJ, McAdams HP, Patz EFJr, et al. Thoracic FDG PET: state of the art. Radiographics. 1998;18:5.
7 Townsend DW, Beyer T. A combined PET/CT scanner: the path to true image fusion. Br J Radiol. 2002;75(Spec No):S24.
8 Steinert HC, von Schulthess GK. Initial clinical experience using a new integrated in-line PET/CT system. Br J Radiol. 2002;75(Spec No):S36.
9 Felson B. Chest roentgenology. Philadelphia: WB Saunders; 1973. viii, 574
10 McCloud TC. Thoracic Radiology. St. Louis: Mosby; 1998. xvi, 541
11 Kawashima A, Fishman EK, Kuhlman JE, Nixon MS. CT of posterior mediastinal masses. Radiographics. 1991;11:1045.
12 American Cancer Society. Cancer facts & figures 2008. Atlanta: American Cancer Society; 2008.
13 The American Thoracic Society and The European Respiratory Society. Pretreatment evaluation of non–small-cell lung cancer. Am J Respir Crit Care Med. 1997;156:320.
14 Patz EFJr. Imaging bronchogenic carcinoma. Chest. 2000;117:90S.
15 Quinn D, Gianlupi A, Broste S. The changing radiographic presentation of bronchogenic carcinoma with reference to cell types. Chest. 1996;110:1474.
16 Rosado-de-Christenson ML, Templeton PA, Moran CA. Bronchogenic carcinoma: Radiologic-pathologic correlation. Radiographics. 1994;14:429.
17 Epstein DM. Bronchioloalveolar carcinoma. Semin Roentgenol. 1990;25:105.
18 Berkmen YM. The many faces of bronchiolo-alveolar carcinoma. Semin Roentgenol. 1977;12:207.
19 Adler B, Padley S, Miller RR, Muller NL. High-resolution CT of bronchioloalveolar carcinoma. AJR Am J Roentgenol. 1992;159:275.
20 Weisbrod GL, Towers MJ, Chamberlain DW, et al. Thin-walled cystic lesions in bronchioloalveolar carcinoma. Radiology. 1992;185:401.
21 Huang D, Weisbrod GL, Chamberlain DW. Unusual radiologic presentations of bronchioloalveolar carcinoma. Can Assoc Radiol J. 1986;37:94.
22 Lee KS, Kim Y, Han J, Ko EJ, Park CK, Primack SL. Bronchioloalveolar carcinoma: clinical, histopathologic, and radiographic findings. Radiographics. 1997;17:1345-1357.
23 Haque AK. Pathology of carcinoma of lung: An update on current concepts. J Thorac Imaging. 1991;7:9.
24 Hartman TE, Tazelaar HD, Swensen SJ, Muller NL. Cigarette smoking: CT and pathologic findings of associated pulmonary diseases. Radiographics. 1997;17:377.
25 Iyoda A, Hiroshima K, Toyozaki T, et al. Clinical characterization of pulmonary large cell neuroendocrine carcinoma and large cell carcinoma with neuroendocrine morphology. Cancer. 2001;91:1992.
26 Carretta A, Ceresoli GL, Arrigoni G, et al. Diagnostic and therapeutic management of neuroendocrine lung tumors: A clinical study of 44 cases. Lung Cancer. 2000;29:217.
27 Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70:606.
28 Bower SL, Choplin RH, Muss HB. Multiple primary bronchogenic carcinomas of the lung. AJR Am J Roentgenol. 1983;140:253.
29 Lee PN. Lung cancer and type of cigarette smoked. Inhal Toxicol. 2001;13:951.
30 Hoffmann D, Djordjevic MV, Hoffmann I. The changing cigarette. Prev Med. 1997;26:427.
31 Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non–small cell lung cancer: A review of the current evidence. Chest. 2003;123:137S.
32 Miller JD, Gorenstein LA, Patterson GA. Staging: The key to rational management of lung cancer. Ann Thorac Surg. 1992;53:170.
33 Silvestri GA, Tanoue LT, Margolis ML, et al. The noninvasive staging of non–small cell lung cancer: The guidelines. Chest. 2003;123:147S.
34 Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23.
35 Toloza EM, Harpole L, Detterbeck F, McCrory DC. Invasive staging of non–small cell lung cancer: A review of the current evidence. Chest. 2003;123:157S.
36 Roberts PF, Follette DM, von Haag D, et al. Factors associated with false-positive staging of lung cancer by positron emission tomography. Ann Thorac Surg. 2000;70:1154-1159.
37 Port JL, Andrade RS, Levin MA, et al. Positron emission tomographic scanning in the diagnosis and staging of non-small cell lung cancer 2 cm in size or less. J Thorac Cardiovasc Surg. 2005;130:1611-1615.
38 Dwamena BA, Sonnad SS, Angobaldo JO, Wahl RL. Metastases from non–small cell lung cancer: Mediastinal staging in the 1990s—Meta-analytic comparison of PET and CT. Radiology. 1999;213:530.
39 von Haag DW, Follette DM, Roberts PF, et al. Advantages of positron emission tomography over computed tomography in mediastinal staging of non–small cell lung cancer. J Surg Res. 2002;103:160.
40 Dunagan D, Chin RJr, McCain T, et al. Staging by positron emission tomography predicts survival in patients with non–small cell lung cancer. Chest. 2001;119:333.
41 Lardinois D, Weder W, Hany TF, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500-2507.
42 Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med. 2004;350:379-392.
43 Ginsberg MS, Grewal RK, Heelan RT. Lung cancer. Radiol Clin North Am. 2007;45:21-43.
44 Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest. 2003;123:137S-146S.
45 Erasmus JJ, Macapinlac HA, Swisher SG. Positron emission tomography imaging in non small-cell lung cancer. Cancer. 2007;110:2155-2168.
46 Patz EFJr, Lowe VJ, Goodman PC, Herndon J. Thoracic nodal staging with PET imaging with 18FDG in patients with bronchogenic carcinoma. Chest. 1995;108:1617-1621.
47 Boiselle PM. MR imaging of thoracic lymph nodes. A comparison of computed tomography and positron emission tomography. Magn Reson Imaging Clin N Am. 2000;8:33.
48 Vansteenkiste JF, Stroobants SG, Dupont PJ, et al. Prognostic importance of the standardized uptake value on (18)F-fluoro-2-deoxy-glucose-positron emission tomography scan in non-small-cell lung cancer: An analysis of 125 cases. Leuven Lung Cancer Group. J Clin Oncol. 1999;17:3201-3206.
49 Duhaylongsod FG, Lowe VJ, Patz EFJr, Vaughn AL, Coleman RE, Wolfe WG. Detection of primary and recurrent lung cancer by means of F-18 fluorodeoxyglucose positron emission tomography (FDG PET). J Thorac Cardiovasc Surg. 1995;110:130-139.
50 Ahuja V, Coleman RE, Herndon J, Patz EFJr. The prognostic significance of fluorodeoxyglucose positron emission tomography imaging for patients with non-small cell lung carcinoma. Cancer. 1998;83:918-924.
51 Saunders CA, Dussek JE, O’Doherty MJ, Maisey MN. Evaluation of fluorine-18-fluorodeoxyglucose whole body positron emission tomography imaging in the staging of lung cancer. Ann Thorac Surg. 1999;67:790.
52 Marom EM, McAdams HP, Erasmus JJ, et al. Staging non-small cell lung cancer with whole-body PET. Radiology. 1999;212:803-809.
53 Bury T, Dowlati A, Paulus P, et al. Whole body 18FDG positron emission tomography in the staging of non-small cell lung cancer. Eur Respir J. 1997;10:2529-2534.
54 Larcos G, Maisey MN. FDG-PET screening for cerebral metastases in patients with suspected malignancy. Nucl Med Commun. 1996;17:197-198.
55 Griffeth LK, Rich KM, Dehdashti F, et al. Brain metastases from non-central nervous system tumors: evaluation with PET. Radiology. 1993;186:37-44.
56 Boland GW, Goldberg MA, Lee MJ, et al. Indeterminate adrenal mass in patients with cancer: evaluation at PET with 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology. 1995;194:131-134.
57 Bury T, Barreto A, Daenen F, Barthelemy N, Ghaye B, Rigo P. Fluorine-18 deoxyglucose positron emission tomography for the detection of bone metastases in patients with non-small cell lung cancer. Eur J Nucl Med. 1998;25:1244-1247.
58 Silvestri GA, Gould MK, Margolis ML, et alAmerican College of Chest Physicians. Noninvasive staging of non-small cell lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132:178S-201S.
59 Macapinlac HA. Clinical applications of positron emission tomography/computed tomography treatment planning. Semin Nucl Med. 2008;38:137-140.
60 Pfister DG, Johnson DH, Azzoli CG, et al. American Society of Clinical Oncology treatment of unresectable non-small cell-lung cancer guideline: update 2003. J Clin Oncol. 2004;22:330-353.
61 Niell HB. Extensive stage small cell lung cancer. Curr Treat Options Oncol. 2001;2:71.
62 Simon GR, Wagner H. Small cell lung cancer. Chest. 2003;123:259S.
63 Komaki R, Chasen MH, Travis WD, et al. Oncodiagnosis panel: 1999. Cancer of the lung: oncodiagnosis. Radiographics. 2001;21:1573.
64 Kamel EM, Zwahlen D, Wyss MT, Stumpe KD, von Schulthess GK, Steinert HC. Whole-body (18)F-FDG PET improves the management of patients with small cell lung cancer. J Nucl Med. 2003;44:1911-1917.
65 Brink I, Schumacher T, Mix M, et al. Impact of [18F]FDG-PET on the primary staging of small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2004;31:1614-1620.
66 Schumacher T, Brink I, Mix M, et al. FDG-PET imaging for the staging and follow-up of small cell lung cancer. Eur J Nucl Med. 2001;28:483-488.
67 Quint LE, Park CH, Iannettoni MD. Solitary pulmonary nodules in patients with extrapulmonary neoplasms. Radiology. 2000;217:257.
68 Ginsberg MS, Griff SK, Go BD, et al. Pulmonary nodules resected at video-assisted thoracoscopic surgery: Etiology in 426 patients. Radiology. 1999;213:277.
69 Seo JB, Im JG, Goo JM, et al. Atypical pulmonary metastases: Spectrum of radiologic findings. Radiographics. 2001;21:403.
70 Gross BH, Glazer GM, Bookstein FL. Multiple pulmonary nodules detected by computed tomography: Diagnostic implications. J Comput Assist Tomogr. 1985;9:880.
71 Ginsberg MS, Panicek DM. Subcentimeter pulmonary nodules detected in patients with sarcoma. Sarcoma. 2000;4:63.
72 Gimenez A, Franquet T, Prats R, et al. Unusual primary lung tumors: A radiologic-pathologic overview. Radiographics. 2002;22:601.
73 Swensen SJ, Jett JR, Hartman TE, et al. Lung cancer screening with CT: Mayo Clinic experience. Radiology. 2003;226:756.
74 Henschke CI, McCauley DI, Yankelevitz DF, et al. Early lung cancer action project: Overall design and findings from baseline screening. Lancet. 1999;354:99.
75 Hillman BJ, Schnall MD. American College of Radiology Imaging Network: Future clinical trials. Radiology. 2003;227:631-632.
76 Laurent F, Latrabe V, Lecesne R, et al. Mediastinal masses: diagnostic approach. Eur Radiol. 1998;8:1148.
77 Azarow KS, Pearl RH, Zurcher R, et al. Primary mediastinal masses. A comparison of adult and pediatric populations. J Thorac Cardiovasc Surg. 1993;106:67.
78 Santana L, Givica A, Camacho C. Best cases from the AFIP: thymoma. Radiographics. 2002;22(Spec No):S95-S102.
79 Santana L, Givica A, Camacho C. Best cases from the AFIP: thymoma. Radiographics. 2002;22:S95.
80 Sakai F, Sone S, Kiyono K, et al. MR imaging of thymoma: radiologic-pathologic correlation. Am J Roentgenol. 1992;158:751.
81 Drevelegas A, Palladas P, Scordalaki A. Mediastinal germ cell tumors: a radiologic-pathologic review. Eur Radiol. 2001;11:1925.
82 Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: Report of the Clinical Advisory Committee meeting—Airlie House, Va, November 1997. J Clin Oncol. 1999;17:3835.
83 Au V, Leung AN. Radiologic manifestations of lymphoma in the thorax. Am J Roentgenol. 1997;168:93.
84 Munker R, Glass J, Griffeth LK, et al. Contribution of PET imaging to the initial staging and prognosis of patients with Hodgkin’s disease. Ann Oncol. 2004;15:1699-1704.
85 Naumann R, Beuthien-Baumann B, Reiss A, et al. Substantial impact of FDG PET imaging on the therapy decision on patients with early-stage Hodgkin’s lymphoma. Br J Cancer. 2004;90:620-625.
86 Jerusalem G, Hustinx R, Beguin Y, Fillet G. Positron emission tomography imaging for lymphoma. Curr Opin Oncol. 2005;17:441-445.
87 Dobert N, Menzel C, Hamscho N, Wordehoff W, Kranert WT, Grunwald F. Atypical thoracic and supraclavicular FDG-uptake in patients with Hodgkin’s and non-Hodgkin’s lymphoma. Q J Nucl Med Mol Imaging. 2004;48:33-38.
88 Yeung HW, Grewal RK, Gonen M, Schoder H, Larson SM. Patterns of (18)F-FDG uptake in adipose tissue and muscle: a potential source of false-positives for PET. J Nucl Med. 2003;44:1789-1796.
89 Dynes MC, White EM, Fry WA, Ghahremani GG. Imaging manifestations of pleural tumors. Radiographics. 1992;12:1191.
90 Bonomo L, Feragalli B, Sacco R, et al. Malignant pleural disease. Eur J Radiol. 2000;34:98.
91 Miller BH, Rosado-de-Christenson ML, Mason AC, et al. From the archives of the AFIP, Malignant pleural mesothelioma: radiologic-pathologic correlation. Radiographics. 1996;16:613.
92 Zellos LS, Sugarbaker DJ. Diffuse malignant mesothelioma of the pleural space and its management. Oncology (Huntingt). 2002;16:907.
93 Carbone M, Kratzke RA, Testa JR. The pathogenesis of mesothelioma. Semin Oncol. 2002;29:2.
94 Heineman EF, Bernstein L, Stark AD, Spirtas R. Mesothelioma, asbestos, and reported history of cancer in first-degree relatives. Cancer. 1996;77:549.
95 Kawashima A, Libshitz HI. Malignant pleural mesothelioma: CT manifestations in 50 cases. AJR Am J Roentgenol. 1990;155:965.
96 Statement on malignant mesothelioma in the United Kingdom. Thorax. 2001;56:250.
97 Hierholzer J, Luo L, Bittner RC, et al. MRI and CT in the differential diagnosis of pleural disease. Chest. 2000;118:604.
98 Rusch VW. Diagnosis and treatment of pleural mesothelioma. Semin Surg Oncol. 1990;6:279.
99 Briselli M, Mark EJ, Dickersin GR. Solitary fibrous tumors of the pleura: Eight new cases and review of 360 cases in the literature. Cancer. 1981;47:2678.
100 de Perrot M. Fibrous tumors of the pleura. Curr Treat Options Oncol. 2000;1:293.
101 Ferretti GR, Chiles C, Cox JE, et al. Localized benign fibrous tumors of the pleura: MR appearance. J Comput Assist Tomogr. 1997;21:115.
102 Jeung MY, Gangi A, Gasser B, et al. Imaging of chest wall disorders. Radiographics. 1999;19:617.
103 Mesurolle B, Qanadli SD, Merad M, et al. Unusual radiologic findings in the thorax after radiation therapy. Radiographics. 2000;20:67.
104 Park KJ, Chung JY, Chun MS, Suh JH. Radiation-induced lung disease and the impact of radiation methods on imaging features. Radiographics. 2000;20:83.
105 Libshitz HI, Shuman LS. Radiation-induced pulmonary change: CT findings. J Comput Assist Tomogr. 1984;8:15.
106 Libshitz HI, Sheppard DG. Filling in of radiation therapy-induced bronchiectatic change: a reliable sign of locally recurrent lung cancer. Radiology. 1999;210:2.
107 Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2:S94-S100.